Abstract
Background & Aims
We performed a systematic review and meta-analyses to estimate treatment efficacy and constipation rate of 5-HT3 antagonists in patients with non-constipated (NC) or diarrhea-predominant (D) -IBS.
Methods
Two reviewers independently searched MEDLINE, EMBASE, and Web of Science (1966 to December 15th 2006) for randomized controlled trials (RCTs) of 5-HT3 antagonists in IBS reporting clinical endpoints of the IBS symptom complex and safety parameters. Study characteristics, markers of methodological quality, and outcomes for the intention-to-treat population for each RCT were extracted independently.
Results
We found 14 eligible RCTs of alosetron (n=3024) or cilansetron (n=1116) vs. placebo (n=3043) or mebeverine (n=304). Random effects meta-analyses found 5-HT3 antagonists more effective than the comparators in achieving global improvement in IBS symptoms (pooled relative risk 1.60, 95% CI 1.49, 1.72; I2=0%) and relief of abdominal pain and discomfort (pooled relative risk 1.30, 95% CI 1.22, 1.39, I2=22%). Benefit was apparent for both agents, in patients of either sex. These agents were more likely to cause constipation (pooled relative risk 4.28, 95% CI 3.28, 5.60, I2=65%); there was less constipation with 5-HT3 antagonists in D-IBS patients than in mixed populations (NC- and D-IBS; ratio of RR 0.65, 95% CI 0.41, 0.99). Nine patients (0.2%) using 5-HT3 antagonists had, at least, possible ischemic colitis versus none in control groups.
Conclusions
5-HT3 antagonists significantly improve symptoms of NC- or D-IBS in men and women. There is increased risk of constipation with 5-HT3 antagonists, although the risk is lower in those with D-IBS.
INTRODUCTION
Irritable bowel syndrome (IBS) is a highly prevalent functional gastrointestinal disorder affecting 3 to 15 % of the general population1–3. It has a substantial impact on morbidity and quality of life4. It is characterized by unexplained abdominal pain, discomfort, and bloating in association with altered bowel habits5. The pathophysiology of IBS is not well understood, but evidence of abnormal gastrointestinal motor function, visceral hypersensitivity, autonomic dysfunction, and psychological factors indicate disturbances within the enteric nervous system and the brain-gut axis.
Serotonin (5-HT) is an important neurotransmitter in the brain-gut axis and is involved in several functions of the gastrointestinal (GI) tract including the peristaltic reflex 6. At least seven different 5-HT receptor types have been described 7. 5-HT3 receptors are present both centrally and peripherally in the brain-gut axis, and 5-HT3 antagonists have been shown to reduce responses to noxious gut stimuli in animals 8–11.
Two 5-HT3 antagonists have been developed to date for the treatment of IBS, alosetron and cilansetron. Alosetron is approved and available in the United States; cilansetron has undergone a large phase III trial program, but is not yet approved. Several studies in healthy individuals and IBS patients have demonstrated significant differences in the effects of these two 5-HT3 antagonists versus placebo with respect to a wide range of outcomes. These include sensory ratings or thresholds for perception in response to gut distention 12, postprandial symptoms 13, gastrointestinal transit 14, 15, and bowel function, including more solid stool consistency and decreased stool frequency and urgency.
Clinical phase II and III trials have demonstrated superiority of alosetron or cilansetron over placebo, and over an alternative IBS treatment (mebeverine) in one study, with respect to the specific primary endpoints used in each study 16–22. However, the primary endpoints varied across studies. Thus, while earlier studies used ‘relief of abdominal pain or discomfort’ as a primary feature of the symptom complex of IBS 5, subsequent studies followed the recommendations of the consensus Rome documents 23 and used ‘global symptom improvement’ to capture the breadth of bothersome symptoms. Current evidence suggests that such binary endpoints of symptom relief are able to assess therapeutic efficacy of drugs in clinical trials of IBS 24.
Few studies have also evaluated the effect of 5-HT3 antagonists on quality of life 18 or patient satisfaction 25.
Since 5-HT3 antagonists delay GI transit 26, the main adverse effect of this drug class is constipation. While earlier studies included IBS patients with non-constipated bowel habits (NC-IBS), later trials focused on diarrhea-predominant IBS (D-IBS).
An earlier meta-analysis of studies with the 5-HT3 antagonist alosetron showed beneficial effects in women with non-constipated IBS 27. Since that publication, further trials using different 5-HT3 antagonists and including male patients have been performed. The hypothesis of this study was that the drug class of 5-HT3 antagonists is superior to placebo or other comparators in improving endpoints of the IBS symptom complex in both men and women with non-constipated IBS.
