Abstract
Stromal cell-derived Factor-1α (SDF-1α) stimulates the migration of bone marrow (BM) cells, similar to Vascular Endothelial Growth Factor (VEGF). We previously demonstrated that inhibition of VEGF165 by small interfering RNA inhibited Ewing’s sarcoma tumor growth, tumor vessel formation and recruitment of BM cells to the tumor. To determine the importance of BM cells in tumor vessel development, we investigated the effects of SDF-1α on VEGF-inhibited TC/siVEGF7-1 Ewing’s tumor neovasculature formation and growth. The effect of SDF-1α on CD34+ progenitor cell chemotaxis was determined in vivo. Using a BM transplantation model with GFP+ transgenic mice as BM donors and nude mice as recipients, we evaluated the effect of SDF-1α on the recruitment of BM-derived cells to VEGF165-inhibited TC/siVEGF7-1 tumors, as well as its effect on neovasculature development, vessel morphology and tumor growth. SDF-1α stimulated the migration of CD34+ progenitor cells to Matrigel plugs in vivo and promoted the retainment of BM-derived pericytes in close association with perfused, functional tumor vessels. Intratumor inoculation of Ad-SDF-1α into TC/siVEGF7-1 tumors resulted in increased SDF-1 and PDGF-BB expression, augmented tumor growth, an increase in the number of large, lumen-bearing vascular structures, and enhanced vessel pericyte coverage, with no change in VEGF165. SDF-1α stimulates BM cell chemotaxis and the association of these cells with functional tumor vessels. Furthermore, SDF-1α enhances tumor neovascularization and growth with no alteration in VEGF165. Our work suggests that SDF-1-mediated vasculogenesis may represent an alternate pathway that could potentially be utilized by tumors to sustain growth and neovasculature expansion after anti-VEGF therapy.
Keywords: SDF-1, bone marrow stem cells, pericytes, vasculogenesis, Ewing’s sarcoma
Introduction
Both angiogenesis and vasculogenesis contribute to the formation and expansion of the tumor vasculature that supports the growth of Ewing’s sarcoma.1,2 We have previously demonstrated that bone marrow (BM) stem/progenitor cells migrate to Ewing’s sarcoma tumors and differentiate into endothelial cells that co-localize with tumor neovessels.1,2 Incorporation of BM cells into the tumor vascular endothelial lining has been similarly reported in Lewis lung carcinoma3, lymphoma3, mammary adenocarcinoma4, and glioma5. While the direct differentiation of stem/progenitor cells into endothelial cells lining blood vessel lumens is known to be important in wound healing and tissue revascularization post ischemia,6 it is unclear whether these cells play a critical role in tumor vasculature expansion and growth.
Vascular endothelial growth factor (VEGF), one key factor produced by tumors, mediates the mobilization and differentiation of endothelial progenitor cells (EPCs)7 and stimulates the formation of functional tumor vessels. Inhibition of VEGF induces tumor regression largely by impacting the tumor vessels. In Ewing’s sarcoma, elevated VEGF levels have been detected in cell lines, primary human tumors, and in the serum of Ewing’s patients.8-10 VEGF expression has been correlated with increased Ewing’s tumor microvessel density as well as poor patient prognosis11. We have previously shown that inhibition of VEGF165 led to growth suppression, decreased vessel density2,12 and reduced migration of bone marrow-derived CD34+ cells to the tumor vascular bed in Ewing’s tumors.2 If BM cells indeed are an important source of endothelial cells and pericytes for tumor vessel development then stimulating the influx of these cells into the tumor area may enhance tumor vessel formation in the VEGF-inhibited tumor and restore tumor growth in the absence of VEGF.
Several cytokines, such as Granulocyte Colony-Stimulating Factor, Placental Growth Factor and Stromal cell-derived Factor-1 (SDF-1), have been shown to induce BM stem/progenitor cell mobilization and chemotaxis. SDF-1 is a α-chemokine that specifically regulates the chemotaxis of CXCR4-expressing cell populations, such as CD34+ stem/progenitors.13 SDF-1 directs hematopoietic stem cell homing to and engraftment within the bone marrow both during embryogenesis and adult life.14,15 In addition, this chemokine has also been shown to stimulate the recruitment of endothelial progenitor cells to sites of ischemia,16-19 thereby contributing to accelerated neovascularization. A cooperative relationship between VEGF and SDF-1 during neovascularization was recently proposed, in which VEGF induces perivascular expression of SDF-1 and mobilizes BM-derived CXCR4+ cells, while SDF-1 retains the incoming stem cells in a perivascular location.20
With respect to tumor growth, both stimulatory and inhibitory roles of SDF-1 have been reported. Disruption of the SDF-1/CXCR4 axis was found to inhibit tumor growth, microvessel density and intratumoral blood flow without affecting VEGF levels.21 In another study, however, the adenovirus-mediated upregulation of SDF-1 in tumors led to accumulation of dendritic cells and subsequent growth inhibition.22 SDF-1 has also been demonstrated to act locally within the hypoxic tumor microenvironment to direct differentiation of recruited BM cells into both endothelial cells and pericytes.23 In the present study, we demonstrate that increasing local SDF-1α led to the accumulation of BM-derived cells, including pericyte-progenitors, at the vascular endothelial lining. Furthermore, we demonstrate that upregulation of SDF-1α in VEGF165-inhibited Ewing’s tumors augmented tumor growth and the formation of large vascular structures with increased pericyte coverage, with no effect on VEGF165.
Materials and methods
Ewing’s sarcoma cells and culture conditions
TC/siVEGF7-1 cells were created by transfection of TC71 cells with a vector-based VEGF165-targeted siRNA expression system.12 VEGF165 expression in TC/siVEGF7-1 cells is decreased by 80% with no alteration in VEGF189 expression. TC/siVEGF7-1 cells produce 98% less VEGF165 protein. TC71 and TC/siVEGF7-1 human Ewing’s sarcoma cells were cultured in Eagle’s modified essential medium supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 1 mM non-essential amino acids, I mM penicillin-streptomycin, and 2-fold vitamin solution (Gibco BRL, Grand Island, NY). The t(11; 22) translocation was detected by reverse-transcriptase polymerase chain reaction.
Mice and murine bone marrow cells
All experiments were approved by the Institutional Animal Care and Use committee of the University of Texas M. D. Anderson Cancer Center. Female nude BALB/cAnN mice, 6 to 8 weeks of age, were purchased from the National Cancer Institute (Bethesda, MD). GFP transgenic mice (Jackson Laboratories strain 003115) were purchased from M.D. Anderson Cancer Center, Genetic Engineering Mouse Facility (GEMF). GFP transgenic mice were used as bone marrow (BM) donors, and nude BALB/cAnN mice served as BM recipients. Bone marrow (BM) cells were isolated from donors by flushing hind femurs with PBS. Freshly-isolated BM cells were resuspended in PBS and kept on ice for no more than 30 minutes prior to being injected into the tail vein of a recipient mouse.
