Abstract
A variety of dicarboxylic acid linkers introduced between the α-amino group of Pro6 and the ε-amino group of Lys10 of the cyclic lactam α-melanocyte-stimulating hormone (α-MSH)-derived Pro6-D-Phe7/D-Nal(2′)7-Arg8-Trp9-Lys10-NH2 pentapeptide template lead to nanomolar range and selective hMC3R agonists and antagonists. Replacement of the Pro6 residue and the dicarboxylic acid linker with 2,3-pyrazine-dicarboxylic acid furnished a highly selective nanomolar range hMC3R partial agonist (analogue 12, c[CO-2,3-pyrazine-CO-D-Phe-Arg-Trp-Lys]-NH2, EC50 = 27 nM, 70% max cAMP) and an hMC3R antagonist (analogue 13, c[CO-2,3-pyrazine-CO-D-Nal(2′)-Arg-Trp-Lys]-NH2, IC50 = 23 nM). Modeling experiments suggest that 2,3-pyrazinedicarboxylic acid stabilizes a β-turn-like structure with the D-Phe/D-Nal(2′) residues, which explains the high potency of the corresponding peptides. Placement of a Nle residue in position 6 produced a hMC3R/hMC5R antagonist (analogue 15, c[CO-(CH2)2-CO-Nle-D-Nal(2′)-Arg-Trp-Lys]-NH2, IC50 = 12 and 17 nM, respectively), similarly to the previously described cyclic γ-melanocyte-stimulating hormone (γ-MSH)-derived hMC3R/hMC5R antagonists. These newly developed melanotropins will serve as critical biochemical tools for elucidating the full spectrum of functions performed by the physiologically important melanocortin-3 receptor.
Introduction
The five known subtypes of human melanocortin receptors (hMC1–5R) are members of the superfamily of seven trans-membrane G-protein-coupled receptors (GPCRs) expressed in various tissues, including skin (hMC1R),1–4 the adrenal cortex (hMC2R),5,6 and throughout the central nervous system (hMC3R, hMC4R, hMC5R).7 The melanocortin system has received much attention in recent years due to its involvement in a large number of important physiological functions, such as skin pigmentation,1–3 control of the immune system,1–4 erectile function,8–12 blood pressure and heart rate,13,14 control of feeding behavior and energy homeostasis,15–21 modulation of aggressive/defensive behavior,22,23 and mediation of pain.24,25 The endogenous melanocortin agonists include α-, β-, and γ-melanocyte-stimulating hormones (MSH) and adrenocorticotropin (ACTH), while Agouti-signaling and Agouti-related proteins have been identified as the endogenous antagonists.26,27
A considerable effort has been made toward the develop-mentofhighlypotenthMC4R-selectiveagonistsandantagonists28–51 due to the involvement of this receptor in the regulation of feeding15–21 and sexual behavior.8–12 At the same time, comparatively little attention has been given to obtaining selective ligands for the hMC3R28–31,52–55 and the hMC5R,30,31,54–62 owing to the dearth of specific evidence on their physiological functions.21 However, recent reports have demonstrated that inactivation of the mouse MC3R leads to increased fat mass, reduced lean mass, and higher feed efficiency than their wild type littermates.63,64 Furthermore, peripheral injections of the hMC3R selective agonist 152 (Figure 1) can stimulate food intake in mice,65 suggesting an important role of this receptor subtype in the regulation of feeding and energy partitioning. In addition, possible involvement of the hMC3R in the regulation of inflammatory responses and cardiovascular function has also been proposed.66 Finally, the emerging evidence points to a potential role of the hMC3R in regulation of erectile function and sexual behavior, which provides further impetus for the development of highly selective hMC3R agonists and antagonists.67 The hMC5R, on the other hand, is found in a variety of peripheral tissues and plays a role in regulating exocrine gland function68 and coordinating central and peripheral signals for aggression.22,23
Figure 1.
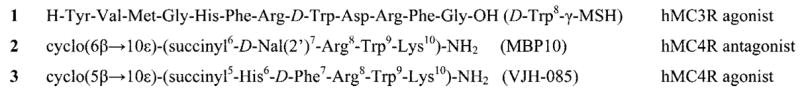
Some leading hMC3R- and hMC4R-selective melanotropin peptide ligands.
Several approaches to the design of hMC3R-selective agonists and antagonists have been described in the literature.69 Among the natural melanocyte-stimulating hormones, γ-MSH exhibits substantial hMC3R selectivity, whereas α-MSH and β-MSH show little selectivity for any specific receptor subtype.52,53 A D-amino acid scan of the γ-MSH sequence revealed the importance of position 8 in hMC3R selectivity and led to the discovery of a highly selective hMC3R agonist 1.52 Structure–activity relationships of γ-MSH have yielded linear peptide analogues with enhanced potency and selectivity, most notably, the nonselective superagonist Ac-Tyr-Val-Nle-Gly His-D-Phe-Arg-Trp-Asp-Arg-Phe-Gly-NH2 (Ac-NDP-γ-MSH-NH2)53 and a potent hMC3R/hMC5R antagonist and hMC4R agonist H-Tyr-Val-Nle-Gly His-D-Nal(2′)-Arg-Trp-Asp-Arg-Phe-Gly NH2 (PB-II-94).31 Recently, our laboratories have produced several potent and selective hMC3R agonists and hMC3R/hMC5R antagonists by placing a bulky hydrophobic Nle residue next to the melanocortin pharmacophore Xaa-Phe-Arg-Trp in a cyclic γ-MSH-derived template.54 Some cyclic α-MSH templates have also been described, where increased selectivity in hMC3R agonists and antagonists was observed. Thus, Kavarana et al.28 have found that enhancing the hydrophobic properties of the cyclic α-MSH analogues, increasing the peptide macrocycle size, resulted in improved hMC3R selectivity. Furthermore, Grieco et al.29 have shown that certain dihedrally constrained amino acid substitutions at position 6 of Ac-Nle4-c[Asp5, D-Nal(2′)7, Lys10]α-MSH(4–10)-NH2 (SHU9119)70 led to potent and highly hMC3R- and hMC4R-selective antagonists. Balse-Srinivasan et al. have reported a series of cyclic disulfide α-MSH/β-MSH hybrid peptides with highly selective hMC3R (Ac-c[Pen-Glu-His-D-Nal(2′)-Arg-Trp-Cys]-Pro-Pro-Lys-Asp-NH2) and hMC5R (Ac-c[Cys-Glu-His-D-Phe-Arg-Trp-D-Cys]-Pro-Pro-Lys-Asp-NH2) antagonists.55
Described herein are a series of novel cyclic α-MSH analogues possessing a variety of constrained linkers introduced between the α-amino group of D-Phe7/D-Nal(2′)7 and the ε-amino group of Lys10 of the cyclic lactam α-MSH-derived D-Phe7/D-Nal(2′)7-Arg8-Trp9-Lys10-NH2 tetrapeptide template, which have been designed and synthesized by solid-phase methods to further pursue SAR trends leading to hMC3R selectivity.
Peptide Design
Ac-Nle4-c[Asp5, D-Phe7, Lys10]α-MSH(4–10)-NH2 (MT-II), a superpotent but nonselective human melano-cortin receptor agonist,71 along with the potent nonselective hMC3R/hMC4R antagonist SHU9119,70 were chosen as templates for the design of more selective melanotropin peptides. Conformationally constrained linkers, including o-phthaloyl-prolyl, glutaryl-prolyl, maleyl-prolyl, 2,6-pyridinyl, 2,3-pyrazi-nyl, and succinyl-norleucinyl, were introduced between the α-amino group of D-phenylalanine or D-(2′)naphthylalanine and the ε-amino group of lysine to achieve higher receptor selectivity (Figure 2). Earlier SAR studies on MT-II and SHU9119-based cyclic α-MSH analogues revealed that Pro6 substitution did not lead to a significant discrimination between the hMC3R and the hMC4R.72 Furthermore, Kavarana et al. has reported that such a substitution in a cyclo(5β → 10ε)-[succinyl5-His6-D-Phe7-Arg8-Trp9-Lys10]-NH2 α-MSH template resulted in a considerable decline in binding affinities as well as agonist activity.28 This study was aimed at further optimization of the conformationally constrained cyclic Pro6-α-MSH template toward achieving higher receptor selectivity. In addition, the effects of steric factors on hMC3R selectivity were also investigated by placing a bulky hydrophobic Nle residue into position 6 of this cyclic α-MSH template. The sequences of the peptides discussed in this report, as well as their physicochemical properties, are summarized in Table 1.
Figure 2.
Design of the α-MSH-derived cyclic lactam scaffold.
Table 1.
