Abstract
The present work documents the first example of an enzyme-catalyzed β-elimination of a thioether from a sulfonium cysteine S-conjugate. β-(S-Tetrahydrothiophenium)-L-alanine (THT-A) is the cysteine S-conjugate of busulfan. THT-A slowly undergoes a non-enzymatic β-elimination reaction at pH 7.4 and 37°C to yield tetrahydrothiophene, pyruvate and ammonia. This reaction is accelerated by a) rat liver, kidney and brain homogenates, b) isolated rat liver mitochondria, and c) pyridoxal 5′-phosphate (PLP). A PLP-dependent enzyme in rat liver cytosol that catalyzes a β-lyase reaction with THT-A was identified as cystathionine γ-lyase. This unusual drug metabolism pathway represents an alternate route for intermediates in the mercapturate pathway.
Introduction
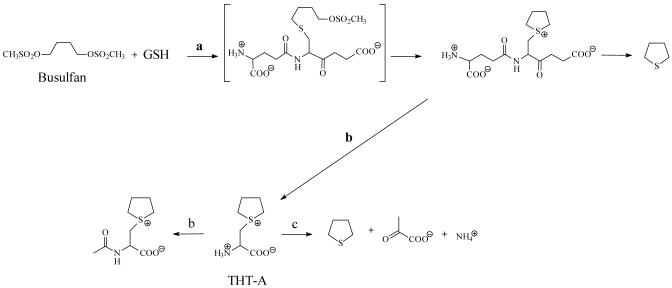
Busulfan is a bifunctional alkylating agent used for the treatment of hematological and other malignancies prior to stem cell transplantation (e.g. Iwamoto et al., 2004). Busulfan is converted to a glutathione S-conjugate (L-γ-glutamyl-β-(S-tetrahydrothiophenium)-L-alanylglycine) by direct interaction with glutathione (Ritter et al., 1999) and enzymatic catalysis by glutathione S-transferases (GSTs), especially GST A1-1 (Ritter et al., 1999, 2002; Czerwinski et al., 1996; Gibbs et al., 1996). The busulfan-glutathione adduct undergoes a base catalyzed β-elimination reaction yielding tetrahydrothiophene (Roberts and Warwick, 1961). Oxidation products of tetrahydrothiophene make up the majority of identified busulfan metabolites, although an enzymatic pathway leading to tetrahydrothiophene formation has not been elucidated.
A hypothetical pathway to tetrahydrothiophene from busulfan could involve intermediates in the mercapturate pathway that convert glutathione S-conjugates to cysteine S-conjugates via the action of γ-glutamyltransferase and dipeptidase/cysteinylglycinase (Meister, 1989). The mercapturate pathway of busulfan metabolism was shown to occur in rats by the detection of the sulfonium mercapturate, N-acetyl-β-(S-tetrahydrothiophenium)-L-alanine in the urine (Hassan and Ehrsson, 1987). Therefore, it is probable that the busulfan-glutathione adduct is converted in vivo to the corresponding cysteine S-conjugate [β-(S-tetrahydrothiophenium)-L-alanine, THT-A] by enzymes of the mercapturate pathway (Fig. 1). Theoretically, THT-A may also be formed non-enzymatically by the condensation of busulfan and cysteine.
Figure 1.
Proposed busulfan metabolism pathways a) Glutathione S-transferase, b) mercapturate pathway leading to N-acetylated metabolite with THT-A as an intermediate, c) β-elimination of tetrahydrothiophene from THT-A catalyzed by PLP-dependent enzymes including cystathionine γ-lyase.
Because THT-A contains a good leaving group (nucleofuge), it is reasonable to predict a facile β-elimination reaction to yield pyruvate, ammonium and tetrahydrothiophene. Although this reaction is likely to occur slowly non-enzymatically, we hypothesize that one or more cysteine S-conjugate β-lyases will accelerate the reaction. These lyases are pyridoxal 5′-phosphate (PLP)-dependent enzymes that catalyze β-elimination reactions with L-cysteine S-conjugates [RSCH2CH(NH3+)CO2-]. The enzyme-catalyzed reaction generates an RSH fragment and aminoacrylate [CH2=CH(NH3+)CO2-]. The aminoacrylate is an enamine that rapidly tautomerizes to the more stable ketimine tautomer, α-iminopropionate [CH3CH(=NH2+)CO2-], followed by hydrolysis to pyruvate [CH3C(O)CO2-] and ammonium. The net reaction is shown in eqn 1. [See Cooper and Pinto (2006) for a recent review].
| (1) |
Cystathionine γ-lyase (γ-cystathionase), although primarily a γ-lyase (Greenberg, 1962), also catalyzes β-elimination reactions. For example, rat liver cystathionine γ-lyase catalyzes a) the formation of S-mercapto-L-cysteine from L-cystine (Cavallini et al., 1960), b) β-elimination of alkane thiols from several cysteine S-conjugates containing non-halogenated alkyl groups attached to the sulfur (Tomisawa et al., 1988), and c) β-elimination of alkyl/allyl thiols/persulfides from various alkyl/allyl cysteine S-conjugates present in garlic extracts (Cooper and Pinto, 2005; Pinto et al., 2006). In the present study, we evaluated the ability of free (i.e. non-protein-bound) PLP, rat liver cystathionine γ-lyase and rat tissue homogenates to catalyze a β-elimination reaction with THT-A.
Materials and Methods
Reagents and enzymes
L-Homoserine, L-alanine, PLP, D,L-propargylglycine, Tris, 2,4-dinitrophenylhydrazine, NADH, ADP, bovine serum albumin, protease-inhibitor cocktail, busulfan, tetrahydrothiophene, and the sodium salts of pyruvate, α-ketoglutarate (KG) and α-keto-γ-methiolbutyrate (KMB) were obtained from Sigma-Aldrich Chemical Company (St. Louis, MO). OmniSol® acetonitrile was HPLC grade and was obtained from EM Science (Gibbstown, NJ). THT-A was synthesized by adapting previously described methods (Roberts and Warwick, 1961). The positive ion electrospray ionization mass spectrum (Finnigan LCQ mass spectrometer) showed a molecular species consistent with the compound mass of THT-A (m/z 176). MS/MS fragmentation gave a daughter ion consistent with the neutral loss of tetrahydrothiophene. The synthesized THT-A used in the experiments contained about 4% molar equivalent of pyruvate as determined by the 2,4-dinitrophenylhydrazone procedure and about 4% tetrahydrothiophene as determined by a CoulArray detection procedure (see below). Aqueous stock solutions of THT-A (50 mM) were stored at -20 °C. Some additional pyruvate formation was noted on storage of THT-A at -20 °C, but the rate was slow (∼1% conversion of THT-A to pyruvate per month). Stock solutions of tetrahydrothiophene (10 mM) were made immediately before use in 50% (v/v) acetonitrile/water. [2H8]-Busulfan obtained from Cambridge Isotopes (Andover, MA) was used as an internal standard for quantitative mass spectrometry analyses.
