Abstract
Objective
Non-opioid analgesics (NOA) are widely used to palliate osteoarthritis (OA) pain, however their role in health-related quality of life (HRQoL) in OA has not been well studied. Here, we assess the relationship of pain, physical function, and HRQoL to NOA use in symptomatic knee OA.
Methods
NOA dose, pain, physical function, and HRQoL were evaluated longitudinally over one year in medial knee OA. Doses provided by subjects’ weekly medication diaries were normalized to equi-analgesic ibuprofen-equivalents (IE). Descriptive analyses at baseline, 1.5, and 12 months, and non- parametric comparisons of NOA with pain, physical function, and HRQoL at 1.5 months and over 12 months were performed.
Results
71 subjects (19 males: 52 females; mean 57 ± 10.5 years) used an overall median of 300 mg/week of IE. 25 subjects reported no analgesic use during the study; of the 46 subjects that reported NOA use, the median intake was 1325 mg/week IE. Whereas age, physical functioning and HRQoL were predictive of NOA dose both at 1.5 months and during the entire study, pain level was not. The median NOA dose declined over 12 months (p = 0.02), however the change was not associated with changes in physical functioning, HRQoL or pain.
Conclusion
Greater age and worse physical function and HRQoL, but not pain severity, are predictive of NOA use in symptomatic knee OA. Longitudinally, NOA use does not change as a function of pain. These data suggest that pain is not the primary determinant of NOA use over time among OA patients.
Keywords: Osteoarthritis, Quality of Life, Pain, Analgesics, Osteoarthritis of the knee
Introduction
Non-opioid analgesics (NOA), such as the non-steroidal anti-inflammatory agents (NSAIDs), the coxibs, and acetaminophen have varied anti-inflammatory and analgesic properties and are widely used to palliate the pain of osteoarthritis (OA). However, despite the availability and usage of a variety of these agents, OA patients continue to suffer chronic pain and a diminished health related quality of life (HRQoL), and the morbidity related to OA remains substantial.
To date the relationships between the use of such analgesic agents and pain, functional status, and quality of life in patients with OA have not been evaluated with controlled trials. It has previously been suggested that patients with relatively good physical functioning may not require regular analgesic therapy, and that relief obtained with analgesic medication may be a more significant indicator of quality of life than of pain levels in patients with OA (1).
OA patients may be started on analgesics for the indication of pain by their primary care physicians, and may inadvertently continue the regular use of these prescriptions despite their pain having improved or abated. In this study, we assessed the relationship between demographics, pain severity, physical functioning, and HRQoL as predictor variables for continued non-opioid analgesic use in patients with symptomatic knee OA, to test the hypothesis that NOA use correlates more closely with indices of well being than with pain in knee OA.
Methods
Subjects were recruited as part of an ongoing, randomized, double-blind, placebo-controlled, three-year longitudinal assessment of a biomechanical intervention for knee OA (ClinicalTrials.gov NCT00076453). to study the clinical effects of altered biomechanics in knee OA. The biomechanical intervention under investigation in the primary study is a custom fabricated laterally wedged orthotic shoe insert, which is compared in a double-blind randomized manner to a neutral orthotic insert; the primary outcomes to be assessed are dynamic loading of the medial knee and pain reduction at three years.