Isolation, expansion and labeling of CD34+ cells
Human CD34+ cells were isolated from umbilical cord blood obtained from the St. Louis Cord Blood Bank (St. Louis, MO) as previously described (2). CD34+ cells were cultured for 5 - 10 days at 37°C in StemSpan SFEM Serum-free Expansion Medium (Stemcell Technologies, Vancouver, British Columbia) supplemented with StemSpan CC100 cytokine cocktail (Stemcell Technologies). Culture-expanded CD34+ cells were analyzed using phycoerythrin (PE)-conjugated mouse anti-human CD34 (BD Biosciences, San Jose, CA) and fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD45 (BD Biosciences. Isotypic mouse IgG1κ antibodies conjugated to either PE or FITC were used as negative controls (BD Biosciences). We have demonstrated that these expanded CD34+ cells differentiate into endothelial cells when cultured in medium containing serum or VEGF and incorporate into new tumor vessels when injected intravenously (24). CD34+ cells were GFP-labeled by culturing for 24 hours with the adenovirus vector Ad5/F35-GFP. Ad5/F35 binds cells in a CAR-independent manner, allowing effective gene delivery to human stem/progenitor cells (25).
Chemotaxis of GFP-labeled CD34+ cells to matrigel implants containing recombinant SDF-1α
Growth factor-reduced BD Matrigel basement membrane matrix alone (BD Biosciences, San Jose, CA), Matrigel with recombinant human VEGF (200 ng/ml; Sigma-Aldrich, St. Louis, MO), or Matrigel with recombinant human SDF-1α (100, 200 or 1000 ng/mL; Stemcell Technologies) was injected subcutaneously on the ventral side of nude mice (300 uL matrigel/implant). Mice received i.v. injections of GFP-labeled CD34+ cells on days 1 and 8 following the introduction of the s.c. implants. Matrigel implants were excised on day 13, frozen-sectioned and analyzed using immunohistochemistry for GFP+ cells.
RT-PCR
Total RNA was extracted from cell lines or tumor tissues using a Trizol reagent (Introgen, Grand Island, NY), as directed by the manufacturer’s instructions. Extracted RNA was treated with DNase I. cDNA was synthesized using a Reverse Transcription System (Promega Corp, Madison, WI). RT products were amplified by PCR using the following specific primers: 1) VEGF: 5′ - CACATAGGAGAGATGAGCTTC - 3′ and 5′ - CCGCCTCGGCTTGTCACAT - 3′; 2) SDF-1: 5′ - ATGAACGCCAAGGTCGTGGTC - 3′ and 5′ - TGGCTGTTGTGCTTACTTGTTT - 3′; 3) GAPDH: 5′ - TGAAGGTCG GAGTCAACGGATTTGGT - 3′ AND 5′ - CATGTGGGCCATGAGGTCCACCAC - 3′; 4) CXCR4: 5′ - TTCTACCCCAATGACTTGTG - 3′ and 5′ - ATGTAGTAAGGCA GCCAACA - 3′; 5) PDGF -BB: 5′ - CCCACAGTGGCTTTTCATTT - 3′ and 5′ - GTGGAGGAGCAGACTGAAGG - 3′. Initial denaturation was performed at 94°C for 5 minutes, followed by denaturation at 94°C for 1 minute, annealing for 1 minute, extension at 72°C for 1 min (30 cycles), and a final elongation at 72°C for 10 minutes. Annealing was performed at 59 °C for VEGF, 55 °C for GAPDH and PDGF-BB and 57 °C for SDF-1 and CXCR4. PCR products were subjected to electrophoresis on a 2% agarose gel with ethidium bromide and visualized under UV light. Real-time PCR was performed using SYBR Green Supermix kit in an iCycler iQ System (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instructions. Real-time PCR products were amplified using the following specific primers: 1) PDGF-BB: 5′ - CCCACAGTGGCTTTTCATTT - 3′ and 5′ - GTGAACGTAGGGGAAGTGGA - 3′; 2) VEGF: 5′ - CGAGGAAGAGAGAGACGGGG - 3′ and 5′ - CCAAAAGCAGGT CACTCACTTTG - 3′; 3) β-actin: 5′ - CTGGAACGGTGAAGGTGACA - 3′ and 5′ - AAGGGACTTCCTGTAACAATGCA - 3′. To quantitate results from real-time PCR, control group gene expression was normalized to 1.
Analysis of BM-derived cell recruitment to TC/siVEGF7-1 tumors with or without SDF-1α
Bone marrow transplantation was performed using GFP transgenic mice as bone marrow (BM) donors, and a nude BALB/cAnN mice as the BM recipient. The recipient nude mouse underwent whole-body irradiation with 8 Gy in one dose using an external cesium source (137Cs Mark 1 irradiator, J.L. Sheperd & Associates, Glendale, CA). Freshly isolated BM cells (3 × 106 cells/0.2 mL PBS) from GFP transgenic mice were injected via the lateral tail vein into the nude recipient within 1 hour following irradiation. Five weeks following engraftment of donor GFP+ BM cells, TC/siVEGF7-1 Ewing’s sarcoma cells in growth factor-depleted matrigel basement membrane matrix were subcutaneously injected in the bilateral flank. Recombinant SDF-1α was resuspended into the tumor-matrigel implant on one side only. Three weeks following tumor cell inoculation, fluorescein-conjugated Lycopersicon esculentum (tomato) lectin (0.4 mg, Vector Laboratories, Burlingame, CA) was intravenously injected to mark the perfused, functional tumor vessels. Two minutes later, euthanasia was performed and the tumors were resected, placed in optimal cutting temperature compound (OCT) (Sakura Finetek USA, Torrance, CA), snap-frozen in liquid nitrogen and stored at -80°C.
Effect of adenovirus-SDF-1α on subcutaneous TC/siVEGF7-1 tumor growth and neovasculature formation
Ad5-CMV-SDF-1α (Ad-SDF-1) was generously provided by Dr. Ronald Crystal (Weill Medical College of Cornell University, New York, NY). Ad5-CMV-control vector (Ad-control) was purchased from the Vector Development Laboratory, Baylor College of Medicine (Houston, TX). TC/siVEGF7-1 cells were injected subcutaneously on the ventral side of nude mice (4 million cells/mouse). Beginning on day 3 after tumor cell inoculation, animals received intratumoral injections of Ad-SDF-1 or Ad-control (2.5 × 108 pfu/50 microliters/administration/mouse). A total of five injections were performed over three weeks. On day 23 following tumor cell inoculation, all tumors were carefully resected, weighed, placed in OCT, snap-frozen in liquid nitrogen, and stored at -80°C. To determine SDF-1 effects on TC/siVEGF7-1 tumor neovasculature, tissue was analyzed for expression of the mouse endothelial marker CD31 and vascular smooth muscle cell (pericyte) marker desmin using IHC (as described below).
