Abstract
Photothrombotic infarcts of the neocortex result in structural and functional alterations of cortical networks, including decreased GABAergic inhibition, and can generate epileptic seizures within one month of lesioning. In our study, we assessed the involvement and potential changes of cortical GABAA receptor (GABAAR) α1 subunits at 1, 3, 7, and 30 days after photothrombosis. Quantitative competitive reverse transcription polymerase chain reaction (cRT-PCR) and semi-quantitative Western blot analysis were used to investigate GABAAR α1 subunit mRNA and protein levels in proximal and distal regions of perilesional cortex and in homotopic areas of young adult Sprague-Dawley rats. GABAAR α1 subunit mRNA levels were decreased ipsilateral and contralateral to the infarct at 7 days, but were increased bilaterally at 30 days. GABAAR α1 subunit protein levels revealed no significant change in neocortical areas of both hemispheres of lesioned animals compared with protein levels of sham-operated controls at 1, 3, 7, and 30 days. At 30 days, GABAAR α1 subunit protein expression was significantly increased in lesioned animals within proximal and distal regions of perilesional cortex compared with distal neocortical areas contralaterally (Student’s t-test, p<0.05). Short- and long-term alterations of mRNA and protein levels of the GABAAR α1 subunit ipsilateral and contralateral to the lesion may influence alterations in cell surface receptor subtype expression and GABAAR function following ischemic infarction and may be associated with formative mechanisms of poststroke epileptogenesis.
Keywords: competitive RT-PCR, Western blot, cerebral ischemia, brain injury, animal model
1. Introduction
A balance of excitatory and inhibitory neurotransmission is required for normal functioning of the central nervous system. Inhibition is mediated by the principal neurotransmitter gamma-aminobutyric acid (GABA), which binds to ionotropic GABAA receptors (GABAARs) and metabotropic GABAB receptors. GABAergic neurons provide inhibitory control in the brain and have an important role in selective neuronal degeneration following ischemia and epilepsy (Mileson et al., 1992; Sperk et al., 2004). Photothrombotic brain infarction results in morphological and physiological changes in multiple perilesional and remote areas of the brain (Neumann-Haefelin et al., 1998, 1999; Liu et al., 2002; Redecker et al., 2002; Frahm et al., 2004a,b, 2006) and an imbalance of excitatory and inhibitory neurotransmission (Schiene et al., 1996; Qu et al., 1998) that reflect various degrees of acute injury of cortex and its subsequent repair, recovery, and reorganization. In addition to augmentation of endogenous protective mechanisms following different pathophysiological conditions, alterations in the kinetics and pharmacology of GABAARs may be associated with the development of spontaneous seizure activity (Coulter, 2001; Treiman, 2001; Nishimura et al., 2005) and may contribute to the process of poststroke epileptogenesis (Kelly et al., 2001; Liu et al., 2002; Kharlamov et al., 2003, 2007; Karhunen et al., 2007); however, studies of the basic mechanisms of ischemia-induced epileptogenesis have had limited development (Kelly, 2002, 2007).
Because large photothrombotic infarcts of the neocortex, variably associated with the epileptic state, resulted in a significant increase of α1 subunit mRNA in the ipsilateral cortex 4 months after lesioning (Liu et al., 2002), we sought to explore these findings further by determining whether photothrombotic infarction triggered alterations of GABAAR α1 subunit mRNA and the corresponding polypeptide expression in different areas of neocortex at earlier time points after lesioning. The α1 subunit of GABAARs is the most common α isoform (McKernan and Whiting, 1996) and is the dominant subunit in importance for the assembly and functioning of GABAARs (Sieghart and Sperk, 2002; Mohler et al., 2002; Sieghart, 2006). The α1 subunit is abundant in the rat cerebral cortex (Wisden et al., 1992; Paysan et al., 1994), shows a distinct pattern of distribution throughout the adult brain (Pirker et al., 2000), is sensitive to benzodiazepine modulation, and mediates sedative and anticonvulsant activity (Fisher et al., 1997; Hevers and Luddens, 1998; Crestani et al., 2002; Kralic et al., 2002; Mohler, 2007). Alterations in or abnormalities of α1 subunit expression may lead to the development of neurological and behavioral disorders (Fisher, 2004; Mohler, 2006); however, the overall importance of α1 subunit changes for the development of epilepsy is not fully determined (Fritschy et al., 1999; Kumar et al., 2006; Loup et al., 2006; Raol et al., 2006a).
In the present study, we investigated GABAAR α1 subunit mRNA expression by a quantitative competitive reverse transcription polymerase chain reaction (cRT-PCR) and protein expression by Western blot analysis with affinity-purified α1 subunit-specific antibodies in young adult Sprague-Dawley rats at 1, 3, 7, and 30 days following photothrombosis. In order to demonstrate the distribution and time course of potential molecular alterations in GABAARs following lesioning, experiments were designed to determine whether α1 subunit mRNA and protein expression was altered: 1) in relation to the time elapsed after lesioning; 2) in proximal and distal areas surrounding the infarct core ipsilaterally; and 3) in homotopic areas of the contralateral cortex.
2. Results
2.1. Brain inspection
Gross inspection of the brains of sham-operated (sham) and naive animals revealed no apparent structural abnormalities, e.g., tumor, hematoma. Brains of lesioned animals had a distinct circular lesion, ~3 mm in diameter, consistently localized to the area of the left sensorimotor cortex. A rim of swollen tissue around the lesioned area was observed up to 7 days post-lesioning. By 30 days, the lesion appeared as a cystic, scarred area with a slightly reduced diameter (Fig.1).
Fig. 1.
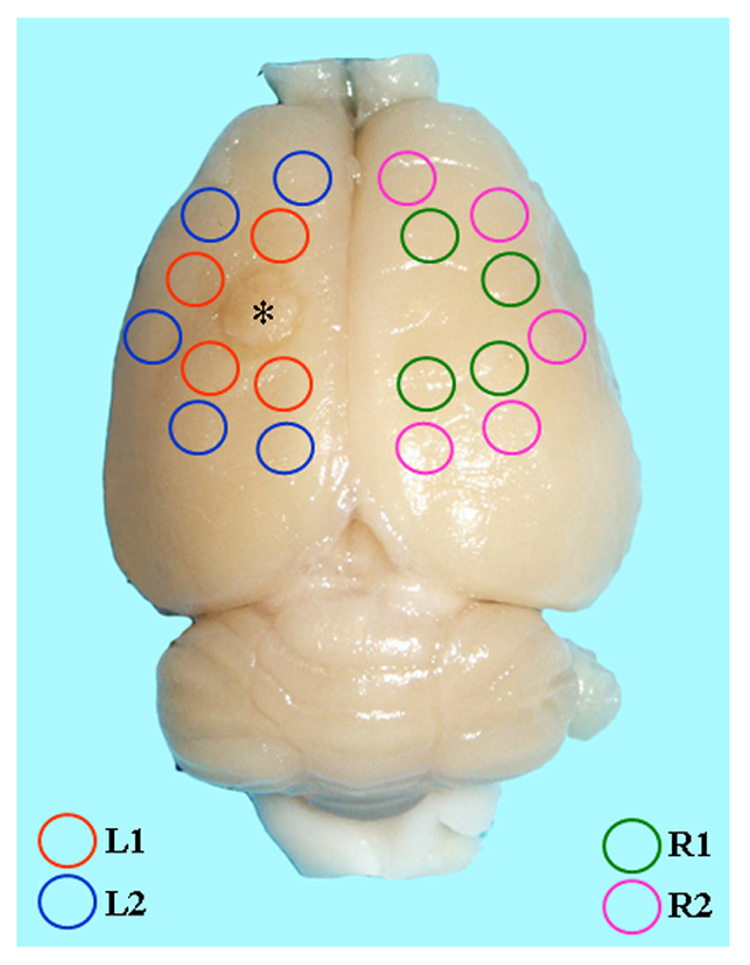
Position of tissue punches in the rat brain at 30 days following photothrombotic infarction. Tissue punches, 3 mm diameter, were made around the lesion in the left (L) ipsilateral hemisphere and homotopic areas of the right (R) contralateral hemisphere. L1, L2, R1, and R2 were designated to reflect the proximal and distal areas of the brain that were analyzed relative to the cortical lesion.