After approval by the Institutional Review Board and provision of informed consent, subjects were included if they had symptomatic knee OA defined both radiographically- grades 2 or 3 OA based on the Kellgren and Lawrence scale (2), as modified by Felson, et al. (3), and symptomatically- at least 20 mm (of a 100 mm visual analog scale) of knee pain while walking on a level surface, corresponding to question #1 of the Western Ontario and MacMaster Universities OA Index (WOMAC) (4). Each subject fulfilled the American College of Rheumatology criteria for the classification of knee OA (5) and had predominantly medial compartment involvement. Subjects were excluded if they had clinically evident OA involving lower extremity joints other than the knees, if they had any inflammatory arthropathy or other significant medical disease, or if they had a body mass index (BMI) > 35 kg/m2; in addition, as the study group was recruited primarily for a biomechanical study, subjects with prior arthroplasty of any lower extremity joint were excluded. The purpose of the primary study was to characterize biomechanical alterations, and the study was explicitly designed not to interfere with the normal OA care provided to these patients by their rheumatologists. Therefore, there were no specific instructions concerning the use of analgesics, except that any use was to be documented in the diaries and at the study visits. The use of complementary and alternative medicine was not excluded but was not to vary during the study period. In addition, hyaluronans were not permitted within 6 months of enrollment, physiotherapy and exercise programs were permitted but the regimen was not to change during the study, and weight was tracked. At each study visit (at baseline, and months 1.5, 3, 6, 9, and 12), subjects underwent Medical Histories and Physical Examinations, and completed the standardized questionnaires.
Medication use
Medications taken by each subject at entry were recorded directly from their prescription bottles and by questioning regarding over-the-counter medications and dietary supplements. To determine medication use during the first year, subjects were each given a medication diary at study entry. They were instructed to record on a weekly basis any analgesic use for knee pain. If pain medication was used for an indication other than knee pain, it was not included in the patient’s total use of pain medication. In addition to the medication diaries, subjects were asked about medication and dosage changes at each of the visits during the 12 month follow-up. For each subject, the medication intake was determined by averaging the weekly medication usage during the course of the 12 month study period, and then normalizing to equi-analgesic doses of ibuprofen (6). These values were then expressed as total mg ibuprofen-equivalents (IE) per week. Subjects for whom medication diaries were unavailable or were incomplete were excluded from the analyses. All of the data were collected for this study during the first year of participation of each subject, in the three-year longitudinal intervention study for knee OA. The values are all expressed as total medication per week (Ibuprofen-equivalents/wk). As subjects did not initiate their use of the medication diaries until the baseline visit, it was not possible to accurately quantify the medication taken in the week prior to baseline. Therefore, the Month 1.5 visit was used for the early timepoint in the analyses. Follow-up at 6 weeks (1.5 months) and 12 months were prospectively chosen as primary endpoints and those data are reported.
Assessment of pain, physical function and HRQoL
Health Outcome measures of interest were pain, physical function and HRQoL. These were assessed using the Health Assessment Questionnaire (HAQ) (7), the Short Form-36 (SF-36) (8), and the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index (4) at study entry, 1.5 months and at 12 months. All three tools include validated assessments of pain, physical function, and HRQoL.
Statistical Analyses
Descriptive statistics of the study subjects and their non-opioid analgesic use, and health outcome measures of pain, physical function, and HRQoL were obtained. The data were tested for normality. A non-parametric test, the Kruskall-Wallis test, was utilized to compare the average non-opioid analgesic dose per week at 1.5 months and over the year as a function of the subjects’ study entry and 12 month measures of pain, physical function, and HRQoL, taking p < 0.05 as significant using a two-tailed test.
Results
A total of 71 subjects (19 male: 52 female), with a mean ± S.D. age of 57 ± 10.5 years participated. The majority of the subjects were either African American (46.5%) or Caucasian (38%). The mean ± S.D. BMI of the study group was 30.1 ± 6.5 kg/m2. The overall median scores (and interquartile ranges (IQR)) at baseline for pain, physical function, and HRQoL, using the HAQ, SF-36, and WOMAC are presented in Table 1. During the course of the study, the median (IQR) intake of non-opioid analgesics (NOA) was 300 mg/week of ibuprofen-equivalents (IE) (0, 3147 mg/wk); however 25 subjects reported that they did not take any analgesics during the study period at all. As a large number of subjects reported medication intake as zero and the data set did not have a normal distribution, the medication dose data were divided into approximate tertiles for the purposes of analysis: zero intake of NOA, between zero and the median intake of the remaining subjects, and greater than the median intake. One-third (n = 25, 35.2%) of the participants did not use any NOA despite a diagnosis of symptomatic knee OA at study entry, another one-third (n = 23, 32.4%) of the participants used between 0 and 1325 mg/week of IE, and the remaining 23 subjects (32%) used 1325 mg/week or higher of IE over the study duration for their knee OA.
