Abstract
We have previously reported on the relevance of the prevalence of CD44+/CD24−/low cells in primary breast tumors. To study regulation of CD24, we queried a number of publicly available expression array studies in breast cancer cells, and found that CD24 was down-regulated upon estrogen treatment. We confirmed this estrogen-mediated repression of CD24 mRNA by qPCR in MCF7, T47D, and ZR75-1 cells. Repression was also seen at the protein level as measured by flow cytometry. CD24 was not down-regulated in the ERα negative MDA-MB-231 cells suggesting that ERα was necessary. This was further confirmed by ERα silencing in MCF7 cells resulting in increased CD24 levels, and by reintroduction of ERα into C4-12 cells resulting in decreased CD24 levels. Estrogen treatment did not alter half-life of CD24 mRNA, and new protein synthesis was not essential for repression, suggesting a primary transcriptional effect. HDAC inhibition by Trichostatin A completely abolished the repression, but decrease of the ERα corepressors NCoR, LCoR, RIP140, SMRT, SAFB1, and SAFB2 by siRNA or overexpression of SAFB2, NCoR, and SMRT had no effect. In silico promoter analyses led to the identification of two EREs in the CD24 promoter, one of which was able to bind ERα as shown by electrophoretic mobility shift assay and chromatin immunoprecipitation assay. Together, our results show that CD24 is repressed by estrogen, and that this repression is a direct transcriptional effect depending on ERα and HDACs.
Keywords: CD24, ERα, breast cancer, estrogen-mediated repression
Introduction
CD24 is a small, heavily glycosylated, mucin-like glycosylphophatidyl-inositol (GPI)-linked cell surface protein that localizes in lipid rafts.1 CD24 has recently generated considerable attention in tumor biology due to its role as a potential breast cancer stem cell marker2 and its function in cell adhesion and metastatic tumor spread. Particularly, CD24 is expressed in a wide variety of human malignancies and has been associated with an unfavorable prognosis in breast cancer.1,3 In experimental animals, CD24 has been suggested to regulate numerous processes associated with tumor growth and metastasis.4 In humans, CD24 has been identified as a molecular marker that allows distinction between luminal epithelial, non-epithelial, and myoepithelial cells.5 Moreover, the marker combination CD44+/CD24−/low has been reported to characterize putative breast cancer stem cells.2 Our own studies demonstrated that the high prevalence of CD44+/CD24−/low cells in primary breast cancers favored distant metastasis, especially to the bone.6 Although the lack of CD24 has been highlighted within the context of putative breast cancer stem cells recent gene expression signatures designed to predict distant metastasis, prognosis and therapeutic planning pointed to a relationship between the breast cancer ERα status and CD24 expression levels [ONCOMINE-CANCER PROFILING DATABASE (http://www.oncomine.org)].7–10 These independent microarray analyses of breast tumors showed a consistent association between CD24 levels and estrogen receptor alpha (ERα) status in that CD24 expression was highest in ERα negative and lowest in ERα positive tumors. Furthermore, microarray and RT-PCR analyses of ZR75-1 breast cancer cells showed that CD24 is down-regulated following estrogen (E2) treatment. The down-regulation of CD24 by E2 is particularly important while considering CD44+/CD24−/low as a breast cancer stem cell marker because CD44 is known to be up-regulated by E2.11,12 These observations prompted us to explore the transcriptional regulation of CD24 by ERα. Our findings show that CD24 RNA and protein expression were repressed upon estrogen treatment and that this repression requires ERα and histone deacetylase (HDAC). We identified the estrogen responsive element (ERE) in the CD24 promoter which is able to recruit ERα upon E2 treatment.
Materials and methods
Cells, culture conditions
Breast cancer cell lines MCF7, MDA-MB-231, T47D, and ZR-75-1 were obtained from ATCC and cultured in DMEM supplemented with 5% fetal bovine serum (FBS), 2mM L-glutamine, 100 IU/ml penicillin, and 100 µg/ml streptomycin at 37°C under 5% CO2. C4-12 and C4-12 ERα cells were modified from MCF7 cells and routinely maintained in αMEM medium without phenol red as described previously.13
E2 treatment and siRNA
For estrogen treatment, cells were switched to Improved MEM medium supplemented with 5% dextran-charcoal-treated FBS for at least 48 hours before and during the treatment. Cells were treated with vehicle (ethanol), 10−8M E2, or 10−8M E2 + 10−6 pure steroidal estrogen receptor antagonist ICI 182,780 (Faslodex, Astrazeneca, Cheshire, UK). For siRNA experiments, non-specific (NS), ERα, p53, LCoR, SAFB1, SAFB2 (all from Dharmacon, IL), RIP140 (GAAGGAAGCUUUGCUAGCU),14 NCoR (AAGAAGGAUCCAGCAUUCGGA),15 or SMRT (AAGGGUAUCAUCACCGCUGUG)16 siRNAs were transfected into MCF7 cells using Lipofectamine 2000 (Invitrogen, CA) or Dharmafect (Dharmacon, IL) according to the manufacturer’s protocol. 36 hours after siRNA transfection, cells were treated with E2 or vehicle for either 8 (SAFB1), 16 (RIP140, LCoR), or 24 (SAFB2, NCoR, SMRT) hours, and the efficacy of the siRNA-mediated knockdown was evaluated by quantitative real-time PCR (qPCR), and by immunoblotting (except RIP140 and LCoR). The antibodies used were ERα (Santa Cruz, CA), NCoR (Santa Cruz, CA), SMRT (Upstate, NY), SAFB1,17 and SAFB2.18 All antibodies were used in a dilution of 1:1000. To understand the effect of overexpression of corepressors on E2 mediated down-regulation of CD24 transcription, we used NCoR, SMRT, and SAFB2 cDNAs. NCoR and SMRT cDNAs were kindly provided by Dr. Myles Brown (Dana-Farber Cancer Institute, Boston, MA). cDNA or the control vector were transfected to MCF7 cells using Lipofectamine 2000 (Invitrogen, CA) for 24 hours. Cells were then treated with either vehicle or E2 for another 24 hours prior to RNA extraction. For the cycloheximide (CHX) experiment, cells were treated with CHX (10mg/ml) in the presence or absence of E2, and RNA was isolated 4 or 8 hours later. For the actinomycin-D (Act-D) experiment, Act-D (2µg/ml) was added 30 min prior to E2 treatment, and RNA was isolated at indicated time points. To inhibit HDAC activity, cells were treated with Trichostatin-A (TSA) (1µM) for 6 hours before E2 treatment, and RNA was isolated 24 hours later.
