Abstract
For more than two decades, HIV has infected millions of people worldwide each year through mucosal transmission. Our knowledge of how HIV secures a foothold at both the molecular and cellular levels has expanded by recent investigations that have applied new technologies and used improved techniques to isolate ex vivo human tissue and generate in vitro cellular models, as well as more relevant in vivo animal challenge systems. Here, we review the current concepts of the immediate events that follow viral exposure at genital mucosal sites where most documented transmissions occur. Furthermore, we discuss the gaps in our knowledge that are relevant to future studies, which will shape strategies for effective HIV prevention.
Introduction
HIV vaccines and microbicides hold promise of preventing the acquisition of HIV-1 and HIV-2, the two viruses that cause AIDS, but the success of designing such agents necessitates a clear understanding of where HIV first encounters its target cells — primarily T cells, macrophages and dendritic cells (DCs) — and how it gains entry at various sites to eventually establish infection. HIV infection has rapidly spread since the early 1980’s to become an epidemic that is maintained by sexual transmission through the lower genital and rectal mucosa, and it is these routes of infection that account for the vast majority of current and new infections (Figure 1, Table 1)1. Here, we have endeavored to synthesize the current knowledge on the acquisition of HIV at these mucosal sites, confining our discussion to the lower genital mucosa. We 1 clearly recognize that other sites of entry, such as blood, placenta and gastrointestinal mucosa, are important considerations but these are beyond the scope of this Review.
Figure 1. HIV invasion sites.
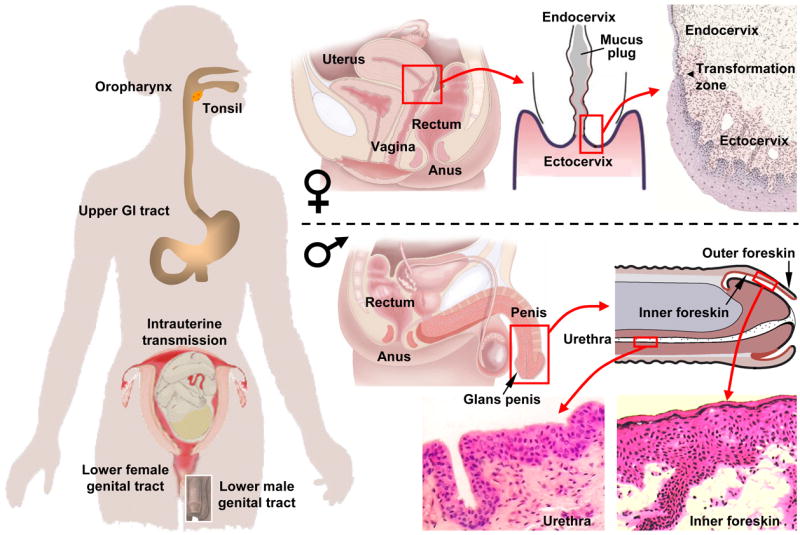
HIV mucosal invasion sites of the lower genital tract, the rectum, and the upper intestinal tract. In women, viral invasion occurs mostly through the non-keratinized squamous epithelium of the vagina and ectocervix, as well as through the single-layer columnar epithelium of the endocervix. The endocervical canal is filled with mucus, providing a barrier against ascent of pathogens. However, ovulation is accompanied by hydration and alkalinization of the mucus plug, possibly decreasing its barrier function. Infection in women can also ensue when HIV-1 invades the single-layer columnar epithelium of the rectum following receptive anal intercourse. In men, viral invasion occurs most frequently through the inner foreskin and the penile urethra as a consequence of penile–vaginal or penile–anal intercourse. Thinly stratified columnar epithelial cells line most of the urethra except for the fossa navicularis near the external meatus (exit hole), which is covered by non-keratinized squamous epithelium. The glans penis and the outer foreskin are protected by keratinized squamous epithelium, which provides a strong mechanical barrier against HIV invasion. By contrast, a thin and poorly keratinized squamous epithelium covers the inner foreskin, rendering this site vulnerable to HIV invasion. Men are also infected by viral invasion through the rectum. In fact, receptive anal intercourse carries the highest per exposure probability of infection among all mucosal transmission sites. The upper gastrointestinal (tract, lined by non-keratinized squamous epithelium in the oropharynx and the esophagus, and by single layer columnar epithelium in the stomach and the small intestine, is another site of mucosal HIV invasion. In adults, transmission in the upper gastrointestinal tract occurs following contact with HIV-containing semen during fellatio, but the efficiency of this route is low. In infants, HIV invasion in the upper gastrointestinal tract occurs after exposure to or ingestion of infected maternal blood and genital secretions during birth as well as infected milk during breast feeding.
Table 1.
| HIV invasion site | Anatomical sub-location | Type of epithelium | Transmission medium | Transmission probability per exposure event | Estimated contribution to HIV cases worldwide |
|---|---|---|---|---|---|
| Female genital tract | Vagina | Squamous, non-keratinized | |||
| Ectocervix | Squamous, non-keratinized | ||||
| Endocervix | Columnar, single layer | Semen | 1 in 200 - 1 in 2,000 | 12.6 million | |
| Other | Various epithelia | ||||
| Male genital tract | Inner foreskin | Squamous, poorly keratinized | |||
| Penile urethra | Columnar, stratified | Cervicovaginal and rectal secretions and desquamations | 1 in 700 - 1 in 3,000 | 10.2 million* | |
| Other | Various epithelia | ||||
| Rectum | Columnar, single layer | Semen | 1 in 20 - 1 in 300 | 3.9 million** | |
| Intestinal tract | Semen | 1 in 2,500 | 1.5 million | ||
| Upper GI tract | Various epithelia | Maternal blood, genital secretions¶ | 1 in 5 - 1 in 10 | 960,000*** | |
| Breast milk¶¶ | 1 in 5 - 1 in 10 | 960,000*** | |||
| Placenta | Chorionic villi | Two layer epithelium(cyto- and syncytiotrophoblast) | Maternal blood¶¶¶ | 1 in 10 - 1 in 20 | 480,000*** |
| Blood stream | Blood products, sharps | 95 in 100 - 1 in 150 | 2.6 million**** |
Includes men having sex with men (MSM), bisexual men and heterosexual men
Includes MSM, bisexual men and women infected via anal receptive intercourse
Mother-to-child transmission:
intrapartum
breastfeeding
intrauterin
Mostly intravenous drug use (IDU), but includes infections by transfusions and health care related accidents
Many elegant studies have provided insights into selective aspects of how HIV and simian immunodeficiency virus (SIV) enter the mucosa through detailed examination of the relevant tissues and target cells following in vivo, ex vivo and in vitro virus exposure. We have organized the discussion in this Review based on the experimental system used from the same mucosal source to alleviate confusion and inconsistencies that often emerge when findings from one system are extrapolated to those of another. When appropriate, we have emphasized the benefits and limitations of the experimental approaches, important considerations in the interpretation of findings and their relevance for future studies.
HIV invasion in the female genital tract
Anatomical sites
An estimated 30–40% of all new HIV-1 infections occur in women through vaginal intercourse, which carries a lower HIV transmission probability per exposure event than anal intercourse or parenteral inoculation (Table 1). Although HIV-1 can infect the vaginal, ectocervical and endocervical mucosa (Figure 1), the relative contribution of each site to the establishment of the initial infection is not known. The multilayered squamous epithelium that covers the vagina and ectocervix, when intact, provides better mechanical protection against HIV invasion than the single-layer columnar epithelium that lines the endocervix. However, the greater surface area of the vaginal wall and ectocervix, which often exceeds 15 times that of the endocervix, provides more potential access for HIV entry, particularly when breaches occur in the epithelial-cell layer. HIV or SIV can establish an initial infection solely in the vagina, as shown in women who lack a uterus at birth2 and in female macaques after surgical removal of the uterus3. In fact, selective transmission of HIV through the vaginal mucosa rather than the cervix may commonly occur, as suggested by a recent large randomized, controlled prevention clinical trial in African women. In this study, no significant reduction in HIV-1 acquisition occurred in women using a diaphragm compared with the control group4. However, the observed potential benefit of blocking HIV-1 exposure to the cervix may have been undermined because the sexual partners reported lower condom usage in the diaphragm intervention group than the control group.
The region where the ectocervix transforms into the endocervix (Figure 1) can be enriched with CD4+ T cells and may be a particularly susceptible site of HIV entry. Whether HIV can cross the endocervical mucus plug, reach the uterine cavity and invade through the mucosa of the upper genital tract has not been well examined. In principle, uterine tissue is susceptible to infection if directly inoculated with HIV5. Uterine simian–HIV (SHIV) infection has been shown in one monkey following vaginal inoculation two days earlier, an interval that is probably too short for stromal or lymphatic spread from the lower genital tract6. This indicated that ascent of virus through the endocervical mucus plug may be possible, but confirmation in humans is lacking. Conceivably, the upper mucosal tract may become more vulnerable to HIV-1 penetration during ovulation, a period when rising estrogen levels alter the endocervical mucus to a less viscous and more alkaline consistency.
