Abstract
Estrogen Receptors can activate transcription in the nucleus, and activate rapid signal transduction cascades in the cytosol. Multiple reports identify Estrogen Receptors at the plasma membrane, while others document the dynamic responses of Estrogen Receptor to ligand binding. However, the function and identity of membrane Estrogen Receptors remains controversial. We have used confocal microscopy and cell fractionation on the hippocampus-derived HT22 cell line and primary cortical neurons transfected with Estrogen Receptor-GFP constructs to address the membrane localization of these receptors. We observe translocation of Estrogen Receptor β to the plasma membrane 5 minutes after exposure to 17β-estradiol, whereas Estrogen Receptor α localization remains unchanged. Membrane localization of Estrogen Receptor β is transient, selective for 17β-estradiol, and is not blocked by ICI182,780. Inhibition of the Mitogen Activated Protein Kinase pathway does not block estrogen-mediated Estrogen Receptor β membrane translocation, and in fact prolongs membrane localization. These data suggest that while both Estrogen Receptor α and Estrogen Receptor β can be present at the neuronal membrane, their presence is differentially regulated.
Keywords: steroid, hormone, neuron, HT22, MAPK, subcellular localization
Introduction
The steroid hormone estrogen plays a critical role in sexual development and behavior (reviewed in (Palanza et al., 1999)). Estrogen binds to Estrogen Receptors (ERs) ERα (Green et al., 1986) and ERβ (Gustafsson, 1999) to regulate transcription. ERs alter transcription by both classical and non-classical means—classical estrogen signaling involves direct binding to Estrogen Response Element (ERE)-containing promoters, whereas non-classical signals occur via non-ERE mechanisms, including SRE, CRE, AP-1, Nf<kappa>B and GC-rich regions (for review, see (Bryant DN et al., 2006; Vasudevan et al., 2001)). Estrogen also has rapid effects (within minutes) in various cell types. These include activation of various components of the Mitogen Activated Protein Kinase (MAPK) pathway (Migliaccio et al., 1996; Razandi et al., 1999), calcium influx (Tesarik and Mendoza, 1995), potassium currents (Li et al., 2000), phospholipase C activation (Imai et al., 1990), IP3 generation (Lieberherr et al., 1993), cAMP accumulation (Aronica et al., 1994), eNOS (Chambliss et al., 2000), and the protein kinase A (PKA) and protein kinase C (PKC) pathways (Kelly et al., 1999; Linford et al., 2000; Wade et al., 2001). The division between rapid versus genomic signaling is somewhat amorphous, as rapid estrogen signaling has important consequences for both ERE and non-ERE-dependent responses (Bjornstrom and Sjoberg, 2005; Lu et al., 2002; Vasudevan et al., 2001; Watters et al., 2000). It is likely that in a complex promoter, estrogen acts on a variety of elements to finely modulate transcription, as is the case with vasopressin (Shapiro et al., 2000).
Membrane ERs were identified 30 years ago (Pietras and Szego, 1977) yet controversy remains over their significance and identity (Pedram et al., 2006; Warner and Gustafsson, 2006). Ligand-bound ERs function in the nucleus to regulate transcription, but can also function at the plasma membrane to activate rapid signals (Razandi et al., 1999; Wade et al., 2001). Whether ERs do so in vivo becomes more complicated with discovery of novel estrogen targets, such as G-protein-coupled receptors (Kelly et al., 1999; Lieberherr et al., 1993; Qiu et al., 2003), GPR30 (Carmeci et al., 1997; Revankar et al., 2005; Vivacqua et al., 2006) and novel ER candidates, such as ER-X (Toran-Allerand, 2004).
Many signaling molecules have pleiotropic effects within a cell. Regulation of receptor localization is one mechanism by which signaling specificity can be attained (Smith and O'Malley, 2004). Consistant with this concept, localization of ERs affects both rapid and genomic signaling. ERα mutants with reduced membrane localization exhibit reduced rapid signaling (reviewed in (Evinger, III and Levin, 2005)), whereas increased cytoplasmic localization promotes non-classical signaling (Bjornstrom and Sjoberg, 2002). Plasma membrane-tethered ERα activates MAPK more robustly and ERE-mediated transcription weakly (Rai et al., 2005; Zhang et al., 2002). Conversely, sequestering ERα in the nucleus abrogates rapid estrogen signaling (Wang et al., 2004). These studies demonstrate that artificially altering ER localization has profound effects on which signaling pathways they can activate.
To clarify activity of “nuclear” receptors at the plasma membrane and to investigate how estrogen might regulate the subcellular localization of ERs, we have followed localization of untagged and GFP-fused ER constructs by confocal microscopy and fractionation. We observe differences between ERα and ERβ with respect to basal location and their response to estrogen.
Experimental Procedures
ER fusion Generation
ERα-GFP: The open reading frame (ORF) of ERα was fused, in frame, to the open reading frame of enhanced green fluorescent protein (pEGFP-N2, Invitrogen). The ERα ORF was generated in a PCR reaction using the HEGO/pcDNA3 plasmid as template, T7 oligonucleotide for the forward primer and an oligonucleotide corresponding to the seven C-terminal amino acids of ERα with the addition of a BamH1 restriction site [5’-aattggatccc GACTGTGGCAGGGAAACC-3’] as the reverse primer. After PCR with Pfu polymerase, the PCR product was digested with EcoR1 and BamH1, gel purified and ligated into EcoR1/BamH1 digested pEGFP-N2. The sequence was confirmed by DNA sequencing and the fusion construct functionally tested for the ability to transcribe an ERE-luciferase reporter construct in response to 17β-estradiol. ERβ-GFP was a gift from the Handa lab (Price, Jr. et al., 2001).
Cell Culture and Treatment
Murine Hippocampus-derived HT22 cells were cultured as described (Mize et al., 2003) and transiently transfected 24 hours before treatment using Transfast (Promega) plus rat ERβ (kindly provided by Dr. George Kuiper, Karolinska Institute, Sweden), ERβ-GFP, ERβ1δ3-GFP (Price, Jr. et al., 2001), mouse ERα or ERα-GFP cDNAs. 16 hrs before cells are exposed to estrogen, media was changed to DMEM without phenol red or FBS. 17β-estradiol (Sigma Aldrich) was prepared fresh in ethanol. ICI 182,780 (Tocris), Pd98059 (Sigma Aldrich), UO126 (Sigma Aldrich) and H89 (Sigma Aldrich) were prepared from a 100× stock in ethanol.
Primary cultures of cortical neurons were prepared from fetal rats on gestational day 18 (E18). Cortices were dissected in HEPES-buffered Hanks balanced salt solution (Sigma H2387), containing sodium bicarbonate (0.35 g/l, Sigma) glucose (6 g/l, Sigma), BSA (0.3 g/l, Sigma) and magnesium sulfate (0.97 g/l, Sigma), and dissociated by trypsin (Invitrogen) treatment and trituration. Cells were seeded in poly-L-lysine (100 µg/ml, Sigma) coated 24-well plates (Corning) in Neurobasal medium (Invitrogen) supplemented with 2% B27 (Invitrogen), Glutamax (Invitrogen), and Penicillin/Streptomycin (Invitrogen) at a density of 1.5×105 cells / cm2. Cultures were maintained for 10 days in vitro (DIV) in a humidified incubator with 5% CO2 in air. On DIV 3 and DIV 7, half of the culture medium was replaced with fresh medium. Cortical neurons were transfected 48 hours prior to fixation using Lipofectamine 2000 (Invitrogen).
