Abstract
Intradermal administration of DNA vaccines encoding luciferase represents a convenient method to assess gene expression in vivo. Gene silencing by intradermal gene gun administration of DNA encoding short hairpin RNA (shRNA) may represent an effective technique for the specific knockdown of gene expression in vivo. In the current study, we characterized luciferase gene expression over time in vivo by noninvasive bioluminescence imaging. Furthermore, we characterized in vivo luciferase gene silencing with DNA encoding shRNA targeting luciferase. We also characterized human papillomavirus type 16 (HPV-16) E7-specific CD8+ T cell immune responses in mice immunized with E7 DNA and DNA encoding shRNA targeting Fas ligand (FasL), a key proapoptotic signaling protein. Our results indicated that coadministration of DNA encoding shRNA targeting luciferase significantly reduced luciferase expression in mice intradermally administered luciferase DNA. Furthermore, we observed that mice vaccinated with E7-expressing DNA coadministered with DNA encoding shRNA targeting FasL generated significantly enhanced E7-specific CD8+ cytotoxic T cell responses as well as potent therapeutic antitumor effects against E7-expressing tumors. Thus, intradermal administration of DNA encoding shRNA represents a plausible approach to silence genes in vivo and a potentially useful tool to enhance DNA vaccine potency.
Introduction
Vaccination with DNA encoding tumor- and/or virus-associated antigens has emerged as an especially promising strategy for generating therapeutic immunity against cancer. DNA is stable, simple to prepare, and safe relative to virus- or bacteria-based vectors (for reviews, see Donnelly et al., 1997; Gurunathan et al., 2000). In addition, intradermal administration of DNA vaccines via gene gun represents an efficient method to deliver DNA to the key professional antigen-presenting cells, Langerhans cells, in the skin. Using this noninvasive vaccine delivery system, antigen-expressing dendritic cells (DCs) mature and become able to migrate to the draining lymph nodes, where they activate T cells, resulting in efficient elimination of infected and cancerous cells (for review, see Guermonprez et al., 2002). Nevertheless, the potency of these vaccines is often limited by signaling pathways that negatively regulate the adaptive immune response. Thus, the silencing of these immunosuppressive signals potentially represents a highly effective strategy for the enhancement of DNA vaccine potency.
RNA interference (RNAi) technology has developed into a powerful method for specific posttranscriptional gene silencing in vitro and may be applied to diminish the expression of immunosuppressive factors in vivo as a complement to traditional DNA vaccines. Multiple species of small interfering RNA (siRNA) have been introduced into a variety of cells and have demonstrated that RNAi can be widely employed as a technique for specific gene knockdown (for reviews, see Caplen, 2004; Leung and Whittaker, 2005; Shankar et al., 2005). One approach leading to gene silencing is by transfection of DNA encoding short hairpin RNA (shRNA), targeting the gene of interest. The transfected cell will be able to generate shRNA, resulting in silencing of the gene of interest.
Despite the great potential of RNAi technology to serve as a tool for therapeutic in vivo gene silencing, the issue of specific and efficient delivery of siRNA to target tissue in vivo poses a formidable obstacle to the implementation of this technique in the clinic. Previously, we have employed intradermal administration of DNA vaccine in conjunction with siRNA targeting the key proapoptotic proteins Bax and Bak (Kim et al., 2005). Using this approach, we observed prolonged survival of transfected DCs, resulting in enhanced human papillomavirus type 16 (HPV-16) E7-specific CD8+ T cell responses against E7-expressing tumors in mice (Kim et al., 2005). Thus, intradermal administration by gene gun represents an efficient method to deliver siRNA targeting molecules of interest into DCs in vivo and thus effectively knock down the expression of specific genes.
Fas ligand (FasL) is a proapoptotic molecule that is produced in abundant amounts by DCs (Lu et al., 1997). FasL may associate with its cognate death receptor, Fas, found on the surface of naive T cells during antigen presentation, as an immunoregulatory mechanism. Fas–FasL interactions at the immunological synapse could then possibly initiate the extrinsic apoptosis pathway in these specific T cells during their activation (a type of activation-induced cell death). We reasoned that downregulation of FasL in DCs, using shRNA, may lead to improved T cell survival, thus enhancing DNA vaccine potency.
In the current study, we first characterized the expression of gene gun-delivered luciferase over time in vivo, using a noninvasive bioluminescence imaging system. Our results indicated that the expression of luciferase in vivo peaked 24 hr after DNA administration. Furthermore, we observed that coadministration of DNA encoding shRNA targeting luciferase significantly reduced the expression of luciferase in mice intradermally administered with luciferase DNA. We then investigated the immunotherapeutic potential of coadministering DNA vaccines containing HPV-16 E7 (Sig/E7/LAMP-1) (Ji et al., 1998) in conjunction with DNA encoding shRNA targeting the apoptosis inducer Fas ligand (FasL). We observed that mice vaccinated with Sig/E7/LAMP-1 DNA and coadministered DNA encoding shRNA targeting FasL generated significantly enhanced E7-specific CD8+ cytotoxic T cell responses, which translated into improved tumor cell killing in vitro as well as potent therapeutic antitumor effects against E7-expressing tumors. Furthermore, we demonstrated that blocking of FasL on the E7 peptide-loaded DCs reduces the apoptosis of E7-specific CD8+ T cells in vitro. Thus, intradermal administration of DNA encoding shRNA targeting FasL represents an excellent approach to increase the potency of DNA vaccines.
Materials and Methods
Mice
Female C57BL/6J mice (6 to 8 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME). Animals were maintained under specific pathogen-free conditions at Johns Hopkins Hospital (Baltimore, MD). All procedures were performed according to approved protocols and in accordance with recommendations for the proper care of laboratory animals.