HIV entry through the female genital epithelium
Both free HIV and SIV virions, and HIV and SIV virions from seminal leukocytes (cell-associated virus) can establish mucosal infection (Figure 2)7–11. This has been shown directly in vivo in female macaques12 and in mice13, and indirectly in humans through genetic sequence comparisons of viral isolates from acutely infected women and from seminal cells and plasma from their infected source partners14. Ex vivo studies using human cervical explants have also confirmed transmission of cell-free and cell-associated HIV-115,16. Initially, cervical mucus can trap seminal cells or free virions17,18. Conceivably, this could facilitate transmission by prolonging mucosal contact time. However, while immobilized, the virions may also become more susceptible to innate antiviral substances.
Figure 2. Pathways of HIV invasion in the mucosa of the vagina and uterine ectocervix, part A.
The human vagina and ectocervix are covered by non-keratinized squamous epithelium. Shearing during sexual intercourse can lead to physical abrasions of the epithelium, in particular in microanatomical regions where the stromal papillae, enriched with stromal DCs, reach close to the luminal surface of the mucosa. Depicted are two stromal papillae containing arterioles, venules and lymphatic vessels. The stromal papilla on the right signifies the afferent arm shuttling blood cells to the mucosa. Monocytic precursor cells differentiate upon arrival either into macrophages or dendritic cells (DCs), and DCs may differentiate further into subsets. Three stromal DC subsets have been identified in human skin, distinguished by BDCA-1, CD1 and CD14 expression patterns65, but their presence and susceptibility to HIV have not been determined in the mucosa. An abrasion of the outer epithelium exposes the stromal papilla tip (left), as well as several epithelial cells located close to or within the basal layer. Infected donor cells and free virions may migrate along such an abrasion, as shown here, and directly contact various target cells in the mucosal epithelium and stroma. Resident mucosal leukocytes such as DCs and T cells tend to cluster in these regions (see Figure 3e), creating susceptible foci for infection. Possible pathways for HIV penetration are depicted on the left side of the illustration and are indicated by letters. Characteristic phenotypic cell receptors and receptors relevant for HIV binding and infection are shown on the top of the figure. a. Trapping of free HIV virions or HIV-infected donor cells in mucus covering the mucosa. b. Attachment of HIV-infected donor cells to the luminal surface of the mucosa and secretion of virions upon contact. c. Penetration of virions into gaps between epithelial cells. d. Capture of penetrating virions by Langerhans cells (LCs) residing within the epithelium, which extend processes toward the vaginal lumen. e. Internalization of virions into endocytic compartments of LCs. f. Fusion of HIV with the surface of intraepithelial CD4+ T cells, followed by productive infection. g. Transcytosis of virions through epithelial cells located close to or within the basal layer of the squamous epithelium (Figure 3a–c). h. Productive infection of basal epithelial cells. i. Internalization of virions into endocytic compartments of basal epithelial cells. j. Immigration of infected donor cells along physical abrasions of the epithelium into the mucosal stroma, where they are taken up by lymphatic or venous microvessels and transported to local lymph nodes or the blood circulation. k. Immigration of free virions along microabrasions into the stroma, where they can make direct contact with stromal DCs. l. Productive infection of stromal DCs by HIV. m. Internalization of virions into endocytic compartments of stromal DCs. n. Passage of virus from stromal DCs to CD4+ T cells across an infectious synapse (see also Figure 4). o. Massive productive infection of mucosal CD4+ T cells activated by contact with antigen-presenting DCs. p. Productive infection of resting mucosal CD4+ memory T cells. q. Binding of HIV and possibly productive infection of stromal macrophages. r. Emigration of productively infected CD4+ T cells and DCs into the submucosa and the draining lymphatic and venous microvessels. T cells may derive from the epithelium or the stroma. Likewise, emigrating DCs may originate from intraepithelial LCs or stromal DCs. DCs and T cells often form conjugates, and HIV may accumulate between the two cells along an infectious synapse. DCs carry virions in endocytic compartments and some are also productively infected, but it remains unclear at which differentiation stage this occurs.
HIV virions that are initially free or following their release from infected donor cells interact with epithelial cells and traverse the epithelium through several pathways, including transcytosis, endocytosis, or productive infection, or they merely penetrate through gaps between epithelial cells (Figure 2). Understanding these events has been hindered by inconsistent findings, largely because experimental systems have used epithelial cell types derived from different anatomical sites, and primary and immortalized cell lines. Several reports demonstrate that HIV-1 binds to and enters epithelial cells from the lower female genital tract19–21. Transcytosis has been shown to occur in both cell lines and primary cells, but not definitively within intact tissue. Upon release, the virions readily infect susceptible leukocytes22,23. Interestingly, cell-associated virions secreted from infected leukocytes appear markedly more efficient in transcytosis than cell-free virions8,11,22. Productive infection may also occur within cervical epithelial cells themselves7, although this remains controversial19,24. Conceivably, HIV-1 can also be transported through the cervicovaginal epithelium to the draining lymphatics by donor lymphocytes and macrophages, as has been suggested in mouse studies7,25.
Our ex vivo experiments using sheets of isolated vaginal epithelium, devoid of mucosal stroma, confirmed that the sequestration of HIV-1 virions in endocytic compartments and in the cytosol of epithelial cells occurs (Figure 3a–c). However, although the experimental conditions permitted HIV-1 access to both the luminal and basal sides of the epithelium, the virions were detected exclusively in the basal and suprabasal epithelial cells (F. Hladik, P. Sakchalathorn, M. J. McElrath, unpublished observations). Therefore, rather than entering and traversing superficial epithelial cells in the vagina and ectocervix, HIV-1 probably disperses through the narrow gaps between them17, as depicted in Figure 2. This route might permit HIV-1 to directly contact and then infect intraepithelial Langerhans cells and CD4+ T cells26 (see later), or it might allow HIV-1 to reach suprabasal or basal epithelial cells that are susceptible to viral sequestration and transcytosis. Importantly, factors in human semen, most notably amyloid fibrils forming from naturally occurring fragments of seminal prostatic acidic phosphatase, can capture virions and promote attachment to epithelial cells and leukocytes, thus increasing infectivity27.
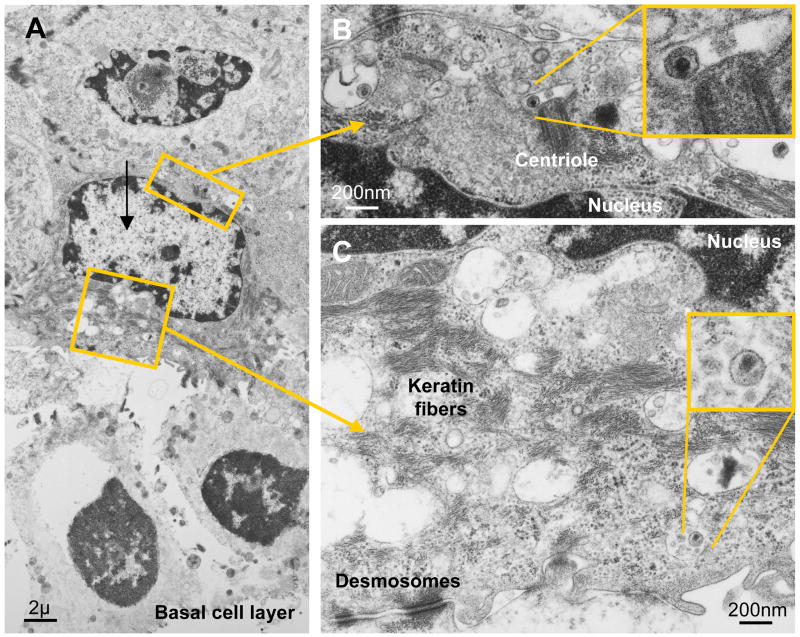
Figure 3. Pathways of HIV invasion in the mucosa of the vagina and uterine ectocervix, part B.
A–C. Likely HIV transcytosis in a vaginal epithelial cell in situ located one layer above the basal cell layer. Virions can be seen in the cytoplasm on both sides of the nucleus. Desmosomes and keratin fibers identify the cell as epithelial.
Several proteins expressed on the surface of epithelial cells may mediate attachment of HIV-1. Two cell surface glycosphingolipids, sulfated lactosylceramide expressed by vaginal epithelial cells28 and galactosylceramide expressed by ectocervical epithelial cells19,29, bind HIV-1 gp120 and foster transcytosis22. Interactions of HIV-1 gp120 with transmembrane heparan sulfate proteoglycans (syndecans) expressed by genital epithelial cells can also contribute to HIV-1 attachment and entry20,23. Recently, glycoprotein 340, a splice variant of salivary agglutinin expressed by cervical and vaginal epithelial cells, was shown to specifically bind to the HIV envelope protein and to enhance the passage of HIV through the epithelium to susceptible leukocytes30. One group found that the β1 subunit of integrins expressed by explant cervical epithelial cells bound virions that were presumably coated with fibronectin, which is abundant in human semen, although this was not observed across all explants17. Detection of HIV-1 chemokine co-receptor expression has been inconsistent: one study did not detect the expression of either CC-chemokine receptor 5 (CCR5) or CXC-chemokine receptor 4 (CXCR4) by cervical epithelial cells19, another reported the expression of CXCR4 by these cells21, and another reported the exclusive expression of CCR529.