Hence, the aim of the present systematic review and meta-analysis was to estimate the effects of 5-HT3 antagonists on ‘relief of abdominal pain or discomfort’ or on ‘global IBS symptom improvement’ and on constipation in patients with non-constipated or diarrhea-predominant irritable bowel syndrome.
METHODS
The present meta-analysis was performed and reported according to the standards of the QUOROM statement 28.
Eligibility Criteria
We included randomized controlled trials evaluating the effect of 5-HT3 antagonists on ‘relief of abdominal pain and discomfort’ or ‘global improvement of IBS symptoms’.
Exclusion criteria were based on type of study (e.g. review, animal study, basic research), study endpoints (e.g. pharmacodynamic endpoints), duplicate publication, and indication (i.e. non-IBS studies). Neither publication status nor language of publication was an exclusion criterion.
Search Strategy
We designed comprehensive computer-based searches of the electronic databases MEDLINE (1966 - December 15, 2006) and EMBASE (1988 - December 15, 2006). Terms used for the computer-based search included: serotonin, 5-HT, alosetron, cilansetron, irritable bowel syndrome, therapy, clinical trial, diarrhea-predominant, and functional bowel disease. Using Web of Science (1990 - December 15, 2006) we sought relevant abstracts in order to identify unpublished trials. We also reviewed the reference sections of included trials. The search strategy, including key words and steps followed, are included in Appendix I (on-line manuscript).
Study Selection
Two investigators (V.A, J.K.), working independently and in duplicate, selected and evaluated study eligibility. κ statistic was used to test for chance-adjusted inter-observer agreement on study eligibility. One investigator (M.C.), who had participated in the planning, design, analysis and interpretation of three of the included trials 19, 29, 30, did not participate in the process of retrieval, trial selection or in tabulation and statistical analysis of the data for this review.
Data Collection
For each study, we assessed the participants’ IBS type according to the declared abnormal bowel function, mean age and gender, treatment regimen used (daily dosage and duration of treatment), number of patients lost to follow up and adequacy of randomization and blinding of patients, clinicians and investigators. Then, we extracted the intention-to-treat data for the efficacy and safety analyses.
For the assessment of the primary efficacy parameters, we used the proportion of patients responding to treatment as defined in the individual trials with regard to either ‘relief of abdominal pain and discomfort’ or ‘global improvement of IBS symptoms’. Most studies used weekly binary assessments of “yes/no-improvement” or “yes/no-adequate relief” to define responders. A few studies used either a visual analog scale (VAS) or a 7-point Likert scale to assess symptom improvement, and the studies included a definition of the cut-off on this scale to define responders 17, 31, 32. For the purpose of our analysis, we incorporated the individual study’s definition of a responder when a VAS or Likert scale was used.
For studies that did not report an overall response rate, but presented separate numbers of responders for different treatment periods (e.g. number of responders in each month), we averaged the period response rates.
For the safety analysis, we used the number of patients reporting constipation and ischemic colitis per treatment group during the overall treatment period.
Quality Assessment
Two independent investigators (V.A., J.K.) evaluated the quality of the studies according to quality criteria suggested for randomized controlled trials (Table 1)33.
Table 1.
Study quality criteria 33 fulfilled by all full paper studies included in the meta-analysis
| Validity | Control group |
| Random allocation | |
| Masking of patients and investigators | |
| Parallel-group design | |
| Validated disease definition for inclusion (Rome I and Rome II criteria) | |
| Validated outcome measures | |
| Attrition bias: to follow up | |
| Adequate power for clinically significant effect size | |
| Definition of “responder” included a priori | |
| Intention to treat analysis | |
| Applicability | Baseline assessment of all treatment groups characteristics |
| Clear description of treatment regimens: dosage, timing, route of administration and duration of treatment | |
| Clear definition of outcome and duration of follow up | |
Author Contact
Two papers of alosetron studies 30, 34, which included NC-IBS and D-IBS patients, reported the constipation results for all included patients but the efficacy results only for the D-IBS population. We contacted GlaxoSmithKline, the pharmaceutical company that conducted all clinical trials with alosetron. GlaxoSmithKline kindly provided us with the efficacy results for the complete study population of these studies as well as with additional information regarding the alosetron study by Krause et al.35, published only in abstract form as of the date of this systematic review.