Cell culture and antibodies
BHK-21 cells (CCL-10; American Type Culture Collection [ATCC], Manassas, VA) and explanted splenocytes were maintained in RPMI 1640 containing l-glutamine (2 mmol/liter), sodium pyruvate (1 mmol/liter), nonessential amino acids (100 μmol/liter), penicillin–streptomycin (100 IU/ml), 2-mercaptoethanol (50 μmol/liter), and 10% fetal bovine serum in a 37°C incubator with 5% CO2. The HPV-16 E7-expressing murine tumor model TC-1 cell line, and its luciferase-expressing derivative line TC-1/Luciferase, have been previously described (Lin et al., 1996; Huang et al., 2007). Briefly, primary lung epithelial cells of C57BL/6J mice were transformed with HPV-16 E6, E7, and an activated ras oncogene. These cells were then transduced with viral particles packaged with a Luciferase/Thy1.1 expression construct on the pMSCVpuro backbone (a kind gift from H. Levitsky, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore, MD). TC-1/Luciferase cells were cultured in the same medium as BHK-21 cells. Phycoerythrin-conjugated anti-mouse FasL antibody (clone MFL3; eBioscience, San Diego, CA) and isotype control (clone eBio299Arm; eBioscience) were used for cell surface staining of FasL, which was performed according to the vendor's protocol. Phycoerythrin-conjugated monoclonal rat anti-mouse CD8 antibody and fluorescein isothiocyanate (FITC)-conjugated anti-mouse interferon (IFN)-γ antibody (BD Biosciences Pharmingen, San Diego, CA) were used for intracellular cytokine staining of explanted splenocytes. For FasL-blocking experiments, the antibodies used were anti-mouse FasL (CD178.1) antibody (Kay-10 clone) (BioLegend, San Diego, CA) and functional-grade mouse IgG2b isotype control (eBioscience). The DC-1 dendritic cell line was kindly provided by K. Rock (University of Massachusetts, Worcester, MA) (Kim et al., 2004).
Preparation of DNA plasmid constructs
Construction of the plasmid encoding firefly luciferase (pcDNA3-Luc), as well as that encoding HPV-16 Sig/E7/LAMP-1, has been described previously. The shRNA constructs pRS-Luc (cat. no. TR30002) and pRS empty vector control (cat. no. TR20003) were purchased from OriGene (Rockville, MD). The FasL-GFP (green fluorescent protein) expression vector and GFP empty vector control were kind gifts from L. Cheng and X. Yu (Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine). FasL MISSION shRNA (TRC no. TRCN0000066640; sequence, CCGGCTTCGTGTATTCCAAAGTATACTCGAGTATACTTTGGAATACACGAAGTTTTTG) and the MISSION nontarget shRNA control vector (cat. no. SHC002; sequence, CCGGCGTGATCTTCACCGACAAGATCTCGAGATCTTGTCGGTGAAGATCACGTTTTT) were purchased from Sigma-Aldrich (St. Louis, MO). DNA plasmids were amplified in Escherichia coli DH5α (pcDNA3-Luc, pRS vector, pRS-Luc, and Sig/E7/LAMP-1) or MAX Efficiency Stbl2 cells (Invitrogen, Carlsbad, CA) (FasL-GFP, GFP-empty vector, and FasL Mission shRNA) and purified as reported by Chen and coworkers, using an endotoxin-free plasmid purification kit (Qiagen, Valencia, CA) (Chen et al., 2000).
In vitro transfection
For the experiments with luciferase shRNA, BHK-21 cells were seeded in a 24-well plate at a density of 5 × 104 cells per well the day before transfection. Lipofectamine 2000 (Invitrogen) was used to transfect the cells with DNA plasmids (0.01 μg of pcDNA3-Luc, combined with a 0.81-μg mixture of pRS-Luc and pRS vector) in quadruplicate for each Luc shRNA dose. Cells were washed, and culture medium was replaced subsequent to overnight incubation and daily luciferase-based bioluminescence imaging. For the experiments with FasL shRNA, BHK-21 cells were seeded in 10-cm dishes at 1.5 × 106 cells per dish. Eight hours later, FasL-GFP expression vector (2.4 μg) combined with FasL shRNA or nonspecific shRNA construct (21.6 μg each) was transfected into the cells, using Lipofectamine 2000. Cell surface expression of FasL was assayed after overnight incubation.
Flow cytometric analysis for FasL expression
BHK-21 cells transfected with combinations of FasL-GFP expression vector and FasL shRNA were scraped after 24 hr of incubation at 37°C, resuspended in FACScan buffer, and surface-stained in the dark with phycoerythrin-conjugated anti-mouse FasL antibody or isotype control for 30 min at 4°C. FasL expression analysis was performed on a BD FACScan flow cytometer with CellQuest software (BD Biosciences Immunocytometry Systems, Mountain View, CA).
DNA vaccination
DNA-coated gold particles were prepared according to a previously described protocol (Chen et al., 2000), and delivered to the shaved abdominal region of C57BL/6J mice with a helium-driven gene gun (Bio-Rad, Hercules, CA) at a discharge pressure of 400 psi. For the kinetic measurement of epidermal luciferase expression, two DNA bullets were administered to noncontiguous areas per mouse (1 μg of pcDNA3-Luc or pcDNA3 vector per bullet). For measurement of luciferase shRNA activity in vivo, one DNA bullet was administered per mouse (0.5 μg of pcDNA3-Luc combined with 0.5 μg of pRS-Luc or pRS vector; 1 μg of total DNA per bullet). For the FasL shRNA experiments, mice were primed with two bullets of DNA (0.1 μg of Sig/E7/LAMP-1 combined with 0.9 μg of FasL shRNA or negative control shRNA per bullet; 2 μg of total DNA per mouse) and boosted with the same dose 1 week later. For the FasL shRNA tumor treatment experiments, mice were vaccinated with two bullets of DNA (0.1 μg of Sig/E7/LAMP-1 combined with 0.9 μg of FasL shRNA or negative control shRNA per bullet, as per immune response experiment) 8 days after tumor inoculation, and boosted three times with the same dose at 4-day intervals.
Luciferase-based bioluminescence imaging
The expression of luciferase in cultured BHK-21 cells was detected with the Xenogen IVIS 200 system (Xenogen, Alameda, CA) and quantitated with the Living Image software package (Xenogen). Briefly, cells were washed twice with phosphate-buffered saline (PBS), and then 1 ml of medium with beetle luciferin (potassium salt, 39 μg/ml; Promega, Madison, WI) was added to the cells and followed immediately by bioluminescence imaging (1-sec exposure). The expression of luciferase in the skin of mice was visualized with the same apparatus and software. C57BL/6J mice were injected intraperitoneally with 0.2 ml of beetle luciferin (3.9 mg/ml in PBS) and placed under general anesthesia, using isoflurane, for 10 min. Luciferase activity was recorded with the IVIS digital camera (30-sec exposure).