Highly purified cystathionine γ-lyase (specific activity, 2.4 U/mg; 1.32 U/mL) was isolated from rat liver cytosol by the method of Pinto et al. (2006) and stored frozen at -20°C. SDS-Polyacrylamide gel electrophoresis indicated a purity of >80%. The enzyme is stable in 10 mM potassium phosphate buffer (pH 7.4) at -20°C for at least a year and to repeated freeze-thawing. Bovine liver glutamate dehydrogenase (40 U/mg in 40% glycerol) was obtained from Boehringer Mannheim (Mannheim, Germany). Rat liver mitAspAT [1.35 mg/mL in 20 mM Tris-HCl buffer, pH 8.3, containing 0.1 mM EDTA, 150 mM NaCl and 0.2% (w/v) sodium azide; 410 U/mg of protein at 37 °C] was a generous gift from Dr. Ana Iriarte, University of Missouri-Kansas, Kansas City, MO. Rat kidney GTK [5 U/mg in 20% glycerol; 0.18 U/mL] was purified from the cytosolic fraction of rat kidneys as described previously (Cooper, 1978).
Enzyme assays
Cystathionine γ-lyase was assayed by a slight modification of the procedure of Cooper and Pinto (2005) in which α-ketobutyrate formed from L-homoserine was measured as the 2,4-dinitrophenylhydrazone. The standard reaction mixture (20 μL) contained 100 mM potassium phosphate buffer (pH 7.4), 20 mM L-homoserine and enzyme. The blank contained enzyme, but no L-homoserine. After incubation at 37 °C, the reaction was terminated by the addition of 10 μL of 5 mM 2,4-dinitrophenylhydrazine in 2 M HCl. After a further 10 min incubation, 170 μL of 1 M NaOH was added and the absorbance at 430 nm was read within 2 min of addition of alkali against a blank carried through the same procedure. The extinction coefficient of α-ketobutyrate 2,4-dinitrophenylhydrazone under these conditions is 15,000 M-1cm-1.
β-Lyase reactions with THT-A were measured in a reaction mixture (20 μL) containing 100 mM potassium phosphate buffer (pH 7.4), 5 mM THT-A and enzyme. After incubation at 37 °C the reaction was stopped by addition of 10 μL of 5 mM 2,4-dinitrophenylhydrazine in 2 M HCl. After an additional 10 min incubation, 170 μL of 1 M NaOH was added and the absorbance at 430 nm was read within 2 min. The extinction coefficient of pyruvate 2,4-dinitrophenylhydrazone under these conditions is 16,000 M-1cm-1. The blank contained no enzyme or enzyme source added just before addition of 2,4-dinitrophenylhydrazine reagent. A blank containing no enzyme took into account the small amount of pyruvate formed non-enzymatically from 5 mM THT-A. We cannot rule out the possibility that some β-lyase activity toward THT-A was lost as a result of the initial freezing of the tissue or mitochondrial homogenates. However, subsequent freeze thawing of rat liver cytosol and mitochondria had no significant effect on the β-lyase activity toward THT-A.
All spectrophotometric measurements were carried out with a SpectraMax 96-well plate spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). A unit of enzyme activity (U) is defined as the amount of enzyme that catalyzes the formation of 1 μmol of product per min at 37 °C.
Reaction of cysteine with busulfan
Busulfan (10 μg) in acetonitrile (50 μL) was added to cysteine (2.0 mg) in 4.9 mL of 100 mM potassium phosphate buffer (pH 7.4). The mixture was vortexed for one min and incubated at 37°C. Aliquots (100 μL) removed at 0, 30, 60, 90, 120, and 180 min, were diluted with 100 μL acetonitrile containing [2H8]-busulfan (5 μg/mL; internal standard), and transferred to HPLC vials. Busulfan was measured according to the method described by Murdter et al. (2001). The chromatography system (Waters 2695) used a Luna C8 analytical column (5 μm particle size, 150 × 2 mm i.d.; Phenomenex, Torrence, CA). Elution was carried out using a gradient of 10 mM ammonium acetate and 10 mL/L acetic acid, and acetonitrile at a flow rate of 0.4 mL/min. Gradients were programmed as follows: 15% acetonitrile at 0 min, increased to 45% over 7 min, decreased to 15% acetonitrile over 0.1 min and then left at 15% acetonitrile for 3 min to re-equilibrate. Detection was carried out using a Waters Micromass ZMD Mass Spectrometer equipped with an electrospray source operating in the positive ion mode. Selected ion monitoring was used to detect the ammonium adduct of busulfan (m/z 264), and [2H8]-busulfan ammonium adduct (m/z 272).
The disappearance of busulfan with time was measured in an incubation mixture containing a 400-fold molar excess of cysteine over busulfan in 100 mM potassium phosphate buffer (pH 7.4; 37 °C). Busulfan decomposed in solution slowly (k1pseudo = 0.0014 min-1) as determined by analysis of products resulting from hydrolysis and adduct formation (khydrolysis = 0.0007 min-1).
Stability of THT-A
The stability of THT-A was evaluated by measuring its rate of disappearance from aqueous solutions (100 μg/mL) at pH 7.4 and 8.0 and 37°C. Aliquots (6 μL) removed at 0, 0.25, 0.5, 0.75, 1.0, 1.5, 2, 3, 4, 5, and 6 h, were diluted in 1.5 mL distilled water and 50-μL aliquots were injected into an LC-MS system consisting of a Waters 2695 HPLC coupled to a Waters 996 photodiode array detector programmed to scan between 200 and 300 nm. Quantification of THT-A was performed using selected ion monitoring at m/z of 176. The mobile phase consisted of methanol-water (50:50, v/v) pumped at 0.4 mL/min through an Agilent Zorbax SB-NC C18 150 × 4.6 mm reverse phase column.