Immunohistochemical analysis and microscopy
For immunofluorescence staining of tumor sections, all frozen tissue sections were fixed in acetone and blocked using 4% fish gelatin in PBS. The intensity of spontaneous green fluorescence emitted by recruited GFP-expressing bone marrow cells within tumor (and matrigel sections) in vivo was too low to detect these cells without utilizing an anti-GFP antibody. No green cells could be detected in the tumor sections. For this reason, GFP-expressing cells within subcutaneous tumors (and matrigels) were detected in cryostat sections by incubating with rabbit anti-GFP (Santa Cruz Biotechnology, Santa Cruz, CA), followed by goat anti-rabbit Alexa Fluor 594 (Molecular Probes, Eugene, OR). This resulted in detection of GFP-expressing cells as red. The lack of significant spontaneous green fluorescence by GFP+ cells and immunostaining of GFP+ cells as red in tissue sections allowed co-immunostaining with other green fluorescent antibodies to stain additional cell markers (such as desmin). Fluorescein-lectin-marked tumor vessel endothelium was imaged directly via fluorescence microscopy. Immunostaining for desmin was performed by incubating with rabbit anti-desmin (abcam, Cambridge, MA), followed by either goat anti-rabbit Alexa Fluor 488 (Molecular Probes) or goat anti-rabbit Alexa 594 (Molecular Probes). Immunostaining for CD31 was performed by incubating with rat anti-mouse CD31 (BD Biosciences, San Jose, CA), followed by goat anti-rat Alexa Fluor 488 (Molecular Probes). When using mouse monoclonal antibodies, slides were preblocked overnight with F(ab) fragment (Jackson ImmunoResearch, West Grove, PA) prior to incubation with primary antibody. When co-staining was performed using two rabbit primary antibodies (e.g., rabbit anti-GFP and rabbit anti-desmin), serial adjacent tumor sections were first immunostained with each antibody alone to look at the cellular staining pattern and confirm that GFP+ cells and desmin+ cells were present. Hoescht 33342 dye (Molecular Probes) was used to stain cell nuclei. Fluorescent images were captured at 10× (eyepiece) and 10× or 20× (objective) with Optimas imaging software (San Diego, CA). SDF-1 expression was evaluated by incubating tumor sections with goat anti-SDF-1 (Santa Cruz Biotechnologies), followed by horseradish peroxidase-conjugated rabbit anti-goat IgG (Jackson ImmunoResearch Laboratories). Expression was detected with diaminobenzidine, and Gill’s hematoxylin was used for counterstaining.
Quantification of enlarged, lumen-bearing vessels
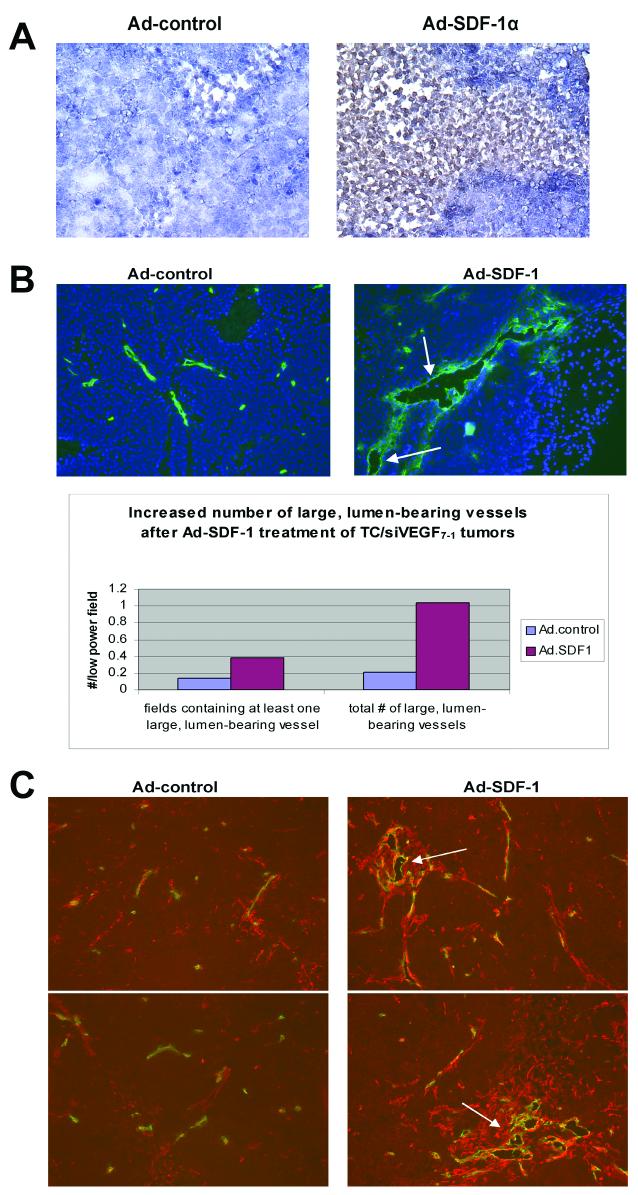
Following CD31 staining, the number of large, lumen-bearing tumor blood vessels was counted by two independent, blinded observers. A total of 66 and 71 low power (10x) viewing fields (LPF) were evaluated for Ad-control-treated and Ad-SDF-1-treated groups, respectively. Results were expressed as both the number of fields containing at least one large, lumen-bearing vessel per LPF analyzed, as well as the total number of such vessels per LFP.
Statistical Analysis
Two-tailed Student’s t test was used to statistically evaluate the difference in final tumor weights or volumes between treated and control groups. A P value of <0.05 was considered statistically significant. Chi-square test was used to analyze the difference in microscopic viewing fields containing at least one large, lumen-bearing vessel between treated and control groups. A two-sided P value <0.05 was considered statistically significant.