Table 1. Baseline pain, physical function, and health-related quality of life scores.
| Health Parameters | Median (IQR) |
|---|---|
| HAQ- Global | 0.6 (0.2, 1.0) |
| HAQ- Pain | 0.9 (0.4, 1.2) |
| HAQ- Disability Index (DI) | 0.5 (0.3, 0.8) |
| SF-36 Bodily Pain (BP) | 57.5 (45, 67.5) |
| SF-36 General Health (GH) | 75 (60, 85) |
| SF-36 Mental Health (MH) | 78 (68, 88) |
| SF-36 Physical Functioning (PF) | 60 (45, 80) |
| SF-36 Role Emotional (RE) | 100 (67, 100) |
| SF-36 Role Physical (RP) | 50 (0, 100) |
| SF-36 Social Functioning (SF) | 80 (68, 100) |
| SF-36 Vitality (V) | 60 (50, 70) |
| SF-36 Mental Component Score (MCS) | 76.5 (63.8, 85.3) |
| SF-36 Physical Component Score (PCS) | 62.5 (46.3, 74.4) |
| WOMAC- Activities of Daily Living (ADL) | 610 (368, 823) |
| WOMAC- Stiffness | 86 (28, 124) |
| WOMAC- Pain | 181 (97, 281) |
Abbreviations: HAQ= Health Assessment Questionnaire: Global Assessment, Pain, Disability Index; score range 0 (best) to 3 (worst) SF-36= Short Form-36: Bodily Pain, General Health, Mental Health, Physical Functioning, Role Emotional, Role Physical, Social Functioning, Vitality, Mental Component Score, Physical Component Score; score range 0 (worst) to 100 (best). WOMAC= Western Ontario and McMaster Universities Osteoarthritis Index: Activities of Daily Living, score range 0 (best) to 1700 (worst); Stiffness, score range 0 (best) to 200 (worst); Pain, score range 0 (best) to 500 (worst).
Analysis of the three groups of NOA intake revealed that they had similar gender and BMI distributions (Table 2), however, the subjects who used more than 1325 mg/week of NOA over the year were older (mean 62.9 ± 10.5 years) than those who used either no NOA (55.2 ± 9.9 years) or between 0-1325 mg/week of NOA (54.3 ± 9.4 years), p = 0.02. Age did not correlate with pain (SF-36 BP, Pain HAQ, WOMAC pain) or with physical function (PF, WOMAC-ADL, global HAQ or HAQ-DI), but did correlate with HRQoL {PCS (r=-0.32, p=0.008) and GH (r=-0.25, p=0.04)}.
Table 2. Medication use and demographics and baseline health scores.
| Demographic and Health Parameters | No medication | <1325 mg/week+ | > 1325 mg/week+ | p-value |
|---|---|---|---|---|
| Age (years) (Expressed as mean ± SD) | 55.2 ± 9.9 | 54.3 ± 9.4 | 62.9 ± 10.5 | 0.02* |
| Race | ||||
| Black | 11 (44%) | 10 (44%) | 11 (50%) | |
| White | 11 (44%) | 8 (35%) | 8 (36%) | |