2.2. cRT-PCR of GABAAR α1 subunit mRNA
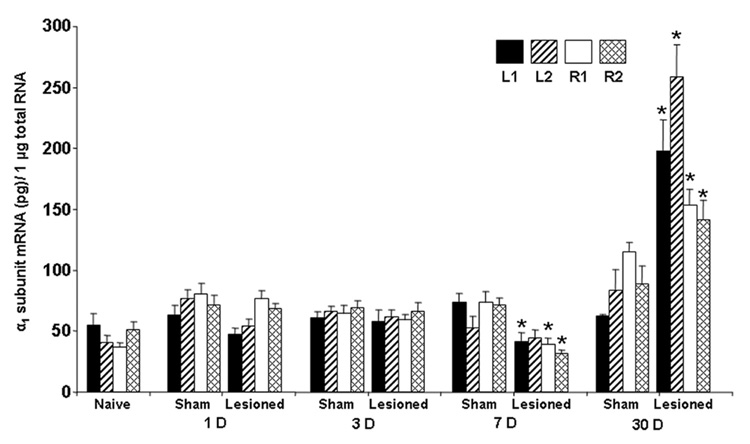
Representative electrophoretic gels and linear regression plots of cRT-PCR for target GABAAR α1 subunit mRNA and the associated internal standard for sham and lesioned animals are presented in Fig. 2. α1 subunit mRNA expression in 3-month old naïve control animals was determined as a reference mRNA value (Table 1, Fig. 3) but was not included in statistical comparisons. Quantitative analysis of α1 subunit mRNA expression following photothrombosis is shown in Table 1. No significant difference in α1 subunit mRNA expression was determined for lesioned animals vs. sham at 1 and 3 days. Significant decreases in α1 mRNA expression were determined in lesioned animals at 7 days for L1 (41.2 ± 7.4 pg; p = 0.0093), R1 (39.0 ± 4.9 pg; p = 0.0062), and R2 (31.2 ± 3.4 pg; p < 0.0001) cortical areas compared with the corresponding areas of shams: L1 (73.4 ± 7.6 pg), R1 (73.7 ± 9.0 pg), and R2 (71.5 ± 5.5 pg), respectively (unpaired Student’s t-test, Table 1, Fig. 3). In contrast, α1 mRNA expression at 30 days was increased in lesioned animals for L1 (197.8 ± 25.6 pg), L2 (258.4 ± 26.5 pg), R1 (153.6 ± 13.2 pg), and R2 (141.4 ± 16.0 pg) compared with shams at the same time point: L1 (62.4 ± 1.6 pg), L2 (83.6 ± 16.9 pg), R1 (115.7 ± 7.5 pg), and R2 (88.4 ± 15.4 pg) (Student’s t-test, p<0.05, where p =0.0019, 0.0001, 0.0314, 0.0381, respectively, and one-way ANOVA with post hoc Tukey-Kramer test, p<0.001, where p=0.0004 for L1, and p<0.0001 for L2, R1, and R2; Table 1, Fig.3). At 7 days following lesioning, α1 mRNA expression for R1 (39.0 ± 4.9 pg) and R2 (31.2± 3.4 pg) were decreased compared with R1 (76.8 ± 6.8 pg) and R2 (68.0 ± 5.1pg) at 1 day, and R1 (58.9 ± 4.7 pg) and R2 (66.3 ± 7.1 pg; ANOVA, p< 0.01; Table 1) at 3 days. Within the sham groups, we found unexpected variability of α1 mRNA expression at 30 days for R1 (115.7 ± 7.5 pg), which was increased compared with R1 at 3 days (64.7 ± 6.6 pg; ANOVA, p< 0.01; Table 1).
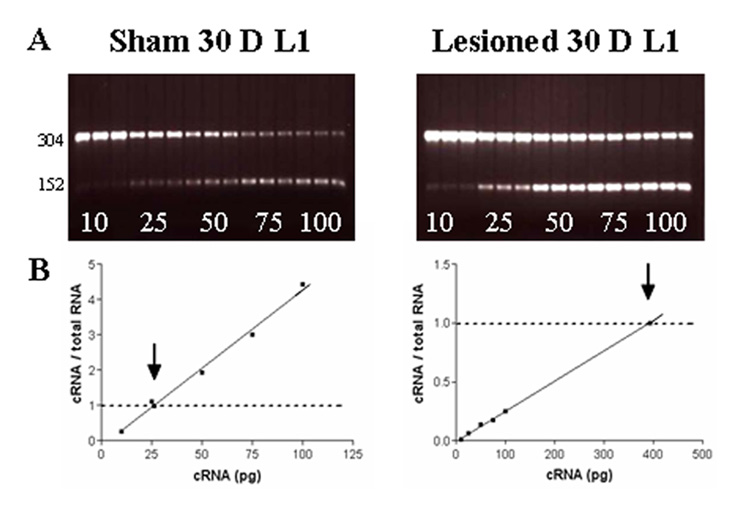
Fig. 2.
Representative electrophoretic gels (A) and linear regression plots (B) of α1 subunit mRNAs for GABAARs from L1-pooled samples of shams and lesioned animals generated by cRT-PCR at 30 days. (A) A series of concentrations of internal standard cRNAs (10, 25, 50, 75, and 100 pg) was added to each sample aliquot of total RNA (1.0 µg). On each gel, the cRT-PCR products are shown in triplicate; upper bands are products of target α1 subunit mRNA, whereas lower bands are Bgl II-digested internal standard PCR products. The increasing concentration of internal standards compete with α1 subunit mRNA for amplification. (B) Linear regression analysis of the ratios of cRNA/total RNA versus the amount of internal standard cRNA added to the reaction to generate the point of equivalency where the ratio is 1 (arrows), which represents the absolute concentration of target GABAAR α1 subunit mRNA.
Table 1.