Intracellular cytokine staining and flow cytometric analysis for antigen-specific immune response
Splenocytes were harvested 1 week after the last vaccination from mice (three per group) immunized with Sig/E7/LAMP-1 DNA in combination with DNA encoding FasL shRNA or nonspecific shRNA. Pooled splenocytes (5 × 106) from each vaccination group were incubated overnight at 37°C with E7 peptide (RAHYNIVTF, 1 μg/ml) in the presence of BD GolgiPlug (1 μg/ml; BD Biosciences Pharmingen). Cells were then washed with FACScan buffer and costained with phycoerythrin-conjugated monoclonal rat anti-mouse CD8 antibody as well as FITC-conjugated IFN-γ antibody, using a BD Cytofix/Cytoperm kit according to the manufacturer's instructions (BD Biosciences Pharmingen). Analysis was performed on a BD FACScan flow cytometer with CellQuest software.
Bioluminescence cytotoxicity assay
Splenocytes were harvested from mice immunized with Sig/E7/LAMP-1 DNA in combination with DNA encoding FasL shRNA or nonspecific shRNA (three per group) 1 week after the last vaccination. Splenocytes of mice from each treatment group were incubated in pools of 5 million cells with E7 peptide (RAHYNIVTF, 1 μg/ml) and interleukin (IL)-2 (20 units/ml) for 5 days, and then collected and combined with TC-1/Luciferase target cells at 10:1 effector:target ratios. After adjustment of cell volumes by centrifugation and resuspension in equal volumes of medium (to achieve 5 × 104 target cells/100 μl), cell mixtures were plated in 100-μl quadruplicate aliquots in 96-well round-bottom plates, lightly centrifuged to bring cell mixtures to the bottom of the wells, and incubated overnight. After incubation, media was drawn from the wells, briefly centrifuged to remove cells and cell debris, and then quantitatively assayed in quadruplicate for luciferase activity by the addition of Steady-Glo reagent (Promega) in light-resistant black plastic 96-well plates. Visualization and signal quantitation were performed with the Xenogen IVIS system and Living Image software package.
In vivo tumor treatment experiment
Mice (five per group) were challenged with TC-1/Luciferase tumor cells (5 × 104 cells per mouse) by subcutaneous injection in the right flank. DNA vaccine treatments (2 μg/dose) were initiated 7 days after tumor inoculation, and boosted three times with the same dose at 4-day intervals. Tumor growth was periodically visualized under general anesthesia with the Xenogen IVIS system, and quantitated with the Living Image software package. Mice were also observed for signs of morbidity (cachexia, failure to groom, and decreased social interaction with cagemates), and euthanized when overtly moribund.
Apoptosis assays
FasL-blocked E7-specific CD8+ T cells (2 × 105) were incubated with or without 1 × 107 E7 peptide-loaded DC-1 cells transfected with FasL-GFP at a 1:50 ratio in the presence of a 5-μg/ml concentration of FasL blocking antibody or isotype control. The percentage of apoptotic cells was characterized by intracellular staining with antibodies specific for activated caspase-3 and analyzed by flow cytometric analysis.
Statistical analysis
All data are expressed as means ± standard deviation (SD) and are representative of at least two different experiments. The statistical significance of group differences was measured by Student t test to generate p values. p Values less than 0.05 were considered to be significant.
Results
Intradermal delivery of DNA encoding luciferase results in maximal levels of luciferase expression in mice 24 hr after DNA administration
We employed a noninvasive bioluminescence imaging system to characterize the expression of luciferase over time. C57BL/6J mice (two per group) were intradermally administered luciferase DNA or control DNA via a gene gun and luciferase activity was monitored over a period of 5 days by bioluminescence imaging. As shown in Fig. 1A, the shaved abdominal region of luciferase-treated mice exhibited significant luminescence intensity (Fig. 1A, left) within 30 min of DNA administration, whereas luciferase activity was undetectable in animals treated with control DNA (Fig. 1A, right). We further characterized the kinetics of luciferase expression in luciferase-treated mice over a period of 5 days. As shown in Fig. 1B, luciferase expression peaked 24 hr after DNA administration and then gradually decreased. After 144 hr, the level of luciferase expression became similar to that observed at 30 min. Taken together, these data indicate that luciferase serves as an excellent marker for monitoring gene expression in vivo with a noninvasive bioluminescence imaging system, and that this expression reaches a maximal level 24 hr after gene administration.
FIG. 1.
Bioluminescence imaging of C57BL/6J mice administered DNA encoding the luciferase gene. C57BL/6J mice (two per group) were intradermally administered two bullets containing 1 μg of plasmid DNA, each encoding luciferase (pcDNA3-Luc) or control (pcDNA3), via gene gun and luciferase activity was monitored over a period of 5 days by bioluminescence imaging. (A) Representative luminescence images demonstrating in vivo luciferase expression 30 min after gene gun administration in luciferase DNA-administered mice (left) and control DNA-administered mice (right). (B) Bar graph of luminescence intensity over a period of 144 hr after gene gun administration. Results are expressed as means and SD. Note: Luciferase activity peaked 24 hr postadministration and then gradually declined.
Transfection of luciferase-expressing cells with DNA encoding shRNA targeting luciferase reduces levels of luciferase activity in vitro in a dose-dependent manner
We performed in vitro bioluminescence imaging to characterize luciferase expression in BHK-21 cells transfected with DNA encoding luciferase in combination with various amounts of DNA encoding shRNA targeting luciferase. As shown in Fig. 2A, expression of luciferase declined with increasing dose of DNA encoding shRNA targeting luciferase, indicating that silencing of the luciferase gene increased in a dose-dependent manner. Luciferase activity becomes virtually undetectable in cells transfected with more than 0.09 μg of DNA encoding luciferase-specific shRNA. We further characterized the kinetics of silencing of luciferase gene expression over a period of 5 days in cells transfected with various amounts of DNA encoding luciferase-specific shRNA. We observed dose-dependent silencing of luciferase gene expression over time. Furthermore, luciferase activity remained substantially suppressed even after 120 hr (Fig. 2B). Thus, these data demonstrate that luciferase can be continuously silenced in a dose-dependent manner in vitro with DNA encoding shRNA targeting luciferase.