Regardless of the mode, the penetration of virus through the cervicovaginal epithelium in vivo occurs rapidly within 30–60 minutes of exposure, as shown in SIV infected macaques31. Once within the epithelium, HIV encounters CD4+ T cells as well as Langerhans cells (LCs). LCs have dendrites that extend and retract through the intercellular spaces32, and even reach up to the epithelium surface33 where HIV can bind directly to these cells (T. Hope, personal communication). Based on observations of gut dendritic cells34,35, this could be particularly true for DCs that are located just beneath the endocervical columnar epithelium. However, direct sampling of luminal pathogens by endocervical DCs or vaginal LCs, which could be exploited by HIV to bypass the epithelial-cell barrier, has not yet been formally demonstrated.
Finally, mechanical microabrasions of the mucosal surface induced by intercourse may allow HIV to directly access target cells, such as DCs, T cells and macrophages, at the basal epithelium and the underlying stroma36. Areas above the stromal papillae, where the epithelium is relatively thin and where LCs on the epithelial-cell side (F. Hladik, L. Ballweber, M. J. McElrath, unpublished observations) and T cells and macrophages on the stromal side29 congregate, appear particularly vulnerable to viral invasion. Consistent with this notion, in vivo SIV infection of the genital mucosa of macaques is initially established in a highly focal manner, and continuous seeding from this nidus of infection is crucial for establishing systemic infection18. Similarly, chemical microabrasions with the use of certain topical microbicides and microabrasions due to genital ulcers caused by sexually transmitted diseases (for example, syphilis, chancroid and those caused by infection by Herpes simplex virus) are also likely to expose vulnerable target cells in the basal epithelium and stroma37.
Importance of cervicovaginal LCs in HIV invasion
LCs are a DC subtype residing within the outer squamous epithelium of the skin or mucosae. For many years, HIV-1 acquisition in the lower genital tract has been assumed to occur through internalization of HIV-1 by LCs. This view was supported by evidence that skin LCs are susceptible to infection by HIV-138–41 and that genital mucosal LCs harbour SIV virions within 24 hours of intravaginal inoculation of macaques31. However, soon after ex vivo organ culture, LCs migrate out of the epithelium15,24,42–44; therefore, examination of LC infection specifically within the human vaginal epithelium has been technically difficult. For example, one landmark study demonstrated that after exposure of human complete cervical mucosa to HIV-1, emigrating DCs had efficiently captured HIV and were capable of transmitting the virus in trans43. However, determining whether the cells originated from the epithelium as LCs or the underlying stroma as DCs was impossible. More recently, we resolved this issue by preparing sheets of vaginal epithelium separated from the underlying stroma, and observed that vaginal LCs efficiently internalized HIV-1 into their cytoplasmic compartments26. As LCs exit the epithelium at the basal side, they transport intact virions, thereby enabling infection to spread beyond the site of viral entry (Figure 2).
The ability of LCs in the cervicovaginal epithelium to produce and release new HIV-1 virions is uncertain. HIV-specific receptors are expressed by these LCs, including CD4, CCR5 and the C-type lectin langerin (CD207), but not CXCR4 and dendritic-cell-specific ICAM3-grabbing non-integrin (DC-SIGN, CD209)26,45–48. Antibodies that bind CD4 and CCR5 partially block the uptake of R5-tropic HIV-1 by LCs, but blocking the binding of C-type lectin to mannan on HIV-1 had little effect on uptake26. Although low-level CD4- and CCR5-mediated productive infection of LCs in human skin explants has been shown40,41,49, we were unable to confirm this finding in our imaging studies of vaginal LCs26. Therefore, if de novo production of virions occurs, it appears relatively inefficient in contrast to the high capacity of vaginal LCs to endocytose HIV-1. Nevertheless, even low levels of productive HIV-1 infection of cutaneous DCs and LCs lead to profound viral replication in co-cultured T cells41,49,50. Therefore, future investigations must conclusively determine if LCs in cervicovaginal epithelium can support productive HIV infection in vivo and if this property is required for the passage of the virus to T cells, as has been reported for other types of DCs51–54.
The relative inefficiency of mannan, a mannose polymer, to block binding and endocytosis of HIV-1 by vaginal LCs was surprising26, because C-type lectins, which recognize mannose containing carbohydrate structures, mediate viral entry in other types of DCs47. However, HIV-1 can bind DC subsets independent of C-type lectins and CCR555–57. So, although HIV is efficiently captured by langerin expressed by epidermal LCs58, HIV appears to largely bypass langerin expressed by vaginal LCs in favour of alternative endocytic routes. This distinction may be highly relevant for mucosal HIV transmission. Langerin expressed by epidermal LCs can direct HIV-1 to Birbeck granules for degradation58. By contrast, by gaining entry to the vaginal LCs independently of langerin, HIV may survive by reaching endocytic compartments, such as early phagosomes, where antigens are preserved for cross-presentation59. This is consistent with our observations that intact virions were still present in LCs that had migrated out of the vaginal epithelium at 60 hours after viral challenge26. Thus, it appears that HIV-1 enters vaginal LCs through a different route than skin LCs, resulting in a distinct fate of the endocytosed virions. More detailed studies are now warranted to uncover which endocytic pathways HIV uses in vaginal LCs, and how this can be harnessed therapeutically.
Infection of DCs in the cervicovaginal stroma
Unlike genital LCs, stromal DCs express both DC-SIGN47,48 and CCR560,61 and have been implicated in SIV and HIV infection, but their exact role in mucosal transmission is not clear. In situ studies in the human explant model have failed to identify DCs in the cervicovaginal stroma as foci for productive HIV infection15,24,42,44. By contrast, SIV-infected DCs were present in the lamina propria of the cervicovaginal mucosa of macaques shortly after intravaginal SIV challenge31,62, as well as in chronically infected animals63. Likewise, HIV-infected DCs were identified in tissue biopsies of the vaginal stroma of asymptomatic HIV-1 infected women64. The failure to reveal infection of stromal DCs in the human explant models may have been due to the relatively low sensitivity of the detection methods employed and the migration of stromal DCs from the tissue, which may drastically decrease the number of infected cells in situ over time. Indeed, when DCs were harvested from the culture supernatants of human cervical explants that were challenged with HIV-1, significant in trans infectivity was detected43 and massive budding of virions was observed among emigrant DCs five days after virus exposure61 (Figure 4b–d). However, it could not be determined if the original source of DCs was from the epithelium proper or from the stromal tissue. In addition, inferring the initial susceptibility of DCs while confined to the mucosal stroma, based on findings from emigrated DCs that undergo phenotypic changes as they exit the mucosa, may be less reliable. Thus, stromal DCs exhibit different HIV-1 receptor expression patterns than LCs, potentially permitting different HIV-1 entry pathways than in the epithelial LCs. Much still remains to be learned about stromal DCs in the human genital mucosa in general, about whether different subsets exist similar to those found in skin dermis65, and about the interaction of these DC subsets with HIV in particular. More sensitive in situ detection methods of HIV infection, as well as assays distinguishing de novo virus production from endocytically engulfed virions, as recently reported66, will be helpful in sorting out the contribution of stromal DCs to HIV-1 propagation.
Figure 4. Significance of DC–T-cell interactions for HIV-1 transmission.
A. Pathways of HIV-1 passage between dendritic cells (DCs) and T cells. DCs can store HIV-1 in three forms for eventual infection of CD4+ T cells. (1) Endocytosed intact virions. Endocytic entry via C-type lectins such as DC-specific ICAM3-grabbing non-integrin (DC-SIGN, CD209) directs HIV to early and late endosomes. From there it enters multivesicular bodies and remains intact, or traffics to lysosomes where it is degraded. (2) Integrated provirus. Entry of HIV-1 by CD4- and co-receptor-mediated fusion leads to productive infection of DCs. (3) Surface-bound intact virions. Binding and trapping of intact virions on the cell surface can also occur by C-type lectins such as DC-SIGN.
