Abstract
Background
Matrix-metalloproteinase-9 (MMP-9) may serve as a biomarker of ventricular remodeling in selected populations, but few studies have assessed its performance in clinical practice. We tested MMP-9 as a biomarker of remodeling and predictor of outcomes in a systolic heart failure cohort derived from clinical practice, and compared its performance to brain natriuretic peptide (BNP).
Methods
Plasma MMP-9 and BNP levels were measured in 395 outpatients with systolic heart failure who participated in the Penn Heart Failure Study. We tested for 1) cross-sectional associations between biomarker levels, left ventricular end-diastolic dimension index (LVEDDI), and ejection fraction (EF), and 2) associations between baseline biomarker levels and risk of subsequent cardiac hospitalization or death over 3 years of follow-up.
Results
MMP-9 had no significant correlation with LVEDDI (rho=0.04, P=NS) or EF (rho=−0.06, P=NS), whereas BNP showed highly significant correlations (LVEDDI: rho= 0.27, P<0.0001; EF: rho=−0.35, P<0.0001). In multivariate linear regression models, MMP-9 again showed no significant associations with LVEDDI (P=0.6) or EF (P=0.14), whereas BNP showed strong independent associations (LVEDDI: P<0.001; EF: P=0.002). Kaplan-Meier analyses showed no difference in hospital-free survival by baseline MMP-9 tertile (P=0.7), whereas higher BNP tertile predicted worse survival (P<0.0001). In multivariate Cox models, baseline MMP-9 level did not predict risk of adverse outcome (hazard ratio for log increase [HR log] 0.98, P = 0.9), whereas BNP was a significant independent predictor (HRlog 1.15, P= 0.02).
Conclusion
Compared to BNP, MMP-9 is a poor clinical biomarker of remodeling and outcome in patients with systolic heart failure derived from clinical practice.
Subject Heads: matrix metalloproteinase, biomarker, heart failure, ventricular remodeling, epidemiology
In response to pathologic stress, the failing left ventricle undergoes a complex process of remodeling that is characterized at the cellular level by cardiac myocyte hypertrophy and interstitial fibrosis (1). Research in animal models has shown that increased expression of brain natriuretic peptide (BNP) is a hallmark of myocyte hypertrophy (2), and subsequent work in human subjects has established circulating BNP as an important biomarker in the clinical management of acute and chronic heart failure (3). By contrast, there is no clinically used biomarker of interstitial fibrosis even though it is a cardinal feature of virtually all forms of heart failure.
Fibrosis is a complex process that is mediated to a large extent by matrix metalloproteinases (MMPs) and endogenous tissue inhibitors of matrix metalloproteinases (TIMPs) (4;5). Accordingly, circulating levels of MMPs and TIMPs have been proposed as potential biomarkers of ventricular remodeling and heart failure. Among the many MMPs and TIMPs, MMP-9 has shown promising associations with echocardiographic parameters in population-based cohorts (6) and in substudies of clinical trials (7). However, there are limited data assessing the performance of MMP-9 as a biomarker in clinical cohorts more representative of patients encountered in clinical practice (8;9). Moreover, no study has compared the performance of MMP-9 with BNP in chronic heart failure patients, even though BNP is in widespread clinical use.
The purpose of this study was to compare the performance MMP-9 and BNP as biomarkers of cardiac remodeling and outcome in a clinical cohort of patients with chronic systolic heart failure. We tested the hypotheses that 1) MMP-9 and BNP levels would each show cross-sectional associations with echocardiographic measures of ventricular remodeling and that 2) baseline MMP-9 and BNP levels would each predict the combined endpoint of subsequent cardiac hospitalization or death. Our findings demonstrate that, in contrast to BNP, MMP-9 shows minimal associations with remodeling or outcome in clinical practice.
Methods
We studied patients with systolic heart failure who participated in the Penn Heart Failure Study (PHFS). PHFS is a single-center, prospective, observational cohort study of outpatients with chronic heart failure referred to the University of Pennsylvania Heart Failure and Transplantation Program. Patients are excluded if, in the judgment of their treating physician, they have a non-cardiac condition likely to result in mortality within the next six months. At the time of enrollment, baseline clinical data are collected using a standardized protocol, case report forms, and a customized Oracle database. Peripheral blood samples are drawn and immediately processed, and plasma is frozen at −80°C for measurement of biomarkers. Cardiac imaging data are obtained from two-dimensional Doppler echocardiograms obtained within one month of the baseline visit. Follow-up events (cardiac hospitalization, heart transplantation, and death) are prospectively ascertained every six months using direct patients encounters and review of medical records. For the current analysis, we selected a subcohort of patients (n=395) with a diagnosis of systolic heart failure as defined by the treating heart failure cardiologist and by an ejection fraction < 40%. All participants gave written informed consent, and the PHFS protocol was approved by our Institutional Review Board.