Immunocytochemistry
To visualize untagged ERs, the following antibodies were used: anti-ERα Ab-16 (Neomarkers RB-1493), anti-ERβ antibodies pa1-310b (1:1000 dilution, Affinity Bioreagents, immunogen: C467-485), and h-150 (1:50 dilution, Santa Cruz Biologicals, immunogen: n-terminal 150 a.a.s). Co-staining with the mitochondrial marker anti-OxPhosII (Molecular Probes 2E3), anti-Caveolin-1 (Signal Transduction Laboratories #C37120) is done at 1:1000 dilution in 0.1% BSA/0.1% Gelatin in PBS (PBG). Cells are grown on poly-L-lysine coated cover slips and fixed following two different protocols: one gentle on cytoplasmic structure-- 2% paraformaldehyde/0.1% Gluteraldehyde 30 min at 37°C, blocked for 1 hr at RT with 10% BSA / 0.1% Cold Water Fish Skin Gelatin in PBS, or by methanol precipitation (MEOH, 90% methanol/10%MES) for 15 min at −20°C. After 3 washes in PBG, primary antibodies are incubated overnight at 4°C, followed by 3 washes in PBG. Alexa-conjugated secondary antibodies (Molecular Probes) are incubated at RT for 1 hr followed by several washes with PBS. Actin staining was done with alexa546-conjugated phalloidin (Molecular Probes) for 40 min at 1:20 dilution in PBS. Plasma membranes were stained with Cellmask Orange plasma membrane stain (Molecular Probes), added to live HT22 cells for 2 min at RT at 0.25 ug/mL in PBS, washed 3× with 10%BSA/0.1% Cold Water Fish Skin Gelatin in PBS and 1× in PBS, then imaged within 30min of the last wash. Single and double-stained images (Figure 2 and Figure 4) were collected on an Olympus FluoView 300 Confocal microscope at the Center for Research on Occupational and Environmental Toxicology (CROET). The aperture setting was 2 and laser intensity at 5% for use with a 60× oil immersion objective. Following image capture, transfected cells were counted for the presence or absence of distinct membrane staining. Triple stained images in Figure 1 were acquired using a Zeiss (Jena, Germany) Axioskop FS with UV illumination out-fitted with DAPI, FITC and TRITC filters and a 40× objective.
Figure 2. Short term estrogen treatment induces translocation of ERβ, but not ERα, to the membrane of HT22 cells and primary cortical neurons.
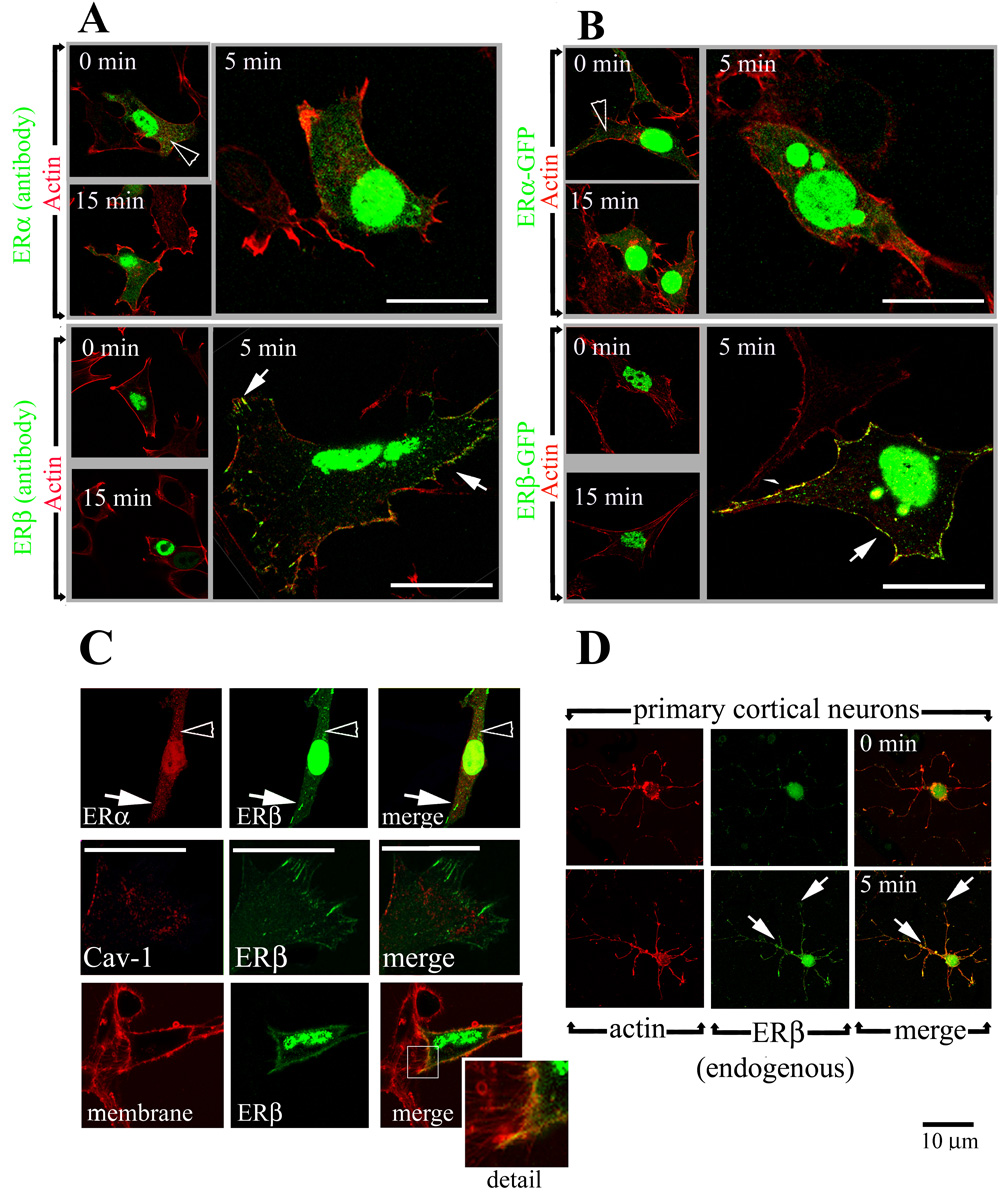
(A) Untagged ERβ (green, lower panels), but not ERα (green, upper panels), translocates to the cell periphery (arrows) following a 5 minute exposure to 10nM 17β-estradiol. In the absence of estrogen, ERβ is almost exclusively nuclear, whereas ERα staining is visible in the cytoplasm (open arrowhead). Cells are counterstained with the actin stain Phalloidin (red) to highlight the cell borders.