| Other | 3 (12%) | 5 (21%) | 3 (14%) | 0.87 |
| Gender | ||||
| Male | 5 (20%) | 7 (30%) | 7 (30%) | |
| Female | 20 (80%) | 16 (70%) | 16 (70%) | |
| BMI (kg/m2) | 28.6 ± 3.6 | 29.4 ± 4.5 | 32.3 ± 9.5 | 0.32 |
| HAQ- Global (Expressed as median (IQR)) | 0.7 (0.4, 1.0) | 0.5 (0.2, 1.0) | 0.7 (0.2, 1.1) | 0.77 |
| HAQ- Pain | 0.9 (0.3, 1.1) | 0.8 (0.4, 1.1) | 0.9 (0.5, 1.4) | 0.45 |
| HAQ- Disability Index (DI) | 0.5 (0.3, 0.8) | 0.4 (0.1, 0.6) | 0.6 (0.5, 0.9) | 0.01* |
| SF-36 Bodily Pain (BP) | 58 (45, 74) | 58 (47, 68) | 58 (40, 68) | 0.55 |
| SF-36 General Health (GH) | 81 (65, 90) | 75 (65, 88) | 65 (55, 75) | 0.02* |
| SF-36 Mental Health (MH) | 80 (70, 88) | 76 (60, 85) | 76 (68, 88) | 0.78 |
| SF-36 Physical Functioning (PF) | 65 (50, 79) | 67 (45, 85) | 55 (35, 65) | 0.07 |
| SF-36 Role Emotional (RE) | 100 (100, 100) | 100 (25, 100) | 100 (33, 100) | 0.22 |
| SF-36 Role Physical (RP) | 67 (0, 88) | 75 (25, 100) | 13 (0, 75) | 0.03* |
| SF-36 Social Functioning (SF) | 90 (78, 100) | 78 (68, 100) | 78 (60, 100) | 0.11 |
| SF-36 Vitality (V) | 65 (53, 75) | 60 (49, 70) | 55 (50, 65) | 0.20 |
| SF-36 Mental Component Score (MCS) | 79.9 (68.7, 89.9) | 75.8 (58.4, 85.3) | 73.4 (55.5, 80.6) | 0.08 |
| SF-36 Physical Component Score (PCS) | 63.3 (47.7, 73.5) | 66.6 (56.9, 80.6) | 50.0 (35.0, 63.1) | 0.02* |
| WOMAC- Activities of Daily Living (ADL) | 484 (258, 699) | 687 (368, 802) | 786 (450, 1090) | 0.15 |
| WOMAC- Stiffness | 80 (34, 124) | 74 (14, 123) | 97 (31, 130) | 0.40 |
| WOMAC- Pain | 152 (81, 279) | 195 (119, 281) | 217 (94, 282) | 0.68 |
Medication intakes are expressed in ibuprofen-equivalent doses (see text for explanation)
significant p-value
Importantly, there was no association between baseline pain severity and quantity of NOA use, as assessed by any of the validated pain scales (HAQ-Pain (p = 0.45), SF-36 Bodily Pain score (p = 0.55), and WOMAC-Pain subscale (p = 0.68)), as shown in Table 2. In addition there was no association between 1.5 months or 12 months pain severity and quantity of NOA used at either the 1.5 months visit or over the 12 months study duration (HAQ-Pain (p = 0.17 and 0.54, for 1.5 months and 12 months, respectively), SF-36 Bodily Pain score (p = 0.07 and 0.29, respectively), and WOMAC-Pain subscale (p = 0.15 and 0.56, respectively)), as shown in Table 3.
TABLE 3. Correlation of medication with health outcome measures (Month 1.5).