Sequences and the Physicochemical Properties of the Cyclic α-MSH Analogues
|
m/z (M + 1)
|
TLC Rfb |
||||||
|---|---|---|---|---|---|---|---|
| No. | Sequence | calcd | obsd (ESI) | HPLC retention time, mina | 1 | 2 | 3 |
| 4 | c[CO-o-C6H4-CO-Pro-D-Phe-Arg-Trp-Lys]-NH2 | 862.4364 | 862.4345 | 17.27 | 0.68 | 0.45 | 0.75 |
| 5 | c[CO-o-C6H4-CO-Pro-D-Nal(2′)-Arg-Trp-Lys]-NH2 | 912.4521 | 912.4536 | 19.64 | 0.69 | 0.46 | 0.78 |
| 6 | c[CO-(CH2)3-CO-Pro-D-Phe-Arg-Trp-Lys]-NH2 | 828.4521 | 828.4544 | 16.97 | 0.61 | 0.35 | 0.72 |
| 7 | c[CO-(CH2)3-CO-Pro-D-Nal(2′)-Arg-Trp-Lys]-NH2 | 878.4677 | 878.464 | 19.41 | 0.64 | 0.38 | 0.74 |
| 8 | c[CO-cis-CH=CH-CO-Pro-D-Phe-Arg-Trp-Lys]-NH2 | 812.4208 | 812.4179 | 17.05 | 0.64 | 0.38 | 0.74 |
| 9 | c[CO-cis-CH=CH-CO-Pro-D-Nal(2′)-Arg-Trp-Lys]-NH2 | 862.4364 | 862.4397 | 18.43 | 0.66 | 0.41 | 0.76 |
| 10 | c[CO-2,6-pyridine-CO-D-Phe-Arg-Trp-Lys]-NH2 | 766.3789 | 766.3821 | 16.45 | 0.65 | 0.52 | 0.77 |
| 11 | c[CO-2,6-pyridine-CO-D-Nal(2′)-Arg-Trp-Lys]-NH2 | 816.3946 | 816.393 | 19.10 | 0.67 | 0.49 | 0.78 |
| 12 | c[CO-2,3-pyrazine-CO-D-Phe-Arg-Trp-Lys]-NH2 | 767.3742 | 767.3763 | 16.24 | 0.62 | 0.4 | 0.73 |
| 13 | c[CO-2,3-pyrazine-CO-D-Nal(2′)-Arg-Trp-Lys]-NH2 | 817.3898 | 817.3906 | 19.02 | 0.65 | 0.42 | 0.76 |
| 14 | c[CO-(CH2)2-CO-Nle-D-Phe-Arg-Trp-Lys]-NH2 | 830.4677 | 830.4704 | 18.80 | 0.68 | 0.45 | 0.78 |
| 15 | c[CO-(CH2)2-CO-Nle-D-Nal(2′)-Arg-Trp-Lys]-NH2 | 880.4834 | 880.4806 | 21.35 | 0.69 | 0.47 | 0.78 |
HPLC column: Vydac 218TP104, 250 × 4.6 mm, 10 μm, 300 Å; HPLC solvent A, 0.1% TFA in water; solvent B, acetonitrile; gradient: 10–90% B in A over 40 min, flow rate 1.0 mL/min.
TLC system 1: n-butanol/acetic acid/water/pyridine (4:1:2:1); TLC system 2: n-butanol/acetic acid/water (4:1:1); TLC system 3: ethyl acetate/acetic acid/water/pyridine (5:1:3:5).
Results and Discussion
Table 2 summarizes the binding affinities and the in vitro biological activities of the cyclic α-MSH analogues. Analogue 4 was found to have modest binding affinities to the hMC3R and hMC4R and exhibited full agonist activity at the hMC3R (EC50 = 95 nM, 100% cAMP stimulation), while being evidently inactive at the hMC1R and hMC5R. D-Nal(2′)7 substitution (analogue 5) resulted in improved binding affinities at all four receptor subtypes and converted a full hMC3R agonist (analogue 4) to a good affinity hMC3R antagonist (IC50 = 32 nM). Interestingly, these results are in contrast with the findings previously reported by Kavarana et al.,28 where structurally similar His6 α-MSH analogues exhibited little or no selectivity between the hMC3R and the hMC4R. It seems plausible that increased conformational constraint within this cyclic peptide template brought about by the Pro6 substitution is responsible for the enhanced hMC3R selectivity. Replacement of the phthalic acid linker with the glutaric acid linker produced analogues 6 and 7, which followed the same trend toward a potent and hMC3R-selective agonist (EC50 = 29 nM) and antagonist (IC50 = 11 nM, pA2 = 9.8), respectively. Comparison of the three-dimensional structures of analogue 6 and MT-II73 revealed a good fit between their secondary structures (Table 3, 7Figure 3), which may explain the high agonist potency of this peptide. Pharmacology of the analogue , on the other hand, seems entirely in parallel with that of structurally similar α-MSH analogue cyclo(5γ → 10ε)-(glutaryl5-His6-D-Nal(2′)7-Arg8-Trp9-Lys10)-NH2 (MK-9), which has been reported to be a highly potent hMC3R antagonist (Ki = 5.9 nM, pA2 = 10.6), with a fair selectivity against the hMC4R and the hMC5R.28 These peptides show an improved antagonist potency and selectivity toward the hMC3R compared to the parent peptide, the nonselective hMC3R/hMC4R antagonist SHU9119 (IC50 = 3.3 and 1.8 nM,70 pA2 = 8.7 and 9.1,29 respectively). Analogues 8 and 9 were obtained by employing a maleic acid linker, which provides a similar conformational constraint to the phthalic acid linker but is less sterically demanding. Analogue 8 showed no activity at the hMC1R, partial agonist activity at the hMC3R (EC50 = 100 nM, 80% cAMP stimulation), and a modest binding affinity at the hMC4R (IC50 = 160 nM) and the hMC5R (IC50 = 790 nM). The D-Nal(2′)7-substituted analogue 9 displayed a reversal of the hMC3R agonist activity to antagonist activity (IC50 = 20 nM), whereas the binding affinities at the hMC4R and hMC5R did not show significant change (IC50 = 520 and 120 nM, respectively). Overall, the Pro6 cyclic α-MSH analogues exhibited higher receptor selectivity, favoring the hMC3R, than the corresponding His6 peptides.28
Table 2.
Binding Affinities and cAMP Activities of Cyclic α-MSH Analogues at hMCRsa
| hMC1R
|
hMC3R
|
hMC4R
|
hMC5R
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | sequence | IC50, nM | EC50, nM | % max effect | IC50, nM | EC50, nM | % max effect | IC50, nM | EC50, nM | % max effect | IC50, nM | EC50, nM | % max |
| MT-II | Ac-Nle-c[Asp-His-D-Phe-Arg-Trp-Lys]-NH2 | 0.2 ± 0.01 | 0.3 ± 0.04 | 100 | 1.25 ± 0.2 | 1.85 ± 0.2 | 100 | 1.07 ± 0.3 | 2.87 ± 0.52 | 100 | 7.47 ± 0.23 | 3.3 ± 0.7 | 100 |
| 4 | c[CO-o-C6H4-CO-Pro-D-Phe-Arg-Trp-Lys]-NH2 | >10000 | NA | 0 | >1000 | 95 ± 11 | 100 | 777 ± 200 | NA | 0 | >1000 | NA | 0 |
| 5 | c[CO-o-C6H4-CO-Pro-D-Nal(2′)-Arg-Trp-Lys]-NH2 | 410 ± 80 | >5000 | 80 | 32 ± 4 | NA | 0 | 430 ± 100 | NA | 0 | 210 ± 30 | NA | 0 |
| 6 | c[CO-(CH2)3-CO-Pro-D-Phe-Arg-Trp-Lys]-NH2 | >10000 | NA | 0 | 385 ± 50 | 29 ± 3 | 100 | >1000 | >2000 | 12 | 870 ± 100 | 570 ± 60 | 40 |
| 7 | c[CO-(CH2)3-CO-Pro-D-Nal(2′)-Arg-Trp-Lys]-NH2 | >10000 | NA | 0 | 11 ± 2 pA2 = 9.8 | NA | 0 | 330 ± 40 | NA | 0 | 27 ± 3 | 23 ± 7 | 40 |
| 8 | c[CO-cis-CH=CH-CO-Pro-D-Phe-Arg-Trp-Lys]-NH2 | >10000 | NA | 0 | >1000 | 100 ± 20 | 80 | 160 ± 20 | NA | 0 | 790 ± 100 | NA | 0 |
| 9 | c[CO-cis-CH=CH-CO-Pro-D-Nal(2′)-Arg-Trp-Lys]-NH2 | >10000 | NA | 0 | 20 ± 3 | NA | 0 | 520 ± 60 | NA | 0 | 120 ± 20 | NA | 0 |
| 10 | c[CO-2,6-pyridine-CO-D-Phe-Arg-Trp-Lys]-NH2 | >10000 | >1000 | 15 | >10000 | NA | 0 | >10000 | NA | 0 | >10000 | NA | 0 |
| 11 | c[CO-2,6-pyridine-CO-D-Nal(2′)-Arg-Trp-Lys]-NH2 | >10000 | NA | 0 | >1000 | NA | 0 | >1000 | NA | 0 | >10000 | NA | 0 |
| 12 | c[CO-2,3-pyrazine-CO-D-Phe-Arg-Trp-Lys]-NH2 | 412 ± 50 | >10000 | 60 | 1100 ± 120 | 27 ± 3 | 70 | >10000 | NA | 0 | >10000 | NA | 0 |
| 13 | c[CO-2,3-pyrazine-CO-D-Nal(2′)-Arg-Trp-Lys]-NH2 | 707 ± 101 | >3000 | 55 | 23 ± 5 | NA | 0 | >1000 | NA | 0 | 230 ± 40 | NA | 0 |
| 14 | c[CO-(CH2)2-CO-Nle-D-Phe-Arg-Trp-Lys]-NH2 | >10000 | NA | 0 | 84 ± 10 | NA | 0 | 930 ± 133 | NA | 0 | 520 ± 70 | >1000 | 40 |
| 15 | c[CO-(CH2)2-CO-Nle-D-Nal(2′)-Arg-Trp-Lys]-NH2 | 2000 ± 200 | >1000 | 20 | 12 ± 3 pA2 = 8.3 | NA | 0 | 300 ± 100 | NA | 0 | 17 ± 2 pA2 = 8.7 | NA | 0 |
IC50 = concentration of peptide at 50% specific binding (N = 4). EC50 = effective concentration of peptide that was able to generate 50% maximal intracellular cAMP accumulation (N = 4). % max effect = % of cAMP produced at 10 μM ligand concentration, in relation to MT-II. NA = 0% cAMP accumulation observed at 10 μM. The peptides were tested at a range of concentration from 10−10 to 10−5 M.
Table 3.