Statistical Analysis
The meta-analytic comparison was based on the crude unadjusted relative risk (RR) of treatment response or constipation. Using a random effects model, we estimated the pooled RRs for improvement of ‘pain and discomfort’, ‘global IBS symptoms’ and pooled RRs for constipation and their corresponding 95% confidence intervals (CI). We used I2 statistic quantifying between-study inconsistency as the proportion of the overall variability across studies that is not due to chance (random error) 36 to evaluate heterogeneity. One convention considers I2 <25% as reflecting small inconsistency and >50 % as large inconsistency across studies 36. While we report meta-analytical estimates for constipation, we report the total number of patients with ischemic colitis across all studies.
Subgroup Analyses
To explore potential causes of between-study inconsistency, we pre-specified several subgroup analyses with tests of interaction 37. We explored subgroups based on study populations (NC-IBS versus D-IBS only), sex (women, men or mixed population), medication used, dose (standard vs. higher than the standard), treatment duration (12 weeks versus long-term, i.e. 24 or 48 weeks), and comparator (vs. placebo or vs. active comparator). We also explored subgroup analyses based on outcome definition (dichotomous ‘yes/no improvement’ versus Likert or visual analog scales with pre-specified cut-offs to define responders) and outcome estimation (reported overall response rates versus calculated average response across periods). Finally, we explored subgroup analyses by publication status (abstract only versus full text manuscript).
RESULTS
Flow of Study Retrieval
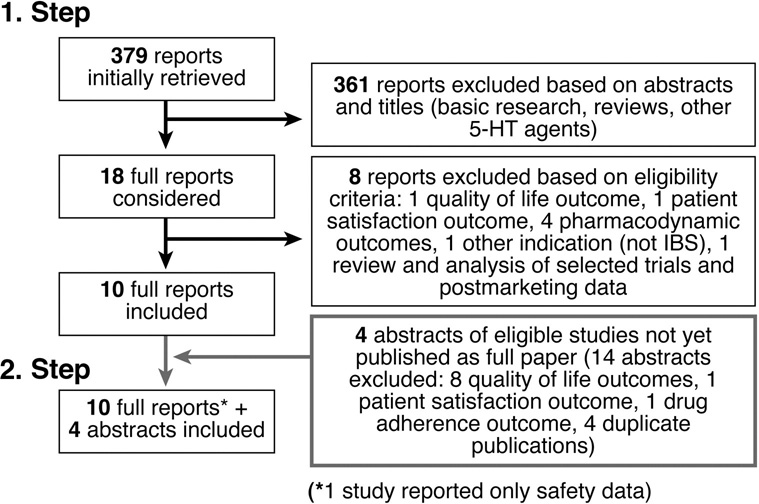
Figure 1 describes the study identification and selection process. Reasons for exclusion included the type of study (review, animal study, basic research, and review and analysis of already included trials and post-marketing data), the study endpoints (pharmacodynamic endpoints, quality of life, patient satisfaction, patient adherence to therapy), duplicate publication (e.g. of other endpoints such as quality of life) of studies that were already included, and the indication (functional dyspepsia instead of IBS).
Figure 1.
Trial selection flow
Ten full reports of randomized controlled trials with the 5-HT3 antagonist alosetron met the inclusion criteria 17, 19, 20, 29–32, 34, 38, 39, one of which reported only safety data 39. We also found 4 eligible abstracts, one trial with alosetron 35 and three trials with cilansetron 21, 22, 40. Overall this review includes 14 trials (n=7984 patients, 3221 randomized to alosetron 1 mg bid, 1116 to cilansetron 2mg tid, 3343 to placebo and 304 to mebeverine 125 mg tid as comparator). The κ statistic for chance-adjusted inter-observer agreement on study eligibility was 0.86.
Study Characteristics
Table 2 summarizes the principal characteristics of the 14 eligible trials. The 10 full reports met all quality criteria (Table 1). For the studies not published as full papers, only partial assessment of quality parameters was possible. Overall, all studies excluded constipation-predominant IBS and focused on recruiting non-constipated IBS patients. Eight studies included only D-IBS patients 17, 21, 22, 29, 32, 35, 38, 40 while 6 studies included both D-IBS and NC-IBS 19, 20, 30, 31, 34, 39. The published manuscript of two of these studies 30, 34 reported only the efficacy outcomes for the D-IBS subpopulation, but GlaxoSmithKline provided results for the complete study population, i.e. D-IBS and NC-IBS. Most of the alosetron studies included only women, consistent with the decision to explore efficacy of alosetron exclusively in women in the phase III program given the lack of efficacy in men in the earlier phase IIB study 19. A later study exclusively tested the efficacy of alosetron in men 38. While the cilansetron studies included both women and men with a planned ratio of 2:1 21, 22, 40; the abstracts did not report the actual proportion in each treatment arm.