Expression of GABAAR α1 subunit mRNAs in pooled samples from proximal and distal neocortical areas of each hemisphere from different animal groups at 1, 3, 7, and 30 days after the procedure.
| Time Point Day | Animal Group | Brain Hemisphere | |||
|---|---|---|---|---|---|
| Ipsilateral | Contralateral | ||||
| L1 | L2 | R1 | R2 | ||
| Naive | 54.9 ± 9.8 | 40.7 ± 6.1 | 36.5 ± 4.0 | 50.9 ± 6.8 | |
| 1 | Sham | 62.7 ± 8.3 | 76.4 ± 7.7 | 80.5 ± 8.5 | 71.4 ± 8.2 |
| Lesioned | 47.1 ± 5.7 | 54.1 ± 6.1 | 76.8 ± 6.8 | 68.0 ± 5.1 | |
| 3 | Sham | 60.6 ± 5.4 | 66.1 ± 4.6 | 64.7 ± 6.6 | 69.0 ± 6.0 |
| Lesioned | 57.6 ± 10.0 | 61.6 ± 6.1 | 58.9 ± 4.7 | 66.3 ± 7.1 | |
| 7 | Sham | 73.4 ± 7.6 | 52.6 ± 9.8 | 73.7 ± 9.0 | 71.5 ± 5.5 |
| Lesioned | 41.2 ± 7.4 * | 44.6 ± 6.5 | 39.0 ± 4.9 *▲ | 31.2 ± 3.4 *▲ | |
| 30 | Sham | 62.4 ± 1.6 | 83.6 ± 16.9 | 115.7 ± 7.5 ▲ | 88.4 ± 15.4 |
| Lesioned | 197.8 ± 25.6 *▲ | 258.4 ± 26.5*▲ | 153.6 ± 13.2 *▲ | 141.4 ± 16.0 *▲ | |
Data represent the mean ± S.E.M. from multiple (3–5) cRT-PCR runs for pooled extract aliquots from each analyzed area and hemisphere from different animal groups and corresponding time after the procedure. Student’s t-test and one-way ANOVA with post hoc Tukey-Kramer multiple comparisons testing were used to assess statistical significance
unpaired Student’s t-test, p < 0.05
ANOVA, p < 0.01.
Fig. 3.
cRT-PCR data analysis of GABAAR α1 subunit mRNA levels in sham and lesioned animals at 1, 3, 7 and 30 days (D) following photothrombosis. Bar graphs represent the mean ± S.E.M. Statistical significances are presented for the sham and lesioned animals at the same time point after the procedure; *, unpaired Student’s t-test, p < 0.05. Multiple comparisons are presented in Table 1.
2.3. Western blots of GABAAR α1 subunit protein
Western blot analysis was performed on crude synaptic membranes obtained from sham and lesioned animals at 1, 3, 7, and 30 days after the procedure. Optical density measurements of the expression of GABAAR α1 subunit protein in pooled sample homogenates from proximal and distal neocortical areas of each hemisphere at the different experimental time points from sham and lesioned animals are presented in Table 2. The signal intensity for GABAAR α1 subunit protein was normalized to the signal intensity of β-actin to control for equal protein loading in the gels. As shown in Fig. 4A, applied antibodies immunolabeled the protein band of ~51 kDa, which corresponds to the size of α1 subunits (De Blas, 1996; Miranda and Barnes, 1997); anti-β-actin antibodies elicited a band of ~42 kDa. Fig. 4B shows the relative percent difference of α1 subunit protein expression in proximal and distal neocortical areas of each hemisphere from lesioned animals compared with shams. No significant difference in GABAAR α1 subunit protein levels was found at any time point after lesioning (Table 2, Fig. 4B). At 30 days in lesioned animals, a significant difference was observed in the GABAAR α1 subunit protein expression in L1 (9.3±3.1%) and L2 (7.8±3.0%) ipsilateral neocortical areas compared with the distal R2 area (1.2±2.8%) of the contralateral hemisphere (Student’s t-test, p < 0.05; Table 2, and Fig. 4B).
Table 2.
Optical density measurements of the expression of GABAAR α1 subunit protein levels in pooled sample homogenates from proximal and distal neocortical areas of each hemisphere from sham and lesioned animals at 1, 3, 7, and 30 days after the procedure.
| Time Point Day | Animal Group | Brain Hemisphere | |||
|---|---|---|---|---|---|
| Ipsilateral | Contralateral | ||||
| L1 | L2 | R1 | R2 | ||
| 1 | Sham | 82.9 ± 14.9 | 92.7 ± 19.6 | 81.5 ± 19.6 | 84.6 ± 22.0 |
| Lesioned | 80.2 ± 10.9 | 94.1 ± 14.2 | 80.9 ± 13.2 | 87.9 ± 16.4 | |
| 3 | Sham | 101.1 ± 20.4 | 94.6 ± 16.0 | 83.4 ± 27.0 | 87.2 ± 27.8 |
| Lesioned | 96.7 ± 18.9 | 98.6 ± 17.3 | 81.2 ± 21.0 | 84.2 ± 21.9 | |
| 7 | Sham | 94.0 ± 7.4 | 98.9 ± 6.9 | 85.6 ± 9.2 | 92.8 ± 8.7 |
| Lesioned | 91.2 ± 6.6 | 96.8 ± 6.2 | 86.7 ± 7.7 | 88.8 ± 8.5 | |
| 30 | Sham | 74.8 ± 10.5 | 71.2 ± 6.9 | 68.1 ± 11.0 | 71.1 ± 13.4 |
| Lesioned | 81.8 ± 5.9* | 76.7 ± 7.7* | 72.5 ± 9.6 | 71.9 ± 10.3 | |
Data represent the mean ± S.E.M. from multiple analyses of densitometric determinations of protein expression obtained from 4–5 independent Western blot runs from sham and lesioned animals at the corresponding time points following the procedure (n=4 animal per group/time point). Comparison of α1 subunit protein expression in sham and lesioned animals revealed no significant change at 1, 3, 7, and 30 days after procedure.
an asterisk represents a significant difference at the 30 day time point for lesioned animals between L1 and L2 ipsilateral neocortical areas compared with the distal R2 area of the contralateral hemisphere (Student’s t-test, p < 0.05).
Fig. 4.
Western blot analysis of GABAA receptor α1 subunit protein. (A) Computerized scan of a representative Western immunoblot illustrates the ratio of the GABAAR α1 protein band (51-kDa; upper band) over the area of β-actin (42-kDa; lower band) in crude extracts from cortical areas of the ipsilateral (L1; L2) and contralateral (R1; R2) hemispheres of sham and lesioned animals at 7 days after procedure. (B) Data for densitometry represent the mean ± S.E.M. percent change from the corresponding control obtained from 4–5 independent series of Western immunoblotting for each animal group and time point after the procedure (n=4 animal per group/time point). An asterisk (*) indicates a statistical significance (Student’s t-test, p<0.05).