Quantitative real-time PCR
RNA was isolated using the “RNeasy RNA isolation kit” (QIAGEN Inc, CA) and reverse transcribed using the SuperScript II Reverse Transcriptase (Invitrogen, CA), and qPCR was performed using an ABI Prism 7700 Sequence Detection System (Applied Biosystems, CA) with either the SYBR Green or the Taqman chemistry. The primers and probes used for the assay are listed in table 1 with primers for NCoR from Keeton et al.15 and primers and probe for SMRT from Wieser et al.19 The relative mRNA levels were calculated using the ΔΔCt method, with β-actin mRNA as a normalizer.
Table 1.
qPCR Primer and probe sequences
| Gene | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| β-actin | CCCTGGCACCCAGCAC | GCCGATCCACACGGAGTAC | ATCAAGATCATTGCTCCTCCTGAGCGC |
| CD24 | TGCTCCTACCCACGCAGATT | GGCCAACCCAGAGTTGGAA | AACAACAACTGGAACTTCAAGTAACTCCTCCCA |
| hERα | AACCGAGATGATGTAGCCAGC | CAGGAACCAGGGAAAATGTG | TGTCGAAGATCTCCACCATGCCCTCT |
| N-CoR | AGCATTCCATCCCTACGGG | TGGACCCCTTCACCAAAGC | |
| RIP140 | TTGGAGGGAGGCTTCATCTG | GCTTTCGTTTCTGCAGTAGGAAGTA | |
| SAFB1 | CCCGAAGATGACTCGGATACAA | TGTAGAAGAGAGTCCACTAACCCAGAA | ACCACAACTGCTGCGACCCTTTTCCT |
| SAFB2 | GATCTCAAGAACCTTTTCAGCAAGTAT | TCGACATGGTGACGAATCCA | CCAAAGTGGTAACGAACGCCCGC |
| SMRT | GGTCAAGTCCAAGAAGCAAGAGAT | GCTTCTATAGGTCATAAGGCCTGTTC | TGAACACCCACAACCGGAATGAGC |
Flow cytometry of membranous and cytoplasmic CD24
Membranous and cytoplasmic CD24 expression was analyzed as previously described by Farahat et al.20 MCF7 cells were treated with E2 or vehicle for 24 and 48 hours, and cells were harvested, washed with PBS, and incubated with phycoerythrin (PE)-conjugated anti-human CD24 monoclonal antibody (clone ML5, BD Biosciences, Pharmingen, San Jose, CA) or isotype-matched monoclonal antibody (IgG) control (BD Biosciences, Pharmingen, San Jose, CA) for 30 minutes. After two washes with PBS, cells were resuspended in 1 ml of PBS and analyzed in an EPICS XL flow cytometer (Beckman Coulter, CA). For cytoplasmic CD24, cells were fixed and permeabilized using Fix and Perm® cell permeabilization kit (Caltag Laboratories, Austria) as per the manufacturer’s instructions prior to CD24 antibody/isotype control staining.
Gel mobility shift assay
Digoxigenin (dig)-labeled double-stranded oligonucleotide probe sequences containing putative EREs at −3229 to −3216 bp (CD24-ERE-A 5’-gatcGATCTTGGGTCACTGCAACCTCCGCCT-3) and at −2003 to −1990 bp (CD24-ERE-B 5’-gatcCCATGTTGGTCAGGTTGGTCTGGAACT-3’) in the CD24 promoter, and a positive control containing a consensus ERE (5’gatcGACAAAGTCAGGTCACAGTGACCTGATCAAG3’) were used21 in gel shift assays performed as previously described.22 ERα protein was synthesized using the ERα cDNA and TNT® T7 Quick Coupled Transcription/Translation System (Promega, Madison WI, USA), and for the supershift we added monoclonal antibody against ERα (DakoCytomation, Denmark A/S).
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed in MCF7 cells as described before23 using ERα antibody (Santa Cruz, CA). In brief, MCF7 cells were cultured in charcoal stripped serum medium and treated with vehicle (ethanol) or E2 for 24 hours. The cells were then cross-linked using formalin, sonicated into 500 to 1000 bp fragments and immunoprecipitated using ERα antibody or IgG control. DNA was extracted and a 264 bp CD24 promoter fragment containing the ERE-A site was amplified using sense primer 5’GCGTGAGTTATTATTGGCTAAGGT3’ and antisense primer 5’GATCACATGGTCAGGAGATCG3’. Similarly, a 202 bp fragment containing the ERE-B site was amplified using sense primer 5’CTCAGCCTCCCAAGTAGCTG3’ and antisense primer 5’TGGTGCGCTCACACCTATAA3’. PCR products were analyzed on 2% agarose gels.