Passage of HIV-1 from DCs to CD4+ T cell occurs most effectively across an infectious synapse, formed by concentration of HIV-1 on the DC side and of HIV receptors such as CD4 and CC-chemokine receptor 5 (CCR5) on the T cell side. HIV is released into the infectious synapse either by exocytosis of stored virions from multivesicular bodies (MVBs) or by budding of newly formed virions following active viral replication. Surface bound virions may also accumulate at the infectious synapse. Migration of HIV toward the T cell may be further enhanced by “surfing” of virions along the outer surface of filopodia or cytonemes that are extended from the T cell toward the DC. Coupling of virions with exosomes as they are being released from MVBs may also increase their infectivity. Exosome-associated virions are likely to be transmitted to CD4+ T cells through membrane binding and fusion, either within the infectious synapse or over longer distances. In parallel to transmission of virus from the DC to the CD4+ T cell, the DC also presents antigenic peptides through MHC class II molecules to the T-cell receptor CD3. During peptide recognition, additional receptor–ligand pairs that are important for T-cell stimulation accumulate in this region and form an immunological synapse. Signals delivered through the immunological synapse lead to T-cell activation, which ultimately causes transcription factors such as nuclear factor-κB (NF-κB) and nuclear factor of activated T cells (NFAT) to translocate into the nucleus of the T cell. There, they bind to the enhancer region of the viral long terminal repeat (LTR) and activate viral gene transcription, driving HIV-1 replication. B–D. Example of infectious synapse formation between DCs and T cells in the human vagina (taken from Hladik F. et al.69). HIV-1 buds from the surface of the productively infected DC toward the contact zone between the DC and the two T cells. Viral budding is also seen along other areas of the DC surface. The DC contains the typical veiled nucleus as well as multiple large mitochondria, and one large cytoplasmic process is formed at the top right. C–D. The two contact zones with the lymphocytes are further magnified, displaying virus budding from the DC into the infectious synapse.
HIV infection of cervicovaginal CD4+ T cells
CD4+ T cells are dispersed throughout the lamina propria of the human vagina, ectocervix and endocervix, often clustering just beneath the basal membrane67,68. They also reside at variable numbers within the vaginal and ectocervical squamous epithelium67,68. The majority are memory T cells that express higher levels of CCR5 than T cells that circulate in peripheral blood26,69–71. One day post HIV-1 inoculation of vaginal, ectocervical and endocervical tissue cultures, infected CD4+ T cells were shown to be confined to the mucosal stroma15,17,24,43. This result was surprising, given the presence of CCR5+ CD4+ T cells within the squamous epithelium. However, by analyzing the fate of fluorescence-tagged virions as early as two hours after viral exposure, we observed that R5-tropic HIV-1 bound to intraepithelial vaginal CD4+ T cells very efficiently, followed by fusion and productive infection26. Therefore, infected T cells must rapidly leave the epithelium, and those found in the stroma may be the same or early progeny of intraepithelial T cells.
Findings in the human explant studies show that HIV-1 very effectively targets CD4+ T cells in the genital mucosa for productive infection15,26,43, and that the initial infection of intraepithelial CD4+ T cells is probably independent of LCs26. The central role for genital CD4+ T cells in early infection and propagation is also evident from SIV challenge experiments in macaques63,72,73. Interestingly, not only does SIV productively infect activated T cells, characterized by HLA-DR and Ki67 expression, but also T cells that are in the HLA-DR- and Ki67-negative resting state72. Consistent with this finding, we observed binding of HIV-1 to both HLA-DR+ and HLA-DR− intraepithelial T cells in our human vaginal explant model (M. J. McElrath, P. Sakchalathorn, L. Ballweber, F. Hladik, unpublished observations). In addition, the contribution of resting CD4+ T cells to viral production is substantial during the very earliest stages of infection74. The fact that vaginal CD4+ T cells are rapidly depleted following intravenous SIV inoculation of macaques6,73, similar to that observed in CD4+ T cells of the gut during acute SIV infection75, further illustrates their high susceptibility to infection in vivo.
Other leukocyte targets for HIV in the female genital tract
Macrophages in the female genital mucosa are also susceptible targets for early HIV-1 infection, as demonstrated in studies using human explant models24,42,44, and in two reports were the major cell type infected by R5-tropic HIV-124,44. Whether or not resident macrophages in the female genital tract constitutively express CCR5 in situ is not known, but most macrophages do so when harvested from supernatants of vaginal organ cultures61, suggesting that the expression of CCR5 by macrophages may occur during the period of activation and emigration from the mucosa76. By contrast, SIV-infected macrophages in genital tissues were either rare72, or undetectable31,62. Likewise, macrophages in the human intestinal mucosa were reported to lack CCR5 expression and to possess low permissibility for HIV-1 infection77. These discrepancies illustrate a potential limitation of organ cultures. If indeed explantation activates stromal macrophages and as a consequence increases surface CCR5 expression, this would lead to an overestimation of their susceptibility to infection in vivo. Of note, in addition to chemokine receptor-mediated fusion, monocyte-derived macrophages can also trap intact virions through syndecans78, or even without specific envelope-receptor interactions through a process known as macropinocytosis79. Once captured, HIV-1 can be archived for several days and then transmitted to T cells in trans80,81. If genital macrophages similarly archive infectious virions, their role in viral propagation once HIV-1 invades the stroma may be significant.
Other leukocyte subpopulations also interact with HIV. For example, productive infection of natural killer (NK) cells has been reported82, as well as transmission of virus through DC-SIGN expressed by B cells to T cells in trans83. The significance of B cells for HIV invasion in the genital mucosa remains unknown. Lastly, monocyte precursor cells enter the mouse dermis in large numbers following intracutaneous infection with Leishmania major and differentiate into stromal DCs84. This finding raises the possibility that the influx of inflammatory cells into the genital tract following HIV-1 exposure may create new potential target cells that normally do not reside in the mucosa, and further heighten the initial local infection.
The role of DCs in enhancement of infection
A growing body of literature suggests that HIV exploits DCs to enhance its infectivity of T cells85. First reported in 1992, DCs, even when seemingly uninfected themselves, invoked vigorous cytopathic infection to CD4+ T cells86. The potential relevance of these findings for the transmission of HIV at mucosal sites was subsequently highlighted by reports of increased HIV-1 replication in DC–T-cell conjugates derived from human skin87 and cervicovaginal mucosa61. The enhancement of HIV transmission by DCs probably occurred by facilitating T-cell activation72,88 as well as de novo T-cell infection.
Four mechanisms for how DCs can augment de novo infection of T cells have been proposed. In classic HIV trans infection47, the DCs are not productively infected but trap and preserve the virus, which is subsequently transferred to T cells across an “infectious synapse”, which is a zone of DC–T-cell contact where HIV itself and the HIV receptors are concentrated89,90. Alternatively, trans infection may occur by HIV association with DC-derived exosomes, which, intriguingly, appear to markedly increase the infectivity of virions that are coupled to them91. In a third pathway, productively infected DCs transmit new viral progeny across the infectious synapse to T cells49,52–54. In this case, the contact zone has also been termed a “virological synapse”, to signify that the donor cell, in analogy to cell-associated HIV transmission between CD4+ T cells92, is productively infected93. Furthermore, efficient retroviral transfer between cells has recently been described, in which retroviruses, including HIV-1, “surf” along the outer surface of filopodia or cytonemes that extend from an uninfected cell and interact through their tips with an infected cell94. These narrow filopodial contact zones may be special cases of virological synapses95, or may be analogous to nanotubules that are formed between immune cells96. In T cells, migration of HIV-1 also occurs within nanotubules130. Nanotubules have been shown to functionally connect DCs with other cells96, but their significance for viral transfer from DCs remains to be determined.
Clear evidence for any of these described modes of viral transmission from DCs to CD4+ T cells in the genital mucosa is still lacking. We have shown that HIV concentrates along the cell–cell junction between emigrant LC–T-cell conjugates from human vaginal epithelium, supporting the formation of an infectious synapse26. No consensus has been reached over whether in trans infection occurs primarily from surface bound virions47,97, internalized virions98 or both57. At any rate, once HIV makes its way into the genital DC–T-cell conjugate, a profound productive infection ensues61. Of note, in these conjugates viral budding was not only observed from the surface of T cells but also of the DCs. Visualization of budding by electron microscopy signifies massive infection. So, in DC–T-cell conjugates not only T cells but also DCs, which by themselves are generally weak producers of virus85, acquire the ability to produce large amounts of viral progeny. This is consistent with findings obtained in co-cultures of monocyte-derived DCs and T cells99. Thus, DC–T-cell cross-talk in the genital tract appears to drive productive HIV infection in both cell types. Moreover, transmission from DCs to T cells may provide a means for HIV to avoid antibody-mediated neutralization100–102. Taken together, unravelling the precise mechanisms by which the interaction between genital DCs and CD4+ T cells enhance HIV transmission will be important in developing effective strategies to counteract this process.
HIV invasion in the male genital tract
Of the nearly 15 million infected men, an estimated 70–75% acquired HIV-1 through vaginal intercourse (Table 1), making the male genital tract the second leading site of HIV invasion following the cervicovaginal mucosa. HIV-1 target cells are abundant in the foreskin, and include CD1a+ LCs and CD4+ T cells in the squamous lining, as well as T cells, macrophages and DCs in the underlying stroma46,103–105. Variable fractions of these cells express CD4, CCR5 and CXCR446,103,104. As in the vagina, LCs do not express DC-SIGN, whereas at least some of the stromal DCs do103. Therefore, similar mechanisms that have been described for viral invasion into the female genital tract above are likely to occur in the male genital tract as well.