3. Discussion
Quantitative and semi-quantitative approaches and subunit-specific internal standards and antibodies were used to demonstrate changes of GABAAR α1 subunit mRNA and protein content in rat neocortex following photothrombotic infarction. The main findings of the study were: 1) GABAAR α1 subunit mRNA expression was decreased ipsilateral and contralateral to the infarct at 7 days, but was increased bilaterally at 30 days; 2) GABAAR α1 subunit protein expression was not changed at any time point after lesioning compared with controls; and 3) at 30 days after photothrombosis, α1 subunit protein expression was increased in the ipsilateral cortex (L1 and L2) compared with the distal (R2) region of the contralateral hemisphere.
Alterations in hemispheric mRNA expression have been reported for several GABAAR subunits in epileptic and non-epileptic animals at 4 months after lesioning (Liu et al., 2002). In order to assess whether GABAAR α1 mRNA and protein expression was altered in a proximal to distal gradient pattern at earlier time points following photothrombosis, we used cRT-PCR and Western blots to analyze arcuate bands of cortex surrounding the infarct and homotopically. Although immunohistochemical techniques can demonstrate distinct laminar and regional distributions of GABAAR α1 protein in intact and injured cortex (Fritschy and Mohler, 1995; Redecker et al., 2002), α1 protein expression levels in this study were assessed from commingled tissue punches taken from different cortical areas; good correlation has been demonstrated between Western immunoblotting and immunohistochemical techniques applied for the analysis of GABAAR subunit proteins (Harris et al., 1994).
Our results indicated significant alterations in GABAAR α1 mRNA expression at 7 and 30 days post-lesioning suggesting a complex time-dependent cascade of brain compensatory mechanisms. The observed decrease in GABAAR α1 mRNA at 7 days could result from either a decrease in the level of mRNA transcription and/or a decrease in mRNA stability. In this study, α1 subunit mRNA transcript and protein expression did not correspond, possibly due to a translation block and/or a post-translational modification reported by others (Neumann-Haefelin et al., 1999). Comparison of the present results of α1 subunit mRNA and protein expression with those of other studies (Neumann-Haefelin et al., 1998, 1999; Redecker et al., 2002) revealed several differences. α1 mRNA levels were shown to increase threefold ipsilaterally at 7 days following photothrombosis of the primary somatosensory cortex (Neumann-Haefelin et al., 1999), whereas a moderate decrease of corresponding protein was observed (Neumann-Haefelin et al., 1998). Using relative optical density measurements of GABAAR α1 subunit immunostaining in single coronal brain sections that encompassed frontal, hindlimb, primary and secondary somatosensory cortex, Redecker et al. (2002) found that GABAAR α1 subunit protein expression was decreased at 1 day ipsilaterally, and bilaterally at 7 days; no change was seen at 30 days. In the present study, we found significant up-regulation of α1 mRNA levels bilaterally in lesioned animals at 30 days compared with sham-operated controls, and a significant increase of corresponding protein expression within the ipsilateral hemisphere compared with the distal area of the contralateral hemisphere.
Due to significant differences in experimental techniques, we anticipated this study to yield results potentially different than those of our previous study (Liu et al., 2002) and those of others (Neumann-Haefelin et al., 1998, 1999; Redecker et al., 2002). Most notably, cortical tissue punches were taken from multiple neocortical areas, which had distinct laminar- and region-specific GABAAR α1 subunit distribution pattern, were pooled and analyzed together. Additionally, differences in the photothrombosis technique, i.e., produced by an argon laser-activated light beam (present study), fiber optic bundle (Neumann-Haefelin et al., 1998, 1999; Redecker et al., 2002), or a halogen lamp beam (Liu et al., 2002), might have had differential effects on GABAAR α1 mRNA and protein expression. Although both Western blotting and immunohistochemistry rely upon immunodetection, there can be variation in absolute protein expression caused by differences in primary antibody specificity, and variations in protein interactions within each technique can impact data acquisition, analysis, and interpretation. Importantly, differences among the studies’ results could be related, in part, to our use of ketamine as an anesthetic. Ketamine, a noncompetitive NMDA receptor antagonist, might inhibit glutamate release (Sakai and Amaha, 2000) and affect the regulatory control of NMDA receptors on GABAAR subunit expression following photothrombosis (Redecker et al., 2002). In addition, we assessed the alterations in α1 protein expression only in crude membrane homogenates; in the brain, there are various pools of GABAAR subunits that are located in neuronal and glial membranes, and in the cytosol (Bovolin et al., 1992; Miranda and Barnes, 1997; Devaud et al., 1997; Kumar et al., 2003). Using the Western blot technique, Kumar et al. (2003) demonstrated increased levels of GABAAR α1 subunit peptide in the cytosolic and clathrin-coated vesicles (CCV) fractions, which contrasted with a decreased level of α1 peptide in the synaptic membrane fraction of cerebral cortex following chronic ethanol consumption. In addition, the authors showed that [3H]flunitrazepam binding was increased in the CCV fraction, and α1 subunit endocytosis was enhanced by chronic ethanol consumption; acute ethanol exposure did not alter α1 subunit peptide expression in the CCV or synaptic fractions. Our study examined only crude membrane homogenates containing α1 subunit protein in both fractions (cytosolic and synaptic) and intracellular compartments, which prevented assessment of possible changes of peptide levels in the different fraction pools. This consideration awaits further study.
Functional consequences associated with alterations of GABAAR subunits following photothrombosis have been described by our laboratory (Liu et al., 2002) and by others (Redecker et al., 2002). GABAARs are the main inhibitory receptors of the brain, the majority of which are heteropentamers composed of α, β, and γ subunits with a 2:2:1 ratio (Tretter et al., 1997) and expressed on cell membranes to mediate neuronal signaling (Sieghart, 2000). GABAARs are well studied in models of temporal lobe epilepsy (Rice et al., 1996; Sperk et al., 2004). Interestingly, Raol et al. (2006b) showed that an increased level of GABAAR α1 subunits in the dentate gyrus can affect the development of spontaneous seizures after status epilepticus. In addition, recent studies have identified the α1 (A294D) and α1 (A322D) genetic mutations of the GABAAR α1 subunit that are associated with juvenile myoclonic epilepsy (Cossette et al., 2002; Gallagher et al., 2004; 2005; 2007; Mizielinska et al., 2006). These mutations affect GABAAR gating, expression, and/or trafficking of the receptor to the cell surface - all pathophysiological mechanisms that result in neuronal disinhibition, cause hyperexcitability throughout the brain, and predispose to epileptic seizures (Fisher, 2004; Krampfl et al., 2005; Macdonald et al., 2006). The mechanism by which the α1 (A322D) mutation reduced total and surface α1 (A322D) protein expression could be inhibition of correct GABAAR folding and assembly and/or alteration of channel gating properties (Gallagher et al., 2005). In addition, the study by Sanders and Myers (2004) revealed that many disease-linked mutations result from loss of protein function and from protein misfolding, which alters protein function, assembly, or subcellular trafficking, but not protein topology. Gallagher et al. (2007) showed that the GABAAR α1 epilepsy mutation A322D reduced total α1(A322D) subunit expression, altered α1 subunit topology, inhibited transmembrane helix formation, and caused proteasomal degradation.