FIG. 2.
Detection of siRNA-mediated gene knockdown in vitro, using a bioluminescence imaging system. BHK-21 cells were transfected with 0.01 μg of pcDNA3-Luc and DNA encoding luciferase-specific shRNA (pRS-Luc) in amounts of 0.00, 0.01, 0.03, 0.09, 0.27, and 0.81 μg at 24- and 48-hr time points. Cells transfected with pcDNA3-Luc and pRS vector alone (0.00 μg of pRS-Luc) were used as the control. (A) Luminescent images of transfected cells at 24 hr (left) and 48 hr (right) posttransfection. (B) Line graph depicting the luminescence intensities over time of cells transfected with 0.01 μg of pcDNA3-Luc and various amounts of pRS-Luc. Results are expressed as the mean luminescence intensity and SD. Note: Luciferase expression in shRNA-transfected cells was suppressed in a dose-dependent manner and was still substantially reduced after 120 hr.
DNA encoding shRNA targeting luciferase greatly decreases levels of luciferase expression in mice administered luciferase-expressing DNA
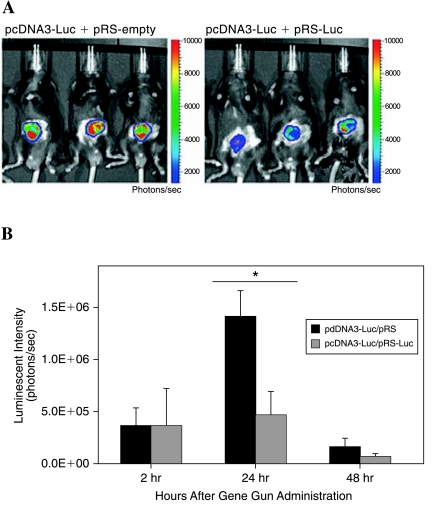
We next sought to determine whether DNA encoding shRNA targeting luciferase could be intradermally administered by gene gun to knock down luciferase expression in vivo as assayed by noninvasive bioluminescence imaging. C57BL/6J mice (five per group) were intradermally administered DNA encoding luciferase and DNA encoding shRNA targeting luciferase (pcDNA3-Luc + pRS-Luc) or DNA encoding luciferase and empty vector DNA (pcDNA3-Luc + pRS-empty). Luciferase activity was monitored over time by bioluminescence imaging. As shown in Fig. 3A, representative bioluminescent images of mice administered DNA encoding luciferase, combined with DNA encoding luciferase-specific shRNA, displayed significantly lower levels of luciferase expression compared with mice administered DNA encoding luciferase combined with empty vector DNA at 24 hr. Furthermore, Fig. 3B indicates the silencing of luciferase expression over time. We observed that the luminescence intensity in mice administered pcDNA3-Luc plus pRS-Luc was significantly lower compared with mice administered pcDNA3-Luc plus pRS-empty vector 24 hr after DNA administration (∼3-fold difference, p < 0.01). These results show that DNA encoding shRNA targeting luciferase can be efficiently administered intradermally with a gene gun to achieve efficient silencing of luciferase in vivo. These encouraging data suggest that we may be able to use intradermal administration via gene gun to modify the properties of DCs, using DNA encoding shRNA targeting immunosuppressive molecules to enhance DNA vaccine potency.
FIG. 3.
Bioluminescence imaging of luciferase activity in vivo. C57BL/6J mice (five per group) were administered intradermally, via gene gun, 0.5 μg of pcDNA3-Luc plus empty pRS vector (left) or 0.5 μg of pcDNA3-Luc plus pRS-Luc (right) and luciferase activity was monitored periodically by bioluminescence imaging. (A) Representative images of luciferase expression in mice 24 hr after gene gun administration. (B) Bar graph depicting luminescence intensity in vaccinated mice 2, 24, and 48 hr after gene gun administration. Results are expressed as mean luminescence intensity and SD. p Values were calculated by Student t test (*p < 0.01). Note: Levels of luciferase expression were significantly lower in mice administered DNA encoding pcDNA3-Luc plus pRS-Luc compared with mice administered DNA encoding pcDNA3-Luc plus pRS-vector control.
Transfection of FasL-expressing cells with DNA encoding shRNA targeting FasL substantially downregulates Fast in vitro
The apoptosis inducer FasL, a protein abundantly expressed on the surface of DCs, may serve as an ideal target for gene silencing to enhance DNA vaccine potency by potentially reducing DC-induced T cell death (Lu et al., 1997). During antigen presentation, FasL expressed by DCs may induce apoptosis in some T cells through association with the Fas death receptor present on the surface of T cells, concomitant with MHC–T cell receptor interactions at the immunological synapse. To determine whether transfection of FasL-expressing cells with DNA encoding shRNA targeting FasL could lead to downregulation of FasL in vitro, we transfected BHK-21 cells with plasmid DNA encoding FasL and DNA encoding shRNA specific for FasL. We then performed flow cytometric analysis to characterize the expression of FasL in transfected cells. As shown in Fig. 4, cotransfection of cells with DNA encoding FasL and DNA encoding FasL-specific shRNA led to significant reduction of FasL expression compared with cells cotransfected with DNA encoding nonspecific shRNA (p < 0.05 by Student t test). Thus, the data indicate that transfection of FasL-expressing cells with DNA encoding shRNA specific for FasL substantially down-regulates FasL expression in vitro.
FIG. 4.
Flow cytometric analysis demonstrating expression of FasL in BHK-21 cells in vitro. BHK-21 cells were transfected with FasL DNA and DNA encoding either nonspecific shRNA or FasL-specific shRNA. Cells were then stained with FasL-specific antibody or an isotype control (data not shown) and subjected to flow cytometric analysis to characterize FasL expression. Cells that did not receive FasL transfection were measured as a negative control. (A) Flow cytometric data representative of three separate analyses. The number shown in each histogram provides an indication of the percentage of gated cells staining positive for FasL. (B) Bar graph depicting relative FasL expression. Data are shown as means and SD. p Values were calculated by Student t test (*p < 0.05). Note: Within the experimental groups transfected with FasL DNA, FasL expression is significantly reduced in cells treated with DNA encoding FasL-specific shRNA compared with cells treated with DNA encoding nonspecific shRNA.