(B) ERβ-GFP (green, lower panels) translocates to the cell periphery after a 5 min exposure to 10nM 17β-estradiol (arrows) whereas ERα-GFP (green , upper panels) localization remains unchanged. Actin is labeled with phalloidin (red).
(C) Co-expression of ERα (antibody staining, red, upper panels) and ERβ-GFP (green) does not alter receptor localization compared to cells expressing either ERα or ERβ (upper panels) following a 5 minute exposure to 10nM 17β-estradiol. No colocalization between ERβ-GFP (green) and Caveolin-1 (red, middle panels) is observed after short-term estrogen exposure. Staining of live cells with the plasma membrane marker Cellmask Orange (red, lower panels) illustrates estrogen induces translocation of ERβ-GFP (green, lower panels) to the plasma membrane (yellow signal denotes colocalization, seen in the merge panel)
(D) Endogenous ERβ (green) translocates to the periphery of primary cortical neurons following a 5 min estrogen treatment (arrows, lower panels). Endogenous ERα was undetectable. Actin staining (red) is used to visualize cell bodies and processes. Scale bars = 10 um; panels without white bars are at black scale bar magnification.
Figure 4. Inhibition of the MAPK pathway prolongs estrogen-stimulated ERβ membrane localization.

(A) In the absence of UO126, membrane staining of ERβ-GFP is detectable after a 5 minute exposure to 17β-estradiol, but returns to a completely nuclear localization by the 30 minute mark, as observed by confocal microscopy in transiently-transfected primary cortical neurons. A 20 minute pre-treatment with 1 uM UO126 does not alter ERβ membrane localization in the absence of estrogen, but prolongs estrogen-stimulated ERβ membrane localization to the 30 minute time point. Localization of ERα-GFP, however, is unchanged with UO126 treatment.
(B) Western blots reveals that UO126 pre-treatment (20 min) leads to increased levels of ERβ within membrane fractions 15 to 30 minutes following estrogen exposure, as well as inhibiting anti-active MAPK staining at all time points. UO126 treatment alone has no effect on membrane ERβ levels (time 0). The PKA-inhibitor H89 has no significant effect on estrogen-stimulated ERβ membrane translocation nor estrogen-stimulated MAPK activation.
Figure 1. Transfected ERs reside in the nucleus of HT22 cells.

(A) Differences between anti-ERβ antibodies are visible when compared by Western blot: A ~55 kD and ~90 kD band can be detected in HT22 cells transfected with untagged ERβ and ERβ-GFP constructs, respectively, using either the Zymed and pa1-310b anti-ERβ antibodies. In contrast, a ~60 kD band is recognized by the h-150 anti-ERβ antibody which does not increase in density (relative to the endogenous control protein Oxidative Phosphorylation Complex II 70 KD subunit) following transfection with untagged ERβ nor shift in size following ERβ-GFP transfection.
(B) Differences between anti-ERβ antibodies are visible when compared by immunocytochemistry: The pa1-310b antibody recognizes both GFP-tagged ERβ and untagged ERβ, found primarily in the nucleus, but detects nothing in either untransfected cells (visualized by DAPI-staining in merged panels, blue). The h-150 antibody stains transfected and untransfected cells equally– specifically, labeling mitochondria, as has been described by the Simpkins lab (Yang, SH et al 2004). Mitochondria are co-stained with the OxPhos subunit II marker (green, lower panels). For comparison, cells transfected with vector alone cells are shown in lower panels stained singly with the three antibodies used (mock transfected).
(C) Antibody detection of tagged and untagged ERα by immunocytochemistry are consistent. Both untagged and GFP-tagged ERα are detected by the anti-ERa antibody AB16. ERα is primarily localized within the nucleus of HT22 cells, although cytoplasmic staining above background is visible. Cells transfected with vector alone and stained with AB16 are shown for comparison (mock).
Membrane Isolation
Cells are grown and treated as described (Mize et al., 2003). Following a 5 min estrogen treatment, membrane fractions are isolated by sucrose centrifugation: cells are washed 2× with ice-cold PBS and scraped into 0.5 mL sucrose buffer (50mM Tris, 1 nM EDTA, 200 mM Sucrose, 4 mM NaHPO4, 1 uM Na3VO4, 1 uM NaF) and homogenized in a 1 mL Dounce homogenizer. Homogenates are spun at 1000×G, supernatants moved to a new tube, pellet resuspended in 0.5 mL sucrose buffer and spun again. Supernatants are combined, while the pellet is resuspended in 50 uL Solubilization buffer (10mM Tris-HCl pH 7.4, 1mM EDTA, 0.5% Triton X-100 plus protease and phosphatase inhibitors) and labeled “nuclear”. Supernatants are spun at 20,000×G for 75 min at 4°C. The pellets are resuspended in 50 uL solubilization buffer and labeled “membrane”. Supernatants are concentrated by TCA precipitation, rinsed 3× in acetone, resuspended in 50 uL solubilization buffer and labeled “cytoplasm”.
Western blot analysis
Lysates are run on Invitrogen NuPage gels, transferred to PVDF membranes as described (Fitzpatrick et al., 2002) and probed using the same antibodies listed above, plus anti-ERα AB15 (1:500, Neomarkers AER611), anti ER-β (1:1000 Zymed, immunogen: 19 amino acids (a.a). at c-terminus), anti-GFP (1:1000, Becton Dickinson JL-8) and as a loading/fraction controls, anti-ERK-2 (1:5000, Cell Signaling #3A7) anti-Actin (1:4000, LabVision), anti-Transferrin Receptor (1:1000, Zymed) anti-Histone H3 (1:1000, Cell Signaling #9715), anti-HSP70 and anti HSP 90 (1:1000, Becton Dickinson). Densitometry was performed with LabWorks software (PC) and the one–way analysis of variance (ANOVA) test was used to determine statistical significance of the data after correction for loading using the control Transferrin Receptor (TR).
Results
Antibody detection of ERβ
We and others have previous shown HT22 cells to be devoid of functional estrogen receptors (Fitzpatrick et al., 2002; Kim et al., 2001; Mize et al., 2003), and only in cells transfected with ERα or ERβ does estrogen activate ERE-transcription and MAPK. However, recent reports have suggested that the HT22 cell line contains endogenous ERβ within the mitochondria (Deecher et al., 2005). In order to resolve the apparent conflict we compared several anti- ERα and anti- ERβ antibodies in a series of transfection experiments.
HT22 cells were transfected with untagged or GFP-tagged rat ERβ and probed for ERβ levels after 24hr by Western blot using three different ERβ antibodies. Both the Zymed and pa1-310b anti-ERβ antibodies do not detect bands above background in lysates from untransfected HT22 cells or RAT2 fibroblasts (Fig 1A). In HT22 cells transfected with untagged ERβ, both antibodies recognize a ~55kD band, and in cells transfected with ERβ-GFP, the apparent molecular weight (m.w.) of this band increases to ~90kD (Figure 1A). In contrast, the h-150 anti-ERβ antibody recognizes a ~60kD band in RAT2 fibroblasts and HT22 cells, and this band does not increase in density in lysates from ERβ-transfected cells, nor does a larger m.w. band appear in ERβ-GFP transfected cell lysates (Figure 1A).