Results
CD24 expression is regulated by ERα
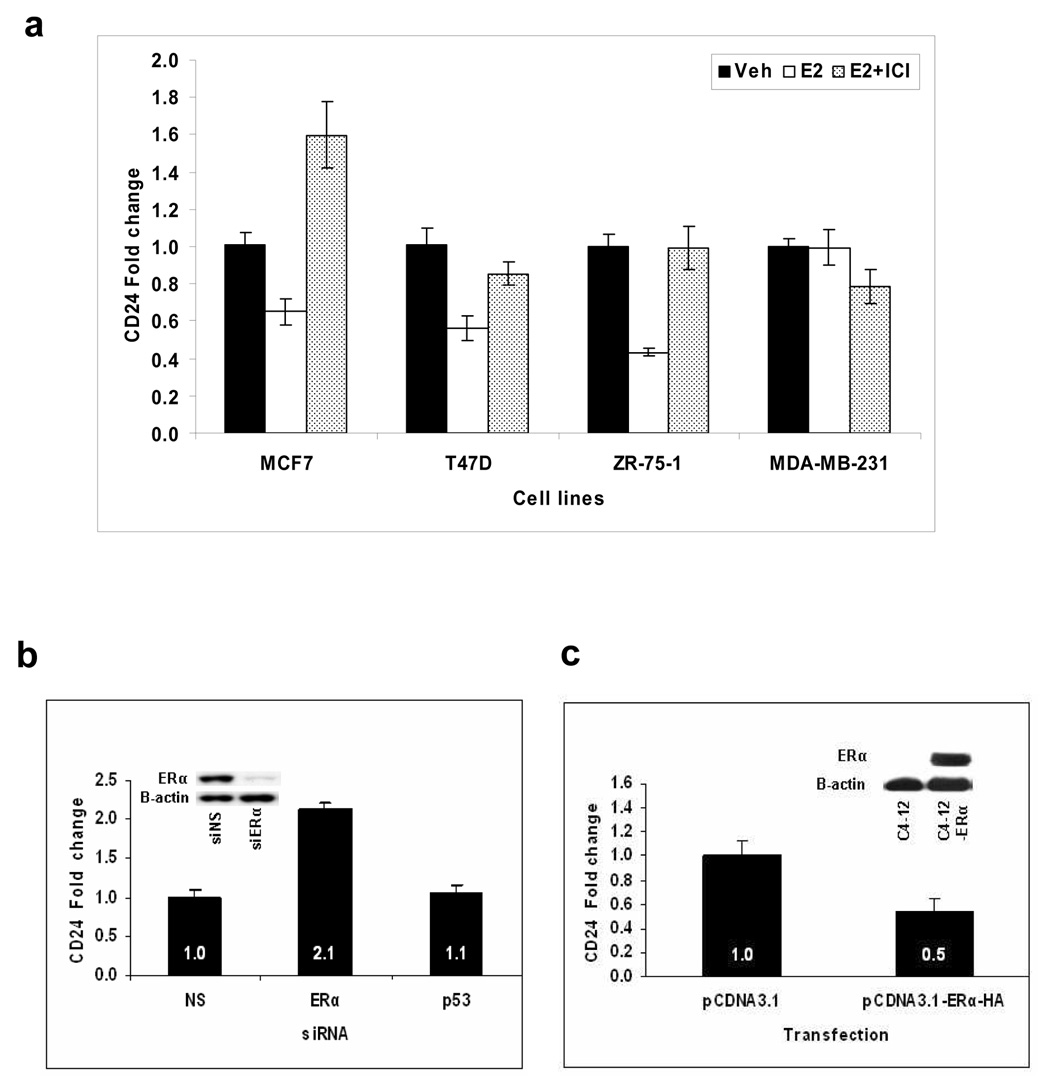
We investigated the possible transcriptional regulation of CD24 by E2 in various ERα positive breast cancer cell lines. As shown in figure 1a, CD24 mRNA expression was down-regulated about 50% after 24 hours of E2 treatment in MCF7, T47D, and ZR75-1 cells. The down-regulation was prevented by the ERα antagonist ICI. Repression was not seen in the ERα negative cell line MDA-MB-231. To further confirm a direct involvement of ERα in the down-regulation of CD24, we decreased ERα levels in MCF7 cells using ERα specific siRNA. The Western blot analysis shows that ERα protein level is remarkably down-regulated with the corresponding siRNA (Fig. 1b). Thus, about 70% down-regulation of ERα protein resulted in a two-fold increase in CD24 levels (Fig. 1b). We used p53 specific siRNA as a negative control for the siRNA transfection experiment and found no significant difference in the CD24 mRNA level compared to the non-specific siRNA. Finally, we compared CD24 expression in ERα negative C4-12 and C4-12-ERα cells in which ERα expression had been restored by stable transfection.13 The Western blot analysis shows the loss of ERα protein expression in C4-12 cells and re-expression after ERα transfection (Fig. 1c). As expected, CD24 expression was two-fold lower in C4-12-ERα cells when compared to vector-transfected C4-12 cells (Fig. 1c), providing further evidence that CD24 repression requires ERα.
Figure 1. ERα-dependent estrogen-mediated repression of CD24 mRNA in breast cancer cell lines as measured by qPCR.
(a) Breast cancer cell lines were treated with vehicle, E2, or E2 and ICI for 24 hours. The vehicle of each experiment is set as 1. (b) Effect of ERα silencing on CD24 expression. MCF7 cells were transfected with non-specific, ERα, or p53 (additional negative control) siRNA, and the CD24 transcript was measured by qPCR. The effect of ERα and control siRNA on ERα protein levels are shown in the corresponding Western blot insert with B-actin protein as the loading control. (c) Transfection of ERα cDNA (ERα-HA) decreased CD24 mRNA. CD24 transcripts were measured by qPCR in ERα-negative/low C4-12 and ERα-positive C4-12-ER cells. In all figures, bars represent mean fold change ± SE. The ERα protein expressions in C4-12 and C4-12-ER cells are shown in the corresponding Western blot insert with B-actin protein as the loading control.