Table 2.
Details of the studies
| Author (year) | Agents | IBS subtype | N | Mean age (SD) | Sex | Dosing# (daily) | Treatment duration (weeks) | Efficacy endpoints used | No. (%) lost to follow up |
|---|---|---|---|---|---|---|---|---|---|
| Camilleri (1999)19 | Alosetron vs. placebo | D-IBS NC-IBS | 370 | 44 (14) | mixed | 2, 8, 12, 16 mg | 12 | Global IBS | 6 (1.6) |
| Jones (1999)20 | Alosetron vs. mebeverine | D-IBS NC-IBS | 623 | 44 (13) | women | 2 mg | 12 | Abd. Pain | 7 (1.1) |
| Bardhan (2000)31 | Alosetron vs. placebo | D-IBS NC-IBS | 462 | 43 (14) | mixed | 0.2, 1, 4 mg | 12 | Abd. Pain | 8 (1.7) |
| Camilleri (2000)29 | Alosetron vs. placebo | D-IBS | 647 | 46 (14) | women | 2 mg | 12 | Abd. Pain | 13 (2) |
| Camilleri (2001)30 | Alosetron vs. placebo | D-IBS NC-IBS* | 626 | 46 (13) | women | 2 mg | 12 | Abd. Pain | 17 (2.7) |
| Lembo (2001)17 | Alosetron vs. placebo | D-IBS | 767 | 48(13) | women | 2 mg | 12 | Global IBS | 15 (2) |
| Wolfe (2001)39 | Alosetron vs. Placebo | D-IBS NC-IBS | 869 | 47 (13) | mixed | 2 mg | 48 | No efficacy endpoint (safety) | 42 (4.8) |
| Chey (2004)34 | Alosetron vs. placebo | D-IBS NC-IBS* | 714 | 46 | women | 2 mg | 48 | Abd. Pain | 18 (2.5) |
| Lembo (2004)32 | Alosetron vs. Placebo | D-IBS | 492 | 49(14) | women | 2 mg | 12 | Global IBS | 15 (3) |
| Chang (2005)38 | Alosetron vs. placebo | D-IBS | 662 | 44(13) | men | 1, 2, 4, 8 mg | 12 | Abd. Pain | 19 (2.9) |
| Miner (A 2004)21 | Cilansetron vs. placebo | D-IBS | 692 | n.r. | mixed | 6 mg | 12 | Abd. Pain Global IBS | n.r. |
| Bradette (A 2004)22 | Cilansetron vs. placebo | D-IBS | 792 | n.r. | mixed | 6 mg | 24 (+12 week analysis) | Abd. Pain Global IBS | n.r. |
| Francisconi (A 2005)40 | Cilansetron vs. placebo | D-IBS | 746 | n.r. | mixed | 6 mg | 12 | Abd. Pain Global IBS | n.r. |
| Krause (A 2006)35 | Alosetron vs. Placebo | D-IBS | 353 | n.r. | women | 0.5, 1, 2 mg | 12 | Abd. Pain Global IBS | n.r. |
NC-IBS: IBS with non-constipated bowel habits, D-IBS: diarrhea-predominant IBS
NC-IBS: Efficacy results were only published for the D-IBS subgroup. This analysis includes the unpublished results for NC-IBS provided by GSK
n.r.= not reported
A= Abstract
patients receiving the dose in bold (i.e. the standard dose of each drug) were included in the final analysis. Sensitivity analysis regarding the Bardhan 31 study, the only one not using the standard dose, did not show a difference between the inclusion of the 4mg vs. the 1 mg group.
The doses used in most studies were standard (alosetron, 1 mg twice a day; cilansetron, 2 mg three times a day); however 4 trials of alosetron used several dosages 19, 31, 35, 38, and one of these dose-response studies (a phase IIb study) did not include what would eventually be the approved standard dose 31. From this study we extracted for analysis the results corresponding to a higher dosage (2 mg twice a day) and a lower dosage (0.5 mg twice a day) relative to the standard dosage, and tested the influence of either choice in our overall results.