Assays
We used an R+D Systems ELISA to quantify MMP-9 in peripheral plasma and the Architect™ BNP immunoassay from Abbott Diagnostics to quantify plasma BNP. All assays were run in duplicate, and standards supplied by the manufacturer and stored plasma pools were used to assess assay performance. MMP-9 showed intra-assay and inter-assay coefficients of variation of 4 and 6 percent, respectively, with a lower limit of detection of 0.15 ng/mL. BNP showed intra-assay and inter-assay coefficients of variation that ranged from 0.9–5.6 percent, and 1.7 – 6.7 percent, respectively, with a lower limit of detection of 10 pg/mL.
Statistical Analysis
MMP-9 and BNP levels were log transformed given their skewed distributions. We used Spearman correlations and multivariate linear regression models to test for cross-sectional associations between biomarker levels, echo-derived ejection fraction (EF), and echo-derived left ventricular end-diastolic dimension indexed to body surface area (LVEDDI). We then used Kaplan-Meier and Cox proportional hazard models to test the ability of baseline biomarker levels to predict the combined endpoint of subsequent cardiac hospitalization or death. In all analyses, we compared the performance of MMP-9 and BNP by using models that contained MMP-9 alone, BNP alone, or both biomarkers together. Age, gender, race (Caucasian vs. non-Caucasian), body mass index (BMI), and heart failure duration were regarded as important confounders and were forced into all multivariate models. In addition, we explored the following variables as potential confounders: New York Heart Association functional class (NYHA class), American College of Cardiology/American Heart Association heart failure stage (ACC/AHA stage), ischemic vs. non-ischemic etiology, history of hypertension, history of diabetes mellitus, mean arterial pressure, pulse pressure, creatinine clearance (estimated from serum creatinine using the Cockroft-Gault method) and use of standard heart failure therapies (beta-blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers, aldosterone antagonists, cardiac resynchronization, and defibrillators). Confounders that were significant at P < 0.1 in univariate models were introduced into multivariate models. All analyses were performed using SAS version 9.1 (Cary, North Carolina).
Results
Table I summarizes the characteristics of our systolic heart failure cohort. Most patients were male, Caucasian, and had non-ischemic heart failure. A substantial degree of cardiac remodeling was present at baseline as indicated by the low mean EF and elevated mean LVEDDI. Most patients had NYHA class II-III heart failure symptoms and were ACC/AHA stage C. Patients received standard therapies including beta-blockers, ACE inhibitors, aldosterone antagonists, cardiac resynchronization, and defibrillators. There were no statistically significant differences in patient characteristics across MMP-9 tertiles.
Table I.