Intradermal administration of Sig/E7/LAMP-1 DNA with DNA encoding shRNA targeting FasL results in a significant increase in the number of E7-specific CD8+ T cells in vaccinated mice
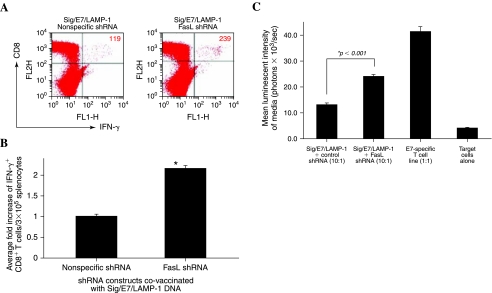
We previously generated a DNA construct containing HPV-16 E7 linked to the sorting signal of lysosome-associated membrane protein (LAMP)-1 to form a chimeric protein, Sig/E7/LAMP-1. We found that intradermal administration of DNA encoding Sig/E7/LAMP-1 could generate enhanced E7-specific CD8+ T cell immune responses in vaccinated mice (Chen et al., 1999). We then sought to determine whether the intradermal administration of Sig/E7/LAMP-1 DNA could be further enhanced by coadministration with DNA encoding shRNA targeting FasL. C57BL/6J mice (three per group) were vaccinated intradermally via gene gun with Sig/E7/LAMP-1 DNA and DNA encoding shRNA targeting FasL, and boosted with the same dose 1 week later. One week after the last vaccination, splenocytes were harvested and characterized for E7-specific CD8+ T cells by intracellular cytokine staining followed by flow cytometric analysis. As shown in Fig. 5A, a significantly enhanced E7-specific CD8+ T cell immune response was observed in mice vaccinated with Sig/E7/LAMP-1 DNA and DNA encoding FasL shRNA compared with mice vaccinated with Sig/E7/LAMP-1 DNA and DNA encoding nonspecific shRNA. A graphical representation of the number of E7-specific IFN-γ+CD8+ T cells in each group is depicted in Fig. 5B (p < 0.005 by Student t test). Thus, the data indicate that intradermal administration of Sig/E7/LAMP-1 DNA with DNA encoding shRNA targeting FasL results in a significant increase in the number of E7-specific CD8+ T cells in vaccinated mice and suggests that RNAi-mediated knockdown of FasL expression in DCs may represent an effective strategy for augmenting vaccine potency.
FIG. 5.
Analysis of IFN-γ-secreting E7-specific CD8+ T cells in mice vaccinated with Sig/E7/LAMP-1 DNA and DNA encoding shRNA targeting FasL. C57BL/6J mice (three per group) were vaccinated intradermally via gene gun with Sig/E7/LAMP-1 DNA in combination with DNA encoding nonspecific shRNA or DNA encoding FasL shRNA. Splenocytes were obtained from vaccinated mice and cultured with E7 peptide (amino acids 49–57) overnight. The cells were then analyzed for CD8 and intracellular IFN-γ staining by flow cytometry. (A) Representative flow cytometric data showing the number of IFN-γ+CD8+ T cells in vaccinated mice (top right quadrant). Data shown are representative of two separate analyses. (B) Bar graph showing the average fold increase in IFN-γ+ CD8+ T cells from each group of vaccinated mice. p Values were calculated by Student t test (*p < 0.005). Note: Vaccination with DNA encoding FasL-specific shRNA increased the number of E7-specific IFN-γ-secreting CD8− T cells by more than 2-fold. (C) CTL assay using luciferase-expressing TC-1 cells as target cells. Splenocytes from the various vaccinated groups were stimulated with E7 peptide (amino acids 49–57) in vitro. Target cells were incubated with the E7 peptide-stimulated splenocytes. Media from the incubated cells were collected and characterized for luminescence activity. Bar graph depicts the luminescence activity of the medium (means and SD).
Splenocytes from mice vaccinated with Sig/E7/LAMP-1 DNA combined with DNA encoding FasL shRNA generate potent cytotoxic activity against E7-expressing tumors
To evaluate whether the increase in the number of E7-specific CD8+ T cells generated by vaccination with Sig/E7/LAMP-1 DNA in combination with FasL shRNA translates into potent cytotoxic activity against E7-expressing tumors, we performed in vitro cytotoxicity assays using in vitro-stimulated splenocytes from vaccinated C57BL/6 mice with luciferase-expressing TC-1 tumors (TC-1/Luciferase). An E7-specific T cell line was used as a positive control and TC-1/Luciferase tumor cells without T cells were used as a negative control. As shown in Fig. 5C, splenocytes obtained from animals vaccinated with Sig/E7/LAMP-1 DNA in combination with DNA encoding FasL shRNA demonstrated significantly enhanced cytolytic activity compared with splenocytes obtained from animals vaccinated with Sig/E7/LAMP-1 DNA in combination with DNA encoding nonspecific shRNA (p < 0.001). Thus, the data suggest that the enhanced E7-specific CD8+ T cells observed in mice vaccinated with Sig/E7/LAMP-1 DNA with DNA encoding FasL-specific shRNA also translated to potent cytolytic activity against an E7-expressing model tumor cell line.