(Expressed as Spearman’s Rank Coefficient (p-value))
| Health Outcome Measure | Medication use Correlation (p) | Yearly average medication Correlation (p) |
|---|---|---|
| HAQ- Global | 0.19 (0.11) | 0.14 (0.26) |
| HAQ- Pain | 0.17 (0.17) | 0.08 (0.54) |
| HAQ- Disability Index (DI) | 0.14 (0.25) | 0.11 (0.39) |
| SF-36 Bodily Pain (BP) | -0.22 (0.07) | -0.13 (0.29) |
| SF-36 General Health (GH) | -0.22 (0.07) | -0.27 (0.03)* |
| SF-36 Mental Health (MH) | -0.04 (0.78) | -0.01 (0.91) |
| SF-36 Physical Functioning (PF) | -0.28 (0.02)* | -0.22 (0.07) |
| SF-36 Role Emotional (RE) | -0.10 (0.40) | -0.13 (0.30) |
| SF-36 Role Physical (RP) | -0.25 (0.04)* | -0.12 (0.32) |
| SF-36 Social Functioning (SF) | -0.14 (0.24) | -0.11 (0.36) |
| SF-36 Vitality (V) | -0.02 (0.85) | -0.01 (0.93) |
| SF-36 Mental Component Score (MCS) | -0.09 (0.45) | -0.08 (0.49) |
| SF-36 Physical Component Score (PCS) | -0.31 (0.01)* | -0.23 (0.06) |
| WOMAC- Activities of Daily Living (ADL) | 0.18 (0.13) | 0.20 (0.10) |
| WOMAC- Stiffness | 0.21 (0.09) | 0.10 (0.40) |
| WOMAC- Pain | 0.17 (0.15) | 0.07 (0.56) |
significant p-value
Evaluation of the parameters of physical function and HRQoL, however, revealed that these domains were strongly associated with the quantity of NOA used by subjects during the 12 month study period. At baseline, those subjects with the most disability, as measured by the HAQ-Disability Index (DI) scores, predicted higher NOA weekly use over the study duration relative to those with less disability (p=0.01). Similar results were also observed with the SF-36 physical function and HRQoL subscales, whereas those who scored poorer on the Physical Component Score, General Health, and Role Physical used significantly more NOA than their peers who scored better in each category, p=0.02, 0.02, and 0.03, respectively. In addition, there was a comparable trend for the SF-36 Mental Component Score, p = 0.08; (Table 2). An inverse relationship between NOA dose at 1.5 months and Physical Functioning (p = 0.02), Role Physical (p = 0.04) and Physical Component Score (p = 0.01) was observed. Similarly, the yearly NOA dose was inversely associated with General Health (p = 0.02) at 1.5 months (Table 3).
Subgroup analyses were performed on the 46 subjects who reported some medication use (not zero) during the study period. Using the sub-sample size of 46 and the previously determined standard errors, there was 80% power to detect correlations as small as 0.399; even using a t-test with equal group sizes, there would be 80% power to detect effect sizes of 0.89. The median (IQR) medication intake in this subpopulation was 1325 mg/week (325, 6650 mg/wk) of IE. Spearman’s rank correlation coefficients (r) between the NOA weekly dose for these 46 subjects with some NOA use over the year and pain, physical function, and HRQoL are shown in Table 4. As with the total study group, there was no correlation in any of the pain subscales between baseline pain levels and total medication intake, even among those subjects who actually used NOA: HAQ-Pain, r = 0.11, p = 0.49; SF-36 Bodily Pain, r = -0.19, p = 0.21; WOMAC Pain, r = -0.002, p = 0.99 (Table 4). In contrast, there was a significant correlation between NOA dose and age (r = 0.42, p = 0.004), as well as NOA dose predicted by baseline physical function and HRQoL indices: HAQ-Disability Index, r = 0.40, p = 0.006; SF-36 General Health, r = -0.35, p = 0.02; SF-36 Physical Function r = -0.30, p = 0.04; SF-36 Role Physical r = -0.48, p = 0.001; SF-36 Physical Component Score, r = -0.45, p = 0.002.
Table 4. Correlation of demographics, health outcome measures and medication use.