To test whether crosstalk with growth factors is involved in the transcriptional down-regulation of CD24 by estrogen, we treated MCF7 cells with E2 in serum-free medium. CD24 expression was still down-regulated (data not shown), suggesting that growth factors are not necessary for estrogen-mediated repression of CD24.
The next question we addressed was whether estrogen-mediated repression of CD24 could also be observed at the protein level. Though CD24 is considered as a membranous protein, significant rates of cytoplasmic CD24 positivity have been reported for a variety of the most common human tumors. Moreover, in several tumor types higher rates of cytoplasmic CD24 expression were significantly associated with shorter patient survival times.1,3 Therefore, we treated MCF7 cells with E2, harvested cells after 24 and 48 hours, and measured membranous and cytoplasmic CD24 protein using flow cytometry. We observed a time dependent decrease in the CD24 membranous protein expression at 24 and 48 hours of E2 treatment compared to the vehicle treated cells (Fig. 2a), which was maintained up to 96 hours of E2 treatment (data not shown). Though the decrease in cyctoplasmic protein expression was not considerable after 24 hours it also significantly decreased after 48 hours of E2 treatment (Fig. 2b). The decreases in mean fluorescence intensities of membranous and cytoplasmic CD24 staining compared with vehicle treated cells were about 42 and 47% respectively after 48 hours. This is an important observation, especially when considering the correlation of cytoplasmic CD24 expression with tumor progression (including breast tumors) and shorter patient survival.1,3
Figure 2. E2-mediated downregulation of CD24 protein in MCF-7 cells.
Membranous (a) and cytoplasmic (b) CD24 protein expression was analyzed by flow cytometry after 24 and 48 hours using PE-labelled CD24 antibody. IgG was used as a negative control. PE= phycoerythrin, MFI= mean fluorescence intensity.
The estrogen-mediated repression of CD24 is a primary transcriptional response that is HDAC dependent
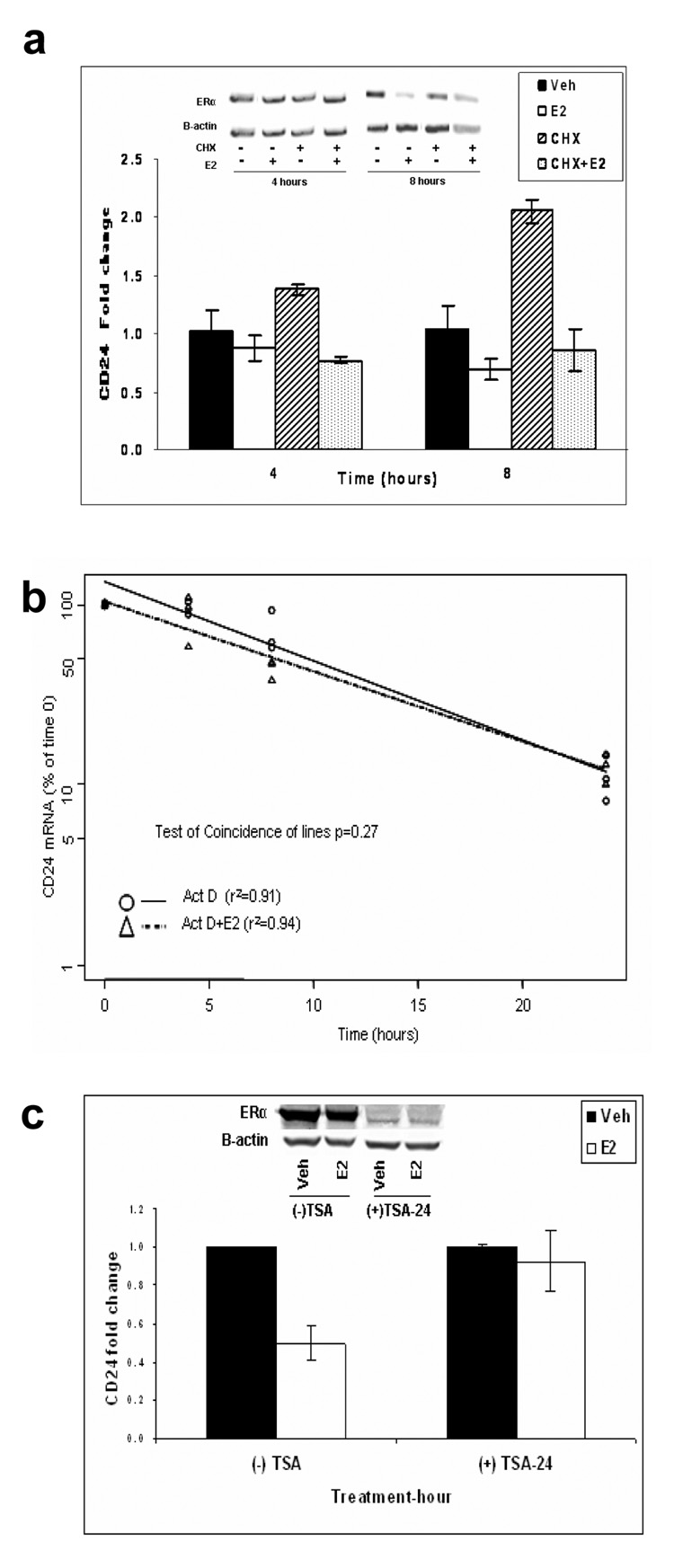
To determine whether CD24 was a primary or secondary E2 responsive gene we treated MCF7 cells with estrogen in the presence of the protein synthesis inhibitor cycloheximide (CHX). As a control, we measured the ERα protein expression and as shown in the insert of figure 3a, protein levels were not significantly altered after CHX treatment. Measuring CD24 transcripts by qPCR showed that repression was not inhibited even in the presence of CHX (Fig. 3a) suggesting that repression was independent of additional protein synthesis. To study whether the observed repression was a result of altered RNA stability, we measured CD24 mRNA stability in the absence or presence of estrogen. MCF7 cells were treated with the transcription inhibitor actinomycin-D and CD24 mRNA stability was estimated by regression analysis of the decline in mRNA levels. There was no significant difference in the presence or absence of E2 (Fig. 3b), suggesting that E2 does not down-regulate CD24 by altering its mRNA half-life, thus providing further evidence that the repression is a direct transcriptional event. To further test this hypothesis, we analyzed whether the estrogen-mediated repression was HDAC dependent. We treated MCF7 cells with the HDAC inhibitor TSA for 6 hours prior to E2 treatment and found that TSA abolished the estrogen-mediated repression of CD24 (Fig. 3c), demonstrating the requirement for HDAC’s in the estrogen mediated down-regulation of CD24, and suggesting that the repression involves active chromatin remodeling. The accompanying Western blot analysis shows that there is a decrease in ERα protein expression after TSA treatment in both vehicle and E2 treated groups as previously published by Pryzbylkowski et al.24 Therefore, these data should be interpreted with caution, however, recent studies from our laboratory clearly proof a critical role of specific HDACs in E2-mediated transcriptional regulation (Zubairy et al. unpublished data), and thus we are confident concluding that HDACs are likely to be involved in estrogen-mediated repression of CD24. Over