Baseline characteristics of the study population by MMP tertile (n = 395)*
| All (n = 395) | Tertile I (n = 130) | Tertile II (n = 135) | Tertile III (n = 130) | P-value† | |
|---|---|---|---|---|---|
| Age (years) | 56 ± 14 | 57 ± 14 | 56 ± 15 | 55 ± 14 | 0.8 |
| Male gender | 276 (70) | 90 (69) | 93 (69) | 93 (72) | 0.9 |
| Race | 0.8 | ||||
| Caucasian | 317 (80) | 107 (72) | 106 (79) | 104 (80) | |
| Non-Caucasian | 72 (18) | 23 (28) | 27 (20) | 22 (17) | |
| Not Reported | 6 (2) | 0 (0) | 2 (1) | 4 (3) | |
| BMI (kg/m2) | 29 ± 6 | 29 ± 7 | 29 ± 6 | 30 ± 6 | 0.4 |
| MAP (mm Hg) | 84 ± 12 | 83 ± 12 | 84 ± 11 | 84 ± 13 | 0.5 |
| Pulse Pressure (mm Hg) | 44 ± 14 | 44 ± 14 | 44 ± 14 | 43 ± 13 | 0.6 |
| EF (%) | 28 ± 13 | 30 ± 13 | 28 ± 12 | 28 ± 14 | 0.3 |
| LVEDD (cm) | 6.4 ± 1.1 | 6.2 ± 1.2 | 6.5 ± 1.1 | 6.5 ± 1.0 | 0.08 |
| LVEDDI (cm/m2) | 3.2 ± 0.6 | 3.1 ± 0.6 | 3.2 ± 0.6 | 3.2 ± 0.6 | 0.3 |
| Etiology | 0.6 | ||||
| Ischemic | 138 (35) | 43 (33) | 51 (38) | 44 (34) | |
| Non-ischemic | 257 (65) | 87 (67) | 84 (62) | 86 (66) | |
| Diabetes | 117 (30) | 34 (26) | 43 (31) | 40 (31) | 0.6 |
| Creatinine Clearance (ml/min) | 88 ± 39 | 88 ± 40 | 83 ± 37 | 93 ± 39 | 0.15 |
| NYHA Class | 0.9 | ||||
| I | 60 (15) | 21 (16) | 22 (16) | 17 (13) | |
| II | 168 (43) | 57 (44) | 59 (44) | 52 (40) | |
| III | 136 (34) | 42 (32) | 44 (32) | 50 (38) | |
| IV | 31 (8) | 10 (7) | 10 (7) | 11 (8) | |
| ACC/AHA Stage | 0.9 | ||||
| B | 26 (6) | 8 (6) | 10 (7) | 8 (6) | |
| C | 339 (86) | 114 (88) | 114 (84) | 111 (85) | |
| D | 30 (8) | 8 (6) | 11 (8) | 11 (8) | |
| Treatment | |||||
| ACE-I or ARB | 356 (90) | 112 (86) | 124 (92) | 120 (92) | 0.18 |
| Beta Blocker | 345 (87) | 113 (87) | 120 (89) | 112 (86) | 0.8 |
| Aldosterone Antagonist | 131 (33) | 44 (34) | 47 (35) | 40 (31) | 0.8 |
| Cardiac resynchronization | 106 (27) | 33 (25) | 37 (27) | 36 (27) | 0.9 |
| Defibrillator | 167 (43) | 53 (41) | 59 (44) | 55 (42) | 0.9 |
| Biomarkers | |||||
| MMP-9 (ng/ml) | 44 (28 – 68) | 24 (19 – 28) | 44 (37 – 50) | 85 (69 – 126) | |
| BNP (pg/ml) | 120 (37 – 373) | 107 (37 – 341) | 93 (33 – 385) | 169 (43 – 452) | 0.7 |
Biomarkers are displayed as median (inter-quartile range). Other data are expressed as number (percent) or mean ± standard deviation. Tertile I, <32.5 ng/mL; Tertile II 32.5 – 57.2 ng/mL; Tertile 3, > 57.2 ng/mL.
From ANOVA or χ2 tests comparing patient characteristics across MMP-9 tertiles.
As shown in Table II, baseline MMP-9 levels had no statistically significant cross-sectional correlations with either EF or LVEDDI. By contrast, BNP showed a positive correlation with LVEDDI and a negative correlation with EF, both of which were highly significant. Univariate linear regression models (Table III) showed no associations between MMP-9 and LVEDDI (P = 0.9), and highly significant positive associations for BNP (P < 0.001). To account for confounders, we built multivariate models by forcing age, gender, race, BMI, and heart failure duration into all models along with additional covariates that had univariate associations at the P < 0.1 level (Table III). After adjusting for these confounders, there was still no significant association between MMP-9 and LVEDDI, whereas the association between BNP and LVEDDI remained strong (P < 0.001). Including both MMP-9 and BNP levels in the same multivariate model also showed no association for MMP-9 and verified an independent positive association between BNP level and LVEDDI (P < .001; Table III). We repeated this series of analyses using EF as the outcome measure of remodeling. As shown in Table IV, these analyses demonstrated no significant associations between MMP-9 and EF, and verified an independent negative association between BNP level and EF (P = 0.002). Taken together, these cross-sectional analyses demonstrate no significant association between MMP-9 level and two commonly used measures of cardiac remodeling in our clinical cohort, whereas BNP showed strong independent associations.
Table II.
Spearman correlations among BNP, MMP-9, and measures of remodeling
P<0.0001
Table III.