Results
Effect of recombinant SDF-1α on the migration of CD34+ cells in vivo
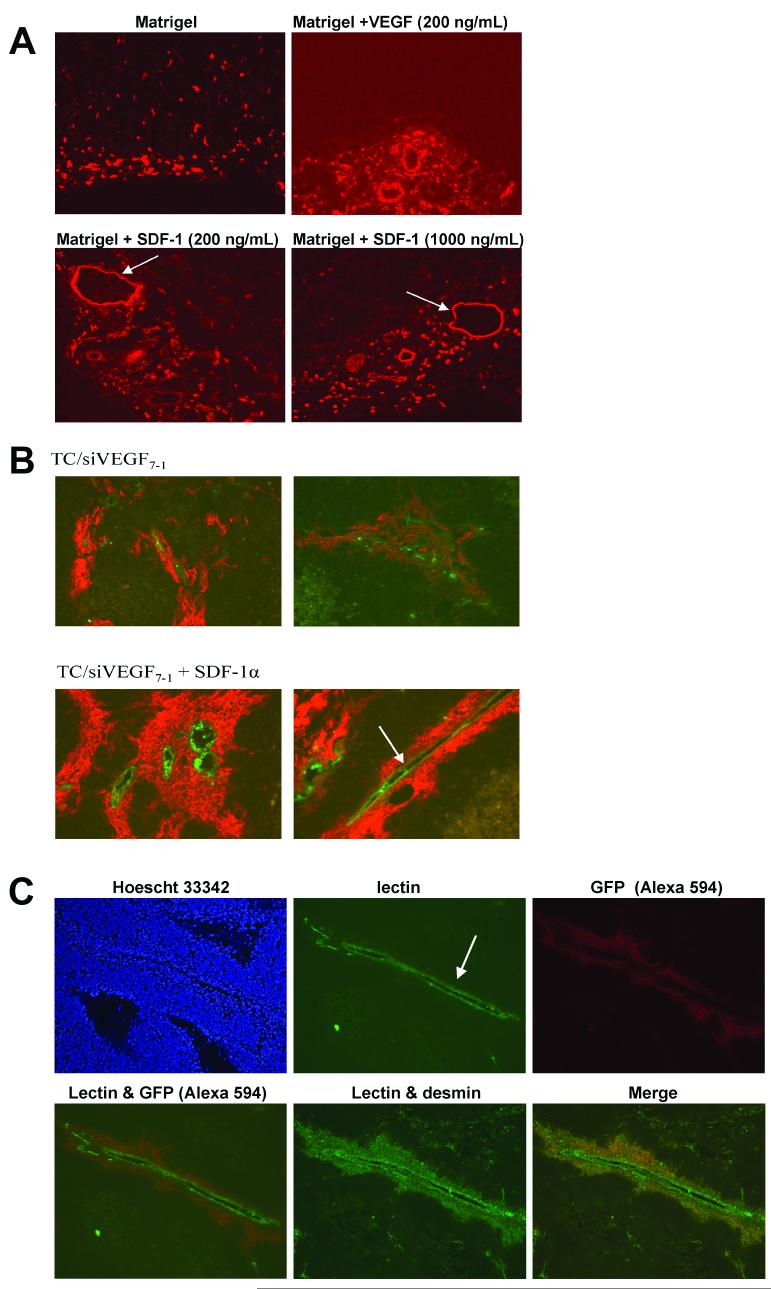
We previously demonstrated that VEGF165-inhibited TC/siVEGF7-1 cells form small tumors in vivo compared with TC71 parental or TC/si-control cells with decreased vascularity2, 12 and less infiltration of BM-derived CD34+ cells into the tumor vascular bed.2 These data suggest that this reduced vascularity and growth rate may be partially linked to the lack of BM cell recruitment into the tumor. Providing another cytokine to the local tumor microenvironment that has chemotactic activity for BM cells (including BM-derived stem cells) will allow us to ascertain whether increasing tumor infiltration with BM cells can rescue tumor growth in the absence of VEGF165. SDF-1α is one cytokine that stimulates the migration of BM cells. We therefore first determined whether recombinant SDF-1α (100, 200 or 1000 ng/mL) stimulated the migration of circulating GFP-labeled CD34+ cells into Matrigel plugs in vivo with subsequent formation of vessels. Matrigel alone (negative control), Matrigel containing 200 ng/ml recombinant human VEGF165 (positive control), or Matrigel containing SDF-1α was injected subcutaneously. GFP-expressing cells within the excised Matrigels were detected in cryostat sections by incubating with rabbit anti-GFP, followed by goat anti-rabbit Alexa594. This strategy was employed because the intensity of spontaneous green fluorescence emitted by recruited GFP-expressing bone marrow cells within the excised Matrigel plugs was too low for detection of these cells. We therefore elected to stain with the anti-GFP antibody. Immunohistochemical analysis of excised Matrigel plugs for GFP+ cells (in red) revealed substantial chemoattraction by SDF-1α at all three concentrations (Fig 1A). Matrigel alone implants demonstrated migrated GFP+ cells predominantly at the periphery, with few single cells extending towards the interior, and no GFP+ vessel-like structures. By contrast, the SDF-1α implants showed numerous GFP+ cells penetrating into the Matrigel interior, similar to what was observed in the VEGF implants. Additionally, at all three SDF-1α concentrations, large vessel structures with lumens were observed (Fig. 1A, arrows). The vascular structures seen in the VEGF-containing matrigel plug were similar but smaller.
Figure 1.
SDF-1 stimulated migration of CD34+ cells in vivo and increased the number of BM-derived pericytes surrounding functional, perfused Ewing’s tumor vessels. (A) Ad5/F35-GFP-labeled CD34+ cells were i.v.-injected into nude mice bearing s.c. Matrigel, Matrigel + recombinant SDF-1 (100, 200 or 1000 ng/mL) or Matrigel + VEGF (200 ng/mL). IHC of excised Matrigel plugs for GFP+ cells (shown in red) revealed substantial chemoattraction by SDF-1 (10x objective). The empty Matrigel implant demonstrated the presence of migrated GFP+ cells predominantly at the periphery, with few single cells extending towards the interior. By contrast, SDF-1-containing Matrigels had larger numbers of GFP+ cells penetrating deeper into the matrigel interior as well as formation of large, lumen-bearing vascular morphologies (arrows). (B) Mice were irradiated and then transplanted with GFP+ BM cells. TC/siVEGF7-1 cells in matrigel with or without recombinant SDF-1α were subcutaneously injected into opposite flanks five weeks post transplantation. Intravascular lectin perfusion staining was used to mark functional tumor vessels (in green; arrow indicates enlarged vascular structure). IHC using anti-GFP was used to detect recruited BM-derived cells (in red). SDF-1α-treated tumors showed an increase in migrated BM cells in close association with lectin-marked tumor vessels (20x objective). (C) In the presence of SDF-1, large, lumen-bearing functional blood vessels were seen (arrow). GFP+ migrated BM-derived cells (in red) formed an extensive, continuous arrangement which surrounded the vessel lumen. These BM-derived cells also expressed desmin, implying vascular smooth muscle cell (pericyte-like) differentiation (10x objective).
SDF-1α stimulates the migration of BM cells to tumor vessels with differentiation into vascular smooth muscle (pericyte-like) cells
Having demonstrated that SDF-1α is a chemotactic stimulus for BM cells in vivo, we next determined the effect of SDF-1α on the tumor vessel formation in TC/siVEGF7-1 tumors. A bone marrow transplantation (BMT) model utilizing GFP+ transgenic mice as BM donors and nude mice as recipients was used to track the migration of BM cells based on GFP expression. To demonstrate successful engraftment, recipient animal BM was collected 6 weeks post transplantation and analyzed by flow cytometry for GFP expression. BM from transplant recipients contained > 69% GFP+ cells (Supplemental Figure 1).