Sub-group analysis of the 46 subjects who took NOA during the study
| Parameter | Spearman’s Rank Correlation (r) with dose | p-value |
|---|---|---|
| Age | 0.42 | 0.004* |
| BMI | -0.02 | 0.90 |
| HAQ- Global | 0.06 | 0.70 |
| HAQ- Pain | 0.11 | 0.49 |
| HAQ- Disability Index (DI) | 0.40 | 0.006* |
| SF-36 Bodily Pain (BP) | -0.19 | 0.21 |
| SF-36 General Health (GH) | -0.35 | 0.02* |
| SF-36 Mental Health (MH) | 0.03 | 0.85 |
| SF-36 Physical Functioning (PF) | -0.30 | 0.04* |
| SF-36 Role Emotional (RE) | 0.03 | 0.87 |
| SF-36 Role Physical (RP) | -0.48 | 0.001* |
| SF-36 Social Functioning (SF) | -0.05 | 0.76 |
| SF-36 Vitality (V) | -0.13 | 0.39 |
| SF-36 Mental Component Score (MCS) | -0.02 | 0.90 |
| SF-36 Physical Component Score (PCS) | -0.45 | 0.002* |
| WOMAC- Activities of Daily Living (ADL) | 0.10 | 0.51 |
| WOMAC- Stiffness | 0.10 | 0.49 |
| WOMAC- Pain | -0.002 | 0.99 |
significant p-value
Longitudinal analysis revealed that there were significant improvements in mean (± SD) pain scores during the course of the 12 months, as measured by SF-36 Bodily Pain, 64.1 ± 18.6 vs. 71.4 ±17.7 (p = 0.04), and WOMAC Pain, 106 ± 116 vs. 77 ± 102, (p = 0.01), subscales at 1.5 months and 12 months respectively (Table 5). Aside from the WOMAC stiffness (p = 0.01) and WOMAC ADL (p=0.004) subscales, there was no significant variability in the other HRQoL and physical function scores over time. In addition, there was a significant reduction in mean (± S.D.) medication use (expressed as ibuprofen-equivalents) by month 12, 3262 ± 7198 vs. 2013 ± 4863 (p = 0.02).
TABLE 5. Correlation between changes in medication dose and health outcome measures between Month 1.5 and Month 12.
| Parameter | Month 1.5 (Mean ± S.D) | Month 12 (Mean ± S.D) | p-value |
|---|---|---|---|
| HAQ- Global | 0.51 ± 0.44 | 0.38 ± 0.34 | 0.16 |
| HAQ- Pain | 0.62 ± 0.54 | 0.55 ± 0.51 | 0.66 |
| HAQ- Disability Index (DI) | 0.45 ± 0.42 | 0.44 ± 0.46 | 0.99 |
| SF-36 Bodily Pain (BP) | 64.1 ± 18.6 | 71.4 ± 17.7 | 0.04* |
| SF-36 General Health (GH) | 72.9 ± 16.3 | 72.2 ± 19.0 | 0.97 |
| SF-36 Mental Health (MH) | 65.1 ± 8.3 | 64.8 ± 8.0 | 0.84 |
| SF-36 Physical Functioning (PF) | 65.9 ± 23.6 | 67.9 ± 22.4 | 0.92 |
| SF-36 Role Emotional (RE) | 75.7 ± 36.3 | 79.0 ± 34.3 | 0.44 |
| SF-36 Role Physical (RP) | 67.9 ± 38.3 | 67.7 ± 36.6 | 0.99 |
| SF-36 Social Functioning (SF) | 83.0 ± 18.5 | 85.0 ± 16.9 | 0.36 |
| SF-36 Vitality (V) | 55.7 ± 9.5 | 53.7 ± 10.4 | 0.69 |
| SF-36 Mental Component Score (MCS) | 69.9 ± 13.7 | 70.6 ± 12.0 | 0.52 |
| SF-36 Physical Component Score (PCS) | 67.5 ± 19.0 | 69.6 ± 18.7 | 0.91 |
| WOMAC- Activities of Daily Living (ADL) | 390 ± 391 | 289 ± 370 | 0.004* |
| WOMAC- Stiffness | 44.6 ± 45.5 | 30.6 ± 42.3 | 0.01* |
| WOMAC- Pain | 106 ± 116 | 77 ± 102 | 0.01* |
| NSAID Dose+ | 3262 ± 7198 | 2013 ± 4863 | 0.02* |
significant p-value
Expressed in mg of ibuprofen-equivalent doses
Despite the significant reductions both in pain scores and in medication intake over time, there was no significant association between medication dose and pain at month 1.5, nor was there an association between change in medication dose and change in pain scores by month 12. (HAQ-Pain (p = 0.95), SF-36 Bodily Pain score (p = 0.66), and WOMAC-Pain subscale (p = 0.29) (Table 6 and Figure 1). In addition, there was no association between changes in NOA intake and changes in physical function or HRQoL.