The stability of THT-A in phosphate buffer was also determined by measuring appearance of pyruvate, ammonium and tetrahydrothiophene. Pyruvate was measured as its 2,4-dinitrophenylhydrazone as described above. Ammonium was measured with glutamate dehydrogenase. To the solution containing ammonium (20 μL) was added 180 μL of a reaction mixture containing 100 mM potassium phosphate buffer (pH 7.4), 0.1 mM EDTA, 10 mM KG, 0.25 mM NADH, 0.01 mM ADP and 4 U of glutamate dehydrogenase. The decrease in absorbance at 340 nm (ε = 6,220 M-1 cm-1) due to oxidation of NADH to NAD+ was continuously measured at 37°C. The reaction was complete in 20 min. Tetrahydrothiophene was determined by HPLC with coulometric detection. Due to its relatively high vapor pressure, precautions were necessary to minimize losses of tetrahydrothiophene to the atmosphere during incubation of reaction mixtures. In order to obtain an estimate of this loss, tetrahydrothiophene (500 nmol) was added to 1.0 mL of 0.1 M potassium phosphate buffer (pH 7.4 or 8.0) and incubated at 37°C in 1.5-mL screw-cap Teflon-coated septum vials (Pierce; Rockford, IL). At intervals, an aliquot (5 μL) of the reaction mixture was removed through the septum by means of a Hamilton syringe and injected directly into an HPLC system. Chromatographic separation of tetrahydrothiophene was achieved on an MD-150 reversephase analytical column (3.0 × 150 mm, 3 micron) from ESA, Inc. (Chelmsford, MA). Elution was carried out with a mobile phase buffer (75 mM citric acid, 25 mM ammonium acetate, 26% acetonitrile, pH 2.6) at a flow rate of 0.8 mL/min. The concentration of tetrahydrothiophene was measured using an ESA model 5600 CoulArray module, equipped with two coulometric array cell modules, each with four working electrodes set at 300, 400, 500, 550, 650, 750, and 825 mV, respectively (ESA, Inc., Chelmsford, MA). Tetrahydrothiophene eluted at 8.23 min. Peak areas were analyzed using ESA, Inc. software. The initial rates of loss of tetrahydrothiophene under these conditions were less than 10% per h.
Preparation of rat tissue homogenates
All experimental procedures involving preparations of rat tissues were performed in the Dementia Division of the Department of Neurology, Weill Medical College of Cornell University at the Burke Medical Research Institute. The protocols were approved by the Weill Medical College of Cornell University Institutional Animal Care and Use Committee (Protocol 0505-367A). Sixmonth old male Fisher × Brown Norway F1 rats were fed ad libitum and had full access to water. The rats were sacrificed by decapitation. Liver, kidneys and forebrains were removed and separately immersed in 50 mL of ice-cold isolation buffer containing 300 mM sucrose, 10 mM HEPES, 0.5 mM EGTA, and 0.5% (w/v) fatty acids-free bovine serum albumin (pH adjusted to 7.4 with Tris base). The tissues were pre-chilled for ∼5-6 min. Each tissue sample was separately cut into small pieces with scissors and homogenized in a ∼10-fold volume of isolation buffer supplemented with proteaseinhibitor cocktail (1/100 dilution). Liver and kidney tissues were homogenized using a Potter homogenizer. The brains were homogenized using a Dounce homogenizer. All steps were conducted at 0-4°C. The homogenates were divided into several aliquots and stored at -20°C. In some experiments, the liver homogenate was fractionated into cytosolic and mitochondrial fractions and stored at -20°C (Krasnikov et al., 2005). The purity of the mitochondria was assessed by measuring the specific activity of the traditional cytosolic marker lactate dehydrogenase in the cytosolic and mitochondrial fractions of the rat liver. The specific activity of lactate dehydrogenase in preparations of rat liver mitochondria obtained by the above procedure in our laboratory is consistently 3-5% that of the cytosolic fraction. Compelling evidence presented by Brooks and colleagues (1999) indicates that this amount of enzyme is endogenous to mitochondria. By contrast, cystathionine γ-lyase is exclusively cytosolic in rat liver (Allsop and Watts, 1975). Cystathionase γ-lyase activity was not detected in the rat liver mitochondrial preparation used in the present study (Table 1). Thus, by this criterion, our rat liver mitochondrial preparation was essentially free of cytosolic elements.
TABLE 1.
Specific activities (nmol mg protein-1 min-1) of cystathionine γ-lyase in selected rat tissuesa
| Homogenate | Cytosol | Mitochondria | |
|---|---|---|---|
| Liver | |||
| No addition | 26.3±0.8 | 88.6±2.9 | <1.0 |
| + 5 mM D,L-Propargylglycine | <1.0a | <1.0b | <1.0 |
| + 0.5 mM KG | 23.3±2.4 | 91.0±3.2 | <1.0 |
| + 0.5 mM KMB | 27.3±3.1 | 88.6±4.8 | <1.0 |
| Kidney | |||
| No addition | 1.59±0.09 | ND | ND |
| Brain | |||
| No addition | 0.27±0.03 | ND | ND |
The standard reaction mixture (20 μL) contained 20 mM L-homoserine, 100 mM potassium phosphate buffer (pH 7.4) and the indicated tissue fraction. After incubation at 37 °C, α-ketobutyrate formation was determined as outlined in the Materials and Methods section. The blank contained phosphate buffer plus tissue fraction incubated at 37 °C (n = 3 or 4). ND, not determined. The amount of protein (μg) in each assay mixture was as follows: liver homogenate (130), liver cytosol (21), liver mitochondria (86), kidney homogenate (76) and brain homogenate (160). The time of incubation (min) was as follows: liver homogenate (10), liver cytosol (10), liver mitochondria (60), kidney homogenate (120) and brain homogenate (120). Since the cystathionine γ-lyase reaction is linear for at least two hours end-point data served as the bases for calculating specific activities.
Significantly different from the “no addition” values (P = 0.05).
All tissue and mitochondrial samples were freeze-thawed several times before assaying for β-lyase activity toward THT-A and for cystathionine γ-lyase activity. We demonstrate below that free PLP can catalyze β-elimination of tetrahydrothiophene from THT-A. Therefore, the possibility exists that the freeze-thawing releases some PLP from proteins in the homogenates and that this free PLP contributes to the β-lyase activity observed with THT-A. To investigate this possibility, aliquots of the freeze-thawed tissue preparations were centrifuged at 1,000 g for 5 min and 0.1 mL of each supernatant fraction was filtered through a centrifugal filter device with a 10-KDa cut-off membrane (Microcon Ultracel YM-10, Millipore Corporation, Bedford, MA). The filtrate and retentate were assayed for β-lyase activity toward THT-A. The filtrate is expected to contain free PLP but not PLP-dependent enzymes.