Targeted depletion of the GABAAR α1 subunit gene in knockout mice resulted in a viable phenotype with more than 50% of total GABAAR loss (Sur et al., 2001; Kralic et al., 2002); absence of the α1 subunit was compensated by up-regulation of other GABAAR subtypes, clustering, and reorganization of GABAergic circuits (Kralic et al., 2006, Schneider Gasser et al., 2007). Importantly, GABAergic inhibition appeared to be functional in GABAAR α1 knockout mice in that the acute and chronic excitotoxic and epileptogenic action of kainic acid was not altered compared with wild-type mice (Schneider Gasser et al., 2007).
In the present study, short- and longer-term alterations of GABAAR α1 subunit mRNA and protein levels could be related to cellular adaptations to the functional disturbances caused by photothrombotic ischemia. Although animals did not undergo video-EEG monitoring in this study, our previous results (Kelly et al., 2001; Kharlamov et al., 2003) and current observations indicate that spontaneous ictal discharges can be recorded from the cortex less than 30 days after lesioning. These discharges can evolve over time and may be associated with alterations of GABAAR structure and function, including changes in subunit composition, cell surface expression, and pharmacological properties; one possible consequence of these adaptations is poststroke epileptogenesis.
In summary, our results indicate alterations of GABAAR α1 subunit mRNA and protein expression in rat neocortex in the first 30 days following photothrombotic infarcts. These alterations may affect the composition, distribution, and pharmacology of GABAARs, and potentially contribute to the mechanisms of poststroke epileptogenesis and epilepsy.
4. Experimental Procedure
4.1. Cortical photothrombosis and brain infarction
All animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Allegheny-Singer Research Institute. Photothrombosis was performed on 3 mo old Sprague-Dawley rats (Taconic Farms Inc., Germantown, NY; n=32) according to the method described by Watson et al. (1985). Briefly, animals were anesthetized by intraperitoneal injection of ketamine (90 mg/kg) and xylazine (10 mg/kg), and placed in a stereotactic frame (David Kopf Instruments, Tujunga, CA). A midline scalp incision was made and the scalp was retracted laterally. Rose bengal (Sigma, St. Louis, MO) was dissolved in 0.9% saline (15 mg/kg of body weight) and injected over 2 min through a catheter placed in the left femoral vein as the brain was illuminated through the intact skull for 10 min by an argon laser-activated light beam (Lexelion laser, model 75, Evergreen Laser Corporation) at a power of 150 mW. The center of the light beam was positioned 1.8 mm posterior to the bregma and 2.8 mm left of midline; the underlying brain area corresponds to the parietal sensorimotor cortex (Paxinos and Watson, 1998). Body temperature was kept constant throughout the surgery at 37°C using a thermo-regulated pad. The skull was cooled to 36–37°C by a fan with continuous airflow to prevent heat-mediated tissue damage. After photostimulation, the femoral catheter was removed, and the abdominal and skull wounds were sutured. Animals were returned to their cages in an environmentally controlled room (23±2°C, 12-h light/12-h dark cycle) with free access to food and water.
Naïve animals (n=6) had no surgery, laser illumination, or rose bengal injection. Sham-operated (sham) animals underwent 10 min of skull illumination with the laser, the laser then was turned off, and rose bengal solution was injected in the femoral vein over 2 min. cRT-PCR and Western blot analysis were conducted on sham (n=32) and lesioned (n=32) animals, which were subdivided after the procedure into 4 time point groups at 1, 3, 7, and 30 days, with 4 animals used at each time point per technique.
4.2. Tissue sampling
Lesioned and sham animals were sacrificed by isoflurane anesthesia and decapitation at the time points described; naïve control animals were sacrificed at 3 mo of age. Brains were dissected and cortical tissue was separated from subcortical areas of the brain. A 3 mm tissue punch was used to sample the area of neocortex immediately surrounding (proximal) the left-sided lesion (L1) and farther (distal) from the lesion (L2), roughly approximating two arcuate tissue bands (Fig.1). Similarly, cortical tissue samples were taken from homotopic cortical areas (R1 and R2). Because of the small tissue punch volume, 4–5 punches from the same arcuate band were pooled together to form a single cortical tissue sample for the animal. Tissue was immediately frozen in liquid nitrogen and preserved at −80°C for subsequent isolation of mRNA or protein.
4.3. Quantitative competitive RT-PCR (cRT-PCR)
Total RNA was extracted from each animal’s pooled cortical tissue sample using TRI Reagent (Molecular Research) (Chomczynski, 1993). Samples were homogenized with a Polytron (Kinematica), mixed with chloroform (0.2 ml/l homogenate), and centrifuged (12,000 × g, 4°C) for 15 min. RNA was precipitated from the aqueous phase by the addition of isopropanol, washed with 75% ethanol, and dissolved in 0.1% DEPC-treated water. The purity of the isolated RNA was assessed by measuring the ratio of absorption at 260 and 280 nm and was found to be >1.8 for all samples. Absence of contaminating DNA was confirmed by PCR analysis of total RNA samples without reverse transcription. cRT-PCR using an internal standard specific for the GABAAR α1 subunit was conducted according to methods described previously (Grayson and Ikonomovic, 1999). Aliquots of total RNA (1.0 µg) were reverse transcribed using Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV RT; Invitrogen, Carlsbad, CA) with various amounts of the GABAA cRNA internal standard in first strand buffer at 37°C for 60 min. The resulting cDNA was heated at 95°C for 10 min to stop the reverse transcription reaction and subsequently put on ice until use for PCR. The PCR reaction was conducted in complementary PCR buffer with sense and antisense primers, dNTPs, Hot Tub Polymerase (Amersham Biosciences, Buckinghamshire, England), and 2 µCi [α-32P]-dCTP (Perkin Elmer Life Sciences, Wellesley, MA) per tube. Using a DNA Thermal Cycler (GeneAmp PCR System 2400), the PCR consisted of 30 cycles, each cycle was 94°C for 45 sec, 60°C for 1 min, and 72°C for 1 min, followed by a final elongation step (72°C for 15 min). Primers for α1 gene were as follow: forward, 5’-AGC TAT ACC CCT AAC TTA GCC AGG-3’, and reverse, 5’-AGA AAG CGA TTC TCA GTG CAG AGG-3’. PCR products were digested for 1 h with 10 units of Bgl II (Invitrogen) and separated by gel electrophoresis (1.8% agarose gel, UltraPure, Invitrogen). Gels were dried and exposed to a phosphor-imaging screen for 24 h. Signal intensities of bands for target RNA products were quantified using a Storm 840 phosphorimager (Molecular Dynamics, CA) and Image Quant software. To ensure the accuracy of the measurements, multiple runs (3–5) were performed for each pooled extract from L1, L2, R1, and R2 cortical areas of each animal and investigated time points after lesioning. Data were analyzed as described by Liu et al. (2002) by using ratio counts of GABAAR α1 subunit internal standard bands against counts of target RNA bands and by plotting these ratios against the known amounts of GABA internal standard added to the test sample using linear regression to create a competitive PCR curve.