Backbone Torsion Angles (°) for the Global Minima of Selected Cyclic α-MSH Analogues Based on MCMM/LMCS-OPLS2005 Calculations Compared with the NMR Structures of MT-II and SHU911973
| Xaa6 |
D-Phe/D-Nal(2′)7 |
Arg8 |
Trp9 |
Lys10 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Φ | Ψ | Φ | Ψ | Φ | Ψ | Φ | Ψ | Φ | Ψ |
| 6 | −73 | 110 | 74 | 9 | −89 | 158 | −59 | −17 | −83 | −14 |
| 7 | −78 | 72 | 74 | 15 | −76 | 105 | −75 | 144 | −145 | 22 |
| 12 | 86 | 0 | −100 | 178 | −52 | −22 | −77 | −15 | ||
| 13 | 76 | 6 | −96 | 154 | −70 | 126 | −151 | 23 | ||
| 14 | −139 | 30 | 91 | −22 | −90 | 118 | −83 | 149 | −140 | 18 |
| 15 | 72 | 18 | 82 | 9 | −83 | 118 | −79 | 129 | −98 | −4 |
| MT-II | −108 | 109 | 84 | 0 | −122 | 90 | −77 | 108 | −101 | 103 |
| SHU9119 | −90 | 49 | 82 | −6 | −99 | 117 | −79 | 111 | −90 | −68 |
Figure 3.
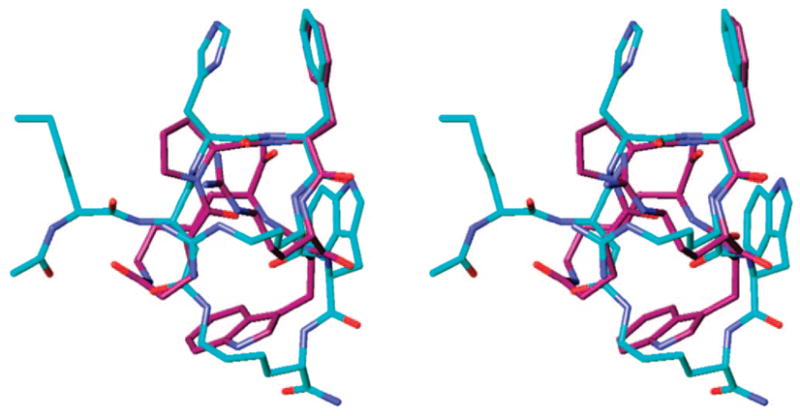
Stereoview of the superimposed global minimum of analogue 6 (purple), obtained by MCMM/LMCS (Monte Carlo Multiple Minima-Low Frequency Mode)-OPLS 2005 simulation with the NMR-derived structure of nonselective super agonist MT-II (blue; rmsd = 1.86 Å, nonhydrogen backbone atoms of the Xaa-D-Phe-Arg-Trp pharmacophore only). Hydrogens are omitted for clarity.
Bednarek et al. have previously reported that deletion of His6 from the sequence of the structurally related cyclic α-MSH analogues results in enhanced receptor selectivity, most notably, yielding a hMC4R selective antagonist 2.34 It was suggested in that report that the tetrapeptide core His-D-Nal(2′)-Arg-Trp was required for high binding affinity toward the hMC3R and hMC5R, whereas a shorter tripeptide core D-Nal(2′)-Arg-Trp was sufficient for high binding affinity toward the hMC4R. To further test this hypothesis, we have used heterocyclic dicarboxylic acid linkers to constrain the tripeptide sequence D-Phe/D-Nal(2′)-Arg-Trp, and the biological activities of the resulting peptides were determined. Analogues 10 and 11, which employed a 2,6-pyridinedicarboxylic acid linker, were found to be inactive at all the receptor subtypes (hMC1, 3–5R). Analogues 12 and 13 contained the 2,3-pyrazinedicarboxylic acid linker, which was structurally better suited to mimic a β-turn motif (Table 3, Figure 4), suggested to be important for melanotropin bioactivities.73 As was revealed by biological evaluation of analogue 12, it showed a weak partial agonist activity at the hMC1R and a potent partial agonist activity at the hMC3R (EC50 = 27 nM, 70% cAMP stimulation), while exhibiting no activity at the hMC4R and hMC5R. Furthermore, analogue 13 retained a weak partial agonist activity at the hMC1R and a marginal binding affinity to the hMC4R. On the other hand, this peptide was found to be a potent hMC3R antagonist (IC50 = 23 nM) and showed a modest hMC5R binding affinity (IC50 = 230 nM). It is evident from our results that, contrary to the earlier hypotheses,34 the tripeptide sequence D-Phe/D-Nal(2′)-Arg-Trp is sufficient for high binding affinity and agonist activity not only at the hMC4R but also at the hMC3R. The observed receptor selectivity in both of these cases seems to be strongly affected by the nature of the linker, including its conformational, steric, and electrostatic properties, which is evident from the remarkable similarity between the 3D conformational structures of peptides 2 and 13, as illustrated by Figure 5. Thus, manipulation of the linker structure is proving to be a powerful tool in the development of highly selective melanotropin peptides.
Figure 4.
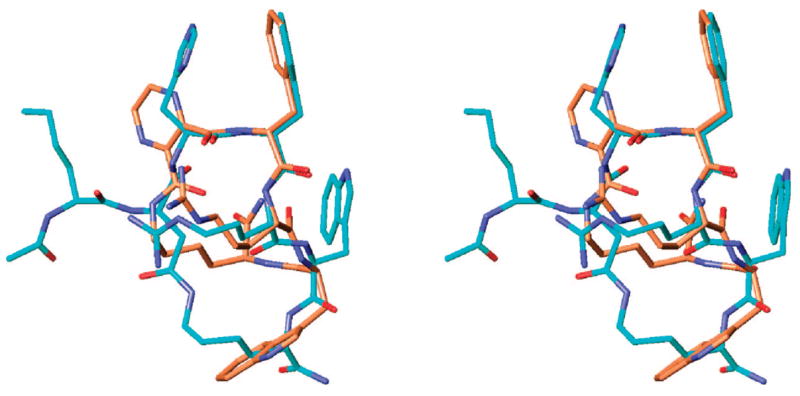
Stereoview of the superimposed global minimum of analogue 12 (gold), obtained by MCMM/LMCS (Monte Carlo Multiple Minima-Low Frequency Mode)-OPLS 2005 simulation with the NMR-derived structure of nonselective super agonist MT-II (blue; rmsd = 1.35 Å, nonhydrogen backbone atoms of the Xaa-D-Phe-Arg-Trp pharmacophore only). Hydrogens are omitted for clarity.
Figure 5.
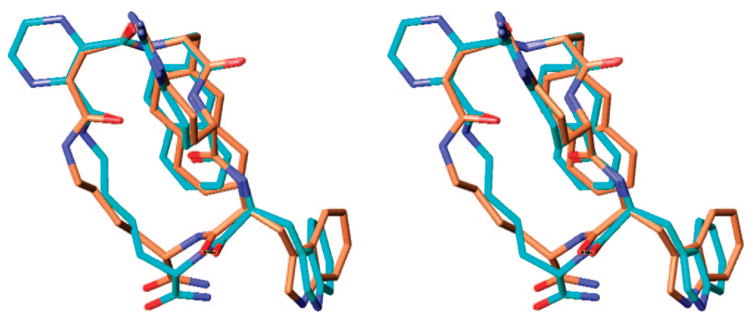
Stereoview of the superimposed global minimum of analogue 13 (blue) with the global minimum of selective hMC4R antagonist 2 (gold), obtained by MCMM/LMCS (Monte Carlo Multiple Minima-Low Frequency Mode)-OPLS 2005 simulations (rmsd = 0.43 Å, nonhydrogen backbone atoms of the Xaa-D-Phe-Arg-Trp pharmacoph-ore only). Hydrogens are omitted for clarity.
Our recent report described the influence of the steric hindrance of the Nle4 residue in cyclic γ-MSH analogues on the hMC3R receptor selectivity.54 To further test the importance of steric factors in the melanocortin receptor selectivity of cyclic α-MSH analogues, a norleucine residue was introduced into position 6 of the hMC4R-selective agonist 3.28 This His6 → Nle6 substitution led to the conversion of the hMC3R/hMC4R agonist 3 (EC50 = 70 and 1.6 nM, respectively) to the hMC3R/hMC4R antagonist (analogue 14, IC50 = 84 and 930 nM, respectively). Interestingly, while the hMC3R binding affinity was largely retained, the drastic loss of the hMC4R binding affinity, as well as agonist activity, provides additional evidence for our hypothesis that steric hindrance of the melanocortin pharmacophore has a strong effect on the receptor–ligand interaction. Furthermore, the D-Nal(2′)7 analogue 15 showed a weak partial agonist activity at the hMC1R and antagonist activities at the hMC3–5R (IC50 = 12 nM, 300 nM and 17 nM, respectively; pA2 = 8.3 (hMC3R) and 8.7 (hMC5R)). This peptide demonstrated a hMC3R/hMC5R antagonist activity trend similar to one observed in cyclic Nle4, D-Nal(2′)6-γ-MSH analogues.54 Figure 6 illustrates the three-dimensional structure of analogue 15, featuring hydrophobic interactions between the side chains of Nle6 and the side chains of Arg8, which may result in partial steric hindrance of the binding space of Arg8, analogous to the structural features of the cyclic Nle4-γ-MSH analogues.54 These results are remarkably consistent with the recent findings by Ballet et al.,74 who reported that placing a conformationally constrained and sterically demanding 4-amino-1,2,4,5-tetrahy-dro-2-benzazepin-3-one (Aba) building block in position 6 of the MT-II/SHU9119 cyclic lactam template produced a good affinity and very selective hMC3R antagonist Ac-Nle-c[Asp-Aba-D-Phe-Arg-Trp-Lys]-NH2 (IC50 = 50 nM) and a hMC3R/hMC5R antagonist Ac-Nle-c[Asp-Aba-D-Nal(2′)-Arg-Trp-Lys]-NH2 (IC50 = 43 and 87 nM, respectively). These findings provide additional support for our hypothesis of the importance of steric factors in hMC3R selectivity.