Protein measurements
Protein concentrations were determined using a micro-Biuret assay kit obtained from Sigma Chemical Company (St. Louis, MO). Bovine serum albumin was used as a standard.
Statistical analysis
For determinations where n ≥3, except where indicated, data are reported as means ± S.E.M. Statistical comparisons were carried out using the Mann-Whitney U-test; a p value of ≤0.05 was considered significant.
Results
Cystathionine γ-lyase catalyzes β-elimination of tetrahydrothiophene from THT-A
Two PLP-containing enzymes, previously shown to catalyze β-elimination reactions with cysteine S-conjugates containing halogenated, electron-withdrawing groups attached at the sulfur, namely GTK and mitAspAT (Cooper and Pinto, 2006), were tested for their ability to catalyze a β-elimination reaction with THT-A. These enzymes serve as well-established reference enzymes that catalyze cysteine S-conjugate β-lyase reactions. However, we were unable to detect any β-lyase activity toward THT-A with GTK and mitAspAT, even when 0.5 mM KMB or 0.5 mM KG was included in the reaction mixture. On the other hand, we showed that highly purified rat liver cystathionine γ-lyase is able to catalyze a β-lyase reaction with THT-A (see below).
The specific activity of the purified rat liver cystathionine γ-lyase (0.55 mg/mL) used in the present studies (20 mM L-homoserine as substrate) is 2,400 nmol/min/mg of protein. The specific activity of this enzyme as a β-lyase with 5 mM THT-A as substrate was found to be 24.0 ± 0.3 nmol/min/mg protein (n = 4). Thus, the rate at which cystathionine γ-lyase catalyzes a β-elimination reaction with 5 mM THT-A is about 1.0% the rate at which the enzyme catalyzes a γ-elimination reaction with 20 mM L-homoserine.
Cystathionine γ-lyase activity in rat tissue homogenates
In order to determine whether cystathionine γ-lyase is the only (or predominant) enzyme able to catalyze a β-elimination reaction with THT-A in rat tissues, the specific activity of cystathionine γ-lyase in rat tissue fractions was compared with that of the β-lyase activity toward THT-A. The specific activity of cystathionine γ-lyase is relatively high in rat liver, but lower in rat kidney and brain (Table 1). The specific activities of cystathionine γ-lyase in kidney and brain homogenates relative to that in the liver homogenate are about 3% and 1%, respectively (Table 1).
Propargylglycine, although not entirely specific for cystathionine γ-lyase, is nevertheless a potent inhibitor of this enzyme (Washtien and Abeles, 1977). Inclusion of 5 mM D,L-propargylglycine in the standard reaction mixture (homoserine as substrate) resulted in no detectable cystathionine γ-lyase activity in rat liver homogenate or cytosol (Table 1).
β-Lyase activity toward THT-A in rat tissue homogenates
β-Lyase activity toward THT-A was found to be present in the liver homogenate, liver cytosol and liver mitochondrial fraction as measured by pyruvate formation (Fig. 2; Table 2). In particular, liver mitochondria exhibit a 5.8 fold greater β-lyase specific activity than do liver homogenates. Moreover, following addition of propargylglycine, the relative specific activity increases to 14.7 fold. This finding suggests the presence of additional enzymes, especially in liver mitochondria, that are capable of catalyzing β-lyase reactions with THT-A. It is important to note that the ability of kidney and brain homogenates relative to that of the liver homogenate to catalyze a β-lyase reaction with THT-A (Table 2) is greater than the relative cystathionine γ-lyase specific activities (Table 1). These findings indicate that kidney and brain also possess enzymes in addition to cystathionine γ-lyase capable of catalyzing β-elimination reactions with THT-A.
Figure 2.
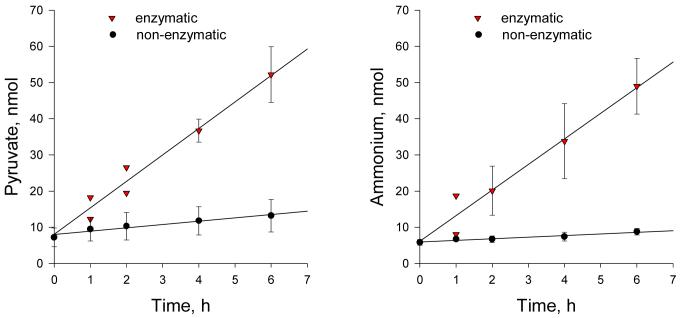
Non-enzymatic and enzymatic formation of pyruvate (panel A) and ammonium (panel B) via β-elimination reactions with THT-A. The reaction mixture containing 5 mM THT-A, 100 mM potassium phosphate buffer (pH 7.4) and cystathionine γ-lyase (600 mU/mL) was incubated at 37 °C (inverted triangles). At intervals, aliquots (0.02 ml) were withdrawn and separately analyzed for ammonium and pyruvate as indicated in the methods section. The y axis shows the amount of pyruvate or ammonium generated per 0.02 ml aliquot. The blank was identical except that enzyme was omitted (filled circles). For most data points n = 3 and the SD are shown. Where only two determinations were made, both values are shown. Ammonium was measured by the glutamate dehydrogenase method (Materials and Methods section). Note that there was a small amount of contaminating ammonia in the blank at the zero time. This value was subtracted to calculate net ammonia production. In the absence of this subtraction, the net change in absorbance due to reductive amination of α-ketoglutarate in the reaction mixture containing enzyme at 1 h and 6 h was about 1.9 and 5.4 times the values obtained in the control reaction mixture that lacked enzyme.
TABLE 2.