Similar results were observed with immunocytochemical experiments. The ERβ antibody, pa1-310b detects no signal above background in untransfected HT22 cells, but does recognize a nuclear protein in cells transfected with either untagged ERβ or ERβ-GFP. This pattern is observed in cells fixed with either paraformaldehyde (PFA) or methanol (MEOH, Figure 1B). Neither C6 glioblastoma cells nor RAT2 fibroblasts show staining with the pa1-310b antibody unless transfected with ERβ or ERβ -GFP (data not shown). The primarily nuclear localization of ERs in HT22 cells is in agreement with previous studies in neurons (Pappas et al., 1995; Price, Jr. et al., 2001; Ylikomi et al., 1992).
Following protocols outlined by Deecher, et al (Deecher et al., 2005), using the anti-ERβ h-150 antibody (1:50 dilution) we observe a mitochondrial signal in untransfected HT22 cells fixed with MEOH (Figure 1B, middle panels, the mitochondrial marker Ox-Phos II is shown in green). h-150 staining is not significantly altered with transfection of untagged ERβ or ERβ-GFP (Figure 1B). Vector alone (mock) transfected cells stained with ERβ or mitochondrial-marker antibodies are shown for comparison (Figure 1B, bottom row). These data leads us to conclude this mitochondrial signal does not represent the same ERβ that capable of mediating genomic and rapid estrogen signals. We have chosen to use GFP-tagged ERs to confirm results observed with antibodies to avoid potential artifacts associated with the use of antibodies.
In contrast to ERβ, there is a wealth of consistent data regarding the visualization of ERα by antibody staining. Figure 1C shows that antibody staining of both untagged and GFP-tagged ERα using the AB16 antibody yields predominantly nuclear staining, with a small amount of cytoplasmic signal that is above background levels (untransfected cells are visualized with DAPI staining, Figure 1C). Mock-transfected cells stained with the AB16 antibody are shown for comparison (Figure 1C, bottom row)
Estrogen induces rapid relocalization of ERβ, but not ERα, to the plasma membrane
HT22 cells were transfected with either GFP-tagged or untagged ERα or ERβ constructs, and ER localization visualized with confocal microscopy. Under estrogen-free conditions, both ERα and ERβ are predominantly localized within the nucleus (ERα: 0/74 cells, ERβ: 0/211 cells show distinct membrane staining), but there appears to be more ERα within the cytoplasm (open arrowheads, Figure 2A–B). ERα localization does not change following estrogen exposure (Figure 2A–B, 0/50 cells show distinct membrane staining). In contrast, we observe ERβ -GFP at the plasma membrane following a 5 minute treatment with 10nM 17β estradiol (Figure 2B, 24/97 cells show distinct membrane staining). The same result is observed in antibody-stained cells transfected with untagged ERβ (Figure 2A, 15/65 cells show distinct membrane staining), demonstrating that the movement to the membrane is not due to the fusion of GFP to the ER. By 15 minutes, the membrane ERβ signal is no longer visible (Figure 2A–B, 1/104 cells show distinct membrane staining). The localization of the GFP protein alone is unaltered by estrogen exposure (data not shown).
To confirm estrogen-dependent ERβ staining is at the plasma membrane, ERβ-GFP transfected HT22 cells were stained with the Cellmask Orange plasma membrane stain and imaged live under the confocal microscope (Fig 2C, bottom panels). There is significant colocalization between ERβ and the plasma-membrane marker (yellow signal in “merge” panel). However, it is important to note that at no point does the GFP signal extend beyond the outer membrane, suggesting ERβ is not inserted into membrane to become a cell-surface receptor (Fig 2C, detail).
ERα and ERβ reportedly form homo and hetero-dimers at the plasma membrane and in the nucleus (Razandi et al., 2004). This raises the possibilities that a heterodimer would behave differently with respect to estrogen-stimulated membrane translocation. To test this, we co-expressed ERα and ERβ in the same cell and found no alteration in either receptors’ subcellular localization following estrogen exposure-- ERβ translocates to the plasma membrane, while ERα localization remains unchanged (Figure 2C, upper row).
It has recently been shown that interaction with Caveolin-1 is critical for the membrane translocation of ERα and ERβ (Galluzzo et al., 2007; Pedram et al., 2007). To test whether membrane ERβ was consitant with caveolar markers, we tested ERβ-GFP transfected HT22 cells with estrogen and counterstained with an anti-Caveolin-1 antibody. While both are present at the cell periphery following estrogen treatment, costaining reveals no significant colocalization between ERβ-GFP at the membrane and Caveolin-1 (Figure 2C, middle row)
To expand our observations from HT22 cells, we collected primary cortical neurons and followed endogenous ERβ localization with antibody staining. Primary cortical neurons (E18) express ERβ (Linford and Dorsa, 2002). In estrogen-free conditions, endogenous ERβ was detectable almost exclusively in the nucleus (Figure 2D, upper row) using the pa1-310b antibody as in Figure 1. Following a 5 min treatment with 10nM 17β - estradiol, we observe ERβ staining in processes (Figure 2D, right panels). Endogenous ERα was undetectable using the methods described for Figure 1 (data not shown). Transfection of cortical neurons with ERβ-GFP confirms estrogen-dependent translocation of ERβ is not due to an antibody artifact (Figure 4).
Fractionation
To confirm immunocytochemistry data, we measured ER levels in cellular fractions by Western blot analysis. Briefly, transfected HT22 cells were homogenized in detergent-free sucrose buffer, nuclei were removed by a brief low-speed centrifugation step, and membranes purified by high-speed centrifugation. This technique produced samples enriched for membrane markers, such as Transferrin Receptor (TR), but devoid of the nuclear marker Histone H3 (Figure 3C). We observe no changes in the levels of ERα in the membrane fraction with any treatment (Figure 3B, n=5). In contrast, membrane ERβ levels increase 2.5 fold (p<0.005, n=5) following a 5 min estrogen treatment (Figure 3A). Levels of the control protein Actin and the membrane marker Transferrin Receptor remain constant, and the nuclear marker Histone H3 is absent, showing that the increase in membrane ERβ is due to neither loading errors nor nuclear contamination (Figure 3A–C).
Figure 3. Cellular fractionation reveals elevated membrane levels of ERβ, but not ERα, following a 5 minute treatment with estrogen.

(A) Levels of membrane ERβ increase following a 5 min estrogen treatment (“+” total cell lysates positive control, “v” 5 min vehicle, “e” 5 min 10nM 17β-estradiol, “ici” 2 hr treatment with 1 uM ICI 182,780, “e+i” ici pretreatment followed by 5 min estrogen exposure). Levels of ERβ staining (with pa1-310b antibody) in membrane fractions of HT22 cells increase following a 5 min estrogen (e) treatment. The control proteins Transferrin Receptor (TR) and Actin remain unchanged. Absence of Histone H3 illustrates that this is not due to contaminant nuclear contents. A 2 hr pre-treatment with ICI does not block estrogen-induced membrane relocalization of ERβ (e+i).