The highly protective effect of circumcision106,107, which has been reviewed comprehensively elsewhere108, suggests that the penile foreskin is particularly vulnerable to HIV infection. The foreskin is lined by stratified squamous epithelium, and the external surface is more heavily keratinized than the internal surface103,104. Consequently, the inner foreskin may be more susceptible to infection, a view that was supported by investigations of penile autopsy tissues103, and corroborated in an in vitro foreskin explant model that showed infectious foci, predominantly containing LCs and CD4+ T cells, at the base of the epithelial-cell layer, exclusively in the inner foreskin104. Alternatively, circumcision may reduce the risk for genital tears, abrasions and ulcer disease, resulting in decreased HIV infection risk108,109.
Although circumcision appears to provide a protective effect against HIV infection, entry sites other than the foreskin must exist for circumcised males. The glans penis has a heavily keratinized squamous epithelium similar to the outer foreskin, and effective viral penetration seems relatively improbable here. By contrast, the penile urethra is a more likely candidate as it is lined by a narrowly stratified, non-keratinized columnar epithelium and contains high numbers of CD4+ and CD8+ T cells, and macrophages, within the epithelium and the lamina propria110. Interestingly, DCs are not observed in the urethral mucosa46,110. The presence of CCR5 and CXCR4 mRNA in urethral swabs indicates that these HIV-1 co-receptors may be expressed by urethral cells111, particularly the predominant CD4+CD45RO+ memory T cells110. Of note, intraurethral infusion of SIV resulted in infection of all six inoculated male macaques112. In HIV-1 infected men, antibiotic treatment of urethral Neisseria gonorrhoeae infection reduces HIV-1 shedding in semen but not the viral load in blood113. This finding, as well as HIV-1 shedding in the ejaculate of vasectomized HIV-positive men114 and in the pre-ejaculatory fluid115,116 suggest a distal source for virus, pointing to the urethra as a site harboring significant numbers of cells susceptible to HIV-1 infection. Taken together, these data suggest that CD4+ T cells and macrophages in the male urethra provide a suitable portal of entry for HIV, although the specific events of urethral invasion are still unknown.
Conclusions
Although studies that focus on mucosal HIV infection continue to be painstaking, many investigators have overcome some of the technical difficulties that have previously precluded active research in this area. Recent investigations summarized here provide invaluable insights into the distinct cellular and molecular interactions of mucosal HIV infection. Clearly, the challenge emerging from these findings is to counteract the rapid acceleration of infection in local reservoirs of the lower genital and gastrointestinal tracts. Two key questions to address in future studies lie in determining whether DC–T-cell interactions that markedly amplify HIV-1 production, which are consistently observed in vitro, are relevant in the mucosal epithelium in vivo, and whether common mechanisms of HIV-1 entry apply to both the lower genital and gastrointestinal tract. This information can guide the development of innovative strategies to protect susceptible target cells from HIV-1 infection in both women and men, such as with barrier protection methods, topical microbicides and mucosal immunization.
Although there are an increasing number of large-scale HIV prevention clinical trials that have recently reported a lack of efficacy, one favourable approach has been male circumcision. The opportunity to gain a more thorough understanding of how HIV-1 invades its target cells in the human foreskin should be exploited. The recent emphasis of CD4+ T-cell depletion by HIV-1 in the gut and vagina during acute infection has sparked a renewed interest in abating infection at the sites where massive replication occurs117. Although important in counteracting HIV disease, CD4+ T-cell depletion appears to be a secondary event that commonly occurs through various routes of transmission. Therefore, the best opportunity to prevent HIV disease clearly lies at the sites of mucosal entry, and investigations to directly counterattack HIV infection must continue to focus on these portals.
Box 1. HIV invasion in the lower gastrointestinal tract
HIV-1 and SIV infection commonly targets the lower gastrointestinal tract as an initial site following receptive anal intercourse in humans and direct inoculation in macaques, and as a secondary infection site following rapid dissemination from mucosal foci18 or acute systemic infection118–120. The rectal mucosa contains simple columnar epithelial cells, and the lamina propria is a rich source of lymphoid cells and lymphoid nodules. Numerous reports have documented the pathogenic effect of the virus in the gastrointestinal tract, and have shown a severe depletion of CD4+ T cells that express CCR5 in the gut, regardless of the route of infection117. The relevant target cells for infection in the lower gastrointestinal tract are thus likely to be primarily CD4+ memory T cells121. At present, detailed features of HIV-1 entry into the lower gastrointestinal tract that may be distinct from the genital tract have not been elucidated, but this remains an important question to address for prevention strategies.
Glossary terms
Simian–HIV (SHIV)
SHIVs are chimeric viruses that are created by inserting the envelope protein (Env), the transcriptional transactivator (Tat) and the regulator of virion gene expression (Rev) of HIV into the SIVMAC239 clone. Depending on the particular HIV Env protein, these SHIVs have different in vivo characteristics. The SHIV chimeric viruses are best used for testing antibodies specific for HIV in non-human primate models.
Transcytosis
A process by which various macromolecules, including HIV-1 virions, are transported across the interior of a cell.
Syndecans
Single transmembrane domain proteins that carry three to five heparan sulfate and chondroitin sulfate chains which allow for interaction with a large variety of ligands including residues on the HIV-1 gp120 protein.
Langerhans cell
A type of dendritic cell that is localized in the squamous epithelial layer of the skin and certain mucosae.
Stromal papillae
Superficial areas of the mucosal stroma that interdigitate with the epithelium.
C-type lectin receptors
A large family of receptors that bind glycosylated ligands and have multiple roles, such as in cell adhesion, endocytosis, natural-killer-cell target recognition and dendritic-cell activation.
R5-tropic HIV-1
An HIV strain that uses CC-chemokine receptor 5 (CCR5) as the co-receptor to gain entry to target cells.
Birbeck granules
Membrane-bound rod- or tennis racket-shaped structures with a central linear density, found in the cytoplasm of Langerhans cells. The formation of Birbeck granules is induced by langerin, an endocytic C-type lectin specific to Langerhans cells.
Phagosomes
Vacuolar compartments that confine bacteria after enforced endocytosis or after phagocytosis. Unless counteracted by a bacterial survival strategy, the phagosome matures into a hostile environment that is designed to kill and digest microorganisms.
Cross-presentation
The initiation of a CD8+ T-cell response to an antigen that is not present within antigen-presenting cells (APCs). This exogenous antigen must be taken up by APCs and then re-routed to the MHC-class-I pathway of antigen presentation.
Lamina propria
Connective tissue that underlies the epithelium of the mucosa and contains various myeloid and lymphoid cells, including macrophages, dendritic cells, T cells and B cells.
Exosomes
Small lipid-bilayer vesicles that are released from activated cells. They comprise either plasma membrane or membrane derived from intracellular vesicles.
Filopodia
Slender cytoplasmic projections, which extend from the leading edge of migrating cells.
Cytonemes
Actin-based filopodial cell extensions.
Nanotubules
Cytonemes that connect blood cells over a distance of several cell diameters and transport membrane proteins, lipids and ions from one of the connected cells to another, thus executing long range intercellular communications.
Macropinocytosis
A mechanism of endocytosis in which large droplets of fluid are trapped underneath extensions (ruffles) of the cell surface. Can be exploited by some pathogens as a route for entry into cells.
Glans penis
Sensitive tip of the penis. When the penis is flaccid it is wholly or partially covered by the foreskin, except in men who have been circumcised.
Biographies
Florian Hladik
Florian Hladik obtained his M.D., Ph.D. and dermatology training at the University of Vienna in Austria. He carried out postdoctoral research at the Johannes Gutenberg University in Mainz, Germany, and the University of Washington, USA. He is currently Research Assistant Professor in the Departments of Gynecology and Medicine, University of Washington, and Affiliate Investigator at the Fred Hutchinson Cancer Research Center in Seattle. His research focuses on mucosal HIV transmission pathogenesis, microbicide development and infectious causes of fetal prematurity.
M. Juliana McElrath
After receiving her M.D., Ph.D. and internal medicine residency training at the Medical University of South Carolina, Dr. McElrath undertook a clinical fellowship in infectious diseases at Columbia University and a faculty position at the Rockefeller University. For nearly two decades she has focused her research efforts on HIV pathogenesis, mucosal immunity and vaccine development in Seattle. She currently is a Member and Co-Director of the Vaccine and Infectious Disease Institute at Fred Hutchinson Cancer Research Center and a Professor of Medicine at the University of Washington.
Online Summary
HIV invasion through the mucosae of the female lower genital tract contributes the largest number of new HIV-1 infections worldwide. The second leading site of viral invasion is the lower male genital tract, followed by invasion via the rectal mucosa in both women and men.
Explant models of human genital tissues have provided new insights into the mechanisms of sexual HIV transmission.