β-Lyase specific activities (nmol mg protein-1 min-1) toward THT-A in selected rat tissuesa
| Homogenate | Cytosol | Mitochondria | |
|---|---|---|---|
| Liver | |||
| No addition | 0.69±0.02 | 1.06±0.29 | 4.01±0.13 |
| + 5 mM D,L-Propargylglycine | 0.19±0.04* | 0.23±0.11* | 2.79±0.17** |
| + 0.5 mM KG | 0.75±0.06 | 1.34±0.14 | 3.66±0.52 |
| + 0.5 mM KMB | 0.65±0.06 | 1.23±0.32 | 2.50±0.33*** |
| Kidney | |||
| No addition | 1.18±0.09 | ND | ND |
| Brain | |||
| No addition | 0.23±0.03 | ND | ND |
The standard reaction mixture (20 μL) contained 5 mM THT-A, 100 mM potassium phosphate buffer (pH 7.4) and the indicated tissue fraction. After incubation at 37 °C, pyruvate was determined by the 2,4-dinitrophenylhydrazone procedure as outlined in the Materials and Methods section. The blank contained phosphate buffer plus tissue fraction incubated at 37 °C. At the end of the incubation period, 2 μL of 50 mM THT-A was added just prior to addition of the 2,4-dinitrophenylhydrazine reagent. A correction was made for the slow non-enzymatic conversion rate of THT-A to pyruvate under the incubation conditions (n = 3 to 6 determinations). ND, not determined. The amount of protein (μg) in each assay mixture was as follows: liver homogenate (324), liver cytosol (53), liver mitochondria (43), kidney homogenate (76) and brain homogenate (160). The time of incubation (min) was as follows: liver homogenate (30), liver cytosol (120), liver mitochondria (120), kidney homogenate (120) and brain homogenate (120).
Significant differences between “no addition” and “addition” values are
P = 0.05
P = 0.025
P = 0.0025.
The β-lyase activity toward THT-A was strongly, but not totally, inhibited by D,L-propargylglycine in the rat liver homogenate and cytosolic fraction. D,L-Propargylglycine also significantly inhibited the β-lyase activity in the mitochondrial fraction, but proportionately less so than that in the cytosol and homogenate (Table 2). Since D,L-propargylglycine completely inhibits cystathionine γ-lyase in rat tissue homogenates (Table 1), these data are also consistent with the hypothesis that enzymes in addition to cystathionine γ-lyase contribute to β-lyase activity with THT-A in rat. Thus, the combined activities of cystathionine γ-lyase and as yet unidentified β-lyases in liver, kidney and brain are expected to contribute substantially to busulfan pharmacokinetics and to the metabolic fate of THT-A in vivo.
The β-lyase activity toward THT-A was not significantly stimulated by added KMB or KG in any of the rat liver fractions (Table 2). Interestingly, there was a highly significant inhibition of the β-lyase reaction by 0.5 mM KMB catalyzed by the liver mitochondrial fraction (Table 2). The reason for this inhibition is not known. The cystathionine γ-lyase activity in the rat liver homogenates and cytosolic fraction is not inhibited by 0.5 mM KMB (Table 1). Purified cystathionine γ-lyase is also not inhibited by 0.5 mM KMB (data not shown). Taken together, these data show that rat liver mitochondria contain at least one enzyme relatively unresponsive to D,L-propargylglycine that catalyzes a β-lyase reaction with THT-A.
Stoichiometry of the cystathionine γ-lyase-catalyzed β-lyase reaction with THT-A
Figure 2 shows the rate of formation of pyruvate and ammonium from THT-A in the presence and absence of cystathionine γ-lyase. A slow non-enzymatic β-elimination reaction of pyruvate and ammonium from THT-A was noted in the presence of 100 mM potassium phosphate buffer (pH 7.4) at 37°C (about 2-4 nmol/h/100 nmol of THT-A; Table 3). Figure 2 shows that a substantial increase in both pyruvate and ammonium occurs when cystathionine γ-lyase is included in the reaction mixture. The enzyme-catalyzed formation of these products is linear for at least 6 h. Moreover, the rate of enzyme-catalyzed ammonium generation is identical to that of pyruvate production within experimental error. After subtracting the blank, the rate of product (pyruvate, ammonium) formation is ∼6.8 nmol per hour per 12 mU of enzyme in the reaction mixture.
TABLE 3.
Formation of pyruvate from THT-A in the presence of cystathionine γ-lyasea
| Addition | Pyruvate (nmol) |
|---|---|
| THT-A only | 15.4±2.7 |
| THT-A + boiled enzyme | 16.1±2.9 |
| THT-A + active enzyme | 22.7±1.1b,c |
| THT-A + active enzyme + 25 mM L-alanine | 15.4±0.1 |
| THT-A + active enzyme + 10 mM D,L-propargylglycine | 15.3±1.7 |
The reaction mixture (20 μl in a small snap-top tube) contained 5 mM THT-A and where indicated cystathionine γ-lyase (2.6 mU), L-alanine and D,L-propargylglycine. Pyruvate was measured after incubation of the reaction mixture for 6 h at 37°C. The relatively high pyruvate concentration in the controls is due to contamination of the THT-A preparation with pyruvate (see Materials and Methods) and some non-enzymatic formation during the incubation period (see the text). N = 3.
Significantly different from the value obtained with boiled enzyme with P = 0.05.
Significantly different from all other determinations with P = 0.01.
As we show below, free PLP can catalyze a β-elimination reaction with THT-A. The cystathionine γ-lyase preparation used in the experiment shown in figure 2 contained no added PLP, and no PLP was added to the reaction mixture. Rat liver cystathionase is a homotetramer (Mr of each subunit ∼44,000) with each subunit containing tightly bound PLP (Oh and Churchich, 1974). To exclude the possibility that the pyruvate and ammonia formed in the upper curves in figure 2 is due to a non-enzymatic reaction with extraneous PLP derived from the cystathionine γ-lyase preparation several control experiments were performed. Table 3 shows that the amount of pyruvate in the reaction mixture containing 5 mM THT-A and active cystathionine γ-lyase was significantly greater than in reaction mixtures containing a) THT-A, b) THA-A plus boiled enzyme, c) THT-A plus active enzyme plus 25 mM L-alanine, or d) THT-A plus active enzyme plus 10 mM D,L-propargylglycine. L-Alanine is a strong competitive inhibitor of rat liver cystathionine γ-lyase (Ki 1.3 mM) relative to substrate L-homoserine (Km 20 mM) (Washtien et al., 1977), and as alluded to above, propargylglycine is a potent irreversible inhibitor of the enzyme (Washtien and Abeles, 1977).
Having unequivocally established that cystathionine γ-lyase catalyzes β-elimination of THT from THT-A, we then showed that the enzyme exhibits saturation kinetics. Double reciprocal plots indicated Km and Vmax values of ∼2.4 mM and ∼25 nmol/min/mg at 37°C in 100 mM potassium phosphate buffer (pH 7.4).