Figure 6.
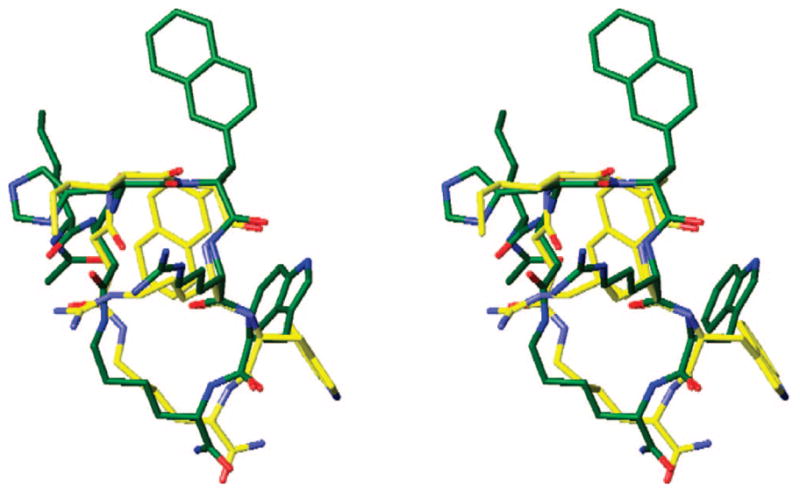
Stereoview of the superimposed global minimum of analogue 15 (yellow), obtained by MCMM/LMCS (Monte Carlo Multiple Minima-Low Frequency Mode)-OPLS 2005 simulation with the NMR-derived structure of hMC3R/hMC4R antagonist SHU9119 (green; rmsd = 0.39 Å, nonhydrogen backbone atoms of the Xaa-D-Nal(2′)-Arg-Trp pharmacophore only). Hydrogens are omitted for clarity.
In summary, the structure–activity relationships of the cyclic lactam α-MSH analogues were established to evaluate the multiple factors that contribute to the melanocortin receptor selectivity and are illustrated by the Figures 7 and 8. In particular, Figure 7 describes the agonist activities of the D-Phe7 analogues, whereas Figure 8 compares the binding affinities of the D-Nal(2′)7 analogues, thus highlighting the trend toward hMC3R selectivity. The Pro6 template yielded several selective hMC3R agonists and antagonists, whereas employment of heterocyclic linkers and deletion of residue 6 from the sequence resulted in substantially augmented hMC3R selectivity. Finally, Nle6 substitution produced a potent hMC3R/hMC5R antagonist, consistent with our earlier findings.54,74
Figure 7.
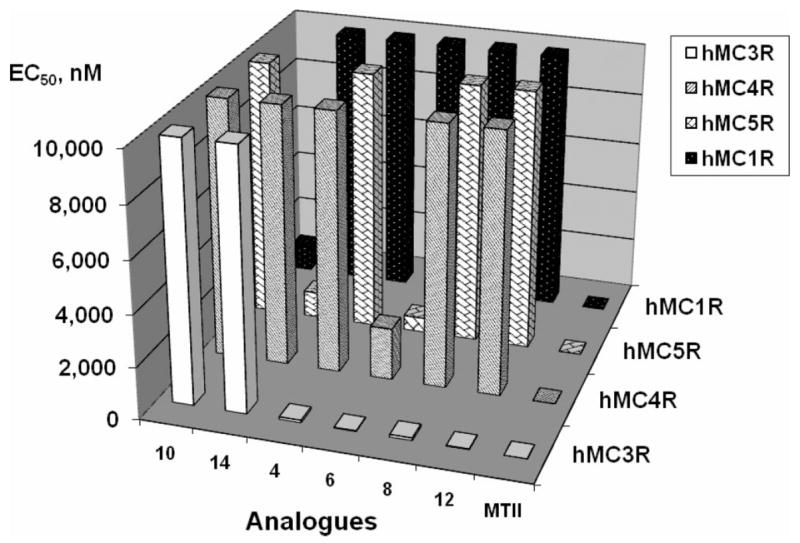
Graphical summary of the agonist activities, expressed in EC50 values (Z axis), of the D-Phe7 analogues (X axis) at the four human melanocortin receptor subtypes (Y axis).
Figure 8.
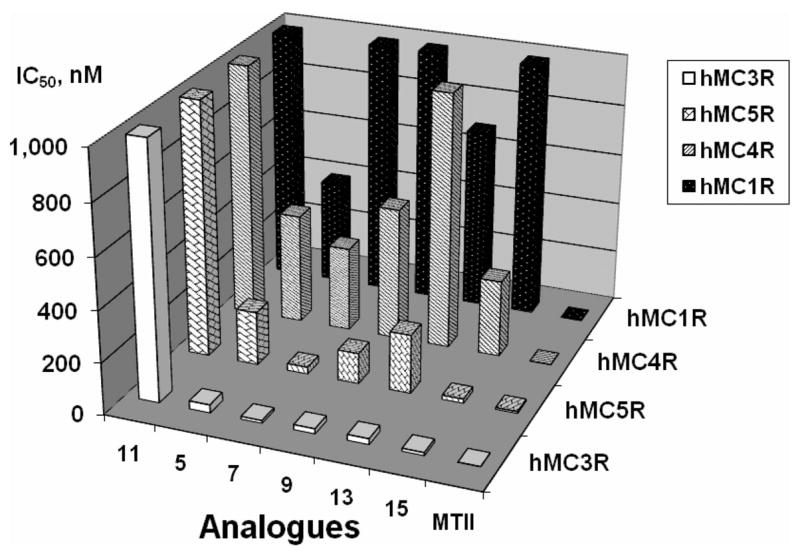
Graphical summary of the binding affinities, expressed in IC50 values (Z axis), of the D-Nal(2′)7 analogues (X axis) at the four human melanocortin receptor subtypes (Y axis).
Conclusions
Our SAR studies of cyclic α-MSH analogues have identified new selective hMC3R agonists (analogues 6 and 12) and hMC3R/hMC5R antagonists (analogues 13 and 15). Molecular modeling experiments suggested that in the Pro6 series the observed hMC3R selectivity is brought about by the increased rigidity of the template. It is noteworthy that replacement of an aliphatic linker in the hMC4R selective antagonist 2 with a heterocyclic pyrazine linker led to a high affinity hMC3R antagonist with good selectivity against the hMC4R (analogue 13). Such a drastic conversion of an hMC4R antagonist into an hMC3R antagonist underlines the importance of the conformational, steric and electrostatic properties of the linker for the melanocortin receptor selectivity. Steric factors were found to be particularly prominent in the Nle6 substitution that produced a potent hMC3R/hMC5R antagonist (analogue 15, pA2 = 8.3 (hMC3R) and 8.7 (hMC5R)). These newly developed melanotropin peptides will facilitate the elucidation of the physiological roles of the hMC3R and the hMC4R in feeding behavior, obesity, sexual dysfunction, and related disorders.
Experimental Section
Materials
Nα-Fmoc-amino acids, peptide coupling reagents, and Rink amide AM resin were obtained from Novabiochem (San Diego, CA), except Nα-Fmoc-Lys(Alloc)-OH, which was purchased from NeoMPS (San Diego, CA). The following side chain protecting groups were used: Trp(Nin-Boc), Arg(Nε-Pbf), and Lys(Nε-Alloc). ACS grade organic solvents were purchased from VWR Scientific (West Chester, PA) and other reagents were obtained from Sigma-Aldrich (St. Louis, MO) and used as commercially available. The polypropylene reaction vessels (syringes with frits) were purchased from Torviq (Niles, MI). The purity of the peptides was checked by analytical reverse-phase HPLC using a Vydac C18 218TP104 column (Western Analytical Products, Murrieta, CA), monitored at 230 and 254 nm, and by thin-layer chromatography (TLC), which was performed using three different solvent systems. Analytical thin-layer chromatography (TLC) was carried out on 0.25 mm glass-backed silica gel 60 F254 plates (EM Science 5715, VWR Scientific). The TLC chromatograms were visualized by UV light and by dipping in potassium permanganate solution followed by heating (hot plate).