4.4. Western blots
Tissue samples were taken in the same manner as described for cRT-PCR, and were homogenized in ice-cold lysis buffer with inhibitors (Complete Mini, Roche Diagnostics GmbH, Mannheim, Germany). The homogenate was incubated on ice for 30 min, transferred to microcentrifuge tubes, and centrifuged (16,000 × g, 4°C) for 30 min to obtain the crude membrane fraction. Protein concentrations were determined using the Bradford assay technique (Bradford, 1976) and a BSA protein assay kit (Pierce, Rockford, II). A standard curve was generated to ensure that the amount of protein used for Western blot analysis was in the linear range of detection. The accuracy of protein loadings was assured by measuring the amount of protein in each sample and the linearity of standard curves for protein (regression square, R2 > 0.9). Based on this curve, aliquots of 20–40 µg/lane of protein were used for Western blot analysis. Tissue proteins were incubated in sample buffer (125 mM Tris-HCl, pH 6.8, 20% glycerol, 0.002% bromphenol blue, 10% β-mercaptoethanol, and 4% SDS) for 5 min at 95°C. Sodium dodecyl sulfate-polyacrilamide gel electrophoresis (SDS-PAGE) was performed on 10% minigels (MiniProtean III Electrophoresis Cell, Bio-Rad, Hercules, CA), and proteins were transferred to polyvinylidene difluoride membranes (PVDF; Immobilon-P; Millipore, Bedford, MA) by electrophoresis in transfer buffer [25 mM Tris, 192 mM glycine (pH 8.3), and 20% methanol] at 100 V during 2–4 h (Mini Trans-Blot Electrophoretic Transfer Cell, Bio-Rad). Kaleidoscope Prestained Standard marker (Bio-Rad) was clearly visible on the gel during electrophoresis and on the membrane following the transfer procedure. Santa Cruz marker, compatible with the secondary antibody, was used as an internal standard for film analysis. Following transfer, the membranes were washed briefly in Tris-buffered saline (TBS, pH 7.4), and immersed in blocking solution consisting of 5% nonfat dry milk (Carnation) and 0.1% Tween 20 (TBST) for 2 h at room temperature. The membranes were incubated with an affinity-purified goat polyclonal antibody against the GABAAR α1 subunit (N-19; 1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) in TBST-milk at 4°C overnight with agitation. After three washes for 10 min in TBST, the membranes were incubated with horseradish peroxidase-conjugated bovine anti-goat secondary antibody (1:5000; Santa Cruz) in TBST-milk for 2 h at room temperature. The membranes were washed three times for 10 min in TBST and one time for 10 min in TBS. PVDF membranes were incubated with ECL Western blotting detection reagents (NEN Life Sciences Products, Inc., Boston, MA) according to the manufacturer’s instructions. Signals were detected using the Kodak X-Omatic autoradiography cassette and ECL films (Hyperfilm ECL, Amersham Pharmacia Biotech). Blots were exposed to ECL film under non-saturating conditions. After completing the analysis of GABAAR α1 protein bands, blotted PVDF membranes were washed with a restore Western blot stripping buffer and incubated with primary monoclonal anti-β-actin antibody (clone AC-15; 1:15000; Sigma, St. Louis, MI) and secondary anti-mouse HRP antibody (1:1000; BD Pharmingen), using protocols similar to those described above. The corresponding β-actin signal was used for a comparative estimation of the amount of protein applied to the gels (Frahm et al., 2006).
Quantification of the results was performed using MCID imaging software (Imaging Research Inc., St. Catharines, Ontario, Canada). The optical density level was obtained for the signal of the specific band, normalized, and expressed as the percentage change from the control value. For background correction, signals obtained in the same film close to the specific band were subtracted. Protein expression was determined from 4–5 independent series of Western immunoblot runs for each animal at each time point with samples loaded in duplicate.
4.5. Data analysis
All experimental results were expressed as mean ± standard error of the mean (S.E.M.). Student’s t-test and one-way ANOVA with post hoc Tukey-Kramer multiple comparisons testing were used to assess statistical significance (p<0.05) for mRNA and protein expression in the proximal and distal tissue bands of the ipsilateral and contralateral hemispheres and between animal groups sampled at the different post-lesioning time points. Analyses were conducted using GraphPad InStat software (GraphPad Software, Inc., San Diego, CA).
Acknowledgments
Supported by AHA Award 0151398U and NINDS RO1NS04601 to KMK.
Abbreviations
- GABA
gamma-aminobutyric acid
- GABAARs
GABAA receptors
- cRT-PCR
competitive reverse transcription-polymerase chain reaction
- mRNA
messenger ribonucleic acid
- DNA
deoxyribonucleic acid
- dNTPs
deoxynucleoside triphosphates
- cRNA
copy ribonucleic acid
- cDNA
copy deoxyribonucleic acid
- DEP
diethyl pyrocarbonate-treated water
- PVDF
polyvinylidene difluoride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bovolin P, Santi MR, Memo M, Costa E, Grayson DR. Distinct developmental patterns of expression of rat α1,α5, γ2S, and γ2L γ-aminobutyric acidA receptor subunit mRNAs in vivo and in vitro. J. Neurochem. 1992;59:62–72. doi: 10.1111/j.1471-4159.1992.tb08876.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532–537. [PubMed] [Google Scholar]
- Cossette P, Liu L, Brisebois K, Dong H, Lortie A, Vanasse M, Saint-Hilaire JM, Carmant L, Verner A, Lu WY, Wang YT, Rouleau GA. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat. Genet. 2002;31:184–189. doi: 10.1038/ng885. [DOI] [PubMed] [Google Scholar]
- Coulter DA. Epilepsy-associated plasticity in gamma-aminobutyric acid receptor expression, function, and inhibitory synaptic properties. Int. Rev. Neurobiol. 2001;45:237–252. doi: 10.1016/s0074-7742(01)45013-6. [DOI] [PubMed] [Google Scholar]
- Crestani F, Assandri R, Tauber M, Martin JR, Rudolph U. Contribution of the alpha1-GABA(A) receptor subtype to the pharmacological actions of benzodiazepine site inverse agonists. Neuropharmacology. 2002;43:679–684. doi: 10.1016/s0028-3908(02)00159-4. [DOI] [PubMed] [Google Scholar]
- De Blas AL. Brain GABAA receptors studied with subunit-specific antibodies. Mol. Neurobiol. 1996;12:55–71. doi: 10.1007/BF02740747. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Fritschy J-M, Sieghart W, Morrow AL. Bidirectional alterations of GABAA receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J. Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Fisher JL. A mutation in the GABAA receptor alpha 1 subunit linked to human epilepsy affects channel gating properties. Neuropharmacology. 2004;46:629–637. doi: 10.1016/j.neuropharm.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Zhang J, Macdonald RL. The role of alpha1 and alpha6 subtype amino-terminal domains in allosteric regulation of gamma-aminobutyric acida receptors. Mol. Pharmacol. 1997;52:714–724. doi: 10.1124/mol.52.4.714. [DOI] [PubMed] [Google Scholar]