Initial attachment of HIV-1 to the mucosa may be aided by cervical mucus and a variety of gp120-binding surface receptors on epithelial cells. HIV-1 penetration into the genital mucosa occurs rapidly after exposure and is possibly enhanced by microabrasions or genital ulcer disease.
In the human vagina, intraepithelial CD4+ T cells and CD1a+ Langerhans cells are the first cells infected by HIV-1.
Vaginal Langerhans cells exhibit a high capacity to endocytose HIV-1 virions. C-type lectins such as DC-SIGN (CD209) or langerin (CD207) appear to play little to no role in mediating this infection pathway.
Genital CD4+ T cells express high levels of CCR5, are rapidly infected by HIV-1 and produce large quantities of viral progeny.
Dendritic cells utilize several pathways to enhance viral propagation to CD4+ T cells for productive infection. Presumably these occur as well in the genital mucosa, but direct evidence is lacking.
The highly protective effect of circumcision indicates that viral invasion in men occurs predominantly through the inner foreskin, where both CD1a+ Langerhans cells and CD4+ cells are abundant. The second leading site of viral invasion in the male genital tract is likely the penile urethra.
References
- 1.2007 AIDS epidemic update. UNAIDS/WHO; Geneva: [Google Scholar]
- 2.Kell PD, Barton SE, Edmonds DK, Boag FC. HIV infection in a patient with Meyer-Rokitansky-Kuster-Hauser syndrome. J R Soc Med. 1992;85:706–707. doi: 10.1177/014107689208501119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller CJ, Alexander NJ, Vogel P, Anderson J, Marx PA. Mechanism of genital transmission of SIV: a hypothesis based on transmission studies and the location of SIV in the genital tract of chronically infected female rhesus macaques. J Med Primatol. 1992;21:64–68. [PubMed] [Google Scholar]
- 4.Padian NS, et al. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. Lancet. 2007 doi: 10.1016/S0140-6736(07)60950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howell AL, et al. Human immunodeficiency virus type 1 infection of cells and tissues from the upper and lower human female reproductive tract. J Virol. 1997;71:3498–3506. doi: 10.1128/jvi.71.5.3498-3506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joag SV, et al. Animal model of mucosally transmitted human immunodeficiency virus type 1 disease: intravaginal and oral deposition of simian/human immunodeficiency virus in macaques results in systemic infection, elimination of CD4+ T cells, and AIDS. J Virol. 1997;71:4016–4023. doi: 10.1128/jvi.71.5.4016-4023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips DM, Tan X, Perotti ME, Zacharopoulos VR. Mechanism of monocyte-macrophage-mediated transmission of HIV. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S67–70. [PubMed] [Google Scholar]
- 8.Alfsen A, Yu H, Magerus-Chatinet A, Schmitt A, Bomsel M. HIV-1-infected blood mononuclear cells form an integrin- and agrin-dependent viral synapse to induce efficient HIV-1 transcytosis across epithelial cell monolayer. Mol Biol Cell. 2005;16:4267–4279. doi: 10.1091/mbc.E05-03-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sourisseau M, Sol-Foulon N, Porrot F, Blanchet F, Schwartz O. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J Virol. 2007;81:1000–1012. doi: 10.1128/JVI.01629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muratori C, et al. Macrophages Transmit Human Immunodeficiency Virus Type 1 Products to CD4-Negative Cells: Involvement of Matrix Metalloproteinase 9. J Virol. 2007;81:9078–9087. doi: 10.1128/JVI.00675-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Herrewege Y, et al. A dual chamber model of female cervical mucosa for the study of HIV transmission and for the evaluation of candidate HIV microbicides. Antiviral Res. 2007;74:111–124. doi: 10.1016/j.antiviral.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Kaizu M, et al. Repeated intravaginal inoculation with cell-associated simian immunodeficiency virus results in persistent infection of nonhuman primates. J Infect Dis. 2006;194:912–916. doi: 10.1086/507308. [DOI] [PubMed] [Google Scholar]
- 13.Khanna KV, et al. Vaginal transmission of cell-associated HIV-1 in the mouse is blocked by a topical, membrane-modifying agent. J Clin Invest. 2002;109:205–211. doi: 10.1172/JCI13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu T, et al. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol. 1996;70:3098–3107. doi: 10.1128/jvi.70.5.3098-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta P, et al. Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol. 2002;76:9868–9876. doi: 10.1128/JVI.76.19.9868-9876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zussman A, Lara L, Lara HH, Bentwich Z, Borkow G. Blocking of cell-free and cell-associated HIV-1 transmission through human cervix organ culture with UC781. AIDS. 2003;17:653–661. doi: 10.1097/00002030-200303280-00002. [DOI] [PubMed] [Google Scholar]
- 17.Maher D, Wu X, Schacker T, Horbul J, Southern P. HIV binding, penetration, and primary infection in human cervicovaginal tissue. Proc Natl Acad Sci U S A. 2005;102:11504–11509. doi: 10.1073/pnas.0500848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller CJ, et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dezzutti CS, et al. Cervical and prostate primary epithelial cells are not productively infected but sequester human immunodeficiency virus type 1. J Infect Dis. 2001;183:1204–1213. doi: 10.1086/319676. [DOI] [PubMed] [Google Scholar]
- 20.Wu Z, Chen Z, Phillips DM. Human genital epithelial cells capture cell-free human immunodeficiency virus type 1 and transmit the virus to CD4+ Cells: implications for mechanisms of sexual transmission. J Infect Dis. 2003;188:1473–1482. doi: 10.1086/379248. [DOI] [PubMed] [Google Scholar]
- 21.Berlier W, et al. Selective sequestration of X4 isolates by human genital epithelial cells: Implication for virus tropism selection process during sexual transmission of HIV. J Med Virol. 2005;77:465–474. doi: 10.1002/jmv.20478. [DOI] [PubMed] [Google Scholar]
- 22.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nature Med. 1997;3:42–47. doi: 10.1038/nm0197-42. This study introduced the concept of HIV-1 transcytosis, a process by which virions are transported intact through the interior of epithelial cells, retaining their infectivity. [DOI] [PubMed] [Google Scholar]
- 23.Bobardt MD, et al. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J Virol. 2007;81:395–405. doi: 10.1128/JVI.01303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenhead P, et al. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol. 2000;74:5577–5586. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ibata B, Parr EL, King NJ, Parr MB. Migration of foreign lymphocytes from the mouse vagina into the cervicovaginal mucosa and to the iliac lymph nodes. Biol Reprod. 1997;56:537–543. doi: 10.1095/biolreprod56.2.537. [DOI] [PubMed] [Google Scholar]
- 26.Hladik F, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. This investigation found that HIV-1 rapidly penetrates intraepithelial vaginal CD1a+ Langerhans cells and CD4+ T cells, and that viral fusion predominates in T cells and viral endocytosis in LCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munch J, et al. Semen-Derived Amyloid Fibrils Drastically Enhance HIV Infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. The authors discovered that amyloidogenic fragments of prostatic acidic phosphatase in semen can serve as strong enhancing factors for HIV infection. [DOI] [PubMed] [Google Scholar]
- 28.Furuta Y, et al. Infection of vaginal and colonic epithelial cells by the human immunodeficiency virus type 1 is neutralized by antibodies raised against conserved epitopes in the envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1994;91:12559–12563. doi: 10.1073/pnas.91.26.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeaman GR, et al. Chemokine receptor expression in the human ectocervix: implications for infection by the human immunodeficiency virus-type I. Immunology. 2004;113:524–533. doi: 10.1111/j.1365-2567.2004.01990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoddard E, et al. gp340 Expressed on Human Genital Epithelia Binds HIV-1 Envelope Protein and Facilitates Viral Transmission. J Immunol. 2007;179:3126–3132. doi: 10.4049/jimmunol.179.5.3126. [DOI] [PubMed] [Google Scholar]
- 31.Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishibu A, et al. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J Invest Dermatol. 2006;126:787–796. doi: 10.1038/sj.jid.5700107. [DOI] [PubMed] [Google Scholar]
- 33.Miller CJ, McChesney M, Moore PF. Langerhans cells, macrophages and lymphocyte subsets in the cervix and vagina of rhesus macaques. Lab Invest. 1992;67:628–634. [PubMed] [Google Scholar]
- 34.Rescigno M, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nature Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 35.Niess JH, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 36.Norvell MK, Benrubi GI, Thompson RJ. Investigation of microtrauma after sexual intercourse. J Reprod Med. 1984;29:269–271. [PubMed] [Google Scholar]
- 37.Weiler AM, et al. Genital ulcers facilitate rapid viral entry and dissemination following intravaginal inoculation with cell-associated SIVmac239. J Virol. 2008 doi: 10.1128/JVI.01947-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tschachler E, et al. Epidermal Langerhans cells--a target for HTLV-III/LAV infection. J Invest Dermatol. 1987;88:233–237. doi: 10.1111/1523-1747.ep12525402. [DOI] [PubMed] [Google Scholar]