TABLE 6. Correlation between change in medication dose and changes in health outcome measures (Month 1.5 to Month 12).
(Expressed as Spearman’s Rank Coefficient (p-value))
| Health Outcome Measure | Change in medication Correlation (p value) |
|---|---|
| HAQ- Global | -0.04 (0.74) |
| HAQ- Pain | -0.008 (0.95) |
| HAQ- Disability Index (DI) | -0.01 (0.93) |
| SF-36 Bodily Pain (BP) | -0.06 (0.66) |
| SF-36 General Health (GH) | -0.008 (0.95) |
| SF-36 Mental Health (MH) | -0.10 (0.46) |
| SF-36 Physical Functioning (PF) | 0.09 (0.50) |
| SF-36 Role Emotional (RE) | 0.04 (0.77) |
| SF-36 Role Physical (RP) | -0.05 (0.72) |
| SF-36 Social Functioning (SF) | -0.06 (0.62) |
| SF-36 Vitality (V) | -0.10 (0.46) |
| SF-36 Mental Component Score (MCS) | -0.01 (0.97) |
| SF-36 Physical Component Score (PCS) | -0.03 (0.80) |
| WOMAC- Activities of Daily Living (ADL) | -0.13 (0.30) |
| WOMAC- Stiffness | -0.05 (0.70) |
| WOMAC- Pain | -0.13 (0.29) |
significant p-value
Figure 1.
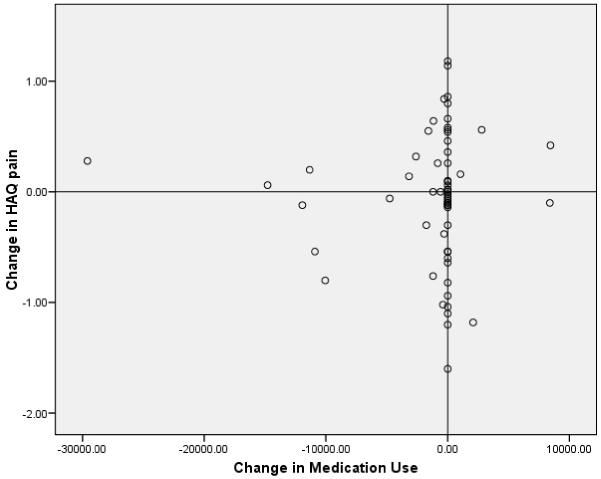
The lack of correlation between change in NOA dose, expressed as Ibuprofen-Equivalents / week, and the change in pain (using the HAQ).
Discussion
The results of this study suggest that although analgesics are generally prescribed to palliate pain, OA patients’ actual use of these agents may relate more to their general level of physical functioning and quality of life than to their current pain or change in pain. In fact, functional status and quality of life appear to be predictors of analgesic use in patients with OA; greater use, especially among older individuals, may be a surrogate of reduced physical functioning, or lower health related quality of life. Thus, whereas analgesics and anti-inflammatory medications are generally prescribed by physicians and medical care providers specifically to palliate pain, these data suggest that patients may actually employ these medications for reasons other than pain relief.
There may be several reasons why patients utilize non-opioid analgesics for indications unrelated specifically to pain palliation. For example, these medications may relieve joint stiffness and thereby improve physical function. However, no association between change in NOA dose with change in stiffness (as measured by WOMAC stiffness), or physical function (WOMAC-ADL) was observed over the study duration. Some patients may continue taking regular doses of NOA after pain has abated either because they do not realize that these medications may be discontinued or they do not recall the specific indication for which the medication was initiated. Thus, although physicians may intend NOA to be used only intermittently for relief of painful exacerbations of OA, patients may continue taking prescribed medications until explicitly counseled to stop; this suggests that at the time of initiating therapy, patients should be carefully educated regarding the appropriate use and discontinuation of NOA in OA. Finally, there may be unrelated pharmacological effects of these medications that may result in improved quality of life through as yet undescribed mechanisms. Nonetheless, the observation that the quantity of analgesic medication consumed by OA patients cross sectionally and over a 12 month period is predicted better by their state of physical function and health related quality of life than by their pain level should be considered by practitioners when prescribing and continuing these medications.