Cross-sectional associations of MMP-9 and BNP with LVEDDI (cm/m2)
| Univariate |
Multivariate* |
|||||||
|---|---|---|---|---|---|---|---|---|
| MMP-9 alone | BNP-Alone | MMP-9 and BNP | ||||||
| Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | |
| MMP-9, log ng/mL | 0.007 | 0.9 | 0.049 | 0.30 | - | - | 0.026 | 0.6 |
| BNP, log pg/mL | 0.081 | <.001 | - | - | 0.076 | <.001 | 0.075 | <.001 |
Adjusted for age, gender, race, BMI, heart failure duration, NYHA class, creatinine clearance, mean arterial pressure, and use of aldosterone antagonists, beta-blockers, and cardiac resynchronization therapy. Other covariates showed minimal associations in univariate models (P > 0.1) and were not included in multivariate models.
Table IV.
Cross-sectional associations of MMP-9 and BNP with EF (%)
| Univariate |
Multivariate* |
|||||||
|---|---|---|---|---|---|---|---|---|
| MMP-9 alone | BNP-Alone | MMP-9 and BNP | ||||||
| Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | |
| MMP-9, log ng/mL | −1.3 | 0.17 | −1.4 | 0.14 | - | - | −1.1 | 0.2 |
| BNP, log pg/mL | −1.7 | <.001 | - | - | −1.6 | <.001 | −1.6 | 0.002 |
Adjusted for age, gender, race, BMI, heart failure duration, ischemic etiology, NYHA class, diabetes mellitus, creatinine clearance, mean arterial pressure, and use of aldosterone antagonists, beta-blockers, and cardiac resynchronization therapy. Other covariates showed minimal associations in univariate models (P > 0.1) and were not included in multivariate models.
We then compared the ability of MMP-9 and BNP to predict clinical outcomes. After biomarker assessments, patients were followed for a minimum of 6 months and a maximum of 3 years. During this interval, there were 180 cardiac hospitalizations, 2 cardiac transplants and 12 deaths. We used Kaplan-Meier analyses to test the ability of tertiles of MMP-9 at baseline to predict hospital-free survival, with transplants treated as censored events. Of note, treating transplants as adverse events showed nearly identical results (data not shown). As shown in Figure 1A, hospital-free survival did not differ by baseline MMP-9 tertiles (P = 0.7). For comparison, we performed the same analysis using baseline BNP tertiles. Unlike for MMP-9, hospital-free survival showed large differences across BNP tertiles, with higher BNP tertile predicting worse outcome (P < 0.0001; Figure 1B). Table V shows the results of Cox proportional hazard models that tested the ability of baseline biomarker levels to predict the combined endpoint of subsequent cardiac hospitalization or death. In all models tested, baseline MMP-9 level showed minimal associations with risk of future events, whereas BNP remained a strong independent predictor.
Figure 1.
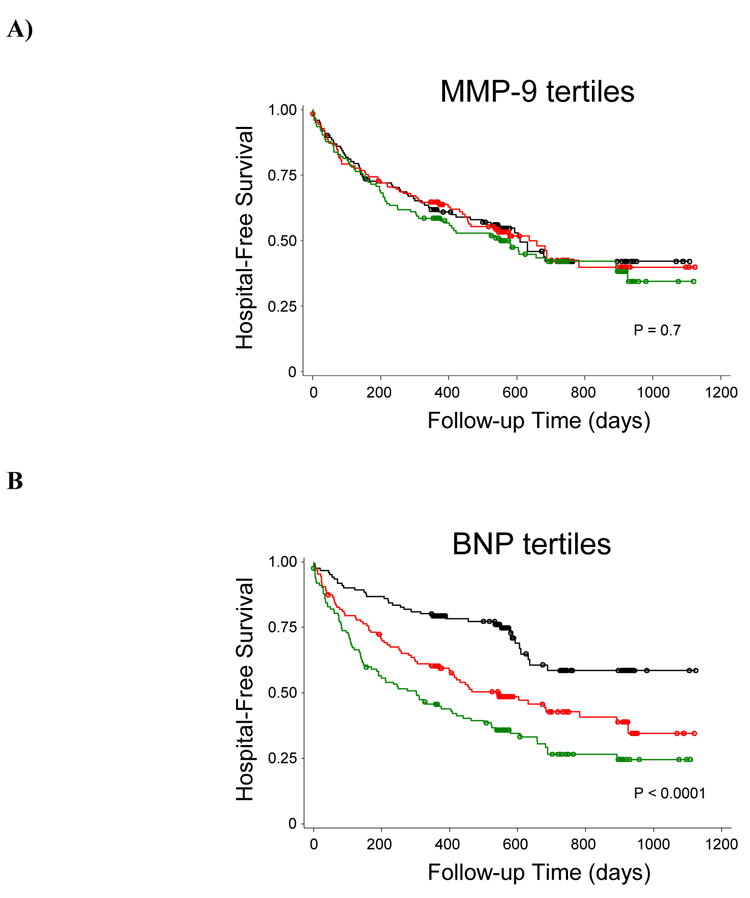
Kaplan-Meier analysis of hospital-free survival by baseline tertile of MMP-9 (A) or BNP (B). Black, lowest tertile; Red, middle tertile; Green, highest tertile. P-values indicate results of logrank tests.