- Frahm C, Haupt C, Weinandy F, Siegel G, Bruehl C, Witte OW. Regulation of GABA transporter mRNA and protein after photothrombotic infarct in rat brain. J. Comp. Neurol. 2004a;478:176–188. doi: 10.1002/cne.20282. [DOI] [PubMed] [Google Scholar]
- Frahm C, Haupt C, Witte OW. GABA neurons survive focal ischemic injury. Neuroscience. 2004b;127:341–346. doi: 10.1016/j.neuroscience.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Frahm C, Siegel G, Grass S, Witte OW. Stable expression of the vesicular GABA transporter following photothrombotic infarct in rat brain. Neuroscience. 2006;140:865–877. doi: 10.1016/j.neuroscience.2006.02.045. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Kiener T, Bouilleret V, Loup F. GABAergic neurons and GABA(A)-receptors in temporal lobe epilepsy. Neurochem. Int. 1999;34:435–445. doi: 10.1016/s0197-0186(99)00040-6. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J. Comp. Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Gallagher MJ, Ding L, Maheshwari A, Macdonald RL. The GABAA receptor alpha1 subunit epilepsy mutation A322D inhibits transmembrane helix formation and causes proteasomal degradation. Proc. Natl. Acad. Sci. U S A. 2007;104:12999–13004. doi: 10.1073/pnas.0700163104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher MJ, Shen W, Song L, Macdonald RL. Endoplasmic reticulum retention and associated degradation of a GABAA receptor epilepsy mutation that inserts an aspartate in the M3 transmembrane segment of the alpha1 subunit. J. Biol. Chem. 2005;280:37995–38004. doi: 10.1074/jbc.M508305200. [DOI] [PubMed] [Google Scholar]
- Gallagher MJ, Song L, Arain F, Macdonald RL. The juvenile myoclonic epilepsy GABA(A) receptor alpha1 subunit mutation A322D produces asymmetrical, subunit position-dependent reduction of heterozygous receptor currents and alpha1 subunit protein expression. J. Neurosci. 2004;24:5570–5578. doi: 10.1523/JNEUROSCI.1301-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DR, Ikonomovic S. Competitive RT-PCR to quantitate steady-state mRNA levels. In: Boulton AA, Baker GB, Bateson AN, editors. In vitro Neurochemical Techniques. Neuromethods. Vol. 34. Totowa, NJ: Humana Press; 1999. pp. 127–151. [Google Scholar]
- Harris BT, Charlton ME, Costa E, Grayson DR. Quantitative changes in alpha 1 and alpha 5 gamma-aminobutyric acid type A receptor subunit mRNAs and proteins after a single treatment of cerebellar granule neurons with N-methyl-D-aspartate. Mol. Pharmacol. 1994;45:637–648. [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol. Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Karhunen H, Bezvenyuk Z, Nissinen J, Sivenius J, Jolkkonen J, Pitkänen A. Epileptogenesis after cortical photothrombotic brain lesion in rats. Neuroscience. 2007;148:314–324. doi: 10.1016/j.neuroscience.2007.05.047. [DOI] [PubMed] [Google Scholar]
- Kelly KM. Poststroke seizures and epilepsy: clinical studies and animal models. Epilepsy Curr. 2002;2:173–177. doi: 10.1046/j.1535-7597.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KM. Animal modeling of poststroke seizures and epilepsy: 5-year update. Epilepsy Curr. 2007;7:1–4. doi: 10.1111/j.1535-7511.2007.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KM, Kharlamov A, Hentosz TM, Kharlamov E, Williamson JM, Bertram EH, Kapur J, Armstrong DM. Photothrombotic brain infarction results in seizure activity in aging F344 and Sprague Dawley rats. Epilepsy Res. 2001;47:189–203. doi: 10.1016/s0920-1211(01)00294-7. [DOI] [PubMed] [Google Scholar]
- Kharlamov EA, Jukkola PI, Schmitt KL, Kelly KM. Electrobehavioral characteristics of epileptic rats following photothrombotic brain infarction. Epilepsy Res. 2003;56:185–203. doi: 10.1016/j.eplepsyres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Kharlamov EA, Kharlamov A, Kelly KM. Changes in neuropeptide Y protein expression following photothrombotic brain infarction and epileptogenesis. Brain Res. 2007;1127:151–162. doi: 10.1016/j.brainres.2006.09.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralic JE, Korpi ER, O'Buckley TK, Homanics GE, Morrow AL. Molecular and pharmacological characterization of GABA(A) receptor alpha1 subunit knockout mice. J. Pharmacol. Exp. Ther. 2002;302:1037–1045. doi: 10.1124/jpet.102.036665. [DOI] [PubMed] [Google Scholar]
- Kralic JE, Sidler C, Parpan F, Homanics GE, Morrow AL, Fritschy JM. Compensatory alteration of inhibitory synaptic circuits in cerebellum and thalamus of gamma-aminobutyric acid type A receptor alpha1 subunit knockout mice. J. Comp. Neurol. 2006;495:408–421. doi: 10.1002/cne.20866. [DOI] [PubMed] [Google Scholar]
- Krampfl K, Maljevic S, Cossette P, Ziegler E, Rouleau GA, Lerche H, Bufler J. Molecular analysis of the A322D mutation in the GABA receptor alpha-subunit causing juvenile myoclonic epilepsy. Eur. J. Neurosci. 2005;22:10–20. doi: 10.1111/j.1460-9568.2005.04168.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kralic JE, O'Buckley TK, Grobin AC, Morrow AL. Chronic ethanol consumption enhances internalization of alpha1 subunit-containing GABAA receptors in cerebral cortex. J. Neurochem. 2003;86:700–708. doi: 10.1046/j.1471-4159.2003.01894.x. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Wen X, Yang Y, Buckmaster PS. GABAA receptor-mediated IPSCs and alpha1 subunit expression are not reduced in the substantia nigra pars reticulata of gerbils with inherited epilepsy. J. Neurophysiol. 2006;95:2446–2455. doi: 10.1152/jn.01173.2005. [DOI] [PubMed] [Google Scholar]
- Liu J, Schmitt KL, Kharlamov EA, Stolarski CJ, Grayson DR, Kelly KM. Quantitative RT-PCR of GABAA α1, β1, γ2S subunits in epileptic rats following photothrombotic infarction of neocortex. Epilepsy Res. 2002;52:85–95. doi: 10.1016/s0920-1211(02)00194-8. [DOI] [PubMed] [Google Scholar]
- Loup F, Picard F, Andre VM, Kehrli P, Yonekawa Y, Wieser HG, Fritschy JM. Altered expression of alpha3-containing GABAA receptors in the neocortex of patients with focal epilepsy. Brain. 2006;129:3277–3289. doi: 10.1093/brain/awl287. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Gallagher MJ, Feng HJ, Kang J. GABA(A) receptor epilepsy mutations. Biochem Pharmacol. 2004;68:1497–1506. doi: 10.1016/j.bcp.2004.07.029. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Mileson BE, Ehrmann ML, Schwartz RD. Alterations in the gamma-aminobutyric acid-gated chloride channel following transient forebrain ischemia in the gerbil. J. Neurochem. 1992;58:600–607. doi: 10.1111/j.1471-4159.1992.tb09761.x. [DOI] [PubMed] [Google Scholar]