Peptide Synthesis
All peptides in this study were synthesized manually by the Nα-Fmoc solid phase methodology,54,58 using bromophenol blue pH indicator to monitor the extent of coupling reactions, as described by Krchnak et al.75,76 Rink amide AM resin (4-(2′,4′-dimethoxyphenyl-Fmoc-aminomethyl)phenoxy resin, 0.5 g, 0.637 mmol/g) was placed into a 50 mL polypropylene syringe with the frit on the bottom and swollen in DMF (20 mL) for 1 h. The Fmoc protecting group on the Rink linker was removed by 25% piperidine in DMF (1 × 5 min and 1 × 15 min). The resin was washed with DMF (4 × 15 mL), then washed with 0.02 M HOBt solution in DMF, stained with 0.05 mM solution of Bromophenol Blue in 0.02 M HOBt/DMF solution, and washed with 0.02 M HOBt/DMF solution (4 × 15 mL). The first Nα-Fmoc amino acid was coupled using preactivated ester (3 equiv of Nα-Fmoc amino acid, 3 equiv of HOBt, and 3 equiv of DIC) in DMF. The coupling mixture was transferred into the syringe with the resin and shaken for 60 min, at which point the blue color of the resin changed to yellow, indicating complete coupling. The resin was washed with DMF (3 × 15 mL) and thrice with DCM (3 × 15 mL), the unreacted amino groups were capped using acetic anhydride (2 mL) and pyridine (2 mL) in DCM (15 mL) for 30 min, and the resin was once again washed with DMF (6 × 15 mL). The peptide sequences were completed by consecutively coupling the appropriate amino acids and then the dicarboxylic acid linkers using the procedure described above. Pyrazinedicarboxylic and succinic acids were converted into their corresponding monoallyl esters prior to appending to the peptides to minimize competing formation of cyclic imides, as previously described.28,58 The other dicarboxylic acids were used as commercially available. The orthogonal allylic protection for the side chain of Lys11 and the linker (if applicable) was removed with 0.1 equiv Pd(PPh3)4/20 equiv PhSiH3 in DCM (2 × 30 min) prior to the peptide cyclization.58,77 The deprotected resin-bound peptide was washed with DCM (6 × 5 mL) and DMF (3 × 5 mL). The peptide cyclizations were accomplished as described previously,54 with 6 equiv DIC, and 6 equiv Cl-HOBt in THF (36 h),78 and were monitored by Kaiser ninhydrin test.79 The DIC/Cl-HOBt treatment was repeated until a negative Kaiser test was obtained. Upon completion of cyclization the resin was treated with 5% solution of sodium diethyldithiocarbamate trihydrate in DMF (20 min) to remove any remaining traces of the Pd catalyst,54 then washed with DMF (5 × 15 mL), DCM (3 × 15 mL), methanol (5 × 15 mL), and diethyl ether (5 × 15 mL), and dried under reduced pressure (16 h). The cyclized peptides were cleaved off the solid support with 82.5% v/v TFA, 5% water, 5% thioanisol, 2.5% 1,2-ethanedithiol, and 5% phenol (5 mL, 3 h), and the crude peptides were precipitated out by the addition of a chilled 3:1 mixture of diethyl ether and petroleum ether (50 mL) to give white precipitates. The resulting peptide suspensions were centrifuged for 10 min at 6500 rpm, and the liquid was decanted. The crude peptides were washed with diethyl ether (4 × 50 mL), and after the final centrifugation, the peptides were dried under vacuum (2 h). The resulting white residues were dissolved in 2 M acetic acid, and the insoluble impurities were removed, by passing the solutions through Gelman Laboratory Acrodisc 13 mm syringe filters with 0.45 μM PTFE membranes (Pall Corporation, East Hills, NY). The clear filtrates were lyophilized, the obtained white powders (50–80 mg) were redissolved in glacial acetic acid (1 mL), and the resulting solutions were diluted with water (4 mL) to a peptide concentration of about 10–15 mg/mL and passed through a Sephadex G-15 column (520 × 30 mm) using 1 M aqueous acetic acid as the eluent. Fractions containing the target peptides, as determined by TLC, were combined and lyophilized. Final purification was accomplished by preparative RP-HPLC on a C18-bonded silica column (Vydac 218TP152022, 250 × 22 mm, 15–20 μm, 300 Å) using a Shimadzu SCL-10A HPLC system. The peptides were eluted with a linear gradient of 20–80% acetonitrile in 0.1% aqueous TFA solution over 50 min with 10 mL/min flow rate. The purified peptides were isolated in 25–30% overall yield. The structures of the pure peptides were confirmed by 1H NMR in DMSO-d6 and by high resolution electrospray ionization (ESI) mass-spectrometry using an IonSpec Fourier transform mass spectrometer with a HiRes ESI source.
Biological Activity Assays. Receptor Binding Assay
Competition binding experiments were carried out using whole HEK293 cells stably expressing human MC1, MC3, MC4, and MC5 receptors. HEK293 cells transfected with hMCRs57,80,81 were seeded on 96-well plates 48 h before assay (50000 cells/well). For the assay, the cell culture medium was aspirated, and the cells were washed once with a freshly prepared MEM buffer containing 100% minimum essential medium with Earle’s salt (MEM, GIBCO) and 25 mM sodium bicarbonate. Next, the cells were incubated for 40 min at 37 °C with different concentrations of unlabeled peptide and labeled [125I]-[Nle4, D-Phe7]-α-MSH (Perkin-Elmer Life Science, 20000 cpm/well, 0.14 nM) diluted in a 125 μL of freshly prepared binding buffer containing 100% MEM, 25 mM HEPES (pH 7.4), 0.2% bovine serum albumin, 1 mM 1,10-phenanthrolone, 0.5 mg/L leupeptin, and 200 mg/L bacitracin. The assay medium was subsequently removed and the cells were washed once with basic medium and then lysed by the addition of 100 μL of 0.1 M NaOH and 100 μL of 1% Triton X-100. The lysed cells were transferred to 12 × 75 mm borosilicate glass tubes, and the radioactivity was measured by a Wallac 1470 WIZARD Gamma Counter.
Adenylate Cyclase Assay
HEK 293 cells transfected with human melanocortin receptors57 were grown to confluence in MEM medium (GIBCO) containing 10% fetal bovine serum, 100 units/mL penicillin and streptomycin, and 1 mM sodium pyruvate. The cells were seeded on 96-well plates 48 h before assay (50000 cells/well). For the assay, the cell culture medium was removed and the cells were rinsed with 100 μL of MEM buffer (GIBCO). An aliquot (100 μL) of the Earle’s balanced salt solution with 0.5 mM isobutylmethylxanthine (IBMX) was placed in each well along for 1 min at 37 °C. Next, aliquots (25 μL) of melanotropin peptides of varying concentration were added, and the cells were incubated for 3 min at 37 °C. The reaction was stopped by aspirating the assay buffer and adding 60 μL of ice-cold Tris/EDTA buffer to each well, then placing the plates in a boiling water bath for 7 min. The cell lysates were then centrifuged for 10 min at 2300 × g. A 50 μL aliquot of the supernatant was transferred to another 96-well plate and placed with 50 μL of [3H] cAMP and 100 μL of protein kinase A (PKA) buffer in an ice bath for 2–3 h. The PKA buffer consisted of Tris/EDTA buffer with 60 μg/mL PKA and 0.1% bovine serum albumin by weight. The incubation mixture was filtered through 1.0 μm glass fiber filters in MultiScreen™-FB 96-well plates (Millipore, Billerica, MA). The total [3H] cAMP was measured by a Wallac MicroBeta TriLux 1450 LSC and Luminescence Counter (PerkinElmer Life Science, Boston, MA) The cAMP accumulation data for each peptide analogue was determined with the help of a cAMP standard curve generated by the same method as described above. The maximal cAMP produced at 10 μM concentration of each ligand was compared to the amount of cAMP produced at 10 μM concentration of the standard agonist MT-II and is expressed in percent (as % max effect) in Table 2. The antagonist properties of the lead compounds were evaluated by their ability to competitively displace the MT-II agonist in a dose-dependent manner at up to 10 μM. The pA2 values were obtained using the Schild analysis method.82
Data Analysis
IC50 and EC50 values represent the mean of two experiments performed in triplicate. IC50 and EC50 estimates and their associated standard errors were determined by fitting the data using a nonlinear least-squares analysis, with the help of GraphPad Prism 4 (GraphPad Software, San Diego, CA).
Computational Procedures
Molecular modeling experiments employed MacroModel version 9.1 equipped with Maestro 7.5 graphical interface (Schrödinger, LLC, New York, NY, 2005), installed on a Linux Red Hat 9.0 system, and were performed as previously described.54 Peptide structures were built into extended structures with standard bond lengths and angles, and they were minimized using the OPLS 2005 force field83 and the Polak-Ribier conjugate gradient (PRCG). Optimizations were converged to a gradient rmsd less that 0.05 kJ/Å mol or continued until a limit of 50000 iterations was reached. Aqueous solution conditions were simulated using the continuum dielectric water solvent model (GB/SA).84 Extended cutoff distances were defined at 8 Å for Van der Waals, 20 Å for electrostatics, and 4 Å for H-bonds.
Conformational profiles of the cyclic peptides were investigated by the hybrid Monte Carlo/Low Frequency Mode (MCMM/LMCS)85 procedure, as implemented in Macromodel using the energy minimization parameters, as described above. MCMM torsional variations and Low Mode parameters were set up automatically within Maestro graphical user interface. A total of 20000 search steps were performed and the conformations with an energy difference of 50 kJ/mol from the global minimum were saved. Interatomic dihedral angles were measured for each peptide analogue using the Maestro graphical user interface and they are described in Table 3. The superimpositions of peptide structures were performed using the α-carbons of the core sequence Xaa-DPhe/D-Nal(2′)-Arg-Trp.
Acknowledgments
This research was supported by grants from the U.S. Public Health Service, National Institutes of Health, DK-17420 and DA-06284. The opinions expressed are those of the authors and not necessarily those of the USPHS.
Footnotes
Abbreviations used for amino acids and designation of peptides follow the rules of the IUPAC-IUB Commission of Biochemical Nomenclature in J. Biol. Chem 1972, 247, 977–983. The following additional abbreviations are used: All, allyl; Alloc, allyloxycarbonyl; Boc, tert-butyloxycarbonyl; Fmoc, fluorenylmethoxycarbonyl; CH3CN, acetonitrile; Cl-HOBt, 1-hydroxy-6-chlorobenzotriazole; DCM, dichloromethane; DIPEA, diisopropylethylamine; DMF, N,N-dimethylformamide; DIC, diisopropyl carbodiimide; HOBt, N-hydroxybenzotriazole; hMCR, human melanocortin receptor; MSH, melanocyte-stimulating hormone; Nal(2′), 2′-naphthylalanine; Pbf, 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl; TFA, trifluoroacetic acid; SPPS, solid-phase peptide synthesis; RP-HPLC, reverse-phase high performance liquid chromatography; hMC3R, human melanocortin-3 receptor; α-MSH, Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly Lys-Pro-Val-NH2; NDP-α-MSH, Ac-Ser-Tyr-Ser-Nle-Glu-His-D-Phe-Arg-Trp-Gly Lys-Pro-Val-NH2.