Table V.
Association of baseline MMP-9 and BNP with risk of subsequent cardiac hospitalization or death
| Univariate |
Multivariate* |
|||||||
|---|---|---|---|---|---|---|---|---|
| MMP-9 alone | BNP-Alone | MMP-9 and BNP | ||||||
| Hazard Ratio | p-value | Hazard Ratio | p-value | Hazard Ratio | p-value | Hazard Ratio | p-value | |
| MMP-9, log ng/mL | 1.14 | 0.19 | 1.02 | 0.8 | - | - | 0.98 | 0.9 |
| BNP, log pg/mL | 1.28 | <.001 | - | - | 1.14 | 0.02 | 1.15 | 0.02 |
Adjusted for age, gender, race, BMI, heart failure duration, ischemic etiology, NYHA class, creatinine clearance, EF, LVEDDI, and use of cardiac resynchronization and defibrillators. Other covariates showed minimal associations in univariate models (P > 0.1) and were not included in multivariate models.
Discussion
In a large systolic heart failure cohort derived from clinical practice, we found no meaningful associations between peripheral MMP-9 levels and baseline measures of ventricular remodeling or risk of adverse clinical outcomes. These results are in direct contrast to those for BNP, which showed strong cross-sectional associations with two different measures of cardiac remodeling and strong associations with risk of adverse outcomes. Our study thus does not support the use of peripheral MMP-9 level as a clinically useful biomarker in chronic systolic heart failure.
These findings contrast with previous reports that suggest a potential role of MMP-9 as a biomarker of cardiac remodeling and heart failure. These differences are largely attributable to differences in the populations under study. For example, Ahmed et al. detected elevated peripheral MMP-9 levels in patients with hypertension and concentric cardiac remodeling compared to normal controls (10). Sundstrom et al. evaluated MMP-9 levels in a healthy population of 699 subjects free of clinical heart failure and previous myocardial infarction (6). They found that, although levels were undetectable in the majority of subjects, there was a cross-sectional association between detectable MMP-9 levels, LV wall thickness, and LV dimensions in men but not in women. These studies suggest that MMP-9 may be a marker for early cardiac remodeling in selected patient populations such as those with hypertensive heart disease or other cardiovascular risk factors prior to the development of clinical heart failure.
More recently, Yan et al. tested the association between plasma MMP-9 and ventricular volumes in 183 patients with systolic heart failure and demonstrated statistically significant correlations with ejection fraction and left ventricular volumes (7). Although their sample size was relatively small, the authors’ ability to detect an association was strengthened by the use of a more homogenous clinical trial population and by the use of quantitatively measured ventricular volumes assessed over time. However, our results indicate that such associations do not necessarily translate into clinically meaningful associations in a less homogenous cohort that is more typical of clinical practice. In addition, Yan et al did not adjust for circulating BNP which, based on our analysis, might overshadow the observed associations with MMP-9. Hence, there may an association between MMP-9 and measures of cardiac remodeling in select circumstances, but our study casts doubt on the clinical utility of that association, especially given the inability of MMP-9 to predict clinical outcomes (Figure 1 and Table V).
From a mechanistic standpoint, our findings are also consistent with the basic biology of natriuretic peptides and the MMP/TIMP systems. As demonstrated in numerous animal and human studies (reviewed in (2)), BNP is specifically expressed by cardiac myocytes during cardiogenesis and is re-induced and released under conditions of mechanical stress and cardiac hypertrophy. Because of its cardiac specificity, BNP levels in the periphery show strong associations with both remodeling and heart failure. By contrast, MMPs are involved in extracellular matrix turnover in a variety of homeostatic and pathologic settings unrelated to cardiac remodeling, including wound healing (11), cancer growth and metastasis (12), inflammation (13), and atherogenesis (14). Given the limited specificity of MMP-9, it is not surprising that peripheral MMP-9 levels show inferior associations with ventricular remodeling and heart failure when compared to peripheral BNP.