the last years, numerous studies have shown that corepressor proteins are involved in the chromatin remodeling process regulating ERα transcription. We therefore set out to decrease expression of the known ligand-dependent ERα corepressors RIP140 and LCoR,25 and S/MAR binding corepressors SAFB1 and SAFB226 to analyze the estrogen mediated repression of CD24. Even the early E2 treatment time point in the absence of SAFB1, or later time points in the absence of LCoR, RIP140, and SAFB2 could not reverse the CD24 repression (Fig. 4a). The same was observed, when we over-expressed SAFB2 (Fig. 4a). We further evaluated the effect of other two well established corepressors NCoR and SMRT which repress through direct interactions with the AF-2 domain of nuclear receptors. Both down-regulation by siRNA and over-expression by transfecting cDNAs of these corepressors could not significantly reverse the E2 mediated repression of CD24 (Fig. 4b). This suggests, that none of the corepressors analyzed are necessary for the down-regulation of CD24 by estrogen.
Figure 3. The estrogen-mediated repression of CD24 is a direct and HDAC-dependent process.
(a) Estrogen-mediated repression of CD24 was measured by qPCR in the absence and presence of CHX as indicated. The effect of CHX and E2 treatment on ERα protein levels are shown in the corresponding Western blot insert with B-actin protein as the loading control. (b) CD24 mRNA stability was determined by measuring CD24 mRNA expression in the presence and absence of E2 in MCF7 cells that were pre-incubated with Act D. The amount of mRNA at time 0 of E2 treatment was taken as 100%. Results are expressed as the mean relative amount of CD24 mRNA at each time point, after correction for the amount of β-actin. A linear regression analysis of the data points is shown for CD24 mRNA level in the presence (Δ) and absence (○) of E2. (c) MCF7 cells were pretreated for 6 hours with TSA or vehicle, and E2-mediated repression of CD24 was measured by qPCR after 24 hours. Bars represent mean fold change ± SE. The effect of TSA and E2 treatment on ERα protein levels are shown in the corresponding Western blot insert with B-actin protein as the loading control.
Figure 4. Corepressors are not affecting the CD24 transcription.
(a) MCF7 cells were pretransfected with non-specific, SAFB1, RIP140, LCoR or SAFB2 siRNA, or SAFB2 cDNA for 24 hours. The cells were then treated with E2 for 8 (SAFB1), 16 (RIP140 and LCoR) and 24 (SAFB2) hours together with the respective control transfected cells and the CD24 transcript was measured by qPCR. (b) MCF7 cells were pretransfected with mock, non-specific, NCoR or SMRT siRNA or cDNA for 24 hours. The cells were then treated with E2 for another 24 hours and the CD24 transcript was measured by qPCR.
ERα directly binds to one of the predicted ERE’s in the CD24 promoter
Analysis of the 5’-flanking region of the CD24 gene on chromosome 6 using Dragon ERE Finder version 227 lead to the identification of two imperfect palindromic ERE’s, ERE-A: GGTCACTGCAACC and ERE-B: GGTCAGGTTGGTC as potential binding sites for ERα as indicated in figure 5a. In addition, the promoter contains consensus p300, AP1, SP1, GATA1, NFκB elements, and TATA boxes (TRANSFAC® 7.0). The specificity and relative affinity of the binding of putative ERE sequences to ERα were determined by non-radioactive electrophoretic mobility shift assays (EMSA). Figure 5b (lane 2) shows efficient binding to the dig-labeled oligonucleotides CD24-ERE-A. A very weak binding observed with CD24-ERE-B was not reliably reproducible (Fig. 5b, lane 5). The binding of CD24-ERE-A was competed by a 100-fold excess of unlabeled ERE-A probe (Fig. 5b, lane 3). Co-incubation with ERα antibody supershifted the ERα-CD24 complex towards a retarded electrophoretic mobility (Fig. 5b, lane 4). A known ERE sequence used as a control showed similar binding and supershift characteristics (Fig. 5b, lanes 8 to 10). These data show that the CD24-ERE-A of the CD24 promoter region binds ERα. From our experiments it follows that the CD24 promoter contains at least one ERE sequence capable of binding ligand-activated ERα.
Figure 5. ERα binds to ERE of the CD24 promotor.