- 39.Zaitseva M, et al. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nature Med. 1997;3:1369–1375. doi: 10.1038/nm1297-1369. [DOI] [PubMed] [Google Scholar]
- 40.Dittmar MT, et al. Langerhans cell tropism of human immunodeficiency virus type 1 subtype A through F isolates derived from different transmission groups. J Virol. 1997;71:8008–8013. doi: 10.1128/jvi.71.10.8008-8013.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawamura T, et al. Candidate microbicides block HIV-1 infection of human immature Langerhans cells within epithelial tissue explants. J Exp Med. 2000;192:1491–1500. doi: 10.1084/jem.192.10.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nature Med. 2000;6:475–479. doi: 10.1038/74743. [DOI] [PubMed] [Google Scholar]
- 43.Hu Q, et al. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J Exp Med. 2004;199:1065–1075. doi: 10.1084/jem.20022212. This investigation clearly demonstrates that DCs migrating from HIV-1 exposed cervical tissue can efficiently disseminate virus. Inhibition of this pathway can occur only by simultaneous blockade of CD4 and mannose-binding C type lectin receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cummins JE, Jr, et al. Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob Agents Chemother. 2007;51:1770–1779. doi: 10.1128/AAC.01129-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turville SG, et al. Diversity of receptors binding HIV on dendritic cell subsets. Nature Immunol. 2002;3:975–983. doi: 10.1038/ni841. [DOI] [PubMed] [Google Scholar]
- 46.Hussain LA, Lehner T. Comparative investigation of Langerhans’ cells and potential receptors for HIV in oral, genitourinary and rectal epithelia. Immunology. 1995;85:475–484. [PMC free article] [PubMed] [Google Scholar]
- 47.Geijtenbeek TB, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. In this report, DC-SIGN (CD209) is identified as an HIV-1 receptor on DCs and promotes efficient infection in trans of CD4+ T cells. [DOI] [PubMed] [Google Scholar]
- 48.Jameson B, et al. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J Virol. 2002;76:1866–1875. doi: 10.1128/JVI.76.4.1866-1875.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawamura T, et al. Significant Virus Replication in Langerhans Cells following Application of HIV to Abraded Skin: Relevance to Occupational Transmission of HIV. J Immunol. 2008;180:3297–3304. doi: 10.4049/jimmunol.180.5.3297. [DOI] [PubMed] [Google Scholar]
- 50.Pope M, Gezelter S, Gallo N, Hoffman L, Steinman RM. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J Exp Med. 1995;182:2045–2056. doi: 10.1084/jem.182.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cameron PU, et al. Preferential infection of dendritic cells during human immunodeficiency virus type 1 infection of blood leukocytes. J Virol. 2007;81:2297–2306. doi: 10.1128/JVI.01795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nobile C, et al. Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes. J Virol. 2005;79:5386–5399. doi: 10.1128/JVI.79.9.5386-5399.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burleigh L, et al. Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells. J Virol. 2006;80:2949–2957. doi: 10.1128/JVI.80.6.2949-2957.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turville SG, et al. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 2004;103:2170–2179. doi: 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]
- 55.Gummuluru S, Rogel M, Stamatatos L, Emerman M. Binding of human immunodeficiency virus type 1 to immature dendritic cells can occur independently of DC-SIGN and mannose binding C-type lectin receptors via a cholesterol-dependent pathway. J Virol. 2003;77:12865–12874. doi: 10.1128/JVI.77.23.12865-12874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boggiano C, Manel N, Littman DR. Dendritic cell-mediated trans-enhancement of human immunodeficiency virus type 1 infectivity is independent of DC-SIGN. J Virol. 2007;81:2519–2523. doi: 10.1128/JVI.01661-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang JH, Janas AM, Olson WJ, Wu L. Functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells. J Virol. 2007;81:8933–8943. doi: 10.1128/JVI.00878-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Witte L, et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nature Med. 2007;13:367–371. doi: 10.1038/nm1541. This study showed that HIV-1 captured by the C type lectin receptor langerin (CD207) was internalized into Birbeck granules and degraded, thus preventing HIV-1 transmission by skin LCs. [DOI] [PubMed] [Google Scholar]
- 59.Savina A, et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 60.Prakash M, Kapembwa MS, Gotch F, Patterson S. Chemokine receptor expression on mucosal dendritic cells from the endocervix of healthy women. J Infect Dis. 2004;190:246–250. doi: 10.1086/422034. [DOI] [PubMed] [Google Scholar]
- 61.Hladik F, et al. Dendritic cell-T-cell interactions support coreceptor-independent human immunodeficiency virus type 1 transmission in the human genital tract. J Virol. 1999;73:5833–5842. doi: 10.1128/jvi.73.7.5833-5842.1999. This is the first reported observation that DCs isolated from the vaginal mucosa internalize HIV-1 into cytoplasmic endosomes and produce new virions that bud from the cell membrane. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spira AI, et al. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. Intravaginal inoculation of macaques with simian immunodeficiency virus (SIV) led to infection of stromal dendritic cells and cells in the draining lymph nodes within two days. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu J, Pope M, Brown C, O’Doherty U, Miller CJ. Immunophenotypic characterization of simian immunodeficiency virus- infected dendritic cells in cervix, vagina, and draining lymph nodes of rhesus monkeys. Lab Invest. 1998;78:435–451. [PubMed] [Google Scholar]
- 64.Bhoopat L, et al. In vivo identification of Langerhans and related dendritic cells infected with HIV-1 subtype E in vaginal mucosa of asymptomatic patients. Mod Pathol. 2001;14:1263–1269. doi: 10.1038/modpathol.3880472. [DOI] [PubMed] [Google Scholar]
- 65.Nestle FO, Nickoloff BJ. Deepening our understanding of immune sentinels in the skin. J Clin Invest. 2007;117:2382–2385. doi: 10.1172/JCI33349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turville SG, Aravantinou M, Stossel H, Romani N, Robbiani M. Resolution of de novo HIV production and trafficking in immature dendritic cells. Nature Methods. 2008;5:75–85. doi: 10.1038/nmeth1137. [DOI] [PubMed] [Google Scholar]
- 67.Johansson EL, Rudin A, Wassen L, Holmgren J. Distribution of lymphocytes and adhesion molecules in human cervix and vagina. Immunology. 1999;96:272–277. doi: 10.1046/j.1365-2567.1999.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edwards JN, Morris HB. Langerhans’ cells and lymphocyte subsets in the female genital tract. Br J Obstet Gynaecol. 1985;92:974–982. doi: 10.1111/j.1471-0528.1985.tb03080.x. [DOI] [PubMed] [Google Scholar]
- 69.Hladik F, Lentz G, Delpit E, McElroy A, McElrath MJ. Coexpression of CCR5 and IL-2 in human genital but not blood T cells: implications for the ontogeny of the CCR5+ Th1 phenotype. J Immunol. 1999;163:2306–2313. [PubMed] [Google Scholar]
- 70.Zhang L, et al. In vivo distribution of the human immunodeficiency virus/simian immunodeficiency virus coreceptors: CXCR4, CCR3, and CCR5. J Virol. 1998;72:5035–5045. doi: 10.1128/jvi.72.6.5035-5045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prakash M, Kapembwa MS, Gotch F, Patterson S. Higher levels of activation markers and chemokine receptors on T lymphocytes in the cervix than peripheral blood of normal healthy women. J Reprod Immunol. 2001;52:101–111. doi: 10.1016/s0165-0378(01)00114-0. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Z, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. The authors identified CD4+ T cells in the rhesus macaque genital mucosa as the predominant targets for SIV infection, and they noted that both activated and resting T cells propagate virus. [DOI] [PubMed] [Google Scholar]
- 73.Veazey RS, Marx PA, Lackner AA. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. J Infect Dis. 2003;187:769–776. doi: 10.1086/368386. [DOI] [PubMed] [Google Scholar]
- 74.Zhang ZQ, et al. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 2004;101:5640–5645. doi: 10.1073/pnas.0308425101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Picker LJ, Watkins DI. HIV pathogenesis: the first cut is the deepest. Nature Immunol. 2005;6:430–432. doi: 10.1038/ni0505-430. [DOI] [PubMed] [Google Scholar]
- 76.Tuttle DL, Harrison JK, Anders C, Sleasman JW, Goodenow MM. Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. J Virol. 1998;72:4962–4969. doi: 10.1128/jvi.72.6.4962-4969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meng G, et al. Lamina propria lymphocytes, not macrophages, express CCR5 and CXCR4 and are the likely target cell for human immunodeficiency virus type 1 in the intestinal mucosa. J Infect Dis. 2000;182:785–791. doi: 10.1086/315790. [DOI] [PubMed] [Google Scholar]