- Miranda JD, Barnes EM., Jr Repression of gamma-aminobutyric acid type A receptor alpha1 polypeptide biosynthesis requires chronic agonist exposure. J. Biol. Chem. 1997;272:16288–16294. doi: 10.1074/jbc.272.26.16288. [DOI] [PubMed] [Google Scholar]
- Mizielinska S, Greenwood S, Connolly CN. The role of GABAA receptor biogenesis, structure and function in epilepsy. Biochem. Soc. Trans. 2006;34:863–867. doi: 10.1042/BST0340863. [DOI] [PubMed] [Google Scholar]
- Mohler H. GABAA receptors in central nervous system disease: anxiety, epilepsy, and insomnia. J. Recept. Signal Transduct. Res. 2006;26:731–740. doi: 10.1080/10799890600920035. [DOI] [PubMed] [Google Scholar]
- Mohler H. Molecular regulation of cognitive functions and developmental plasticity: impact of GABAA receptors. J. Neurochem. 2007;102:1–12. doi: 10.1111/j.1471-4159.2007.04454.x. [DOI] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Rudolph U. New benzodiazepine pharmacology. J. Pharmacol. Exp. Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Staiger JF, Redecker C, Zilles K, Fritschy JM, Mohler H, Witte OW. Immunohistochemical evidence for dysregulation of the GABAergic system ipsilateral to photochemically induced cortical infarcts in rats. Neuroscience. 1998;87:871–879. doi: 10.1016/s0306-4522(98)00124-9. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Bosse F, Redecker C, Muller HW, Witte OW. Upregulation of GABAA-receptor alpha1- and alpha2-subunit mRNAs following ischemic cortical lesions in rats. Brain Res. 1999;816:234–237. doi: 10.1016/s0006-8993(98)01162-7. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Schwarzer C, Gasser E, Kato N, Vezzani A, Sperk G. Altered expression of GABA(A) and GABA(B) receptor subunit mRNAs in the hippocampus after kindling and electrically induced status epilepticus. Neuroscience. 2005;134:691–704. doi: 10.1016/j.neuroscience.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Paysan J, Bolz J, Mohler H, Fritschy JM. GABAA receptor alpha 1 subunit, an early marker for area specification in developing rat cerebral cortex. J. Comp. Neurol. 1994;350:133–149. doi: 10.1002/cne.903500110. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Qu M, Buchkremer-Ratzmann I, Schiene K, Schroeter M, Witte OW, Zilles K. Bihemispheric reduction of GABAA receptor binding following focal cortical photothrombotic lesions in the rat brain. Brain Res. 1998;813:374–380. doi: 10.1016/s0006-8993(98)01063-4. [DOI] [PubMed] [Google Scholar]
- Raol YH, Lund IV, Bandyopadhyay S, Zhang G, Roberts DS, Wolfe JH, Russek SJ, Brooks-Kayal AR. Enhancing GABA(A) receptor alpha 1 subunit levels in hippocampal dentate gyrus inhibits epilepsy development in an animal model of temporal lobe epilepsy. J. Neurosci. 2006a;26:11342–11346. doi: 10.1523/JNEUROSCI.3329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raol YH, Zhang G, Lund IV, Porter BE, Maronski MA, Brooks-Kayal AR. Increased GABA(A)-receptor alpha1-subunit expression in hippocampal dentate gyrus after early-life status epilepticus. Epilepsia. 2006b;47:1665–1673. doi: 10.1111/j.1528-1167.2006.00640.x. [DOI] [PubMed] [Google Scholar]
- Redecker C, Wang W, Fritschy JM, Witte OW. Widespread and long-lasting alterations in GABA(A)-receptor subtypes after focal cortical infarcts in rats: mediation by NMDA-dependent processes. J. Cereb. Blood Flow. Metab. 2002;22:1463–1475. doi: 10.1097/01.WCB.0000034149.72481.BD. [DOI] [PubMed] [Google Scholar]
- Rice A, Rafiq A, Shapiro SM, Jakoi ER, Coulter DA, DeLorenzo RJ. Long-lasting reduction of inhibitory function and gamma-aminobutyric acid type A receptor subunit mRNA expression in a model of temporal lobe epilepsy. Proc. Natl. Acad. Sci. U S A. 1996;93:9665–9669. doi: 10.1073/pnas.93.18.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai F, Amaha K. Midazolam and ketamine inhibit glutamate release via a cloned human brain glutamate transporter. Can. J. Anaesth. 2000;47:800–806. doi: 10.1007/BF03019485. [DOI] [PubMed] [Google Scholar]
- Sanders CR, Myers JK. Disease-related misassembly of membrane proteins. Annu. Rev. Biophys. Biomol. Struct. 2004;33:25–51. doi: 10.1146/annurev.biophys.33.110502.140348. [DOI] [PubMed] [Google Scholar]
- Schiene K, Bruehl C, Zilles K, Qu M, Hagemann G, Kraemer M, Witte OW. Neuronal hyperexcitability and reduction of GABAA-receptor expression in the surround of cerebral photothrombosis. J. Cereb. Blood Flow. Metab. 1996;16:906–914. doi: 10.1097/00004647-199609000-00014. [DOI] [PubMed] [Google Scholar]
- Schneider Gasser EM, Duveau V, Prenosil GA, Fritschy JM. Reorganization of GABAergic circuits maintains GABAA receptor-mediated transmission onto CA1 interneurons in alpha1-subunit-null mice. Eur. J. Neurosci. 2007;25:3287–3304. doi: 10.1111/j.1460-9568.2007.05558.x. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Unraveling the function of GABA(A) receptor subtypes. Trends Pharmacol. Sci. 2000;21:411–413. doi: 10.1016/s0165-6147(00)01564-9. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure, pharmacology, and function of GABAA receptor subtypes. Adv. Pharmacol. 2006;54:231–263. doi: 10.1016/s1054-3589(06)54010-4. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr. Top. Med. Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Sperk G, Furtinger S, Schwarzer C, Pirker S. GABA and its receptors in epilepsy. Adv. Exp. Med. Biol. 2004;548:92–103. doi: 10.1007/978-1-4757-6376-8_7. [DOI] [PubMed] [Google Scholar]
- Sur C, Wafford KA, Reynolds DS, Hadingham KL, Bromidge F, Macaulay A, Collinson N, O'Meara G, Howell O, Newman R, Myers J, Atack JR, Dawson GR, McKernan RM, Whiting PJ, Rosahl TW. Loss of the major GABA(A) receptor subtype in the brain is not lethal in mice. J. Neurosci. 2001;21:3409–3418. doi: 10.1523/JNEUROSCI.21-10-03409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Ehya N, Fuchs K, Sieghart W. Stoichiometry and assembly of a recombinant GABAA receptor subtype. J. Neurosci. 1997;17:2728–2737. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42 Suppl. 3:8–12. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x. [DOI] [PubMed] [Google Scholar]
- Watson BD, Dietrich WD, Busto R, Wachte MS, Ginsburg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann. Neurol. 1985;17:497–504. doi: 10.1002/ana.410170513. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J. Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]