(B) Levels of membrane ERα do not change with a 5 min estrogen treatment. Levels of ERα and the control proteins Transferrin Receptor and Actin do not change with either a 5 min E2 or 2hr ICI treatment.
(C) Fractionation separates nuclear and membrane markers. HT22 cells transfected with ERα or ERβ were fractionated and analyzed by Western blot for various protein markers. The membrane marker Transferrin Receptor is enriched in membrane fractions but absent from cytoplasmic fractions, while the nuclear marker Histone H3 is found only in nuclear fractions. Both ERα and ERβ are localized primarily to the nuclear fraction, but a small pool can be detected in the membrane fraction.
(D) 17β -estradiol, but not 17α-estradiol nor progesterone, increase membrane ERβ. A 5 min treatment with 10nM 17β -estradiol, but not 100nM 17α estradiol (17α) nor 100nM progesterone (pr), led to an increase in membrane ERβ.
Translocation of ERβ is specific to 17β-estradiol and is not blocked by ICI 182,780
ICI 182,780 (ICI) is a Selective Estrogen Receptor Modulator (SERM). While it can block estrogen-stimulated ERE-transcription, it can act as an antagonist or an agonist of non-classical signaling in a cell-type specific manner (Fitzpatrick et al., 2002; Mize et al., 2003; Paech et al., 1997; Wade et al., 2001; Wang et al., 2004). We therefore asked whether ICI would block movement of ERβ to the plasma membrane. We found that pre-treatment for 2 hours with 1uM ICI does not block estrogen-stimulated ERβ translocation (2.7 fold increase over vehicle, Figure 3A). ICI alone has no significant effect on ERβ localization (Figure 3A), and does not alter levels of ERα in membrane fractions (Figure 3B). A short (20 min) pre-treatment with ICI does not induce ERβ membrane translocation (data not shown).
Other steroids are capable of binding and activating ERs. We therefore tested the enantiomer 17α-estradiol and progesterone for the ability to induce ERβ translocation. We found that translocation of ERβ is specific to 17β-estradiol, as neither 17α-estradiol nor progesterone treatment are capable of increasing membrane ERβ levels (Figure 3D).
Inhibition of the MAPK pathway prolongs estrogen-stimulated ERβ membrane localization
Membrane localization of ERα has been shown to be important in estrogen-mediated activation of the MAPK pathway (Xu et al., 2003; Zhang et al., 2002). In addition, phosphorylation of ERα by MAPK has been shown to regulate nuclear localization of active ERα (Takahashi K, 2003) and is necessary for full transcriptional activity (for review, see (Lannigan, 2003; Lu et al., 2002)). To test whether inhibition of the MAPK pathway affects membrane localization, we pre-treated ERα-GFP and ERβ-GFP transfected cells with 1uM UO126 or Pd98059 for 20 minutes, then treated cells with 10nM estrogen for 0 to 30 minutes, and measured membrane localization by confocal microscopy and fractionation. Confocal microscopy reveals that UO126 treatment alone has no effect on either ERα or ERβ staining (Figure 4A). However, following estrogen exposure, ERβ moves and remains at the cell membrane well after the 15 minute time point in cells treated with UO126, but not vehicle (Figure 4A). ERα staining remains unchanged with UO126 treatment. Similar results were observed with cell fractionation experiments—ERβ levels in membrane fractions are elevated in UO126 (and PD98059, data not shown)-treated cells at the 15 and 30-minute post-estrogen exposure time points (n=3, Figure 4B). Anti-phospho-ERK staining reveals that UO126 inhibits ERK activity, and that peak ERβ membrane levels precede peak phospho-ERK levels. As a control, cells were treated with the PKA inhibitor H89, which had no effect on estrogen-stimulated ERβ membrane localization or ERK activation (n=3, Figure 4B).
Discussion
We report that ERβ rapidly and transiently moves to the plasma membrane after—not prior to-- exposure to estrogen, in both HT22 cells and primary cortical neurons. The significance of membrane ERs remains controversial. One argument used against the functional significance of membrane ERs is that estrogen freely crosses the plasma membrane, making surface-ERs unnecessary for binding either ligand or DNA (Gustafsson, 2006). Nevertheless, membrane ERα has been detected in endometrial cells, breast cancer cells (Pietras and Szego, 1977; Powell et al., 2001) and neurons (Honda et al., 2000; Norfleet et al., 2000; Pappas et al., 1995; Watson et al., 2002), while ERβ has been detected in extra-nuclear sites of the hippocampus (Kalita et al., 2005; Milner et al., 2005) and at the plasma membrane in platelets (Moro et al., 2005). Our data suggest that in vivo observations of membrane ERs represent ERs engaged in signaling. The possibility remains that ERβ binds estrogen at the cell surface and is retained there upon ligand-binding. The more likely model, we believe, is that ERβ moves to the plasma membrane after binding estrogen in the cytoplasm or nucleus, and that translocation brings active ERβ into proximity with downstream rapid signal transduction molecules.
This report suggests there can be differences between the ability of ERα and ERβ to localize to the plasma membrane in a given cell type. For instance, ERα rapidly translocates to the plasma membrane in MCF-7 cells (Song 2004) and adipocytes (Nagira et al., 2006) and to caveolae in Cos-7 cells (Razandi et al., 2002). We observe estrogenmediated membrane translocation of ERβ, but not ERα, in the HT22 cell line and primary cortical neurons. While the significance of this remains unclear, it is attractive to attribute differences in localization to observed signaling differences between ERα and ERβ. For instance, we observe more ERα at the membrane in estrogen-free conditions. Consistent with this timing difference, we have previously shown that ERα-mediated MAPK activation occurs more rapidly than ERβ-mediated MAPK activation in stably-transfected HT22 cells (Wade et al., 2001). This suggests that the kinetic properties of signals generated via ERα or ERβ may be quite different, and may result in very different downstream consequences.
Membrane localization of ERs may also vary in different cell types. ERα and ERβ have been shown to translocate to caveolae in Cos7 and MCF7 cells (Evinger, III and Levin, 2005; Razandi et al., 2003), as well as HeLa and DLD-1 cells (Galluzzo et al., 2007). We report no colocalization between ERβ and Cav-1. It remains possible that HT22 cells lack a necessary protein for this association. Furthermore, ERβ may associate with caveolae that do not contain Cav-1 (Boulware MI et al., 2007). However, the differences in time-course should be pointed out. We observe ERβ translocation within 5 minutes after estrogen exposure, and while estrogen-dependent palmitoylation of ERβ can be observed within 10 minutes, association with caveolae occurs 6 hours after estrogen exposure (Galluzzo et al., 2007). Further experiments will be necessary to clarify whether these data represent distinct or shared mechanisms.