- 78.Saphire AC, Bobardt MD, Zhang Z, David G, Gallay PA. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J Virol. 2001;75:9187–9200. doi: 10.1128/JVI.75.19.9187-9200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marechal V, et al. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J Virol. 2001;75:11166–11177. doi: 10.1128/JVI.75.22.11166-11177.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharova N, Swingler C, Sharkey M, Stevenson M. Macrophages archive HIV-1 virions for dissemination in trans. EMBO J. 2005;24:2481–2489. doi: 10.1038/sj.emboj.7600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Groot F, Welsch S, Sattentau QJ. Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood. 2008 doi: 10.1182/blood-2007-12-130070. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 82.Harada H, Goto Y, Ohno T, Suzu S, Okada S. Proliferative activation up-regulates expression of CD4 and HIV-1 co-receptors on NK cells and induces their infection with HIV-1. Eur J Immunol. 2007;37:2148–2155. doi: 10.1002/eji.200737217. [DOI] [PubMed] [Google Scholar]
- 83.Rappocciolo G, et al. DC-SIGN on B lymphocytes is required for transmission of HIV-1 to T lymphocytes. PLoS Pathog. 2006;2:e70. doi: 10.1371/journal.ppat.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 85.Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nature Rev Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cameron PU, et al. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. This is the first study demonstrating HIV-1 transmission from DCs to CD4+ T cells. [DOI] [PubMed] [Google Scholar]
- 87.Pope M, et al. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. This investigation showed that DCs and T cells derived from human epithelium form stable conjugates which can enhance HIV replication. [DOI] [PubMed] [Google Scholar]
- 88.Geijtenbeek TB, et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 89.McDonald D, et al. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–1297. doi: 10.1126/science.1084238. The investigators introduce the concept of an infectious synapse between DCs and T cells to which HIV is recruited on the DC side and CD4 and CCR5 on the T cell side, thus promoting efficient in trans infection. [DOI] [PubMed] [Google Scholar]
- 90.Arrighi JF, et al. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J Exp Med. 2004;200:1279–1288. doi: 10.1084/jem.20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wiley RD, Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc Natl Acad Sci U S A. 2006;103:738–743. doi: 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jolly C, Sattentau QJ. Human immunodeficiency virus type 1 assembly, budding, and cell-cell spread in T cells take place in tetraspanin-enriched plasma membrane domains. J Virol. 2007;81:7873–7884. doi: 10.1128/JVI.01845-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jolly C, Sattentau QJ. Retroviral spread by induction of virological synapses. Traffic. 2004;5:643–650. doi: 10.1111/j.1600-0854.2004.00209.x. [DOI] [PubMed] [Google Scholar]
- 94.Sherer NM, et al. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nature Cell Biol. 2007;9:310–315. doi: 10.1038/ncb1544. Retroviruses are transmitted between cells not only across large-surface interfaces (infectious synapses) but also through movement along the outer surface of thin filopodial bridges that are extended from the non-infected to the infected cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hope TJ. Bridging efficient viral infection. Nature Cell Biol. 2007;9:243–244. doi: 10.1038/ncb0307-243. [DOI] [PubMed] [Google Scholar]
- 96.Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 97.Cavrois M, Neidleman J, Kreisberg JF, Greene WC. In Vitro Derived Dendritic Cells trans-Infect CD4 T Cells Primarily with Surface-Bound HIV-1 Virions. PLoS Pathog. 2007;3:e4. doi: 10.1371/journal.ppat.0030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kwon DS, Gregorio G, Bitton N, Hendrickson WA, Littman DR. DC-SIGN-mediated internalization of HIV is required for trans- enhancement of T cell infection. Immunity. 2002;16:135–144. doi: 10.1016/s1074-7613(02)00259-5. [DOI] [PubMed] [Google Scholar]
- 99.Granelli-Piperno A, Finkel V, Delgado E, Steinman RM. Virus replication begins in dendritic cells during the transmission of HIV-1 from mature dendritic cells to T cells. Curr Biol. 1999;9:21–29. doi: 10.1016/s0960-9822(99)80043-8. [DOI] [PubMed] [Google Scholar]
- 100.van Montfort T, Nabatov AA, Geijtenbeek TB, Pollakis G, Paxton WA. Efficient capture of antibody neutralized HIV-1 by cells expressing DC-SIGN and transfer to CD4+ T lymphocytes. J Immunol. 2007;178:3177–3185. doi: 10.4049/jimmunol.178.5.3177. [DOI] [PubMed] [Google Scholar]
- 101.Ganesh L, et al. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J Virol. 2004;78:11980–11987. doi: 10.1128/JVI.78.21.11980-11987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen P, Hubner W, Spinelli MA, Chen BK. Predominant mode of HIV transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J Virol. 2007 doi: 10.1128/JVI.00381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McCoombe SG, Short RV. Potential HIV-1 target cells in the human penis. AIDS. 2006;20:1491–1495. doi: 10.1097/01.aids.0000237364.11123.98. [DOI] [PubMed] [Google Scholar]
- 104.Patterson BK, et al. Susceptibility to human immunodeficiency virus-1 infection of human foreskin and cervical tissue grown in explant culture. Am J Pathol. 2002;161:867–873. doi: 10.1016/S0002-9440(10)64247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Donoval BA, et al. HIV-1 target cells in foreskins of African men with varying histories of sexually transmitted infections. Am J Clin Pathol. 2006;125:386–391. [PubMed] [Google Scholar]
- 106.Meier AS, Bukusi EA, Cohen CR, Holmes KK. Independent association of hygiene, socioeconomic status, and circumcision with reduced risk of HIV infection among Kenyan men. J Acquir Immune Defic Syndr. 2006;43:117–118. doi: 10.1097/01.qai.0000224973.60339.35. [DOI] [PubMed] [Google Scholar]
- 107.Gray RH, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 108.Quinn TC. Circumcision and HIV transmission. Curr Opin Infect Dis. 2007;20:33–38. doi: 10.1097/QCO.0b013e328012c5bc. [DOI] [PubMed] [Google Scholar]
- 109.Wawer MJ, et al. Rates of HIV-1 Transmission per Coital Act, by Stage of HIV-1 Infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 110.Pudney J, Anderson DJ. Immunobiology of the human penile urethra. Am J Pathol. 1995;147:155–165. [PMC free article] [PubMed] [Google Scholar]
- 111.McClure CP, et al. HIV coreceptor and chemokine ligand gene expression in the male urethra and female cervix. AIDS. 2005;19:1257–1265. doi: 10.1097/01.aids.0000180096.50393.96. [DOI] [PubMed] [Google Scholar]
- 112.Miller CJ, et al. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J Virol. 1989;63:4277–4284. doi: 10.1128/jvi.63.10.4277-4284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cohen MS, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 114.Krieger JN, et al. Vasectomy and human immunodeficiency virus type 1 in semen. J Urol. 1998;159:820–825. discussion 825–826. [PubMed] [Google Scholar]
- 115.Pudney J, Oneta M, Mayer K, Seage G, 3rd, Anderson D. Pre-ejaculatory fluid as potential vector for sexual transmission of HIV-1. Lancet. 1992;340:1470. doi: 10.1016/0140-6736(92)92659-4. [DOI] [PubMed] [Google Scholar]
- 116.Ilaria G, et al. Detection of HIV-1 DNA sequences in pre-ejaculatory fluid. Lancet. 1992;340:1469. doi: 10.1016/0140-6736(92)92658-3. [DOI] [PubMed] [Google Scholar]
- 117.Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nature Immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 118.Wang X, et al. Massive infection and loss of CD4+ T cells occurs in the intestinal tract of neonatal rhesus macaques in acute SIV infection. Blood. 2007;109:1174–1181. doi: 10.1182/blood-2006-04-015172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mattapallil JJ, et al. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 120.Mehandru S, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nature Rev Immunol. 2005;5:783–792. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 122.CDC HIV/AIDS Fact Sheet. Centers for Diseases Control and Prevention; Atlanta: 2007. HIV/AIDS among men who have sex with men. [Google Scholar]
- 123.The Global HIV/AIDS pandemic. Morb Mort Wkly Rep. 2006;55:841–844. [PubMed] [Google Scholar]
- 124.Page-Shafer K, et al. Risk of HIV infection attributable to oral sex among men who have sex with men and in the population of men who have sex with men. AIDS. 2002;16:2350–2352. doi: 10.1097/00002030-200211220-00022. [DOI] [PubMed] [Google Scholar]
- 125.Aceijas C, Stimson GV, Hickman M, Rhodes T. Global overview of injecting drug use and HIV infection among injecting drug users. AIDS. 2004;18:2295–2303. doi: 10.1097/00002030-200411190-00010. [DOI] [PubMed] [Google Scholar]
- 126.Lehman DA, Farquhar C. Biological mechanisms of vertical human immunodeficiency virus (HIV-1) transmission. Rev Med Virol. 2007:381–403. doi: 10.1002/rmv.543. [DOI] [PubMed] [Google Scholar]
- 127.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nature Rev Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 128.Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 129.Halperin DT. Heterosexual anal intercourse: prevalence, cultural factors, and HIV infection and other health risks, Part I. Aids Patient Care STDS. 1999;13:717–730. doi: 10.1089/apc.1999.13.717. [DOI] [PubMed] [Google Scholar]
- 130.Sowinski S, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nature Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]