(a) Representation of the 5'-flanking promoter region of the human CD24 gene with possible transcriptional binding sites. Locations of the putative ERE sites, transcription start site, and primer positions used for the ChIP analysis are indicated. The promoter contains consensus AP1, SP1, GATA1, and NFκB elements, and TATA boxes. (b) Electrophoretic mobility shift analysis (EMSA) was performed with dig-labelled oligonucleotides for ERE-A (lanes 2 to 4), ERE-B (lanes 5 to 7), and ERE-positive control (lanes 8 to 10) using in vitro translated ERα protein. Lane 1 is the negative control without ERα protein. Excess of corresponding unlabeled oligonucleotides was added for competition assay (lanes 3, 6, and 9) and preincubated with ERα antibody for supershift (lanes 4, 7, and 10). Specific binding sites are marked by arrows. (c) In vivo binding of ERα to ERE-A of CD24 promoter. Chromatin Immunoprecipitation (ChIP) assay was performed using cross-linked DNA-protein complexes isolated from MCF-7 cells. PCR was performed with primers flanking the ERE-A region. Input DNA was used as positive control and IgG as negative control. A control PCR was carried out specific to the ERE region in the pS2 promoter.
The recruitment of E2 bound ERα to the CD24-ERE-A was further confirmed by ChIP assays in MCF7 cells. As shown in figure 5c, chromatin immunoprecipitation of MCF7 cells with ERα specific antibody in the presence or absence of E2 treatment showed an enrichment of ERα at CD24-ERE-A after the E2 treatment. This was not observed with the mouse IgG control antibody. A well established ERE in the pS2 promoter which is known for the recruitment of ligand bound ERα was used as a positive control. There was no reliable evidence observed for the ERα recruitment to the CD24-ERE-B region.
Discussion
Our study suggests that the estrogen-mediated repression of CD24 does involve a classical ERE-mediated mechanism. Over the past decade, it has become increasingly clear that the recruitment of a number of corepressors and corepressor complexes, which often include HDAC’s to convert transcriptionally active into inactive chromatin by histone deacetylation, are a part of ER-mediated transcriptional and biological activities. For example, Stossi et al reported the down-regulation of cyclin G2 expression by ERα through a corepressor/HDAC-mediated mechanism.28 However, in contrast to that observation with cyclin G2 we could not reliably identify a significant role for any of the established corepressors we analyzed in the repression of CD24, though HDACs are involved. Yet, these results do not exclude the involvement of other unexamined corepressors.
The role of CD24 in normal mammary gland and breast tumors is one of the recent interests in breast cancer research. However, our current knowledge of signaling pathways related to CD24 is limited. Although putative human breast cancer stem cells have been suggested to express the CD44+/CD24−/low marker combination2,29 and CD24 has been implicated in the regulation of tumor growth and metastasis4 this is calling attention to a possible conundrum. The current literature supports the notion that the marker combination CD44+/CD24−/low characterizes low prevalent breast cancer stem cells of tumorigenic and self renewing potential.2 However, our own previous clinical data showed their high prevalence in primary breast tumors favoring distant metastasis particularly to the bone.6 Since no correlation with local recurrence, local metastasis, tumor size, or progression was observed, these cells might be critical for the initial tumor cell proliferation but also be able to adapt to a new microenvironment. This adaptation may involve an increased CD24 expression suggesting that CD44+/CD24−/low and high expressing CD24 cells are different phenotypic entities. The latter is in line with recent findings by Shipitsin et al. who reported that CD24+ and CD44+ cells from individual tumors were clonally related but not always identical.30 It is interesting, to note that while CD44 is up-regulated by E2-bound ERα,11,12 CD24 is simultaneously down-regulated. This suggests a possible functional regulation of putative breast cancer stem cells by ERα.
CD24 has been suggested to be directly relevant to tumor progression and metastasis.4 CD24 is a ligand for P-selectin, a lectin that is expressed at the surface of activated platelets and endothelial cells and is implicated in tumor thrombi and metastasis formation.31 CD24 expression was found to significantly correlate with the in vitro invasiveness of established breast cancer cell lines.32 With ERα being a prominent molecule in breast cancer, our data support the notion that ERα signaling could be involved in regulating the dissemination of tumor cells and formation of metastases through the CD24/P-selectin pathway. Our findings and interpretations are in line with the clinical observation of a less favorable prognosis of breast tumors lacking ERα. Of note, a number of gene expression signatures recently provided correlations between ERα status and CD24 expression where ERα negativity was correlated with higher CD24 expression as analyzed by the ONCOMINE-CANCER PROFILING DATABASE (http://www.oncomine.org).7–10 It may be speculated that breast tumors lacking or losing ERα may no longer repress CD24 expression and therefore may more readily engage the CD24/P-selectin and metastasis pathway. This may be particularly important when considering the high percentage of breast cancer treatment failure with selective estrogen receptor modulators (SERMs).
Several reports currently suggest, that cytoplasmic expression of CD24 in different types of tumors is correlated to bad disease prognosis.1,3,6 Our finding of reduced cytoplasmic CD24 expression after estrogen treatment may be particularly important in this context. The mechanism of cytoplasmic expression of CD24 is not well established. However, it is suggested, that cytoplasmic CD24 expression is probably resulting from overproduction of the protein, disturbance of the protein distribution, or degradation within the cell.33 A clinical significance of E2 mediated down-regulation of CD24 expression may not be restricted to breast cancer. For example clinical studies indicate that the incidence of colon cancer is lower in women than in man. Moreover, data from the Women’s Health Initiative (WHI) indicate a significantly reduced incidence of colon cancer in post-menopausal women receiving combined hormone replacement therapy.34 However, further studies in colorectal cancer clinical samples or cell lines in connection with CD24 may be necessary to confirm this possibility.