Differential binding to scaffolding proteins could result in the observed differences in localization of different ER subtypes in the same cell type, as well as differences between the same subtype in different cell lines. Other membrane-resident proteins known to bind ERα and function in rapid estrogen signaling include shc (Song et al., 2002), EGFR (Driggers and Segars, 2002), PI3K (p85 subunit, (Simoncini et al., 2000)), c-src (Ballare et al., 2003; Migliaccio et al., 2002), IGF-R1 (Song et al., 2002), MNAR (Barletta et al., 2004) and the scaffolding protein striatin (Lu et al., 2004). Whether ERβ binds to these proteins remains to be reported, so it is possible that differences between the ability of ERα and ERβ to translocate to the plasma membrane is due to differential association with different scaffolding proteins.
The SERM ICI-182,780 does not block translocation of ERβ to the plasma membrane in response to estrogen. ICI is an antagonist of ERα and ERβ -mediated activation of ERE-dependent transcription, but its activity in non-classical and non-genomic signals is more complex. ICI blocks ER-mediated activation of the MAPK pathway in HT22 cells (Fitzpatrick et al., 2002; Mize et al., 2003) but not in RAT2 fibroblasts (Wade et al., 2001) and has been shown to activate AP-1, CRE and SRE signaling (Paech et al., 1997). Our data suggests that, in HT22 cells, ERβ-mediated non-genomic signaling occurs downstream of estrogen-stimulated ERβ membrane translocation (Figure 5).
Figure 5. Model of estrogen-stimulated ERβ membrane translocation and time-course.

| 0’ | Estrogen can cross the cell membrane and bind to inactive ERβ monomers. Estrogen binding induces membrane translocation. |
| 5’ | Active ER dimers activate rapid signaling cascades, such as MAPK, at the plasma membrane. |
| 15’ | Full estrogen-dependent MAPK activity is reached, and MAPK feeds back to the ER, (possibly phosphorylating ERβ directly or phosphorylating a chaperone protein) inducing ER to leave the plasma membrane. |
| ERβ can enter the nucleus and alter gene transcription |
The MAPK inhibitors UO126 and Pd98059 do not block membrane translocation in response to estrogen, further suggesting that ER translocation occurs before activation of rapid signaling. In fact, blockade of MAPK activity prolongs membrane localization of ERβ following estrogen exposure. Phosphorylation of ERα by MAPK has been shown to be necessary for the import of active ERα into the nucleus (Lu et al., 2002) and for full transcriptional activation of classical estrogen signaling (for review, see (Bjornstrom and Sjoberg, 2005; Lannigan, 2003)). MAPK can phosphorylate ERβ, increasing interaction with transcriptional co-activators (Tremblay et al., 1997). Our data does not distinguish between direct phosphorylation of ERβ by MAPK versus phosphorylation of another protein, such as an ERβ-binding chaperone. Furthermore, it is important to note that the mechanism of nuclear import likely differs between ERα and ERβ (Bjornstrom and Sjoberg, 2004). Regardless, this data suggests membrane translocation of ERβ is not only important for the activation of rapid, non-genomic estrogen signals, but plays a role in classical estrogen signaling.
Razandi et al have shown that membrane ERs can function as dimers in transfected CHO cells (Razandi et al.,2004). Here we report two pieces of evidence that suggest ERβ translocates to the neuronal plasma membrane as a monomer. First, there is no alteration in receptor localization following co-transfection of ERα and ERβ. Secondly, ICI—which is capable of blocking ER dimerization (Fawell et al., 1990; Weatherman et al., 2002)-- does not inhibit membrane translocation. We hypothesize ERβ translocates to the plasma membrane in response to estrogen where it subsequently forms an active dimer to initiate rapid signal transduction cascades (Figure 5).
We and others have previously reported the HT22 cell line is devoid of functional estrogen receptors, yet recent reports have suggested the presence of endogenous ERβ within mitochondria. Here we report that functional ERβ is neither endogenous to HT22 cells nor localized to the mitochondria in HT22 cells. We do not suggest that ERβ cannot be a mitochondrial protein, and we do not rule out the possibility that there is an endogenous ERβ splice variant or homologue of unknown function. Whatever the identity of the epitope detected by one ERβ antibody in untransfected HT22 cell mitochondria, it does not appear to be the same as full length rat ERβ, nor is it capable of activating MAPK nor ERE-dependent transcription, nor does it mediate the neuroprotective effects of estrogen (Fitzpatrick et al., 2002). Our data consistent with numerous reports that ERs are primarily localized to the nucleus in neurons (Pappas et al., 1995; Price, Jr. et al., 2001; Ylikomi et al., 1992).
Estrogen binds to ERα and ERβ to exert a wide variety of effects on different cell types. Ligand-bound ERs can activate ERE-dependent transcription, ERE-independent (i.e. nonclassical) gene transcription, and activate a wide variety of rapid signaling pathways (i.e., non-genomic signals). The results of the Women’s Health Initiative on Hormone Replacement Therapy (Writing Group for the Women's Health Initiative Investigators, 2002) underscore the complexity of ER function. Understanding the molecular and cellular details of ER function will allow for more precise targeted drug therapies in the future.
Acknowledgements
This research was supported by a grant from the Oregon Partnership for Alzheimer’s Research (OPAR) under the Oregon Income Tax Check-off Alzheimer's Research Fund to LCS, NIH grant NS20311-23 to DMD, and NICHD BIRCWH grant K12HD043488 to DNB.