TC/siVEGF7-1 cells in growth factor-depleted Matrigel with or without recombinant SDF-1α (1000ng/mL) were subcutaneously injected into opposite flanks five weeks after BM engraftment. Three weeks following TC/siVEGF7-1 cell injection, fluorescein-conjugated tomato lectin was i.v.-injected into tumor-bearing mice in order to label functional, perfused blood vessels. Intravascular perfusion staining with lectin allowed us to both identify the endothelial cells within functional vessels and, in conjunction with IHC detection of GFP-expressing cells, determine whether BM cells were recruited to these perfused vessels. Tumors were then immediately excised and examined by immunohistochemistry. As with the Matrigel studies described above, the intensity of spontaneous green fluorescence emitted by GFP-expressing bone marrow cells recruited to tumors in vivo was too low to detect these cells without utilizing an anti-GFP antibody. In fact, tumors containing recruited BM-derived cells were indistinguishable from control tumors (from nontransplanted animals) on the basis of green fluorescence alone, without using anti-GFP immunostaining (data not shown). For this reason, GFP-expressing cells within tumors were detected in cryostat sections by incubating with rabbit anti-GFP, followed by a secondary antibody conjugated to the Alexa594 fluorophore. GFP+ cells were therefore red. Following i.v. infusion of tumor-bearing mice with fluorescein-conjugated lectin, perfused (functional) tumor vessel endothelium displayed very strong spontaneous green fluorescence (Fig. 1C top row, middle panel). The TC/siVEGF7-1 tumors containing recombinant SDF-1α demonstrated the presence of enlarged, perfused (lectin-marked) tumor vessels (Fig. 1B and 1C, arrows). These functional vascular structures were surrounded by numerous recruited GFP+ BM progenitor cells (Fig. 1B and 1C, GFP+ cells in red). These BM-derived GFP+ cells did not appear to integrate into the vascular endothelial lining. Rather, they formed a continuous, multilayered arrangement surrounding and adjacent to the lectin-marked vessel lumen (Fig. 1B). Spontaneous green fluorescence by these periluminal GFP-expressing cells was not observed (see lectin only image, Fig. 1C top row, middle panel). Therefore co-immunostaining with anti-GFP (Alexa594) to detect BM-derived cells and anti-desmin (Alexa488) to detect pericytes was used. Colocalization of GFP and desmin indicated that the BM-derived cells bordering the lectin+ tumor vessels also expressed desmin (Fig. 1C), consistent with pericyte differentiation. These desmin+ cells were derived from BM cells that had migrated into the tumor since they also were GFP+. Adjacent serial tumor sections were single stained for either anti-GFP or anti-desmin alone. These single immunostained sections confirmed that both GFP+ and desmin+ cells were present (data not shown).
Treatment of TC/siVEGF7-1 tumors with Ad-SDF-1α augments tumor growth and the formation of large blood vessels with increased pericyte coverage
Since TC/siVEGF7-1 tumors demonstrate decreased recruitment of BM cells and SDF-1α enhanced the chemotaxis of BM cells into the tumor, we examined the effect of intratumoral Ad-SDF-1α treatment on TC/siVEGF7-1 tumor growth and neovascularization. Beginning three days following TC/siVEGF7-1 tumor cell inoculation, mice received a total of five intratumoral injections of Ad-SDF-1α or Ad-control over a three-week period. Animals were euthanized, tumors harvested and assessed by IHC and RT-PCR for SDF-1α expression. TC/siVEGF7-1 tumors treated with Ad.SDF-1α demonstrated enhanced SDF-1 expression (Fig. 2A). Enhanced SDF-1 expression was confirmed by RT-PCR performed on RNA extracted from tumor tissues (data not shown). In addition, there was a five-fold increase in the number of large, lumen-bearing CD31+ blood vessels after Ad-SDF-1α treatment (Fig. 2B, vessels indicated by arrows). TC/siVEGF7-1 tumors treated with Ad-SDF-1α displayed a statistically significant increase in the number of low-power microscopic fields containing at least one large, lumen-bearing blood vessel (P = 0.0023). SDF-1α -treated tumors also demonstrated an increase in desmin+ cells surrounding the CD31+ tumor vessels (Fig. 2C, desmin = red; CD31 = green). Clusters of enlarged, lumen-bearing vessels in SDF-1-treated tumors were observed within the networks of desmin+ cells, which extended from the vessel lumens deeper into the tumor microenvironment (Fig. 2C, arrows denote clusters of desmin+ cells). Ad-SDF-1-treated tumors similarly displayed an increased number of perivascular cells expressing the markers alpha-smooth muscle actin and NG2 (data not shown). TC/siVEGF7-1 tumors treated with Ad-SDF-1α displayed significantly increased growth (P = 0.046, Fig. 3). Immunohistochemical staining demonstrated that tumor cells were responsible for the enlarged size rather than migrated non-malignant cells (data not shown).
Figure 2.
Treatment of TC/siVEGF7-1 tumors with adenovirus-SDF-1α induced local SDF-1α production and an increase in large tumor blood vessels with enhanced pericyte coverage. Mice with TC/siVEGF7-1 tumors were treated with intratumoral adenovirus-SDF-1α (Ad-SDF-1α) or adenovirus-empty vector control (Ad-control). Treatment with Ad.SDF-1α resulted in increased SDF-1 expression (A, 10x objective) and increased CD31+ enlarged, lumen-bearing blood vessels (arrows) (B, 10x objective) with a higher density of desmin+ pericytes (C, 10x objective).
Figure 3.
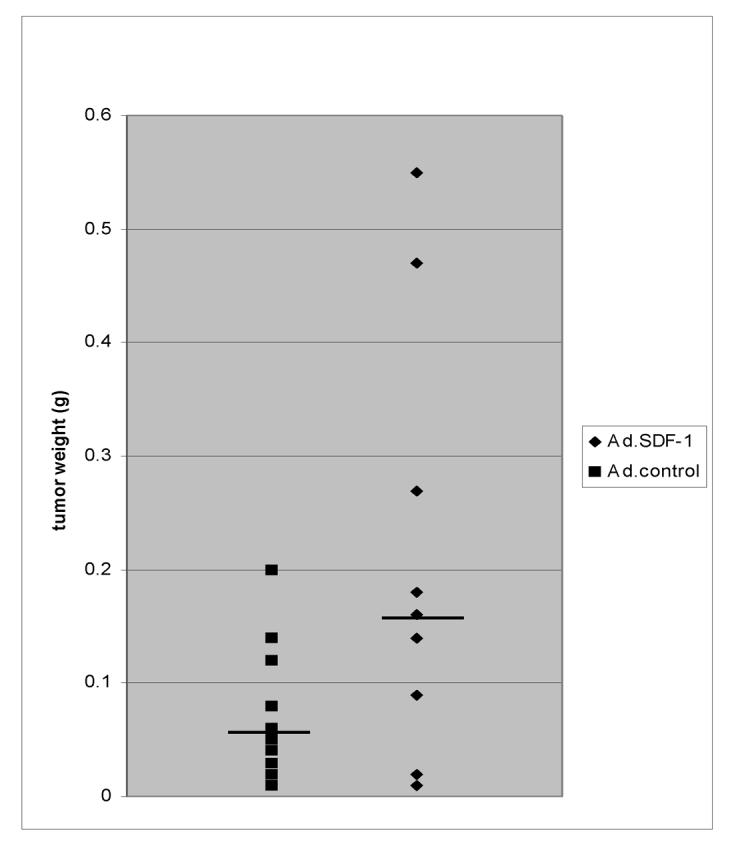
Ad-SDF-1α enhances the growth of VEGF-inhibited TC/siVEGF7-1 tumors. TC/siVEGF7-1 tumors were treated with intratumoral Ad-SDF-1α or Ad-control. Ad-SDF-1α-treated tumors demonstrated a statistically significant augmentation in tumor growth compared to Ad-control-treated tumors (p < 0.05). Horizontal bars indicate median tumor weights.