Non-enzymatic β-elimination reaction of THT-A in phosphate buffers and in the presence of PLP
The rates of decomposition of 5 mM THT-A at pH 7.4 and pH 8.0 at 37 °C were determined by an HPLC/MS method measuring the disappearance of THT-A. Although the products of decomposition of THT-A were the same as those of the enzymatic process (i.e. pyruvate, ammonium and tetrahydrothiophene), the rate of decomposition was slow at both pH values (< 5%/h). Within experimental error, the rate of appearance of tetrahydrothiophene in 0.1 M potassium phosphate buffer (pH 7.4) at 37°C was similar to the rate of appearance of pyruvate and ammonium noted in the blanks shown in Figure 2A and B.
The non-enzymatic decomposition of THT-A to pyruvate in phosphate buffer (pH 7.4) was found to be significantly accelerated in the presence of 2.5 μM PLP (Table 4). This study confirmed the ability of free PLP by itself to enhance the beta-elimination of THT from THT-A.
TABLE 4.
PLP-catalyzed formation of pyruvate and ammonium from THT-Aa
| Time (h) | Pyruvate (nmol) | Ammonium (nmol) | |
|---|---|---|---|
| No PLP | 0 | 47.6±1.0 | 48.8±2.4 |
| 1 | 65.0±2.6 | 63.0±6.4 | |
| 2 | 88.0±4.0 | 84.8±3.8 | |
| Plus PLPb | 0 | 51.0±1.0 | 60.4±12.2 |
| 1 | 168±1 | 185±9 | |
| 2 | 248±3 | 264±6 |
The reaction mixture (0.2 mL) contained 100 mM potassium phosphate buffer pH 7.4, 5 mM THT-A, and where indicated 2.5 μM PLP. After incubation at 37 °C at the times indicated, 10-μL aliquots were analyzed for pyruvate and ammonium (n = 3).
The blank contained PLP and phosphate buffer, but no THT-A.
Discussion
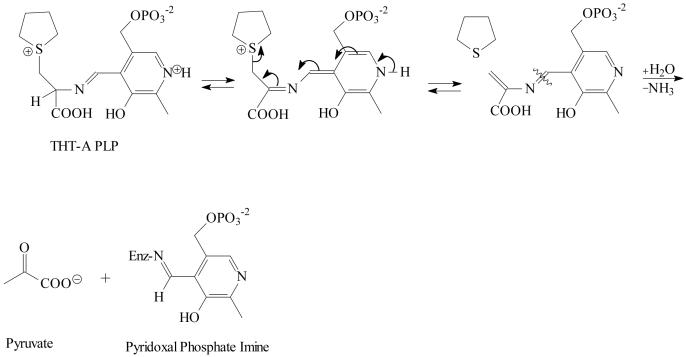
Glutathionylation of busulfan generates an unstable sulfonium ion adduct that is a metabolite along a pathway to the major intermediate metabolite, tetrahydrothiophene (Roberts and Warwick, 1961). We hypothesize that although a portion of the busulfan glutathione adduct could be converted non-enzymatically to tetrahydrothiophene, the major metabolic fate of the adduct in liver and other organs in vivo involves THT-A that is formed by the consecutive action of γ-glutamyltransferase and dipeptidase/cysteinylglycinase in the mercapturate pathway. THT-A is a cysteine S-conjugate that has structural properties similar to those of β-lyase substrates. Most mammalian cysteine S-conjugate β-lyases described thus far are PLP-containing aminotransferases that catalyze β-eliminations as side reactions [reviewed by Cooper and Pinto (2006)]. For these enzymes, a transamination reaction usually competes with the β-lyase reaction. A proposed pathway for the PLP-catalyzed β-elimination of THT-A is shown in Figure 3. Condensation of THT-A with enzyme-bound PLP forms an aldimine intermediate. Loss of the α -proton of the amino acid produces an intermediate described as a quinonoid-carbanion structure (Metzler et al., 1988). Extending the conjugation of the system by elimination of tetrahydrothiophene is likely to be a process that is favored over imine hydrolysis. In the final step, the PLP catalyst is regenerated from the ketimine intermediate, in Schiff base linkage to the ε-amino group of an active site lysine residue, yielding aminoacrylate (not shown), which is hydrolyzed to pyruvate and ammonium.
Figure 3.
Proposed intermediates in the PLP-catalyzed cystathionine γ-lyase mediated conversion of THT-A to tetrahydrothiophene, pyruvate and ammonium. Rearrangement of the THT-A PLP Schiff base eliminates tetrahydrothiophene from the complex. Hydrolysis provides an eneamine analogue of pyruvate (not shown) that tautomerizes and hydrolyzes to give pyruvate. PLP is bound to the enzyme (Enz) through a lysine residue.
A half transamination results in the conversion of coenzyme PLP to its pyridoxamine 5′-phosphate form. The enzyme in the pyridoxamine 5′-phosphate form cannot support a β-lyase reaction. For the enzyme to catalyze a β-lyase reaction at an optimal rate, an α-keto acid substrate (or additional PLP) must be present in the assay mixture to ensure maximal presence of the PLP form of the coenzyme in the active site (Stevens et al., 1986; Cooper and Pinto, 2006). To determine whether β-lyase-catalyzed reactions with THT-A are dependent on an α-keto acid, in some experiments the β-lyase reaction mixture was supplemented with 0.5 mM KMB or 0.5 mM KG. KMB is a good α-keto acid substrate of GTK, an enzyme that catalyzes a strong β-lyase reaction with the halogenated cysteine S-conjugates, S-(1,1,2,2-tetrafluoroethyl)-L-cysteine and S-(1,2-dichlorovinyl)-L-cysteine (Cooper and Pinto, 2006; Stevens et al., 1986; Commandeur et al., 2000). KG is a good α-keto acid substrate of most other mammalian aminotransferases, including mitAspAT, an enzyme that also exhibits β-lyase activity toward TFEC and DCVC (Cooper et al., 2002). β-Elimination reactions with THT-A in the presence of rat tissue homogenates were not significantly stimulated by addition of KG or KMB (Table 2). Thus, either the enzymes responsible for the elimination reaction are not aminotransferases or THT-A possesses such a good leaving group that an aminotransferase reaction cannot compete effectively with a β-lyase reaction at the active site. Moreover, in the present work we were unable to detect β-lyase activity toward THT-A in the presence of GTK or mitAspAT. This finding eliminates GTK and mitAspAT as catalysts in the β-elimination of tetrahydrothiophene from THT-A in rat tissue homogenates. The finding does not, however, eliminate the possibility of other aminotransferases or other PLP-dependent enzymes in the β-elimination of tetrahydrothiophene from THT-A. Indeed, the present work shows that the PLP enzyme cystathionine γ-lyase contributes to the β-elimination of tetrahydrothiophene from THT-A and that as yet unidentified enzymes in tissue homogenates also contribute to the overall β-elimination.