Although our study does not support the use of circulating MMP-9 as a biomarker, we cannot exclude a causal role for myocardial MMPs and TIMPs in the pathogenesis of remodeling. We did not study MMPs within the myocardium directly, nor did we alter MMP function via the use of pharmacologic agents. Such experiments have been conducted in animal models and do suggest a pathogenic role for MMPs. For example, activation of MMPs via genetic deletion of TIMP-3 causes dilated cardiomyopathy in mice (15), whereas inhibition of MMPs using transgenic (16) and pharmacological approaches (17–21) attenuates ventricular remodeling. The only published clinical trial of MMP inhibition to attenuate remodeling in humans showed no effect vs. placebo, but the agent used inhibited MMPs broadly and was limited by musculoskeletal toxicity (22). Thus, the role of MMPs and TIMPs as mediators of remodeling in human heart failure or as therapeutic targets remains uncertain.
Several limitations warrant mention. First, we measured MMP levels and not MMP activity. Since MMP activity within the myocardium is probably a better predictor of matrix remodeling, one could argue that circulating MMP activity might be a superior biomarker than MMP level. However, circulating MMP activity would undoubtedly be influenced by circulating TIMPs and by MMPs released from extra-cardiac sources. Second, we did not analyze longitudinal changes in MMP-9 levels over time, and we cannot draw conclusions regarding temporal changes in cardiac structure or its relation to MMP-9. Third, we limited the echocardiographic parameters studied to the clinically used measures of ejection fraction and left ventricular dimensions. As shown by Yan et al. (7), it is possible that MMP-9 levels associate with other echocardiographic parameters, such as quantitatively assessed ventricular volumes or measures of diastolic function. However, based on our findings, we can safely conclude that these associations would be smaller in magnitude that those for BNP. Finally, it is possible that use of previously frozen plasma or diurnal variation in MMP-9 level could introduce variability into our data that might mask associations of interest, although studies in normal subjects done by Tayebjee et al indicate that diurnal variation is unlikely (23).
In conclusion, our findings do not support the use of circulating MMP-9 levels as a clinical biomarker in systolic heart failure and verify the prognostic value of BNP in clinical practice. Further research in diverse patient populations is needed to clarify the utility of MMPs and other biomarkers of remodeling and heart failure. Moreover, further defining the mechanistic role of MMPs in human heart failure will require trials of specific MMP inhibitors or techniques that allow in vivo measurement of MMPs within the myocardium in human subjects.
Sources of Funding
Supported by research grants from the National Institutes of Health (NIH K23HL071562 and R01HL088577) and Abbott Diagnostics. Assays were performed in collaboration with the University of Pennsylvania Diabetes and Endocrinology Research Center RIA/Biomarkers Core (NIH DK19525). Dr. Vorovich was supported by the NIDDK Medical Student Research Program.