SDF-1α -mediated TC/siVEGF7-1 growth enhancement is associated with upregulation of tumor PDGF-BB expression but not VEGF165 expression
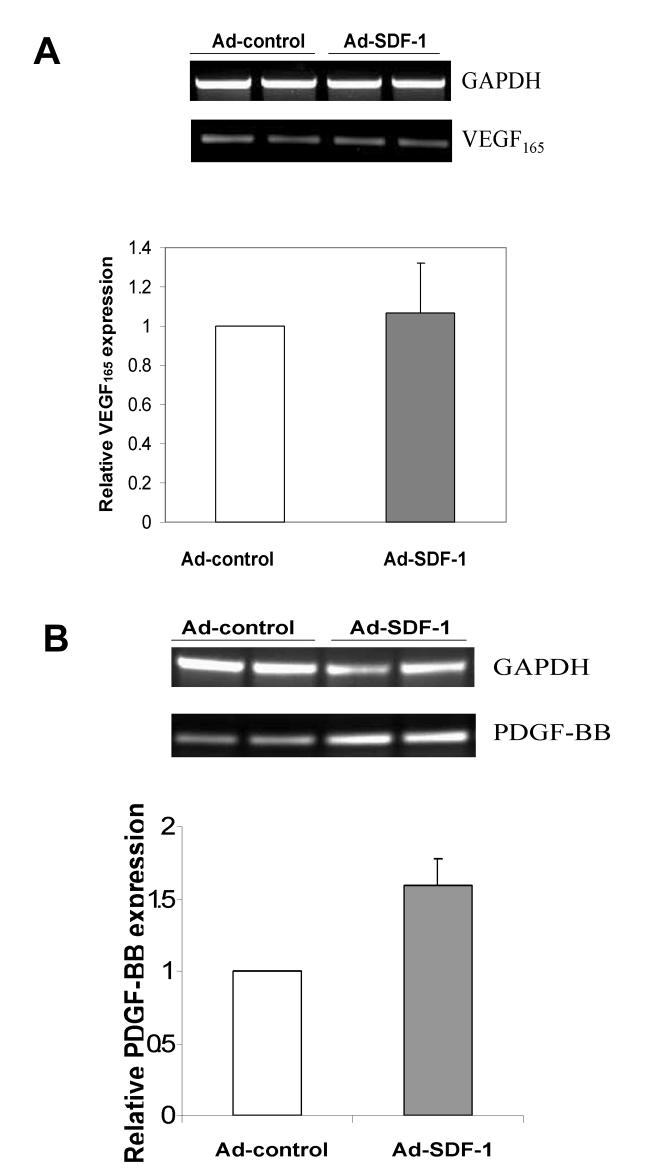
To assure that the effects of Ad-SDF-1α on tumor growth and neovasculature formation in TC/siVEGF7-1 tumors were not the result of increased levels of VEGF165, TC/siVEGF7-1 tumors treated with Ad-SDF-1α or Ad-control were evaluated by RT-PCR for VEGF165 expression. No difference was observed in VEGF165 expression levels of Ad-SDF-1α-treated and Ad-control-treated TC/siVEGF7-1 tumors (Fig. 4A, top). Real-time PCR confirmed this finding (Fig. 4A, bottom; Ad-control group gene expression normalized to 1). Furthermore, no increase in VEGF189 or VEGF121 expression was observed in Ad-SDF-1α-treated TC/siVEGF7-1 tumors (Supplemental Figure 2B). Immunohistochemical analysis of Ad-SDF-1α-treated and Ad-control-treated TC/siVEGF7-1 tumors for VEGF confirmed these findings by demonstrating no difference in VEGF protein expression (Supplemental Figure 2A). PDGF signaling plays a key role in the interaction between pericytes and vascular endothelial cells in tumor neovessels.26, 27 For example, PDGF-B-deficient embryos were found to be unable to recruit PDGFRβ+ pericyte progenitors, leading to insufficient pericyte coverage of capillary endothelium.27 Because of our finding of increased pericyte coverage in SDF-1α-treated TC/siVEGF7-1 tumors, we determined whether there was any change in PDGF-BB expression. RT-PCR analysis revealed that SDF-1α-treated TC/siVEGF7-1 tumors demonstrate increased expression of PDGF-BB compared to Ad-control-treated tumors (Fig. 4B, top). A 1.6-fold augmentation in PDGF-BB expression after Ad-SDF-1α treatment was determined by real-time PCR (Fig. 4B, bottom; Ad-control group gene expression normalized to 1).
Figure 4.
Treatment of TC/siVEGF7-1 tumors with Ad-SDF-1α increased PDGF-BB with no change in VEGF165. RNA was extracted from TC/siVEGF7-1 tumors treated with Ad-SDF-1α or Ad-control and analyzed via RT-PCR and real-time PCR. Ad-control group gene expression was normalized to 1. Ad-SDF-1α upregulated PDGF-BB (B) but not VEGF165 expression (A).
Discussion
We previously demonstrated that inhibition of VEGF165 in Ewing’s sarcoma cells led to suppressed in vivo tumor growth and decreased vessel density,2, 12 as well as reduced infiltration of BM-derived progenitor cells.2 In the present study, we investigated the relationship between BM cell recruitment and tumor growth by utilizing another cytokine, SDF-1α, to stimulate the migration of BM cells to VEGF165-inhibited tumors. We utilized bone marrow transplantation with GFP+ donors, which enabled us to track BM-derived cells on the basis of GFP expression. SDF-1α promoted the chemotaxis of BM cells to the tumor area, as well as the retainment of these migrated cells to a periendothelial residence, in close association with functional, perfused tumor blood vessels. Many of these periendothelial, GFP+ BM-derived cells co-expressed desmin, suggesting a role as pericyte precursors for tumor vessels. In the vicinity of large, perfused lectin+ tumor vessels, BM-derived cells that had undergone pericyte differentiation were found not only in direct contact with the vascular endothelium, but also as large, multilayered networks that extended outwards from the vessel lumen into the tumor tissue. Upregulation of SDF-1α in VEGF165-inhibited tumors resulted in augmented in vivo growth, an increased number of enlarged blood vessels, and enhanced vessel pericyte coverage, with a corresponding upregulation in PDGF-BB.
In this study, we used total bone marrow cells obtained from GFP+ mice (129 background) to reconstitute the BM of Balb/cAnN nude mice prior to the introduction of tumor cells. Given this experimental design, one could argue that graft versus host disease (GVHD) could account for the migration of BM cells to the tumor. However, the classic pathologic and clinical findings associated with GVHD were not seen in the transplanted mice. H & E sections of the tumors from mice receiving GFP+ mouse BM showed no apoptosis, an expected finding in GVHD. Decreased tumor establishment was not seen in the transplanted mice compared to the untransplanted mice. Finally, transplanted mice did not develop diarrhea or cutaneous lesions, hallmarks of GVHD.
The mechanism by which SDF-1α stimulates BM cell chemotaxis is yet to be fully understood. DiVietro et al., recently reported that SDF-1α stimulates the firm adhesion of lymphocytes to VCAM-1 by modulating integrin VLA-4 (α4β1) affinity, thereby stabilizing VCAM-1/VLA-4 interaction.28 α4β1 antagonists have been previously shown to inhibit tumor infiltration of CD34+ progenitor cells.29 These findings suggest that SDF-1α may recruit BM cells to tumors by enhancing stem/progenitor adhesion within tumor vessels.