NOA use may have declined during the study period for a variety of reasons, such as a placebo effect from the primary intervention study, which included several study visits, or other unmeasured variables (including the biomechanical intervention) or a change in HRQOL. A significant decrease in WOMAC-ADL, pain and stiffness scores was observed as well as a decrease in total NOA usage, however the change in NOA did not correlate with the changes in WOMAC ADL, pain or stiffness (Table 6). If the decreased NOA use were due to the biomechanical intervention, then a relationship between change in NOA and change in pain should have been observed.
Another limitation of this study was that a significant number of the subjects (>33%) did not use any analgesic medications at all; this may have been a result of the nature of the study, as biomechanical interventions may have special appeal to individuals who do not wish to take oral medications. This limitation was addressed through subgroup analysis of those subjects who did use analgesics, based on medication dose, and the results were similar.
The strengths of this study include its generalizability. The participants were not primed by the investigators about any aspect of NOA use, and were unaware of this study goal at the time of their study visit; in addition, it was not a medication study. Hence, the results may better represent the actual pattern of NOA use by OA patients in practice, because they are devoid of responder and acquiescence bias. Furthermore, the study subjects were in relatively good health without substantial co-morbidities, thus avoiding the confounding nature of NOA use for extraneous indications. As a result, the patterns of NOA use in this study may be ascribed to OA, and the results may provide novel insights into NOA use for OA.
The results of this study suggest that among knee OA patients, analgesic intake may not be solely related to issues of pain; moreover, among OA patients with increased disability or poorer health related quality of life, the use of pain medications may be related more to these reasons than to the need for pain relief. Our results indicate the need for physicians to periodically review indications and to educate patients about continued use of NOA, especially in the geriatric population. Further studies, including larger group sizes and employing qualitative patient interviewing are necessary to fully elucidate the roles of physical function, quality of life, and feelings of well-being in the decision of OA patients to employ continued analgesia in their care, and thereby to improve the management of analgesic therapy in OA.
Acknowledgments
Role of the funding source.
This study was funded through a grant from the NIH, 1P50 AR048941. The sponsoring agency had no involvement in the study design, data collection or interpretation, or in the writing of or decision to submit this manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest.
There are no products discussed, and there was no corporate involvement in any aspect of this study. None of the authors has a potential conflict of interest related to any material in this report.
References
- 1.Briggs A, Scott E, Steele K. Impact of Osteoarthritis and Analgesic Treatment on Quality of Life of an Elderly Population. Ann Pharmacother. 1999;33:154–59. doi: 10.1345/aph.18411. [DOI] [PubMed] [Google Scholar]
- 2.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman BN, Aliabadi P, Levy D. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995;38:1500–5. doi: 10.1002/art.1780381017. [DOI] [PubMed] [Google Scholar]
- 4.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheum. 1988;5:1833–40. [PubMed] [Google Scholar]
- 5.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Arthritis Rheum. Vol. 29. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association; 1986. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee; pp. 1039–49. [DOI] [PubMed] [Google Scholar]
- 6.Crofford LJ. Nonsteroidal Anti-Inflammatory Drugs. In: Harris ED Jr, Budd RC, Genovese MC, Firestein GS, Sargent JS, Sledge CB, editors. Harris: Kelley’s Textbook of Rheumatology. Elsevier - Saunders; St. Louis, MO.: 2005. pp. 839–854. [Google Scholar]
- 7.Pincus T, Swearingen C, Wolfe F. Toward a Multidimensional Health Assessment Questionnaire (MDHAQ): assessment of advanced activities of daily living and psychological status in the patient-friendly health assessment questionnaire format. Arthritis Rheum. 1999;42:2220–2230. doi: 10.1002/1529-0131(199910)42:10<2220::AID-ANR26>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]