Supporting Information Available: 1H NMR spectra of peptide analogues 4–15 in DMSO-d6 and the list of chemical shifts and coupling constants. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hadley ME, editor. The Melanotropic Peptides. I–III CRC Press; Boca Raton, FL: 1988. [Google Scholar]
- 2.Cone RD. The Melanocortin System. Ann NY Acad Sci. 2003;994:1–387. [Google Scholar]
- 3.Cone RD, editor. The Melanocortin Receptors. Humana Press; Totowa, NJ: 2000. [Google Scholar]
- 4.Vaudry H, Eberle AN. The Melanotropic Peptides. Ann NY Acad Sci. 1993;680:1–687. [Google Scholar]
- 5.Gispen WH, Isaacson RL. ACTH-Induced Excessive Grooming in the Rat. Pharmacol Ther. 1981;12:209–246. doi: 10.1016/0163-7258(81)90081-4. [DOI] [PubMed] [Google Scholar]
- 6.Gantz I, Fong TM. The Melanocortin System. Am J Physiol Endocrinol Metab. 2003;284:E468–E474. doi: 10.1152/ajpendo.00434.2002. [DOI] [PubMed] [Google Scholar]
- 7.Chhajlani B. Distribution of cDNA for Melanocortin Receptor Subtypes in Human Tissues. Biochem Mol Biol Int. 1996;38:73–80. [PubMed] [Google Scholar]
- 8.Wessells H, Gralnek D, Dorr R, Hruby VJ, Hadley ME, Levine N. Effect of an α-Melanocyte Stimulating Hormone Analogue on Penile Erection and Sexual Desire in Men with Organic Erectile Dysfunction. Urology. 2000;56:641–646. doi: 10.1016/s0090-4295(00)00680-4. [DOI] [PubMed] [Google Scholar]
- 9.Wessells H, Fuciarelli K, Hansen J, Hadley ME, Hruby VJ, Dorr R, Levine N. Synthetic Melanotropic Peptide Initiates Erections in Men with Psychogenic Erectile Dysfunction: Double-Blind Placebo Controlled Crossover Study. J Urol. 1998;160:389–393. [PubMed] [Google Scholar]
- 10.Bertolini A, Vergoni W, Gessa GL, Ferrari W. Induction of Sexual Excitement by Action of Adrenocorticotropic Hormone in Brain. Nature. 1969;221:667–669. doi: 10.1038/221667a0. [DOI] [PubMed] [Google Scholar]
- 11.Bertolini A, Vergoni W, Gessa GL, Ferrari W. Erection and Ejaculation: A Central Effect of ACTH-Like Peptides in Mammals. In: Sandler M, Gessa GL, editors. Sexual Behavior: Pharmacology and Biochemistry. Raven Press; New York: 1975. pp. 247–257. [Google Scholar]
- 12.Van der Ploeg LHT, Martin WJ, Howard AD, Nargund RP, Austin CP, Guan X, Drisko J, Cashen D, Sebhat I, Patchett AA, Figueroa DJ, DiLella AG, Connolly BM, Weinberg DH, Tan CP, Palyha OC, Pong S-S, MacNeil T, Rosenblum C, Vongs A, Tang R, Yu H, Sailer AW, Fong TM, Huang C, Tota MR, Chang RS, Stearns R, Tamvakopoulos C, Christ G, Drazen DL, Spar BD, Nelson RJ, MacIntyre DE. A Role for the Melanocortin 4 Receptor in Sexual Function. Proc Natl Acad Sci USA. 2002;99:11381–11386. doi: 10.1073/pnas.172378699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li SJ, Varga K, Archer P, Hruby VJ, Sharma SD, Kesterson RA, Cone RD, Kunos G. Melanocortin Antagonists Define Two Distinct Pathways of Cardiovascular Control by α- and γ-Melanocyte Stimulating Hormones. J Neurosci. 1996;16:5182–5188. doi: 10.1523/JNEUROSCI.16-16-05182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni XP, Butler AA, Cone RD, Humphreys MH. Central Receptors Mediating the Cardiovascular Actions of Melanocyte Stimulating Hormones. J Hypertens. 2006;24:2239–2246. doi: 10.1097/01.hjh.0000249702.49854.fa. [DOI] [PubMed] [Google Scholar]
- 15.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of the Melanocortinergic Neurons in Feeding and the Agouti Obesity Syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 16.Vergoni AV, Poggioloi R, Bertolini A. Corticotropin Inhibits Food Intake in Rats. Neuropeptides. 1986;7:153–158. doi: 10.1016/0143-4179(86)90091-0. [DOI] [PubMed] [Google Scholar]
- 17.Vergoni AV, Poggioloi R, Marrama D, Bertolini A. Inhibition of Feeding by ACTH-(1–24): Behavioral and Pharmacological Aspects. Eur J Pharmacol. 1990;179:347–355. doi: 10.1016/0014-2999(90)90175-6. [DOI] [PubMed] [Google Scholar]
- 18.Ramos EJB, Meguid MM, Campos ACL, Coelho JCU. Neuropeptide Y, α-Melanocyte-Stimulating Hormone, and Monoamines in Food Intake Regulation. Nutrition. 2005;21:269–279. doi: 10.1016/j.nut.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Yang YK, Harmon CM. Recent Developments in Our Understanding of Melanocortin System in the Regulation of Food Intake. Obes Rev. 2003;4:239–248. doi: 10.1046/j.1467-789x.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- 20.Ellacott KLJ, Cone RD. The Central Melanocortin System and the Integration of Short- and Long-term Regulators of Energy Homeostasis. Recent Prog Horm Res. 2004;59:395–408. doi: 10.1210/rp.59.1.395. [DOI] [PubMed] [Google Scholar]
- 21.Zimanyi IA, Pelleymounter MA. The Role of Melanocortin Peptides and Receptors in Regulation of Energy Balance. Curr Pharm Des. 2003;9:627–641. doi: 10.2174/1381612033391234. [DOI] [PubMed] [Google Scholar]
- 22.Morgan C, Thomas RE, Cone RD. Melanocortin-5 Receptor Deficiency Promotes Defensive Behavior in Male Mice. Horm Behav. 2004;45:58–63. doi: 10.1016/j.yhbeh.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Morgan C, Thomas RE, Ma W, Novotny MV, Cone RD. Melanocortin-5 Receptor Deficiency Reduces a Pheromonal Signal for Aggression in Male Mice. Chem Senses. 2004;29:111–115. doi: 10.1093/chemse/bjh011. [DOI] [PubMed] [Google Scholar]
- 24.Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KVS, Lariviere WR, Groce MK, Wallace MR, Kaplan L, Staud R, Ness TJ, Glover TL, Stankova M, Mayorov AV, Hruby VJ, Grisel JE, Fillingim RB. The Melanocortin-1 Receptor Gene Mediates Female-Specific Mechanisms of Analgesia in Mice And Humans. Proc Natl Acad Sci US A. 2003;100:4867–4872. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vrinten DH, Kalkman CJ, Adan RAH, Gispen WH. Neuropathic Pain: A Possible Role for the Melanocortin System. Eur J Pharmacol. 2001;429:61–69. doi: 10.1016/s0014-2999(01)01306-1. [DOI] [PubMed] [Google Scholar]
- 26.Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik RP, Wilkison WO, Cone RD. Agouti Protein Is an Antagonist of the Melanocyte-Stimulating Hormone Receptor. Nature. 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 27.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Agouti-Related Protein Is an Endogenous Antagonist of the Melanocortin-4 Receptor In Vitro and In Vivo. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 28.Kavarana MJ, Trivedi D, Cai M, Ying J, Hammer M, Cabello C, Grieco P, Han G, Hruby VJ. Novel Cyclic Templates of α-MSH Give Highly Selective and Potent Antagonists/Agonists for Human Melanocortin-3/4 Receptors. J Med Chem. 2002;45:2644–2650. doi: 10.1021/jm020021z. [DOI] [PubMed] [Google Scholar]
- 29.Grieco P, Lavecchia A, Cai M, Trivedi D, Weinberg D, MacNeil T, Van der Ploeg LHT, Hruby VJ. Structure–Activity Studies of the Melanocortin Peptides: Discovery of Potent and Selective Affinity Antagonists for the hMC3 and hMC4 Receptors. J Med Chem. 2002;45:5287–5294. doi: 10.1021/jm0202526. [DOI] [PubMed] [Google Scholar]
- 30.Cai M, Mayorov AV, Ying J, Stankova M, Trivedi D, Cabello C, Hruby VJ. Design of Novel Melanotropin Agonists and Antagonists With High Potency and Selectivity for Human Melanocortin Receptors. Peptides. 2005;26:1481–1485. doi: 10.1016/j.peptides.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Balse-Srinivasan P, Grieco P, Cai M, Trivedi D, Hruby VJ. Structure–Activity Relationships of γ-MSH Analogues at the Human Melanocortin MC3, MC4, and MC5 Receptors. Discovery of Highly Selective hMC3R, hMC4R, and hMC5R Analogues. J Med Chem. 2003;46:4965–4973. doi: 10.1021/jm030119t. [DOI] [PubMed] [Google Scholar]
- 32.Nijenhuis WAJ, Kruijtzer JAW, Wanders N, Vrinten DH, Garner KM, Schaaper WMM, Meloen RH, Gispen WH, Liskamp RM, Adana RAH. Discovery and In Vivo Evaluation of New Melanocortin-4 Receptor-Selective Peptides. Peptides. 2003;24:271–280. doi: 10.1016/s0196-9781(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 33.Bednarek MA, MacNeil T, Tang R, Kalyani RN, Van der Ploeg LHT, Weinberg DH. Potent and Selective Peptide Agonists of α-Melanotropin Action at Human Melanocortin Receptor 4: Their Synthesis and Biological Evaluation In Vitro. Biochem Biophys Res Commun. 2001;286:641–645. doi: 10.1006/bbrc.2001.5444. [DOI] [PubMed] [Google Scholar]