Abbreviations
- E2
17B-estradiol
- ER
Estrogen Receptor
- ERE
Estrogen Receptor Element
- FBS
Fetal Bovine Serum
- GFP
Green Fluorescent Protein
- ICI
ICI 182,780
- MAPK
Mitogen Activated Protein Kinase
- MEOH
Methanol
- mw
Molecular Weight
- PFA
Paraformaldehyde
- PKA
Protein Kinase A
- PKC
Protein Kinase C
- SERM
Selective Estrogen Receptor Modulator
- wt
Wild Type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aronica SM, Kraus WL, Katzenellenbogen BS. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8517–8521. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballare C, Uhrig M, Bechtold T, Sancho E, Di Domenico M, Migliaccio A, Auricchio F, Beato M. Two domains of the progesterone receptor interact with the estrogen receptor and are required for progesterone activation of the c-Src/Erk pathway in mammalian cells. Mol. Cell Biol. 2003;23:1994–2008. doi: 10.1128/MCB.23.6.1994-2008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barletta F, Wong CW, McNally C, Komm BS, Katzenellenbogen B, Cheskis BJ. Characterization of the interactions of estrogen receptor and MNAR in the activation of cSrc. Mol Endocrinol. 2004;18:1096–1108. doi: 10.1210/me.2003-0335. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;37:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M. Signal transducers and activators of transcription as downstream targets of nongenomic estrogen receptor actions. Mol Endocrinol. 2002;16:2202–2214. doi: 10.1210/me.2002-0072. [DOI] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M. Estrogen receptor-dependent activation of AP-1 via non-genomic signalling. Nucl. Recept. 2004;2:3. doi: 10.1186/1478-1336-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- Bryant DN, Sheldahl L, Marriott LK, Shapiro RA, Dorsa DM. Multiple Pathways Transmit Neuroprotective Effects of Gonadal Steroids. Endocrine. 2006;29(2):199–208. doi: 10.1385/ENDO:29:2:199. [DOI] [PubMed] [Google Scholar]
- Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a Gene (GPR30) with Homology to the G-Protein-Coupled Receptor Superfamily Associated with Estrogen Receptor Expression in Breast Cancer*1, *2, *3. Genomics. 1997;45:607–617. doi: 10.1006/geno.1997.4972. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RGW, Shaul PW. Estrogen Receptor {alpha} and Endothelial Nitric Oxide Synthase Are Organized Into a Functional Signaling Module in Caveolae. Circulation Research. 2000;87:44e–452e. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- Deecher DC, Daoud P, Bhat RA, O'Connor LT. Endogenously expressed estrogen receptors mediate neuroprotection in hippocampal cells (HT22) J Cell Biochem. 2005;95:302–315. doi: 10.1002/jcb.20413. [DOI] [PubMed] [Google Scholar]
- Driggers PH, Segars JH. Estrogen action and cytoplasmic signaling pathways. Part II: the role of growth factors and phosphorylation in estrogen signaling. Trends Endocrinol Metab. 2002;13:422–427. doi: 10.1016/s1043-2760(02)00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evinger AJ, III, Levin ER. Requirements for estrogen receptor alpha membrane localization and function. Steroids. 2005;70:361–363. doi: 10.1016/j.steroids.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Fawell SE, Lees JA, White R, Parker MG. Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor. Cell. 1990;60:953–962. doi: 10.1016/0092-8674(90)90343-d. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick JL, Mize AL, Wade CB, Harris JA, Shapiro RA, Dorsa DM. Estrogen-mediated neuroprotection against beta-amyloid toxicity requires expression of estrogen receptor alpha or beta and activation of the MAPK pathway. J Neurochem. 2002;82:674–682. doi: 10.1046/j.1471-4159.2002.01000.x. [DOI] [PubMed] [Google Scholar]
- Galluzzo P, Caiazza F, Moreno S, Marino M. Role of ERbeta palmitoylation in the inhibition of human colon cancer cell proliferation. Endocr. Relat Cancer. 2007;14:153–167. doi: 10.1677/ERC-06-0020. [DOI] [PubMed] [Google Scholar]
- Green S, Walter P, Greene G, Krust A, Goffin C, Jensen E, Scrace G, Waterfield M, Chambon P. Cloning of the human oestrogen receptor cDNA. J Steroid Biochem. 1986;24:77–83. doi: 10.1016/0022-4731(86)90035-x. [DOI] [PubMed] [Google Scholar]
- Gustafsson JA. Estrogen receptor beta--a new dimension in estrogen mechanism of action. J Endocrinol. 1999;163:379–383. doi: 10.1677/joe.0.1630379. [DOI] [PubMed] [Google Scholar]
- Honda K, Sawada H, Kihara T, Urushitani M, Nakamizo T, Akaike A, Shimohama S. Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen in cultured cortical neurons. J Neurosci Res. 2000;60:321–327. doi: 10.1002/(SICI)1097-4547(20000501)60:3<321::AID-JNR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Imai A, Iida K, Tamaya T. Phospholipase C in human endometrial fibroblasts and its regulation by estrogens. Comp Biochem. Physiol B. 1990;97:617–621. doi: 10.1016/0305-0491(90)90169-t. [DOI] [PubMed] [Google Scholar]
- Kalita K, Szymczak S, Kaczmarek L. Non-nuclear estrogen receptor beta and alpha in the hippocampus of male and female rats. Hippocampus. 2005;15:404–412. doi: 10.1002/hipo.20066. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Lagrange AH, Wagner EJ, Ronnekleiv OK. Rapid effects of estrogen to modulate G protein-coupled receptors via activation of protein kinase A and protein kinase C pathways. Steroids. 1999;64:64–75. doi: 10.1016/s0039-128x(98)00095-6. [DOI] [PubMed] [Google Scholar]
- Kim H, Bang OY, Jung MW, Ha SD, Hong HS, Huh K, Kim SU, Mook-Jung I. Neuroprotective effects of estrogen against beta-amyloid toxicity are mediated by estrogen receptors in cultured neuronal cells. Neurosci Lett. 2001;302:58–62. doi: 10.1016/s0304-3940(01)01659-7. [DOI] [PubMed] [Google Scholar]
- Lannigan DA. Estrogen receptor phosphorylation. Steroids. 2003;68:1–9. doi: 10.1016/s0039-128x(02)00110-1. [DOI] [PubMed] [Google Scholar]
- Li R, Shen Y, Yang LB, Lue LF, Finch C, Rogers J. Estrogen enhances uptake of amyloid beta-protein by microglia derived from the human cortex. J Neurochem. 2000;75:1447–1454. doi: 10.1046/j.1471-4159.2000.0751447.x. [DOI] [PubMed] [Google Scholar]
- Lieberherr M, Grosse B, Kachkache M, Balsan S. Cell signaling and estrogens in female rat osteoblasts: a possible involvement of unconventional nonnuclear receptors. J Bone Miner. Res. 1993;8:1365–1376. doi: 10.1002/jbmr.5650081111. [DOI] [PubMed] [Google Scholar]
- Linford N, Wade C, Dorsa D. The rapid effects of estrogen are implicated in estrogen-mediated neuroprotection. J Neurocytol. 2000;29:367–374. doi: 10.1023/a:1007113323582. [DOI] [PubMed] [Google Scholar]
- Linford NJ, Dorsa DM. 17[beta]-Estradiol and the phytoestrogen genistein attenuate neuronal apoptosis induced by the endoplasmic reticulum calcium-ATPase inhibitor thapsigargin. Steroids. 2002;67:1029–1040. doi: 10.1016/s0039-128x(02)00062-4. [DOI] [PubMed] [Google Scholar]
- Lu Q, Ebling H, Mittler J, Baur WE, Karas RH. MAP kinase mediates growth factor-induced nuclear translocation of estrogen receptor alpha. FEBS Lett. 2002;516:1–8. doi: 10.1016/s0014-5793(02)02432-8. [DOI] [PubMed] [Google Scholar]
- Lu Q, Pallas DC, Surks HK, Baur WE, Mendelsohn ME, Karas RH. Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor alpha. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17126–17131. doi: 10.1073/pnas.0407492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Bottero D, Varricchio L, Nanayakkara M, Rotondi A, Auricchio F. Sex steroid hormones act as growth factors. J Steroid Biochem. Mol. Biol. 2002;83:31–35. doi: 10.1016/s0960-0760(02)00264-9. [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Mize AL, Shapiro RA, Dorsa DM. Estrogen receptor-mediated neuroprotection from oxidative stress requires activation of the mitogen-activated protein kinase pathway. Endocrinology. 2003;144:306–312. doi: 10.1210/en.2002-220698. [DOI] [PubMed] [Google Scholar]