Efficacy Endpoints
Relief of abdominal pain and discomfort
Meta-analyses
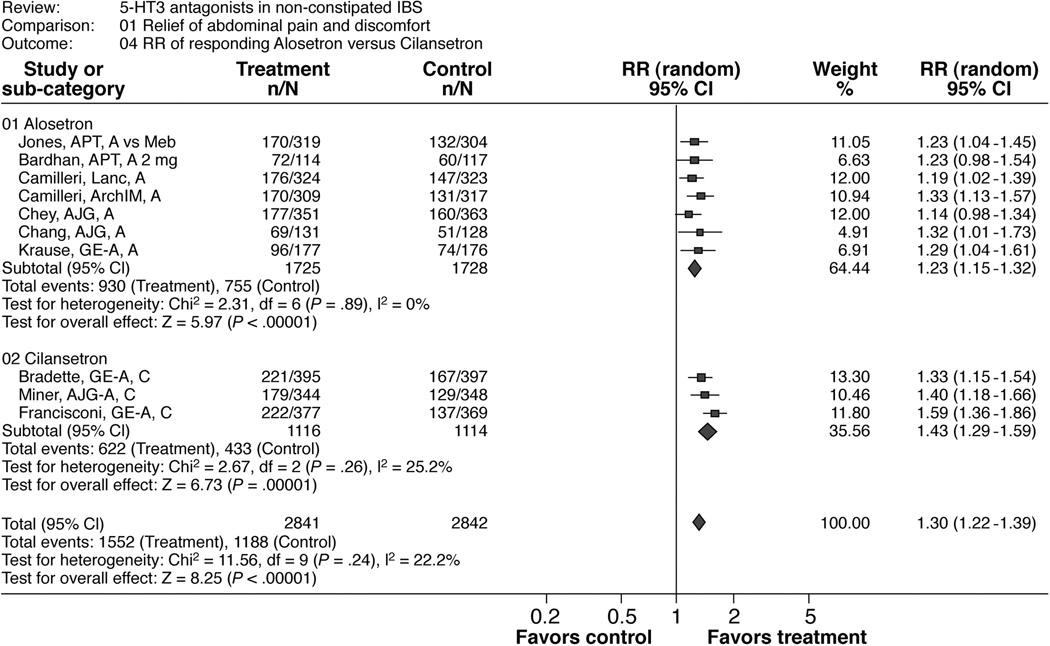
Table 3 and Figure 2 show the results of this meta-analysis and of the subgroup analyses by drugs (alosetron and cilansetron). The overall pooled estimated RR was 1.30 (1.22, 1.39) in favor of 5-HT3 antagonist treatment. The calculated number needed to treat (NNT) was 7.7 and the overall risk difference was 0.13 (0.1, 0.16). The results were consistent across studies (I2=22%). Also, the results were consistent across choice of dose for the Bardhan et al trial 31.
Table 3.
Meta-analyses of the beneficial effects of alosetron and cilansetron in patients with IBS with subgroup analysis by agent.
| Outcomes Intervention Studies | Treatment group (No. with outcome / Total) | Control group (No. with outcome / Total) | RR (95% CI) | Risk Difference (95% CI) |
|---|---|---|---|---|
| Relief of abdominal pain and discomfort | ||||
| Alosetron | ||||
| Jones 20 | 170 / 319 | 132 / 304 | 1.23 (1.04 1.45) | 0.10 (0.02 ,0.18) |
| Bardhan 31 | 72 / 114 | 60 / 117 | 1.23 (0.98 1.54) | 0.12 (−0.01 ,0.25) |
| Camilleri 29 | 176 / 324 | 147 / 323 | 1.19 (1.02 1.39) | 0.09 (0.01 ,0.16) |
| Camilleri 30 | 170 / 309 | 131 / 317 | 1.33 (1.13 1.57) | 0.14 (0.06 ,0.21) |
| Chey 34 | 177 / 351 | 160 / 363 | 1.14 (0.98 1.34) | 0.06 (−0.01 ,0.14) |
| Chang 38 | 69 / 131 | 51 / 128 | 1.32 (1.01 1.73) | 0.13 (0.01 ,0.25) |
| Krause 35 | 96 / 177 | 74 / 176 | 1.29 (1.04 1.61) | 0.12 (0.02 ,0.23) |
| Random effects RR (I2 = 0%) | 1.23 (1.15 1.32) | 0.10 (0.07 ,0.14) | ||
| Cilansetron | ||||
| Bradette 22 | 221 / 395 | 167 / 397 | 1.33 (1.15 ,1.54) | 0.14 (0.07 ,0.21) |
| Miner 21 | 177 / 344 | 129 / 348 | 1.40 (1.18 ,1.66) | 0.15 (0.08 ,0.22) |
| Francisconi 40 | 222 / 377 | 137 / 369 | 1.59 (1.36 ,1.86) | 0.22 (0.15 ,0.29) |
| Random effects RR (I2 = 25%) | 1.43 (1.29 ,1.59) | 0.17 (0.12 ,0.22) | ||
| Overall pooled effect (I2 = 22%) | 1.30 (1.22 ,1.39) | 0.13 (0.10 ,0,16) | ||
| CI = confidence interval; RR = relative risk | ||||
| Global improvement of IBS symptoms | ||||
| Alosetron | ||||
| Camilleri 19 | 29 / 52 | 26 / 68 | 1.46 (0.99 ,2.15) | 0.18 (0.00 ,0.35) |
| Lembo 17 | 361 / 509 | 110 / 258 | 1.66 (1.43 ,1.94) | 0.28 (0.21 ,0.35) |
| Lembo 32 | 156 / 246 | 105 / 246 | 1.49 (1.25 ,1.77) | 0.21 (0.12 ,0.29) |