- 34.Bednarek MA, MacNeil T, Kalyani RN, Tang R, Van der Ploeg LHT, Weinberg DH. Selective, High Affinity Peptide Antagonists of α-Melanotropin Action at Human Melanocortin Receptor 4: Their Synthesis and Biological Evaluation In Vitro. J Med Chem. 2001;44:3665–3672. doi: 10.1021/jm010165y. [DOI] [PubMed] [Google Scholar]
- 35.Yang YK, Fong TM, Dickinson CJ, Mao C, Li JY, Tota MR, Mosley R, Van der Ploeg LHT, Gantz I. Molecular Determinants of Ligand Binding to the Human Melanocortin-4 Receptor. Biochemistry. 2000;39:14900–14911. doi: 10.1021/bi001684q. [DOI] [PubMed] [Google Scholar]
- 36.Ying J, Gu X, Cai M, Dedek M, Vagner J, Trivedi D, Hruby VJ. Design, Synthesis, and Biological Evaluation of New Cyclic Melanotropin Peptide Analogues Selective for the Human Melanocortin-4 Receptor. J Med Chem. 2006;49:6888–6896. doi: 10.1021/jm060768f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiöth HB, Mutulis F, Muceniece R, Prusis P, Wikberg JES. Discovery of Novel Melanocortin-4 Receptor Selective MSH Analogues. Br J Pharmacol. 1998;124:75–82. doi: 10.1038/sj.bjp.0701804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayer JP, Hsiung HM, Flora DB, Edwards P, Smith DP, Zhang XY, Gadski RA, Heiman ML, Hertel JL, Emmerson PJ, Husain S, O’Brien TP, Kahl SD, Smiley DL, Zhang L, DiMarchi RD, Yan LZ. Discovery of a β-MSH-Derived MC-4R Selective Agonist. J Med Chem. 2005;48:3095–3098. doi: 10.1021/jm0501432. [DOI] [PubMed] [Google Scholar]
- 39.Odagami T, Tsuda Y, Kogami Y, Koujia H, Okada Y. Design of Cyclic Peptides With Agonist Activity at Melanocortin Receptor-4. Bioorg Med Chem Lett. 2006;16:3723–3726. doi: 10.1016/j.bmcl.2006.04.050. [DOI] [PubMed] [Google Scholar]
- 40.Arasasingham PN, Fotsch C, Ouyang X, Norman MH, Kelly MG, Stark KL, Karbon B, Hale C, Baumgartner JW, Zambrano M, Cheetham J, Tamayo NA. Structure–Activity Relationship of (1-Aryl-2-piperazinylethyl)piperazines: Antagonists for the AGRP/Melanocortin Receptor Binding. J Med Chem. 2003;46:9–11. doi: 10.1021/jm0255522. [DOI] [PubMed] [Google Scholar]
- 41.Richardson TI, Ornstein PL, Briner K, Fisher MJ, Backer RT, Biggers CK, Clay MP, Emmerson PJ, Hertel LW, Hsiung HM, Husain S, Kahl SD, Lee JA, Lindstrom TD, Martinelli MJ, Mayer JP, Mullaney JT, O’Brien TP, Pawlak JM, Revell KD, Shah J, Zgombick JM, Herr RJ, Melekhov A, Sampson PB, King CHR. Synthesis and Structure–Activity Relationships of Novel Arylpiperazines as Potent and Selective Agonists of the Melanocortin Subtype-4 Receptor. J Med Chem. 2004;47:744–755. doi: 10.1021/jm0304109. [DOI] [PubMed] [Google Scholar]
- 42.Sebhat IK, Martin WJ, Ye Z, Barakat K, Mosley RT, Johnston DBR, Bakshi R, Palucki B, Weinberg DH, MacNeil T, Kalyani RN, Tang R, Stearns RA, Miller RR, Tamvakopoulos C, Strack AM, McGowan E, Cashen DE, Drisko JE, Hom GJ, Howard AD, MacIntyre DE, van der Ploeg LHT, Patchett AA, Nargund RP. Design and Pharmacology of N-[(3R)-1,2,3,4-Tetrahydroisoquinolinium-3-ylcarbonyl]-(1R)-1-(4-chlorobenzyl)-2-[4-cyclohexyl-4-(1H-1,2,4-triazol-1-ylmethyl)piperi-din-1-yl]-2-oxoethylamine (1), a Potent, Selective, Melanocortin Subtype-4 Receptor Agonist. J Med Chem. 2002;45:4589–4593. doi: 10.1021/jm025539h. [DOI] [PubMed] [Google Scholar]
- 43.Ruel R, Herpin TF, Iben L, Luo G, Martel A, Mason H, Mattson G, Poirier B, Ruediger EH, Shi D, Thibault C, Yu G, Zimanyi IA, Poindexter GS, Macor JE. β-Alanine Dipeptides as MC4R Agonists. Bioorg Med Chem Lett. 2003;13:4341–4344. doi: 10.1016/j.bmcl.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 44.Vos TJ, Caracoti A, Che JL, Dai M, Farrer CA, Forsyth NE, Drabic SV, Horlick RA, Lamppu D, Yowe DL, Balani S, Li P, Zeng H, Joseph IBJK, Rodriguez LE, Maguire MP, Patane MA, Claiborne CF. Identification of 2-{2-[2-(5-Bromo-2-methoxyphenyl)-ethyl]-3-fluorophenyl}-4,5-dihydro-1H-imidazole (ML00253764), a Small Molecule Melanocortin 4 Receptor Antagonist that Effectively Reduces Tumor-Induced Weight Loss in a Mouse Model. J Med Chem. 2004;47:1602–1604. doi: 10.1021/jm034244g. [DOI] [PubMed] [Google Scholar]
- 45.Hogan K, Peluso S, Gould S, Parsons I, Ryan D, Wu L, Visiers I. Mapping the Binding Site of Melanocortin 4 Receptor Agonists: A Hydrophobic Pocket Formed by I3.28(125), I3.32(129), and I7.42(291) is Critical for Receptor Activation. J Med Chem. 2006;49:911–922. doi: 10.1021/jm050780s. [DOI] [PubMed] [Google Scholar]
- 46.Chen C, Pontillo J, Fleck BA, Gao Y, Wen J, Tran JA, Tucci FC, Marinkovic D, Foster AC, Saunders J. 4-{(2R)-[3-Aminopropionylamido]-3-(2,4-dichlorophenyl)propionyl}-1-{2-[(2-thienyl)ethylaminomethyl]phenyl}piperazine as a Potent and Selective Melanocortin-4 Receptor Antagonists - Design, Synthesis, and Characterization. J Med Chem. 2004;47:6821–6830. doi: 10.1021/jm049278i. [DOI] [PubMed] [Google Scholar]
- 47.Tian X, Field TB, Switzer AG, Mazur AW, Ebetino FH, Wos JA, Berberich SM, Jayasinghe LR, Obringer CM, Dowty ME, Pinney BB, Farmer JA, Crossdoersen D, Sheldon RJ. Design, Synthesis, and Evaluation of Proline and Pyrrolidine Based Melanocortin Receptor Agonists. A Conformationally Restricted Dipeptide Mimic Approach. J Med Chem. 2006;49:4745–4761. doi: 10.1021/jm060384p. [DOI] [PubMed] [Google Scholar]
- 48.Sun H, Greeley DN, Chu XJ, Cheung A, Danho W, Swistok J, Wang Y, Zhao C, Chen L, Fry DC. A Predictive Pharmacophore Model of Human Melanocortin-4 Receptor As Derived From the Solution Structures of Cyclic Peptides. Bioorg Med Chem. 2004;12:2671–2677. doi: 10.1016/j.bmc.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 49.Yamano Y, Kamon R, Yoshimizu T, Toda Y, Oshida Y, Chaki S, Yoshioka M, Morishima I. The Role of the DRY Motif of Human MC4R for Receptor Activation. Biosci Biotechnol Biochem. 2004;68:1369–1371. doi: 10.1271/bbb.68.1369. [DOI] [PubMed] [Google Scholar]
- 50.Tucci FC, Tran JA, Jiang W, Pontillo J, Marinkovic D, Whit NS, Arellano M, Fleck BA, Wen J, Saunders J, Foster AC, Chen C. Synthesis of Piperazinephen-1-ylethylamines as Potent and Selective Antagonists of the Human Melanocortin-4 Receptor. Lett Drug Des Discovery. 2006;3:311–315. [Google Scholar]
- 51.Jiang W, Tucci FC, Chen CW, Arellano M, Tran JA, White NS, Marinkovic D, Pontillo J, Fleck BA, Wen J, Saunders J, Madan A, Foster AC, Chen C. Arylpropionylpiperazines as Antagonists of the Human Melanocortin-4 Receptor. Bioorg Med Chem Lett. 2006;16:4674–4678. doi: 10.1016/j.bmcl.2006.05.088. [DOI] [PubMed] [Google Scholar]
- 52.Grieco P, Balse PM, Weinberg D, MacNeil T, Hruby VJ. D-Amino Acid Scan of γ-Melanocyte-Stimulating Hormone: Importance of Trp8 on Human MC3 Receptor Selectivity. J Med Chem. 2000;43:4998–5002. doi: 10.1021/jm000211e. [DOI] [PubMed] [Google Scholar]
- 53.Cai M, Mayorov AV, Cabello C, Stankova M, Trivedi D, Hruby VJ. Novel 3D Pharmacophore of α-MSH/γ-MSH Hybrids Leads to Selective Human MC1R and MC3R Analogues. J Med Chem. 2005;48:1839–1848. doi: 10.1021/jm049579s. [DOI] [PubMed] [Google Scholar]
- 54.Mayorov AV, Cai M, Chandler KB, Petrov RR, Van Scoy AR, Yu Z, Tanaka DK, Trivedi D, Hruby VJ. Development of Cyclic γ-MSH Analogues with Selective hMC3R Agonist and hMC3R/hMC5R Antagonist Activities. J Med Chem. 2006;49:1946–1952. doi: 10.1021/jm0510326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balse-Srinivasan P, Grieco P, Cai M, Trivedi D, Hruby VJ. Structure–Activity Relationships of Novel Cyclic α-MSH/β-MSH Hybrid Analogues that Lead to Potent and Selective Ligands for the Human MC3R and Human MC5R. J Med Chem. 2003;46:3728–3733. doi: 10.1021/jm030111j. [DOI] [PubMed] [Google Scholar]