Intradermal administration of Sig/E7/LAMP-1 DNA with DNA encoding shRNA targeting FasL generates potent antitumor therapeutic effects against E7-expressing TC-1/Luciferase tumors in treated mice
To determine the therapeutic antitumor effects of intradermal administration of Sig/E7/LAMP-1 DNA with DNA encoding FasL shRNA, we performed in vivo tumor treatment experiments. C57BL/6 mice (five per group) were challenged with TC-1/Luciferase tumor cells (5 × 104 cells per mouse) by subcutaneous injection in the right flank, and monitored for tumor growth over time by noninvasive bioluminescence imaging. Luminescence intensity has been shown to correlate with tumor load (Hung et al., 2007a). Eight days after tumor challenge, tumor-bearing mice were treated with Sig/E7/LAMP-1 DNA vaccine combined with DNA encoding FasL shRNA, or nonspecific shRNA, via gene gun. The mice received three boosters with the same dose intradermally at 4-day intervals. As shown in Fig. 6, whereas untreated tumor-bearing mice demonstrated a continued significant increase in tumor luminescence intensity over time (p = 0.036), mice treated with Sig/E7/LAMP-1 DNA vaccine and DNA encoding FasL shRNA demonstrated a continued significant decrease in tumor luminescence intensity over time (p = 0.042). Furthermore, mice treated with Sig/E7/LAMP-1 DNA vaccine and DNA encoding nonspecific shRNA demonstrated some therapeutic effect as shown by the decrease in tumor luminescence intensity over time, but the decrease in tumor luminescence intensity was not statistically significant (p = 0.184). Taken together, these results suggest that treatment with the DNA vaccine combined with DNA encoding FasL-specific shRNA generates more potent therapeutic antitumor effects against E7-expressing tumors.
FIG. 6.
In vivo treatment experiment involving tumor-challenged mice vaccinated with a combination of Sig/E7/LAMP-1 DNA and DNA encoding shRNAs. C57BL/6J mice (five per group) were challenged subcutaneously with TC-1/Luciferase tumor cells (5 × 104 cells per mouse). Eight days after tumor challenge, mice were treated with mixtures of Sig/E7/LAMP-1 DNA vaccine combined with DNA encoding a nonspecific shRNA, or FasL shRNA, delivered by gene gun to the shaved abdomen, and then boosted with the same vaccine three times at 4-day intervals. Tumor growth was monitored regularly by luminescence imaging with the Xenogen IVIS system. Bioluminescence signals were acquired for 30 sec. (A) Luminescence imaging depicting tumor load in animals bearing subcutaneously injected TC-1/Luciferase tumor cells 7 days after tumor challenge (1 day before commencement of gene gun vaccination therapy), and 15 days (for untreated) or 31 days after tumor challenge. (B) Line graphs depicting growth progression of tumors in individual mice from each treatment group from day 2, until day 15 (no vaccine group), or day 31, as measured by quantification of tumor bioluminescence intensity.
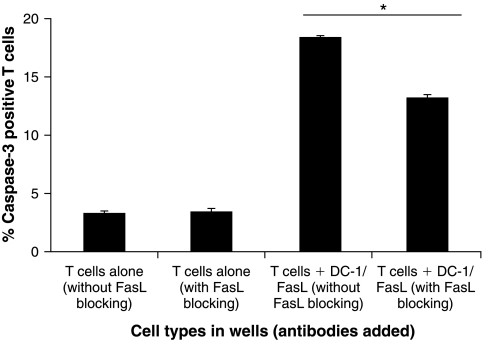
Blocking of FasL on E7 peptide-loaded DCs reduces the apoptosis of E7-specific CD8+ T cells
We further characterized the role of FasL expressed on DCs in the induction of apoptotic cell death of E7-specific CD8+ T cells, using an in vitro system. We incubated FasL-expressing E7 peptide-loaded DCs with E7-specific CD8+ T cells in the presence of FasL-blocking antibody or isotype control. E7-specific CD8+ T cells alone with FasL-blocking antibody or isotype control were used as controls. The percentage of apoptotic cells was characterized with antibodies specific for activated caspase-3 and analyzed by flow cytometric analysis. As shown in Fig. 7, E7-specific CD8+ T cells incubated with FasL-expressing E7 peptide-loaded DCs in the presence of FasL-blocking antibody demonstrated a significantly lower percentage of apoptosis compared with cells incubated in the absence of FasL-blocking antibody (p < 0.005). Our data indicate that blocking of FasL on DCs leads to reduced apoptosis of E7-specific CD8+ T cells. Thus, these data support our hypothesis that the downregulation of FasL on DCs, using shRNA, may lead to improved T cell survival, thus enhancing DNA vaccine potency.
FIG. 7.
Characterization of apoptotic cell death of E7-specific CD8− T cells after incubation with E7 peptide-loaded DC-1 cells expressing FasL. E7-specific CD8+ T cells (2 × 105) were incubated in a 24-well plate with or without 1 × 107 E7 peptide-loaded DC-1 cells transfected with FasL-GFP at a 1:50 ratio in the presence of FasL-blocking antibody or isotype control (without FasL blocking). The percentage of apoptotic cells was characterized with antibodies specific for activated caspase-3 and analyzed by flow cytometric analysis. Bar graph depicts the average percentage of activated caspase-3-positive T cell detected in wells containing the indicated cells and blocking antibodies (means and SD). p Values were calculated by Student t test. Data are representative of two separate analyses.
Discussion
In the current study, we have demonstrated an effective method for quantifying the kinetics of luciferase expression in vivo with a noninvasive bioluminescence imaging system. Our results indicated that the expression of luciferase in vivo peaked 24 hr after DNA administration via gene gun. Furthermore, we observed that intradermal administration of luciferase DNA with DNA encoding shRNA targeting luciferase could significantly reduce the expression of luciferase in mice. In addition, we observed that mice vaccinated with Sig/E7/LAMP-1 DNA coadministered DNA encoding shRNA targeting FasL generated significantly enhanced E7-specific CD8+ cytotoxic T cell responses as well as potent therapeutic antitumor effects against E7-expressing tumors. Furthermore, we demonstrated that blocking of FasL on E7 peptide-loaded DCs reduces the apoptosis of E7-specific CD8+ T cells in vitro. Thus, we have shown that intradermal administration of DNA encoding shRNA is capable of silencing the gene of interest. This strategy may potentially be used to modify the properties of dendritic cells to enhance the potency of DNA vaccines.