The interaction between ERα and CD24 is obviously complex and it is well known from the literature, that down-regulation of CD24 does not always associate with ERα expression. For example, ERα positive cell lines including MCF7 are not all completely negative for CD24, and all ERα negative cell lines are not highly positive for CD24.32 In the same way, both ERα positive and negative tumors express CD24.3,6 Therefore, other regulatory mechanisms such as epigenetic silencing or involvement of other transcription factors may in addition be involved in the expression of CD24. Interestingly, our own preliminary data suggest that CD24 can be subject to promoter methylation in breast cancer and also its mRNA expression can be initiated in CD24 negative breast cancer cell lines by demethylation (own unpublished data). However, since our current observations are limited to cellular studies, these results should be interpreted with caution. Altogether, the current data provide a significant contribution to the understanding of the transcriptional regulation of CD24 in breast cancer. Since the functional relevance of CD24 has not been well characterized and its target genes are not fully revealed at present, further research will be necessary to better understand the signal transduction pathway of CD24 and the related factors involved in this interaction.
Acknowledgements
This work was supported by the Robert Bosch Foundation of Medical Research, Stuttgart, Germany (HB), and by the U.S. National Institute of Health R01 (CA97213) (SO), (CA079911) (GMD), P01 (CA30195) and Spore pilot grant (CA58183) (SO). BK was an overseas scholar from Department of Biotechnology, Ministry of Science and Technology, Government of India, SZ received a graduate fellowship from the Department of Defense Breast Cancer Research Program (BC043880), and MR was a Marie Curie fellow of the European Commission under the Program HPMT-CT-2001-00269 Fighting Breast Cancer Tuebingen/Stuttgart. We appreciate Dr. Rene Meyer’s help with the testing of the SAFB1/SAFB2 siRNA, Prof. Susan G. Hilsenbeck for statistical analysis and Dr. Sanjay Bansal’s help in analyzing ERE.
Abbreviations
- Act-D
actinomycin-D
- ChIP
chromatin immunoprecipitation assay
- CHX
cycloheximide
- CoR
corepressors
- E2
estrogen
- EMSA
eloctrophoretic mobility shift assay
- ER
estrogen receptor
- ERE
estrogen responsive element
- HDAC
histone deacetylase
- LCoR
Ligand dependent nuclear receptor corepressor
- NCoR
Nuclear receptor corepressor 1
- qPCR
Quantitative real-time PCR
- RIP140
Receptor interacting protein 140
- SAFB1
Scaffold attachment factor B1
- SAFB2
Scaffold attachment factor B2
- SMRT
Silencing mediator of retinoid and thyroid hormone receptors
- TSA
Trichostatin
Footnotes
Statements of significance:
We provide first evidence and mechanism for a transcriptional regulation of CD24 by ERα in breast cancer cells. Our in vitro data highlight the direct interaction between the CD24 promoter and ERα which ultimately results in an altered cyctoplasmic and membranous CD24 protein expression. Thus, our findings contribute to a mechanistic explanation for the understanding of the less favorable prognosis of patients with ERα negative breast cancer.
References
- 1.Kristiansen G, Sammar M, Altevogt P. Tumour biological aspects of CD24, a mucin-like adhesion molecule. J Mol Histol. 2004;35:255–262. doi: 10.1023/b:hijo.0000032357.16261.c5. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hajj M, Wicha MS, ito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kristiansen G, Winzer KJ, Mayordomo E, Bellach J, Schluns K, Denkert C, Dahl E, Pilarsky C, Altevogt P, Guski H, Dietel M. CD24 expression is a new prognostic marker in breast cancer. Clin Cancer Res. 2003;9:4906–4913. [PubMed] [Google Scholar]
- 4.Baumann P, Cremers N, Kroese F, Orend G, Chiquet-Ehrismann R, Uede T, Yagita H, Sleeman JP. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res. 2005;65:10783–10793. doi: 10.1158/0008-5472.CAN-05-0619. [DOI] [PubMed] [Google Scholar]
- 5.Sleeman KE, Kendrick H, Ashworth A, Isacke CM, Smalley MJ. CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res. 2006;8:R7. doi: 10.1186/bcr1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H. Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11:1154–1159. [PubMed] [Google Scholar]
- 7.Ivshina AV, George J, Senko O, Mow B, Putti TC, Smeds J, Lindahl T, Pawitan Y, Hall P, Nordgren H, Wong JE, Liu ET, et al. Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer. Cancer Res. 2006;66:10292–10301. doi: 10.1158/0008-5472.CAN-05-4414. [DOI] [PubMed] [Google Scholar]
- 8.Miller LD, Smeds J, George J, Vega VB, Vergara L, Ploner A, Pawitan Y, Hall P, Klaar S, Liu ET, Bergh J. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci U S A. 2005;102:13550–13555. doi: 10.1073/pnas.0506230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van d V, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, Jatkoe T, Berns EM, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 11.Sorbello V, Fuso L, Sfiligoi C, Scafoglio C, Ponzone R, Biglia N, Weisz A, Sismondi P, De BM. Quantitative real-time RT-PCR analysis of eight novel estrogen-regulated genes in breast cancer. Int J Biol Markers. 2003;18:123–129. doi: 10.1177/172460080301800205. [DOI] [PubMed] [Google Scholar]
- 12.Durst B, Sorg RV, Roder G, Betz B, Beckmann MW, Niederacher D, Bender HG, Dall P. The influence of hormones on CD44 expression in endometrial and breast carcinomas. Oncol Rep. 2001;8:987–993. [PubMed] [Google Scholar]