Rat liver cystathionine γ-lyase exhibits relatively high Km values toward L-homoserine and L-cystathionine (20 mM and 3 mM, respectively) (Greenberg, 1962; Washtien et al., 1977). These values are far higher than the concentrations of L-homoserine and L-cystathionine in most mammalian tissues. For example, the concentration of L-cystathionine in human brain is about 1-2 mM, but is about 20 μM in human kidney and liver (Tallan et al., 1958). The concentration of L-homoserine in rat liver is also very low (Chatagner, 1974). Assuming that the concentration of homoserine in human liver is also low and that the properties of human cystathionine γ-lyase are similar to those of the rat liver enzyme, then the human enzyme is far from saturated with L-homoserine or L-cystathionine in the liver. As noted above, L-alanine binds to rat liver cystathionine γ-lyase with a Ki of 1.3 mM (Washtien et al., 1977). The concentration of L-alanine in rat liver is ∼1 mM (Brosnan et al., 1970). Thus, assuming that human liver is similar to rat liver, some cystathionine γ-lyase active sites may be available for binding of THT-A in the liver of patients administered busulfan.
Since PLP catalyzes a β-elimination reaction with THT-A (Table 4), part of the propargylglycine-insensitive β-lyase activity with THT-A may be due in part to the presence of free PLP in the rat tissue homogenates. However, our experiments with low-Mr fractions obtained from ultramicrofiltrates of rat tissue preparations, suggest that although some conversion of THT-A to tetrahydrothiophene may occur spontaneously and via catalysis by low-Mr effectors (including perhaps free PLP), the predominant route for formation of tetrahydrothiophene from THT-A in vivo is enzymatic. In future work, it will be important to determine which enzymes, in addition to cystathionine γ-lyase are responsible for formation of tetrahydrothiophene from THT-A.
Several biologically important reactions involve conversion of sulfonium compounds to thioethers. Examples include 1) formation of S-adenosyl-L-homocysteine following transfer of methyl moieties to acceptor molecules, 2) formation of 5′-methylthioadenosine from S-adenosyl-L-methionine catalyzed by Escherichia coli S-adenosyl-L-methionine cyclotransferase (Mudd, 1959); 3) spontaneous elimination of S-adenosyl-L-methionine following transamination of S-adenosylmethionine with 7-oxo-8-aminopelargonic acid (Stoner and Eisenberg, 1975); 4) formation of 5′-methylthioadenosine from decarboxylated S-adenosyl-L-methionine during polyamine biosynthesis (e.g. Wallace et al., 2003), and 5) enzyme-catalyzed or spontaneous formation of biogeochemically important dimethylsulfide from S-methyl-L-methionine and dimethylsulfoniopropionate (Cooper and Hanson, 1994). To the best of our knowledge, however, the present work is the first to document an enzyme-catalyzed β-elimination reaction of a thioether from a sulfonium cysteine S-conjugate. We suggest that the enzyme-catalyzed β-elimination of tetrahydrothiophene (a thioether) from the cysteine S-conjugate of busulfan (a sulfonium compound) is an example of a less well known drug metabolism pathway that should be taken into consideration in the development of new drugs that have the potential to form sulfonium cysteine S-conjugates.
Acknowledgments
Support
The work reported herein was supported in part by the National Institutes of Health grants RO1 ES08421 (to A.J.L.C.) and 5-PO1-CA47741 (to W.P.P.), and by National Institute of Justice grant IJ-CX-K014 (to P.S.C.)
Abbreviations
- HPLC
high performance liquid chromatography
- GSH
glutathione
- GST
glutathione S-transferase
- GTK
glutamine transaminase K
- KG
α-ketoglutarate
- KMB
α-keto-γ-methiolbutyrate
- mitAspAT
mitochondrial aspartate aminotransferase
- MS
mass spectrometry
- PLP
pyridoxal 5′-phosphate
- THT-A
β-(S-tetrahydrothiophenium)-L-alanine [(2S)-2-amino-3-(2,3,4,5-tetrahydrothiophen-1-yl)propanoic acid].
References
- Allsop J, Watts RW. Methionine adenosyltransferase, cystathionine β-synthase and cystathionine γ-lyase activity of rat liver subcellular particles, human blood cells and mixed white cells from bone marrow. Clin Sci Mol Med Suppl. 1975;48:509–512. doi: 10.1042/cs0480509. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Dubouchard H, Brown M, Sicurello JP, Butz CE. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci USA. 1999;96:1129–1134. doi: 10.1073/pnas.96.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan JT, Krebs HA, Williamson DH. Effects of ischaemia on metabolite concentrations in rat liver. Biochem J. 1970;117:91–96. doi: 10.1042/bj1170091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallini D, DeMarco C, Mondovi B, Mori BS. The cleavage of cystine by cystathionase and the transsulfuration of hypotaurine. Enzymologia. 1960;22:167–173. [PubMed] [Google Scholar]
- Chatagner F. Occurrence of homoserine in the livers of ethionine or methioninetreated rats. Nature. 1964;203:1177–1178. doi: 10.1038/2031177b0. [DOI] [PubMed] [Google Scholar]
- Commandeur JNM, Andreadou I, Rooseboom M, Out M, De Leur LJ, Groot E, Vermeulen NPE. Bioactivation of selenocysteine Se-conjugates by a highly purified rat renal cysteine conjugate β-lyase/glutamine transaminase K. J Pharmacol Exp Ther. 2000;294:753–761. [PubMed] [Google Scholar]
- Cooper AJL. Purification of soluble and mitochondrial glutamine transaminase K from rat kidney. Use of a sensitive assay involving transamination between L-phenylalanine and α-keto-γ-methiolbutyrate. Anal Biochem. 1978;89:451–460. doi: 10.1016/0003-2697(78)90374-3. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Hanson AD. Advances in enzymology of the biogeochemical sulfur cycle. Chemtracts: Biochem Mo. Bio. 1994;11:71–113. [Google Scholar]