The expression of FasL and its death receptor Fas has been reported in both T cells and DCs. Interactions between FasL expressed by DCs and Fas on the surface of T cells during antigen presentation may result in activation-induced T cell death through the extrinsic apoptosis pathway. This potentially represents a mechanism of immunological control to prevent hyperactivation of T cells in response to an infection or autoantigen. In this event, knockdown of FasL in DCs might reduce signaling through Fas during antigen presentation, thereby protecting these T cells from DC FasL-mediated killing. We were unable to observe significant increases in the number E7-specific CD8+ T cells when mice were covaccinated with E7 DNA and DNA encoding shRNA targeting Fas (data not shown). These results suggest that downregulation of FasL in DCs strengthens the antigen-specific immune response predominantly by preventing the death of T cells and not necessarily DCs themselves, because downregulation of Fas showed no such immune enhancement. It has been suggested that DCs are not susceptible to the Fas-associated apoptotic pathway (Rescigno et al., 2000) and die mainly through the intrinsic apoptosis pathway and the action of granzymes and perforins secreted by T cells during antigen presentation.
The encouraging results from the current study suggest that RNAi methodology may be further applied in a similar context to inhibit the expression of molecules by DCs that suppress the generation of T cell-mediated immune responses (for review, see Mao et al., 2007). For example, the immunosuppressive tryptophan-degrading enzyme indoleamine-2,3-dioxygenase (IDO) is secreted in considerable amounts by DCs (Munn et al., 2002; Lee et al., 2003). The presence of IDO in tumor-draining lymph nodes depletes tryptophan availability in the local microenvironment, which may inhibit the mitosis and function of nearby T cells (Munn et al., 2002) or induce profound anergy in these cells (Munn et al., 2004). In addition, endogenous molecules produced by DCs may impair the activation of T cells, as has been shown for the suppressor of cytokine signaling-1 (SOCS1) protein. SOCS1 disrupts signaling pathways associated with IFN-γ, IL-2, IL-6, IL-7, IL-12, and IL-15 in T cells (Shen et al., 2004) and is also believed to suppress antigen presentation to T cells (Kubo et al., 2003). Several studies have shown that administration of siRNA targeting SOCS1 into DCs loaded with tumor antigen causes an increase in the number of tumor-specific T cells and inhibits the growth of established tumors in mice (Shen et al., 2004; Yang et al., 2006; Zhou et al., 2006). Thus, knockdown of such immunosuppressive molecules in DCs by RNA interference technology represents a potentially plausible approach to enhance the potency of DNA vaccines.
Another strategy to enhance DNA vaccine potency, using intradermal administration via a gene gun, involves the use of RNAi targeting key proapoptotic proteins. We have previously shown that intradermal immunization of mice with E7 DNA together with DNA encoding several antiapoptotic proteins, including Bcl-2, Bcl-xL, XIAP (X-linked inhibitor of apoptosis), and SPI-6 (serine proteinase inhibitor-6), could strongly protect DCs from T cell-induced apoptosis and generate potent immunity against E7-expressing tumors (Kim et al., 2003, 2004). To circumvent concerns of oncogenicity associated with the administration of DNA encoding antiapoptotic proteins, we have employed E7 DNA vaccines in conjunction with siRNA targeting key proapoptotic proteins Bax and Bak to significantly enhance HPV E7-specific CD8+ T cell immune responses in vaccinated mice (Kim et al., 2005). Thus, it may be of interest to further investigate the knockdown of other key proapoptotic proteins such as caspase-3, −8, and −9 in DCs to enhance DNA vaccine potency.
More recently, we have developed a strategy to enhance antigen-specific CD4+ T cell immune responses in mice by employing a DNA vaccine encoding the invariant chain molecule (Ii chain), in which the CLIP region is replaced with a CD4+ helper T cell epitope, PADRE (Pan-DR-epitope) (Ii-PADRE DNA). We have demonstrated that coadministration of DNA vaccines with Ii-PADRE DNA led to enhancement of CD4+ T cells, resulting in enhanced antigen-specific CD8+ T cell immune responses and potent protective and therapeutic antitumor effects (Hung et al., 2007b). Because the strategy to enhance CD4+ helper T cells is different from the strategies to avoid apoptosis of T cells, such a strategy may potentially be used in combination with the current strategy in order to further enhance the potency of DNA vaccines.
In future, it will be important to examine both the molecular mechanisms controlling T cell activation by DCs as well as the apoptotic process in DCs. It may also be of interest to couple these strategies with techniques for prolonging the life span of DCs and/or attenuating immunoinhibitory pathways that negatively regulate T cell activation. Thus, this study demonstrates the effectiveness and clinical feasibility of employing RNAi technology as an immunotherapeutic complement to enhance the potency of DNA vaccines.
Acknowledgments
The authors thank Ms. Liangme He for assistance with plasmid construction, Dr. Richard Roden and Mr. Shaw-Wei Tsen for helpful discussion and critical review of the manuscript, and Drs. Xiaobing Yu and Linzhao Cheng for supplying expression vectors for the FasL transfection experiments. The authors are also grateful to Ms. Lucy Wangaruro for excellent secretarial support and Ms. Archana Monie for preparation of the manuscript. This work was supported by National Cancer Institute SPORE in Cervical Cancer P50 CA098252 and 1 RO1 CA114425-01 as well as the Johns Hopkins University Provost's Undergraduate Research Awards program (C.-P.M.).
Author Disclosure Statement
No competing financial interests exist for any of the authors.