- 13.Oesterreich S, Zhang P, Guler RL, Sun X, Curran EM, Welshons WV, Osborne CK, Lee AV. Re-expression of estrogen receptor alpha in estrogen receptor alpha-negative MCF-7 cells restores both estrogen and insulin-like growth factor-mediated signaling and growth. Cancer Res. 2001;61:5771–5777. [PubMed] [Google Scholar]
- 14.White KA, Yore MM, Deng D, Spinella MJ. Limiting effects of RIP140 in estrogen signaling: potential mediation of anti-estrogenic effects of retinoic acid. J Biol Chem. 2005;280:7829–7835. doi: 10.1074/jbc.M412707200. [DOI] [PubMed] [Google Scholar]
- 15.Keeton EK, Brown M. Cell cycle progression stimulated by tamoxifen-bound estrogen receptor-alpha and promoter-specific effects in breast cancer cells deficient in N-CoR and SMRT. Mol Endocrinol. 2005;19:1543–1554. doi: 10.1210/me.2004-0395. [DOI] [PubMed] [Google Scholar]
- 16.Yoon HG, Chan DW, Huang ZQ, Li J, Fondell JD, Qin J, Wong J. Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1. EMBO J. 2003;22:1336–1346. doi: 10.1093/emboj/cdg120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanova M, Dobrzycka KM, Jiang S, Michaelis K, Meyer R, Kang K, Adkins B, Barski OA, Zubairy S, Divisova J, Lee AV, Oesterreich S. Scaffold attachment factor B1 functions in development, growth, and reproduction. Mol Cell Biol. 2005;25:2995–3006. doi: 10.1128/MCB.25.8.2995-3006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Townson SM, Dobrzycka KM, Lee AV, Air M, Deng W, Kang K, Jiang S, Kioka N, Michaelis K, Oesterreich S. SAFB2, a new scaffold attachment factor homolog and estrogen receptor corepressor. J Biol Chem. 2003;278:20059–20068. doi: 10.1074/jbc.M212988200. [DOI] [PubMed] [Google Scholar]
- 19.Wieser F, Schneeberger C, Hudelist G, Singer C, Kurz C, Nagele F, Gruber C, Huber JC, Tschugguel W. Endometrial nuclear receptor co-factors SRC-1 and N-CoR are increased in human endometrium during menstruation. Mol Hum Reprod. 2002;8:644–650. doi: 10.1093/molehr/8.7.644. [DOI] [PubMed] [Google Scholar]
- 20.Farahat N, van der PD, Praxedes M, Morilla R, Matutes E, Catovsky D. Demonstration of cytoplasmic and nuclear antigens in acute leukaemia using flow cytometry. J Clin Pathol. 1994;47:843–849. doi: 10.1136/jcp.47.9.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore JT, McKee DD, Slentz-Kesler K, Moore LB, Jones SA, Horne EL, Su JL, Kliewer SA, Lehmann JM, Willson TM. Cloning and characterization of human estrogen receptor beta isoforms. Biochem Biophys Res Commun. 1998;247:75–78. doi: 10.1006/bbrc.1998.8738. [DOI] [PubMed] [Google Scholar]
- 22.Arai K, Matsumoto Y, Nagashima Y, Yagasaki K. Regulation of class II beta-tubulin expression by tumor suppressor p53 protein in mouse melanoma cells in response to Vinca alkaloid. Mol Cancer Res. 2006;4:247–255. doi: 10.1158/1541-7786.MCR-05-0183. [DOI] [PubMed] [Google Scholar]
- 23.Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9:601–610. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 24.Pryzbylkowski P, Obajimi O, Keen JC. Trichostatin A and 5 Aza-2' deoxycytidine decrease estrogen receptor mRNA stability in ER positive MCF7 cells through modulation of HuR. Breast Cancer Res Treat. 2007 Sep 21; doi: 10.1007/s10549-007-9751-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Christian M, White R, Parker MG. Metabolic regulation by the nuclear receptor corepressor RIP140. Trends Endocrinol Metab. 2006;17:243–250. doi: 10.1016/j.tem.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Dobrzycka KM, Townson SM, Jiang S, Oesterreich S. Estrogen receptor corepressors -- a role in human breast cancer? Endocr Relat Cancer. 2003;10:517–536. doi: 10.1677/erc.0.0100517. [DOI] [PubMed] [Google Scholar]
- 27.Bajic VB, Tan SL, Chong A, Tang S, Strom A, Gustafsson JA, Lin CY, Liu ET. Dragon ERE Finder version 2: A tool for accurate detection and analysis of estrogen response elements in vertebrate genomes. Nucleic Acids Res. 2003;31:3605–3607. doi: 10.1093/nar/gkg517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stossi F, Likhite VS, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen-occupied estrogen receptor represses cyclin G2 gene expression and recruits a repressor complex at the cyclin G2 promoter. J Biol Chem. 2006;281:16272–16278. doi: 10.1074/jbc.M513405200. [DOI] [PubMed] [Google Scholar]
- 29.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 30.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, Halushka MK, Sukumar S, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Aigner S, Sthoeger ZM, Fogel M, Weber E, Zarn J, Ruppert M, Zeller Y, Vestweber D, Stahel R, Sammar M, Altevogt P. CD24, a mucin-type glycoprotein, is a ligand for P-selectin on human tumor cells. Blood. 1997;89:3385–3395. [PubMed] [Google Scholar]
- 32.Schindelmann S, Windisch J, Grundmann R, Kreienberg R, Zeillinger R, Deissler H. Expression profiling of mammary carcinoma cell lines: correlation of in vitro invasiveness with expression of CD24. Tumour Biol. 2002;23:139–145. doi: 10.1159/000064030. [DOI] [PubMed] [Google Scholar]
- 33.Chou YY, Jeng YM, Lee TT, Hu FC, Kao HL, Lin WC, Lai PL, Hu RH, Yuan RH. Cytoplasmic CD24 expression is a novel prognostic factor in diffuse-type gastric adenocarcinoma. Ann Surg Oncol. 2007;14:2748–2758. doi: 10.1245/s10434-007-9501-x. [DOI] [PubMed] [Google Scholar]
- 34.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]