Footnotes
Conflict of Interest Disclosures
Dr. Cappola has received research grants from Abbott Diagnostics and GlaxoSmithKline.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111(21):2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 2.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 3.Latini R, Masson S, de Angelis N, et al. Role of brain natriuretic peptide in the diagnosis and management of heart failure: current concepts. J Card Fail. 2002;8(5):288–299. doi: 10.1054/jcaf.2002.0805288. [DOI] [PubMed] [Google Scholar]
- 4.Li YY, McTiernan CF, Feldman AM. Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res. 2000;46(2):214–224. doi: 10.1016/s0008-6363(00)00003-1. [DOI] [PubMed] [Google Scholar]
- 5.Wilson EM, Spinale FG. Myocardial remodeling and matrix metalloproteinases in heart failure: turmoil within the interstitium. Ann Med. 2001;33(9):623–634. doi: 10.3109/07853890109002108. [DOI] [PubMed] [Google Scholar]
- 6.Sundstrom J, Evans JC, Benjamin EJ, et al. Relations of plasma matrix metalloproteinase-9 to clinical cardiovascular risk factors and echocardiographic left ventricular measures: the Framingham Heart Study. Circulation. 2004;109(23):2850–2856. doi: 10.1161/01.CIR.0000129318.79570.84. [DOI] [PubMed] [Google Scholar]
- 7.Yan AT, Yan RT, Spinale FG, et al. Plasma matrix metalloproteinase-9 level is correlated with left ventricular volumes and ejection fraction in patients with heart failure. J Card Fail. 2006;12(7):514–519. doi: 10.1016/j.cardfail.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 8.George J, Patal S, Wexler D, et al. Circulating matrix metalloproteinase-2 but not matrix metalloproteinase-3, matrix metalloproteinase-9, or tissue inhibitor of metalloproteinase-1 predicts outcome in patients with congestive heart failure. Am Heart J. 2005;150(3):484–487. doi: 10.1016/j.ahj.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Wilson EM, Gunasinghe HR, Coker ML, et al. Plasma matrix metalloproteinase and inhibitor profiles in patients with heart failure. J Card Fail. 2002;8(6):390–398. doi: 10.1054/jcaf.2002.129659. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed SH, Clark LL, Pennington WR, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113(17):2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 11.Moses MA, Marikovsky M, Harper JW, et al. Temporal study of the activity of matrix metalloproteinases and their endogenous inhibitors during wound healing. J Cell Biochem. 1996;60(3):379–386. doi: 10.1002/(SICI)1097-4644(19960301)60:3%3C379::AID-JCB9%3E3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 12.Liotta LA, Tryggvason K, Garbisa S, et al. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284(5751):67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- 13.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4(8):617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 14.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90(3):251–262. [PubMed] [Google Scholar]
- 15.Fedak PW, Smookler DS, Kassiri Z, et al. TIMP-3 deficiency leads to dilated cardiomyopathy. Circulation. 2004;110(16):2401–2409. doi: 10.1161/01.CIR.0000134959.83967.2D. [DOI] [PubMed] [Google Scholar]
- 16.Creemers EE, Davis JN, Parkhurst AM, et al. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2003;284(1):H364–H371. doi: 10.1152/ajpheart.00511.2002. [DOI] [PubMed] [Google Scholar]
- 17.Spinale FG, Coker ML, Krombach SR, et al. Matrix metalloproteinase inhibition during the development of congestive heart failure : effects on left ventricular dimensions and function. Circ Res. 1999;85(4):364–376. doi: 10.1161/01.res.85.4.364. [DOI] [PubMed] [Google Scholar]
- 18.Peterson JT, Hallak H, Johnson L, et al. Matrix metalloproteinase inhibition attenuates left ventricular remodeling and dysfunction in a rat model of progressive heart failure. Circulation. 2001;103(18):2303–2309. doi: 10.1161/01.cir.103.18.2303. [DOI] [PubMed] [Google Scholar]
- 19.Chancey AL, Brower GL, Peterson JT, et al. Effects of matrix metalloproteinase inhibition on ventricular remodeling due to volume overload. Circulation. 2002;105(16):1983–1988. doi: 10.1161/01.cir.0000014686.73212.da. [DOI] [PubMed] [Google Scholar]
- 20.King MK, Coker ML, Goldberg A, et al. Selective matrix metalloproteinase inhibition with developing heart failure: effects on left ventricular function and structure. Circ Res. 2003;92(2):177–185. doi: 10.1161/01.res.0000052312.41419.55. [DOI] [PubMed] [Google Scholar]
- 21.Morita H, Khanal S, Rastogi S, et al. Selective matrix metalloproteinase inhibition attenuates progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Am J Physiol Heart Circ Physiol. 2006;290(6):H2522–H2527. doi: 10.1152/ajpheart.00932.2005. [DOI] [PubMed] [Google Scholar]
- 22.Hudson MP, Armstrong PW, Ruzyllo W, et al. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction: results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) trial. J Am Coll Cardiol. 2006;48(1):15–20. doi: 10.1016/j.jacc.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 23.Tayebjee MH, Lip GY, Blann AD, et al. Effects of age, gender, ethnicity, diurnal variation and exercise on circulating levels of matrix metalloproteinases (MMP)-2 and -9, and their inhibitors, tissue inhibitors of matrix metalloproteinases (TIMP)-1 and -2. Thromb Res. 2005;115(3):205–210. doi: 10.1016/j.thromres.2004.08.023. [DOI] [PubMed] [Google Scholar]