References
- Caplen N.J. Gene therapy progress and prospects. Downregulating gene expression: The impact of RNA interference. Gene Ther. 2004;11:1241–1248. doi: 10.1038/sj.gt.3302324. [DOI] [PubMed] [Google Scholar]
- Chen C.H. Ji H. Suh K.W. Choti M.A. Pardoll D.M. Wu T.C. Gene gun-mediated DNA vaccination induces antitumor immunity against human papillomavirus type 16 E7-expressing murine tumor metastases in the liver and lungs. Gene Ther. 1999;6:1972–1981. doi: 10.1038/sj.gt.3301067. [DOI] [PubMed] [Google Scholar]
- Chen C.H. Wang T.L. Hung C.F. Yang Y. Young R.A. Pardoll D.M. Wu T.C. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–1042. [PubMed] [Google Scholar]
- Donnelly J.J. Ulmer J.B. Shiver J.W. Liu M.A. DNA vaccines. Annu. Rev. Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- Guermonprez P. Valladeau J. Zitvogel L. Thery C. Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- Gurunathan S. Klinman D.M. Seder R.A. DNA vaccines: Immunology, application, and optimization. Annu. Rev. Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- Huang B. Mao C.P. Peng S. He L. Hung C.F. Wu T.C. Intradermal administration of DNA vaccines combining a strategy to bypass antigen processing with a strategy to prolong dendritic cell survival enhances DNA vaccine potency. Vaccine. 2007;25:7824–7831. doi: 10.1016/j.vaccine.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung C.F. Tsai Y.C. He L. Coukos G. Fodor I. Qin L. Levitsky H. Wu T.C. Vaccinia virus preferentially infects and controls human and murine ovarian tumors in mice. Gene Ther. 2007a;14:20–29. doi: 10.1038/sj.gt.3302840. [DOI] [PubMed] [Google Scholar]
- Hung C.F. Tsai Y.C. He L. Wu T.C. DNA vaccines encoding Ii-PADRE generates potent PADRE-specific CD4+ T-cell immune responses and enhances vaccine potency. Mol. Ther. 2007b;15:1211–1219. doi: 10.1038/sj.mt.6300121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H. Chang E.Y. Lin K.Y. Kurman R.J. Pardoll D.M. Wu T.C. Antigen-specific immunotherapy for murine lung metastatic tumors expressing human papillomavirus type 16 E7 oncoprotein. Int. J. Cancer. 1998;78:41–45. doi: 10.1002/(sici)1097-0215(19980925)78:1<41::aid-ijc8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Kim T.W. Hung C.F. Ling M. Juang J. He L. Hardwick J.M. Kumar S. Wu T.C. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. J. Clin. Invest. 2003;112:109–117. doi: 10.1172/JCI17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.W. Hung C.F. Boyd D.A. He L. Lin C.T. Kaiserman D. Bird P.I. Wu T.C. Enhancement of DNA vaccine potency by coadministration of a tumor antigen gene and DNA encoding serine protease inhibitor-6. Cancer Res. 2004;64:400–405. doi: 10.1158/0008-5472.can-03-1475. [DOI] [PubMed] [Google Scholar]
- Kim T.W. Lee J.H. He L. Boyd D.A. Hardwick J.M. Hung C.F. Wu T.C. Modification of professional antigen-presenting cells with small interfering RNA in vivo to enhance cancer vaccine potency. Cancer Res. 2005;65:309–316. [PubMed] [Google Scholar]
- Kubo M. Hanada T. Yoshimura A. Suppressors of cytokine signaling and immunity. Nat. Immunol. 2003;4:1169–1176. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- Lee J.R. Dalton R.R. Messina J.L. Sharma M.D. Smith D.M. Burgess R.E. Mazzella F. Antonia S.J. Mellor A.L. Munn D.H. Pattern of recruitment of immunoregulatory antigen-presenting cells in malignant melanoma. Lab. Invest. 2003;83:1457–1466. doi: 10.1097/01.lab.0000090158.68852.d1. [DOI] [PubMed] [Google Scholar]
- Leung R.K. Whittaker P.A. RNA interference: From gene silencing to gene-specific therapeutics. Pharmacol. Ther. 2005;107:222–239. doi: 10.1016/j.pharmthera.2005.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K.Y. Guarnieri F.G. Staveley-O'Carroll K.F. Levitsky H.I. August J.T. Pardoll D.M. Wu T.C. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- Lu L. Qian S. Hershberger P.A. Rudert W.A. Lynch D.H. Thomson A.W. Fas ligand (CD95L) and B7 expression on dendritic cells provide counter-regulatory signals for T cell survival and proliferation. J. Immunol. 1997;158:5676–5684. [PubMed] [Google Scholar]
- Mao C.P. Hung C.F. Wu T.C. Immunotherapeutic strategies employing RNA interference technology for the control of cancers. J. Biomed. Sci. 2007;14:15–29. doi: 10.1007/s11373-006-9131-5. [DOI] [PubMed] [Google Scholar]
- Munn D.H. Sharma M.D. Lee J.R. Jhaver K.G. Johnson T.S. Keskin D.B. Marshall B. Chandler P. Antonia S.J. Burgess R. Slingluff C.L., Jr. Mellor A.L. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- Munn D.H. Sharma M.D. Hou D. Baban B. Lee J.R. Antonia S.J. Messina J.L. Chandler P. Koni P.A. Mellor A.L. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J. Clin. Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno M. Piguet V. Valzasina B. Lens S. Zubler R. French L. Kindler V. Tschopp J. Ricciardi-Castagnoli P. Fas engagement induces the maturation of dendritic cells (DCs), the release of interleukin (IL)-1β, and the production of interferon gamma in the absence of IL-12 during DC-T cell cognate interaction: A new role for Fas ligand in inflammatory responses. J. Exp. Med. 2000;192:1661–1668. doi: 10.1084/jem.192.11.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar P. Manjunath N. Lieberman J. The prospect of silencing disease using RNA interference. JAMA. 2005;293:1367–1373. doi: 10.1001/jama.293.11.1367. [DOI] [PubMed] [Google Scholar]
- Shen L. Evel-Kabler K. Strube R. Chen S.Y. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat. Biotechnol. 2004;22:1546–1553. doi: 10.1038/nbt1035. [DOI] [PubMed] [Google Scholar]
- Yang R. Yang X. Zhang Z. Zhang Y. Wang S. Cai Z. Jia Y. Ma Y. Zheng C. Lu Y. Roden R. Chen Y. Single-walled carbon nanotubes-mediated in vivo and in vitro delivery of siRNA into antigen-presenting cells. Gene Ther. 2006;13:1714–1723. doi: 10.1038/sj.gt.3302808. [DOI] [PubMed] [Google Scholar]
- Zhou H. Zhang D. Wang Y. Dai M. Zhang L. Liu W. Liu D. Tan H. Huang Z. Induction of CML28-specific cytotoxic T cell responses using co-transfected dendritic cells with CML28 DNA vaccine and SOCS1 small interfering RNA expression vector. Biochem. Biophys. Res. Commun. 2006;347:200–207. doi: 10.1016/j.bbrc.2006.06.093. [DOI] [PubMed] [Google Scholar]