Inhibition of SDF-1 signaling has been shown to disrupt BM cell recruitment and neovascularization. For example, blocking CXCR4 using a specific monoclonal antibody suppressed VEGF-induced mobilization of CXCR4+VEGFR1+ cells as well as reduced recovery of vascular perfusion after femoral artery ligation.19 Similarly, CXCR4 neutralization suppressed in vivo growth, CD31+ capillary density and intratumoral blood flow of subcutaneous gastrointestinal tumors.21 Because CXCR4 is expressed by a number of different tumor cell types, including Ewing’s sarcoma30, it is likely that inhibition of the SDF-1/CXCR4 axis could disrupt tumor growth by inhibition of both SDF-1 paracrine and autocrine signaling. While decreased BM stem/progenitor migration and tumor neovessel formation after inhibition of SDF-1/CXCR4 signaling has been reported, the capacity of SDF-1α to compensate for VEGF suppression in tumors has not been described. This is the first report demonstrating that enhanced SDF-1 expression can significantly augment the growth of a VEGF165-inhibited tumor. Tumor growth stimulation correlated with an upregulation in PDGF-BB expression and an increase in pericyte-like/vascular smooth muscle cells, a substantial fraction of which were derived from BM precursor cells. This increased pericyte volume in Ad-SDF-1-treated tumors may have contributed to the increased expression of PDGF-BB observed. By contrast, augmented tumor growth with upregulated SDF-1 expression did not correlate with increased nonspecific migration of immune cells, such as monocytes, NK cells and macrophages. Ad-control- and Ad-SDF-1-treated tumors did not display any significant differences in expression of the immune cell markers CD14, NCAM and F4/80, suggesting that a difference in immunogenicity between the two vectors (or nonspecific immune cell infiltration) was not responsible for the differences in overall tumor growth observed.
Pericytes are known to play a vital role in the regulation of blood vessel formation, maturation and stability.31 Pericytes in tumor blood vessels have been reported to provide an escape mechanism to anti-angiogenic therapy by promoting endothelial cell survival, through direct cell-to-cell contact, as well as by upregulating the endothelial survival factor angiopoietin-1 (Ang-1).32 The majority of clinical trials using anti-angiogenic agents have focused on inhibition of VEGF. Our work suggests that enhanced SDF-1α signaling may represent an alternate pathway that could potentially be utilized by tumors to sustain growth and neovasculature expansion after anti-VEGF therapy. Importantly, the SDF-1α -mediated growth rescue of VEGF-inhibited Ewing’s tumors did not necessitate an upregulation of VEGF165. This finding is consistent with a previous demonstration that neutralization of CXCR4 suppressed tumor growth and angiogenesis without affecting tumor VEGF.21 It should be noted that forced overexpression of SDF-1α did not recover the growth of VEGF165-inhibited TC/siVEGF7-1 tumors to the level of parental (VEGF165-intact) TC71 tumors. This is not unexpected since VEGF is a potent angiogenic factor and plays key roles in BM cell mobilization and chemotaxis. Therefore, replacing SDF-1 for VEGF may not restore the VEGF-mediated effects on the local endothelial cells. Still, the capacity of SDF-1α to significantly augment VEGF165-inhibited tumor growth suggests that SDF-1α may be one of several cytokines responsible for the limited success of VEGF suppression as a sole anti-angiogenic strategy in clinical practice. In addition, the mechanism by which upregulation of SDF-1α leads to increased levels of PDGF-BB in Ewing’s tumors is yet to be characterized and will be an important area of future investigation.
We also demonstrate that upregulation in tumor SDF-1α results in the formation of enlarged, lumen-bearing, functional blood vessels, implying that this chemokine may influence vascular remodeling via a direct action on endothelial cells. It has been previously shown that endothelial cell tube formation in vitro is associated with time-dependent changes in the distribution of CXCR4 present on the cell surface.33 In addition, CXCR4-deficient (or SDF-1-deficient) mice have been shown to be unable to properly form large blood vessels in the gastrointestinal tract.34 Endothelial cells of the superior mesenteric artery in SDF-1-/- embryos were found to be incapable of intussusception and filopodial extension, which are important processes contributing to angiogenesis.35 SDF-1-mediated changes in surface CXCR4 distribution/activity may impact cell adhesion molecules, promoting the end-to-end arrangement of endothelial cells into large vascular structures. VEGF, in contrast, may promote vessel branching, favoring the arrangement of endothelial cells into tighter, smaller-lumened structures. Because both VEGF and SDF-1 influence the activity of endothelial cells, it is possible that final vessel morphology is dictated by the relative levels of these two cytokines within the local microenvironment. Thus, SDF-1 effect on overall vessel morphology might be more pronounced in the absence of VEGF, as was the case in the SDF-1-supplemented matrigel implants (Fig. 1A) and Ad-SDF-1-treated TC/siVEGF7-1 tumors (Fig. 2B) utilized in our experiments. While large vessels within tumors are often regarded as aberrant, their lectin+ staining in our work indicates that these vessels were in fact perfused (functional), and therefore contributing to tumor cell nourishment. The extensive pericyte networks surrounding these large diameter vessels may be important in maintaining their integrity/stability within the tumor microenvironment. Furthermore, multiple concentric layers of pericytes/smooth muscle cells coating the endothelial lining of large diameter vessels may be important in regulating blood flow through the larger vessel lumens and aid in withstanding the larger blood pressures exterted therein. While the SDF-1α -mediated stimulation of enlarged blood vessel formation correlated with augmented tumor growth in our study, an underlying mechanism for this relationship is not yet understood.
In summary, our findings suggest that the effects of SDF-1α on tumor neovascularization include augmented chemotaxis of BM cells, retainment of BM-derived pericytes in close association with the vessel endothelial lining, enhanced overall pericyte coverage of tumor neovessels, and remodeling of vascular endothelium into larger, functional structures. These processes in combination act to promote the growth of Ewing’s tumors, even in the face of markedly reduced VEGF165. Taken together, this data supports the hypothesis that BM-derived cells play a critical role in the expansion of the Ewing’s tumor vasculature, and that vasculogenesis may be a mechanism by which tumors can circumvent the effects of anti-angiogenic therapy targeted at VEGF.