- Moro L, Reineri S, Piranda D, Pietrapiana D, Lova P, Bertoni A, Graziani A, Defilippi P, Canobbio I, Torti M, Sinigaglia F. Nongenomic effects of 17beta-estradiol in human platelets: potentiation of thrombin-induced aggregation through estrogen receptor beta and Src kinase. Blood. 2005;105:115–121. doi: 10.1182/blood-2003-11-3840. [DOI] [PubMed] [Google Scholar]
- Nagira K, Sasaoka T, Wada T, Fukui K, Ikubo M, Hori S, Tsuneki H, Saito S, Kobayashi M. Altered subcellular distribution of estrogen receptor alpha is implicated in estradiol-induced dual regulation of insulin signaling in 3T3-L1 adipocytes. Endocrinology. 2006;147:1020–1028. doi: 10.1210/en.2005-0825. [DOI] [PubMed] [Google Scholar]
- Norfleet AM, Clarke CH, Gametchu B, Watson CS. Antibodies to the estrogen receptor-alpha modulate rapid prolactin release from rat pituitary tumor cells through plasma membrane estrogen receptors. FASEB J. 2000;14:157–165. doi: 10.1096/fasebj.14.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Palanza P, Morellini F, Parmigiani S, vom Saal FS. Prenatal exposure to endocrine disrupting chemicals: effects on behavioral development. Neuroscience & Biobehavioral Reviews. 1999;23:1011–1027. doi: 10.1016/s0149-7634(99)00033-0. [DOI] [PubMed] [Google Scholar]
- Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER. Nature of Functional Estrogen Receptors at the Plasma Membrane. Mol Endocrinol. 2006 doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- Powell CE, Soto AM, Sonnenschein C. Identification and characterization of membrane estrogen receptor from MCF7 estrogen-target cells. The Journal of Steroid Biochemistry and Molecular Biology. 2001;77:97–108. doi: 10.1016/s0960-0760(01)00040-1. [DOI] [PubMed] [Google Scholar]
- Price RH, Jr, Butler CA, Webb P, Uht R, Kushner P, Handa RJ. A Splice Variant of Estrogen Receptor {beta} Missing Exon 3 Displays Altered Subnuclear Localization and Capacity for Transcriptional Activation. Endocrinology. 2001;142(5):2039–2049. doi: 10.1210/endo.142.5.8130. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid Signaling of Estrogen in Hypothalamic Neurons Involves a Novel G-Protein-Coupled Estrogen Receptor that Activates Protein Kinase C. J. Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai D, Frolova A, Frasor J, Carpenter AE, Katzenellenbogen BS. Distinctive actions of membrane-targeted versus nuclear localized estrogen receptors in breast cancer cells. Mol Endocrinol. 2005;19:1606–1617. doi: 10.1210/me.2004-0468. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol. Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol. 2004;18:2854–2865. doi: 10.1210/me.2004-0115. [DOI] [PubMed] [Google Scholar]
- Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a Structural Determinant Necessary for the Localization and Function of Estrogen Receptor {alpha} at the Plasma Membrane. Mol. Cell. Biol. 2003;23:1633–1646. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs Associate with and Regulate the Production of Caveolin: Implications for Signaling and Cellular Actions. Molecular Endocrinology. 2002;16:100–115. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Shapiro RA, Xu C, Dorsa DM. Differential Transcriptional Regulation of Rat Vasopressin Gene Expression by Estrogen Receptor {alpha} and {beta} Endocrinology. 2000;141:4056–4064. doi: 10.1210/endo.141.11.7796. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, O'Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr. Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- Song RXD, McPherson RA, Adam L, Bao Y, Shupnik M, Kumar R, Santen RJ. Linkage of Rapid Estrogen Action to MAPK Activation by ER{alpha}-Shc Association and Shc Pathway Activation. Molecular Endocrinology. 2002;16:116–127. doi: 10.1210/mend.16.1.0748. [DOI] [PubMed] [Google Scholar]
- Takahashi K OMYMHKMSA-IEMATSTKMYKH. Both estrogen and raloxifene cause G1 arrest of vascular smooth muscle cells. J Endocrinol. 2003;178:319–329. doi: 10.1677/joe.0.1780319. [DOI] [PubMed] [Google Scholar]
- Tesarik J, Mendoza C. Nongenomic effects of 17 beta-estradiol on maturing human oocytes: relationship to oocyte developmental potential. J Clin Endocrinol Metab. 1995;80:1438–1443. doi: 10.1210/jcem.80.4.7714121. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Minireview: A Plethora of Estrogen Receptors in the Brain: Where Will It End? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol Endocrinol. 1997;11:353–365. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Kow LM, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. PNAS. 2001;98:12267–12271. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Ando S, Maggiolini M. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol. 2006;20:631–646. doi: 10.1210/me.2005-0280. [DOI] [PubMed] [Google Scholar]
- Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen Receptor (ER){{alpha}} and ER{beta} Exhibit Unique Pharmacologic Properties When Coupled to Activation of the Mitogen-Activated Protein Kinase Pathway. Endocrinology. 2001;142:2336–2342. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- Wang MM, Traystman RJ, Hurn PD, Liu T. Non-classical regulation of estrogen receptor-alpha by ICI182,780. J Steroid Biochem Mol Biol. 2004;92:51–62. doi: 10.1016/j.jsbmb.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Warner M, Gustafsson JA. Nongenomic effects of estrogen: why all the uncertainty? Steroids. 2006;71:91–95. doi: 10.1016/j.steroids.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Watson CS, Campbell CH, Gametchu B. The dynamic and elusive membrane estrogen receptor-alpha. Steroids. 2002;67:429–437. doi: 10.1016/s0039-128x(01)00172-6. [DOI] [PubMed] [Google Scholar]
- Watters JJ, Chun TY, Kim YN, Bertics PJ, Gorski J. Estrogen Modulation of Prolactin Gene Expression Requires an Intact Mitogen-Activated Protein Kinase Signal Transduction Pathway in Cultured Rat Pituitary Cells. Molecular Endocrinology. 2000;14:1872–1881. doi: 10.1210/mend.14.11.0551. [DOI] [PubMed] [Google Scholar]
- Weatherman RV, Chang CY, Clegg NJ, Carroll DC, Day RN, Baxter JD, McDonnell DP, Scanlan TS, Schaufele F. Ligand-selective interactions of ER detected in living cells by fluorescence resonance energy transfer. Mol Endocrinol. 2002;16:487–496. doi: 10.1210/mend.16.3.0813. [DOI] [PubMed] [Google Scholar]
- Writing Group for the Women's Health Initiative Investigators. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: Principal Results From the Women's Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Xu Y, Traystman RJ, Hurn PD, Wang MM. Neurite-localized estrogen receptor-alpha mediates rapid signaling by estrogen. J Neurosci Res. 2003;74:1–11. doi: 10.1002/jnr.10725. [DOI] [PubMed] [Google Scholar]
- Ylikomi T, Bocquel MT, Berry M, Gronemeyer H, Chambon P. Cooperation of proto-signals for nuclear accumulation of estrogen and progesterone receptors. EMBO J. 1992;11:3681–3694. doi: 10.1002/j.1460-2075.1992.tb05453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Maier B, Santen RJ, Song RX. Membrane association of estrogen receptor alpha mediates estrogen effect on MAPK activation. Biochem Biophys. Res Commun. 2002;294:926–933. doi: 10.1016/S0006-291X(02)00348-0. [DOI] [PubMed] [Google Scholar]