- 56.Chen C, Yu J, Fleck BA, Hoare SRJ, Saunders J, Foster AC. Phenylguanidines as Selective Nonpeptide Melanocortin-5 Receptor Antagonists. J Med Chem. 2004;47:4083–4088. doi: 10.1021/jm0400496. [DOI] [PubMed] [Google Scholar]
- 57.Cai M, Cai C, Mayorov AV, Xiong C, Cabello CM, Soloshonok VA, Swift JR, Trivedi D, Hruby VJ. Biological and Conformational Study of Substituted Prolines in MT-II Template: Steric Effects Leading to Human MC5 Receptor Selectivity. J Pept Res. 2004;63:116–131. doi: 10.1111/j.1399-3011.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 58.Mayorov AV, Han SY, Cai M, Hammer MR, Trivedi D, Hruby VJ. Effects of Macrocycle Size and Rigidity on Melanocortin Receptor-1 and -5 Selectivity in Cyclic Lactam α-MSH Analogues. Chem Biol Drug Des. 2006;67:329–335. doi: 10.1111/j.1747-0285.2006.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cain JP, Mayorov AV, Cai M, Wang H, Tan B, Chandler KB, Lee YS, Petrov RR, Trivedi D, Hruby VJ. Design, Synthesis, and Biological Evaluation of a New Class of Small Molecule Peptide Mimetics Targeting the Melanocortin Receptors. Bioorg Med Chem Lett. 2006;16:5462–5467. doi: 10.1016/j.bmcl.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bednarek MA, MacNeil T, Tang R, Fong TM, Cabello MA, Maroto M, Teran A. Potent and Selective Agonists of α-Melanotropin (αMSH) Action at Human Melanocortin Receptor 5; Linear Analogs of α-Melanotropin. Peptides. 2007;28:1020–1028. doi: 10.1016/j.peptides.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 61.Bednarek MA, MacNeil T, Tang R, Fong TM, Cabello MA, Maroto M, Teran A. Potent and Selective Peptide Agonists of α-Melanocyte Stimulating Hormone (αMSH) Action at Human Melanocortin Receptor 5; their Synthesis and Biological Evaluation In Vitro. Chem Biol Drug Des. 2007;69:350–355. doi: 10.1111/j.1747-0285.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 62.Bednarek MA, MacNeil T, Tang R, Fong TM, Cabello MA, Maroto M, Teran A. Potent and Selective Agonists of Human Melanocortin Receptor 5: Cyclic Analogues of α-Melanocyte-Stimulating Hormone. J Med Chem. 2007;50:2520–2526. doi: 10.1021/jm0614275. [DOI] [PubMed] [Google Scholar]
- 63.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pellymounter MA, Dekoning J, Baetscher M, Cone RD. A Unique Metabolic Syndrome Causes Obesity In The Melanocortin-3 Receptor-Deficient Mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 64.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao LH, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LHI. Inactivation of the Mouse Melanocortin-3 Receptor Results in Increased Fat Mass and Reduced Lean Body Mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 65.Marks DL, Hruby VJ, Brookhart G, Cone RD. The Regulation of Food Intake by Selective Stimulation of the Type 3 Melanocortin Receptor (MC3R) Peptides. 2006;27:259–264. doi: 10.1016/j.peptides.2005.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Getting SJ, Perretti M. MC3-R as a Novel Target for Anti-inflammatory Therapy. Drug News Perspect. 2000;13:19–27. [PubMed] [Google Scholar]
- 67.King SH, Mayorov AV, Balse-Srinivasan PVJ, Vanderah T, Wessells H. Melanocortin Receptors, Melanotropic Peptides, and Penile Erection. Curr Top Med Chem. 2007;7:1098–1106. [PMC free article] [PubMed] [Google Scholar]
- 68.Chen WB, Kelly MA, Opitz Araya X, Thomas RE, Low MJ, Cone RD. Exocrine Gland Dysfunction in MC5-R Deficient Mice: Evidence for Coordinated Regulation of Exocrine Gland Function by Melanocortin Peptides. Cell. 1997;91:789–798. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 69.Hruby VJ, Cai M, Cain JP, Mayorov AV, Dedek M, Trivedi D. Design, Synthesis and Biological Evaluation of Ligands Selective for the Melanocortin-3-Receptor. Curr Top Med Chem. 2007;7:1107–1119. doi: 10.2174/156802607780906645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hruby VJ, Lu D, Sharma SD, Castrucci A, de L, Kesterson RA, Al-Obeidi FA, Hadley ME, Cone RD. Cyclic Lactam Alpha-Melanotropin Analogues of Ac-Nle4-cyclo[Asp5, D-Phe7, Lys10] Alpha-Melanocyte-Stimulating Hormone-(4–10)-NH2 with Bulky Aromatic Amino Acids at Position 7 Show High Antagonist Potency and Selectivity at Specific Melanocortin Receptors. J Med Chem. 1995;38:3454–3461. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- 71.Al-Obeidi F, Hadley ME, Pettitt BM, Hruby VJ. Design of a New Class of Superpotent Cyclic α-Melanotropins Based on Quenched Dynamic Simulations. J Am Chem Soc. 1989;111:3413–3416. [Google Scholar]
- 72.Grieco P, Balse-Srinivasan P, Han G, Weinberg D, MacNeil T, Van der Ploeg LHT, Hruby VJ. Extensive Structure–Activity Studies of Lactam Derivatives of MT-II and SHU-9119: Their Activity and Selectivity at Human Melanocortin Receptors 3, 4, and 5. J Pept Res. 2003;62:199–206. doi: 10.1034/j.1399-3011.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 73.Ying J, Kövér KE, Gu X, Han G, Trivedi DB, Kavarana MJ, Hruby VJ. Solution Structures of Cyclic Melanocortin Agonists and Antagonists by NMR. Biopolymers Pept Sci. 2003;71:696–716. doi: 10.1002/bip.10596. [DOI] [PubMed] [Google Scholar]
- 74.Ballet S, Mayorov AV, Cai M, Tymecka D, Chandler KB, Palmer ES, Van Rompaey K, Misicka A, Tourwé D, Hruby VJ. Novel Selective Human Melanocortin-3 Receptor Ligands: Use of the 4-Amino-1,2,4,5-tetrahydro-2-benzazepin-3-one (Aba) Scaffold. Bioorg Med Chem Lett. 2007;17:2492–2498. doi: 10.1016/j.bmcl.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krchnak V, Vagner J, Safar P, Lebl M. Amino-Acids and Peptides. Part CCVI. Noninvasive Continuous Monitoring Of Solid-Phase Peptide-Synthesis by Acid-Base Indicator. Collect Czech Chem Commun. 1988;53:2542–2548. doi: 10.1111/j.1399-3011.1988.tb01276.x. [DOI] [PubMed] [Google Scholar]
- 76.Krchnak V, Vagner J. Color-Monitored Solid-Phase Multiple Peptide Synthesis under Low-Pressure Continuous-Flow Conditions. Pept Res. 1990;3:182–193. [PubMed] [Google Scholar]
- 77.Thieriet N, Alsina J, Giralt E, Guibé F, Albericio F. Use of Alloc-Amino Acids in Solid-Phase Peptide Synthesis. Tandem Deprotection-Coupling Reactions Using Neutral Conditions. Tetrahedron Lett. 1997;38:7275–7278. [Google Scholar]
- 78.Sabatino G, Mulinacci B, Alcaro MC, Chelli M, Rovero P, Papini AM. Assessment of New 6-Cl-HOBt Based Coupling Reagents for Peptide Synthesis. Part 1: Coupling Efficiency Study. Lett Pept Sci. 2003;9(2–3):119–123. [Google Scholar]
- 79.Kaiser E, Colescott RL, Bossinger CD, Cook PI. Color Test for Detection of Free Terminal Amino Groups in the Solid-Phase Synthesis of Peptides. Anal Biochem. 1970;34:595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- 80.Haskell-Luevano C, Miwa H, Dickinson C, Hruby VJ, Yamada T, Gantz I. Binding and cAMP Studies of Melanotropin Peptides with the Cloned Human Peripheral Melanocortin Receptor, hMC1R. Biochem Biophys Res Commun. 1994;204:1137–1142. doi: 10.1006/bbrc.1994.2581. [DOI] [PubMed] [Google Scholar]
- 81.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, Delvalle J, Yamada T. Molecular Cloning of a Novel Melanocortin Receptor. J Biol Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]
- 82.Schild HO. pA, a New Scale for the Measurement of Drug Antagonism. Br J Pharmacol. 1947;2:189–206. doi: 10.1111/j.1476-5381.1947.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaminski GA, Friesner RA, Tirado-Rives J, Jorgensen WL. Evaluation and Reparametrization of the OPLS-AA Force Field for Proteins via Comparison with Accurate Quantum Chemical Calculations on Peptides. J Phys Chem B. 2001;105:6474–6487. [Google Scholar]
- 84.Still WC, Tempczyk A, Hawlely RC, Hendrickson T. A General Treatment of Solvation for Molecular Mechanics. J Am Chem Soc. 1990;112:6127–6129. [Google Scholar]
- 85.Kolossváry I, Guida WC. Low-Mode Conformational Search Elucidated. Application to C39H80 and Flexible Docking of 9-Dea-zaguanine Inhibitors to PNP. J Comput Chem. 1999;20:1671. [Google Scholar]