Supplementary Material
Acknowledgments
Grant support: NIH, Grant number: R01 CA103986 (ESK), Core Grant CA 16672
References
- 1.Bolontrade MF, Zhou RR, Kleinerman ES. Vasculogenesis plays a role in the growth of Ewing’s sarcoma in vivo. Clin Cancer Res. 2002;8:3622–7. [PubMed] [Google Scholar]
- 2.Lee TH, Bolontrade MF, Worth LL, Guan H, Ellis LM, Kleinerman ES. Production of VEGF165 by Ewing’s sarcoma cells induces vasculogenesis and the incorporation of CD34+ stem cells into the expanding tumor vasculature. Int J Cancer. 2006;119:839–46. doi: 10.1002/ijc.21916. [DOI] [PubMed] [Google Scholar]
- 3.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 4.Dwenger A, Rosenthal F, Machein M, Waller C, Spyridonidis A. Transplanted bone marrow cells preferentially home to the vessels of in situ generated murine tumors rather than of normal organs. Stem Cells. 2004;22:86–92. doi: 10.1634/stemcells.22-1-86. [DOI] [PubMed] [Google Scholar]
- 5.Santarelli JG, Udani V, Yung YC, Cheshier S, Wagers A, Brekken RA, Weissman I, Tse V. Incorporation of bone marrow-derived Flk-1-expressing CD34+ cells in the endothelium of tumor vessels in the mouse brain. Neurosurgery. 2006;59:374–82. doi: 10.1227/01.NEU.0000222658.66878.CC. [DOI] [PubMed] [Google Scholar]
- 6.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–12. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 7.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–72. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavlakovic H, Von Schutz V, Rossler J, Koscielniak E, Havers W, Schweigerer L. Quantification of angiogenesis stimulators in children with solid malignancies. Int J Cancer. 2001;92:756–60. doi: 10.1002/1097-0215(20010601)92:5<756::aid-ijc1253>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 9.Holzer G, Obermair A, Koschat M, Preyer O, Kotz R, Trieb K. Concentration of vascular endothelial growth factor (VEGF) in the serum of patients with malignant bone tumors. Med Pediatr Oncol. 2001;36:601–4. doi: 10.1002/mpo.1136. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Z, Zhou RR, Guan H, Bucana CD, Kleinerman ES. E1A gene therapy inhibits angiogenesis in a Ewing’s sarcoma animal model. Mol Cancer Ther. 2003;2:1313–9. [PubMed] [Google Scholar]
- 11.Fuchs B, Inwards CY, Janknecht R. Vascular endothelial growth factor expression is upregulated by EWS-ETS oncoproteins and Sp1 and may represent an independent predictor of survival in Ewing’s sarcoma. Clin Cancer Res. 2004;10:1344–53. doi: 10.1158/1078-0432.ccr-03-0038. [DOI] [PubMed] [Google Scholar]
- 12.Guan H, Zhou Z, Wang H, Jia S-F, Liu W, Kleinerman ES. A small interfering RNA targeting vascular endothelial growth factor inhibits Ewing’s sarcoma growth in a xenograft mouse model. Clin Cancer Res. 2005;11:2662–9. doi: 10.1158/1078-0432.CCR-04-1206. [DOI] [PubMed] [Google Scholar]
- 13.Jo DY, Rafii S, Hamada T, Moore MA. Chemotaxis of primitive hematopoietic cells in response to stromal cell-derived factor-1. J Clin Invest. 2000;105:101–11. doi: 10.1172/JCI7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–94. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 15.Lapidot T. Mechanism of human stem cell migration and repopulation of NOD/SCID and B2mnull NOD/SCID mice. The role of SDF-1/CXCR4 interactions. Ann N Y Acad Sci. 2001;938:83–95. doi: 10.1111/j.1749-6632.2001.tb03577.x. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–8. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 17.De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, Truffa S, Biglioli P, Napolitano M, Capogrossi MC, Pesce M. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–82. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 18.Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, Sata M, Ichiki T, Takeshita A, Egashira K. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–61. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 19.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–67. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–89. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 21.Guleng B, Tateishi K, Ohta M, Kanai F, Jazag A, Ijichi H, Tanaka Y, Washida M, Morikane K, Fukushima Y, Yamori T, Tsuruo T, et al. Blockade of the stromal cell-derived factor-1/CXCR4 axis attenuates in vivo tumor growth by inhibiting angiogenesis in a vascular endothelial growth factor-independent manner. Cancer Res. 2005;65:5864–71. doi: 10.1158/0008-5472.CAN-04-3833. [DOI] [PubMed] [Google Scholar]
- 22.Fushimi T, O’Connor TP, Crystal RG. Adenoviral gene transfer of stromal cell-derived factor-1 to murine tumors induces the accumulation of dendritic cells and suppresses tumor growth. Cancer Res. 2006;66:3513–22. doi: 10.1158/0008-5472.CAN-05-1493. [DOI] [PubMed] [Google Scholar]
- 23.Aghi M, Cohen KS, Klein RJ, Scadden DT, Chiocca EA. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res. 2006;66:9054–64. doi: 10.1158/0008-5472.CAN-05-3759. [DOI] [PubMed] [Google Scholar]
- 24.Reddy K, Lee T, Zhou Z, Jia SF, Guan H, Kleinerman ES. CD34+ cells augment the growth of Ewing’s sarcoma tumors that are deficient in VEGF.; Proceedings of the AACR Special Conference: Anti-angiogenesis and Drug Delivery to Tumors: Bench to Bedside and Back; Waltham, MA. 2005.pp. 9–13. [Google Scholar]
- 25.Yotnda P, Onishi H, Heslop HE, Shayakhmetov D, Lieber A, Brenner M, Davis A. Efficient infection of primitive hematopoietic stem cells by modified adenovirus. Gene Ther. 2001;8:930–7. doi: 10.1038/sj.gt.3301488. [DOI] [PubMed] [Google Scholar]
- 26.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–55. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 27.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–5. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 28.DiVietro JA, Brown DC, Sklar LA, Larson RS, Lawrence MB. Immobilized stromal cell-derived factor-1α triggers rapid VLA-4 affinity increases to stabilize lymphocyte tethers on VCAM-1 and subsequently initiate firm adhesion. J Immunol. 2007;178:3903–11. doi: 10.4049/jimmunol.178.6.3903. [DOI] [PubMed] [Google Scholar]
- 29.Jin H, Aiyer A, Su J, Borgstrom P, Stupack D, Friedlander M, Varner J. A homing mechanism for bone marrow-derived progenitor cell recruitment to the neovasculature. J Clin Invest. 2006;116:652–62. doi: 10.1172/JCI24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chansky HA, Barahmand-Pour F, Mei Q, Kahn-Farooqi W, Zielinska-Kwiatkowska A, Blackburn M, Chansky K, Conrad EU, 3rd, Bruckner JD, Greenlee TK, Yang L. Targeting of EWS/FLI-1 by RNA interference attenuates the tumor phenotype of Ewing’s sarcoma cells in vitro. J Orthop Res. 2004;22:910–7. doi: 10.1016/j.orthres.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Lamagna C, Bergers G. The bone marrow constitutes a reservoir of pericyte progenitors. J Leukoc Biol. 2006;80:677–81. doi: 10.1189/jlb.0506309. [DOI] [PubMed] [Google Scholar]
- 32.Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes HP, Menger MD, Ullrich A, Vajkoczy P. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18:338–40. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- 33.Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002;99:2703–11. doi: 10.1182/blood.v99.8.2703. [DOI] [PubMed] [Google Scholar]
- 34.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–4. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 35.Ara T, Tokoyoda K, Okamoto R, Koni PA, Nagasawa T. The role of CXCL12 in the organ-specific process of artery formation. Blood. 2005;105:3155–61. doi: 10.1182/blood-2004-07-2563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.