- Cooper AJL, Bruschi S, A Iriarte A, Martinez-Carrion M. Mitochondrial aspartate aminotransferase catalyses cysteine S-conjugate β-lyase reactions. Biochem J. 2002;368:253–261. doi: 10.1042/BJ20020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJL, Pinto JT. Aminotransferase, L-amino acid oxidase and β-lyase reactions involving L-cysteine S-conjugates found in allium extracts. Relevance to biological activity? Biochem Pharmacol. 2005;69:209–220. doi: 10.1016/j.bcp.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Pinto JT. Cysteine S-conjugate β-lyases. Amino Acids. 2006;30:1–15. doi: 10.1007/s00726-005-0243-4. [DOI] [PubMed] [Google Scholar]
- Czerwinski M, Gibbs JP, Slattery JT. Busulfan conjugation by glutathione S-transferases α, μ, and π. Drug Metab Dispos. 1996;24:1015–1019. [PubMed] [Google Scholar]
- Gibbs JP, Czerwinski M, Slattery JT. Busulfan-glutathione conjugation catalyzed by human liver cytosolic glutathione S-transferases. Cancer Res. 1996;56:3678–3681. [PubMed] [Google Scholar]
- Greenberg DM. Cystathionine and homoserine cleavage. Methods Enzymol. 1962;5:936–942. [Google Scholar]
- Hassan M, Ehrsson H. Urinary metabolites of busulfan in the rat. Drug Metab Dispos. 1987;15:399–402. [PubMed] [Google Scholar]
- Iwamoto T, Hiraku Y, Oikawa S, Mizutani H, Kojima M, Kawanishi S. DNA intrastrand cross-link at the 5′-GA-3′ sequence formed by busulfan and its role in the cytotoxic effect. Cancer Sci. 2004;95:54–458. doi: 10.1111/j.1349-7006.2004.tb03231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnikov BF, Kim S-Y, McConoughey SJ, Ryu H, Xu H, Stavrovskaya I, Iismaa SE, Mearns BM, Ratan RR, Blass JP, Gibson GE, Cooper AJL. Transglutaminase activity is present in highly purified non-synaptosomal mouse brain and liver mitochondria. Biochemistry. 2005;44:7830–7843. doi: 10.1021/bi0500877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. In: Metabolism and function of glutathione, in Glutathione: Chemical, Biochemical and Medical Aspects - Part A. Dolphin D, Poulson R, Avromavić O, editors. John Wiley and Sons, Inc.; New York: 1989. pp. 367–474. [Google Scholar]
- Metzler CM, Harris AG, Metzler DE. Spectroscopic studies of quinonoid species from pyridoxal 5′-phosphate. Biochemistry. 1988;27:4923–4933. doi: 10.1021/bi00413a050. [DOI] [PubMed] [Google Scholar]
- Mudd SH. The mechanism of the enzymatic cleavage of S-adenosylmethionine to α-amino-γ-butyrolactone. J Biol Chem. 1959;234:1784–1786. [PubMed] [Google Scholar]
- Murdter TE, Coller J, Claviez A, Schonberger F, Hofmann U, Dreger P, Schwab M. Sensitive and rapid quantification of busulfan in small plasma volumes by liquid chromatography-electrospray mass spectrometry. Clin Chem. 2001;47:1437–1442. [PubMed] [Google Scholar]
- Oh KJ, Churchich JE. Binding of pyridoxal 5′-phosphate to cystathionase. J Biol Chem. 1973;248:7370–7375. [PubMed] [Google Scholar]
- Pinto JT, Krasnikov BF, Cooper AJL. Redox-sensitive proteins are potential targets of garlic derived mercaptocysteine derivatives. J Nutr. 2006;136:S835–S841. doi: 10.1093/jn/136.3.835S. [DOI] [PubMed] [Google Scholar]
- Ritter CA, Bohnenstengel F, Hofmann U, Kroemer HK, Sperker B. Determination of tetrahydrothiophene formation as a probe of in vitro busulfan metabolism by human glutathione S-transferase A1-1: use of highly sensitive gas chromatographic-mass spectrometric method. J Chromatog B. 1999;730:25–31. doi: 10.1016/s0378-4347(99)00170-x. [DOI] [PubMed] [Google Scholar]
- Ritter CA, Sperker B, Grube M, Dressel D, Kunert-Keil C, Kroemer HK. Overexpression of glutathione S-transferase A1-1 in ECV 304 cells protects against busulfan mediated G2-arrest and induces tissue factor expression. Br J Pharmacol. 2002;137:1100–1106. doi: 10.1038/sj.bjp.0704972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JJ, Warwick GP. The mode of action of alkylating agents. III. The formation of 3-hydroxytetrahydrothiophene-1:1-dioxide from 1:4-dimethanesulphonyloxybutane (myleran), S-β-L-alanyl-tetrahydrothiophenium mesylate, tetrahydrothiophene and tetrahydrothiophene-1:1-dioxide in the rat, rabbit and mouse. Biochem Pharmacol. 1961;6:217–227. [Google Scholar]
- Stevens JL, Robbins JD, Byrd RA. A purified cysteine conjugate β-lyase from rat kidney cytosol. Requirement for an α-keto acid or an amino acid oxidase for activity and identity with soluble glutamine transaminase K. J Biol Chem. 1986;263:3395–3401. [PubMed] [Google Scholar]
- Stoner GL, Eisenberg GL. Purification and properties of 7,8-diaminopelargonic acid aminotransferase. An enzyme in the biotin biosynthetic pathway. J Biol Chem. 1975;250:4029–4036. [PubMed] [Google Scholar]
- Tallan HS, Moore S, Stein WH. l-Cystathionine in human brain. J Biol Chem. 1958;230:707–716. [PubMed] [Google Scholar]
- Tomisawa H, Ichimoto N, Ichihara S, Fukazawa H, Tateishi M. Involvement of cystathionase in the formation of alkane-thiols from corresponding cysteine conjugates. Xenobiotica. 1988;18:1029–1037. doi: 10.3109/00498258809042225. [DOI] [PubMed] [Google Scholar]
- Wallace HM, Fraser AV, Hughes A. A perspective of polyamine metabolism. Biochem J. 2003;376:1–14. doi: 10.1042/BJ20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washtien W, Abeles RH. Mechanism of inactivation of γ-cystathionase by the acetylenic substrate analogue propargylglycine. Biochemistry. 1977;16:2485–2491. doi: 10.1021/bi00630a026. [DOI] [PubMed] [Google Scholar]
- Washtien W, Cooper AJL, Abeles RH. Substrate proton exchange catalyzed by γ-cystathionase. Biochemistry. 1977;16:460–463. doi: 10.1021/bi00622a019. [DOI] [PubMed] [Google Scholar]