| Krause 35 | 80 / 177 | 55 / 176 | 1.45 (1.10 ,1.90) | 0.14 (0.04 ,0.24) |
| Random effects RR (I2 = 0%) | 1.55 (1.40 ,1.72) | 0.21 (0.14 ,0.28) | ||
| Cilansetron | ||||
| Bradette 22 | 221 / 395 | 151 / 397 | 1.47 (1.26 ,1.71) | 0.18 (0.11 ,0.25) |
| Miner 21 | 169 / 344 | 98 / 348 | 1.74 (1.43 ,2.13) | 0.21 (0.14 ,0.28) |
| Francisconi 40 | 207 / 377 | 111 / 369 | 1.83 (1.52 ,2.19) | 0.25 (0.18 ,0.32) |
| Random effects RR (I2 = 46%) | 1.66 (1.44 ,1.90) | 0.21 (0.17 ,0.25) | ||
| Overall pooled effect (I2 = 22%) | 1.60 (1.49 ,1.72) | 0.22 (0.18 ,0.25) | ||
CI = confidence interval
IBS = irritable bowel syndrome
RR = relative risk
Figure 2.
Patients responding to alosetron or cilansetron regarding “relief of abdominal pain and discomfort”
Subgroup analyses
There were three significant subgroup-treatment interactions (Table 5a). In these three interaction tests, the composition of the comparison groups largely overlapped. First, there was a lower RR in the alosetron subgroup (1.23 [1.15, 1.32]) compared to the cilansetron subgroup (1.43 [1.29, 1.59]) with a relative risk ratio (RR-ratio) of 0.86 [0.76, 0.98]. Second, there was a lower RR for studies including women only (1.23 [1.14, 1.32]) compared to studies including both genders or only men (1.39 [1.28, 1.51]) with a RR-ratio of 0.88 [0.76, 0.98]. Third, there was a lower RR for full papers (1.23 [1.14, 1.32]) compared to studies published as abstracts only (1.41 [1.29, 1.54]) with a RR-ratio of 0.87 [0.78, 0.98]. The test for interaction was not significant for the other subgroup analyses (Table 5a).
Table 5.
Subgroup analyses for the efficacy endpoints
| a) Endpoint: relief of abdominal pain and discomfort | |||
|---|---|---|---|
| Subgroups | Subgroup 1 (No of studies) RR [95%CI] | Subgroup 2 (No of studies) RR [95%CI] | Test of Interaction RR-Ratio[95%CI] |
| Drugs | Alosetron (7) 1.23 [1.15, 1.32] | Cilansetron (3) 1.43 [1.29, 1.59] | 0.86 [0.76, 0.98] |
| Endpoint definition | Yes/No response (9) 1.31 [1.22, 1.40] | Responder cut-off: 10% change in pain severity (1) 1.23 [0.98, 1.54] | 1.07 [0.84, 1.35] |
| Endpoint assessment | Reported average (6) 1.34 [1.21, 1.47] | Calculated average (4) 1.25 [1.15, 1.37] | 1.07 [0.94, 1.22] |
| Gender | Only female (5) 1.23 [1.14, 1.32] | Mixed gender (5) 1.39 [1.28, 1.51] | 0.88 [0.79, 0.99] |
| Treatment duration | 12 weeks (8) 1.33 [1.23, 1.42] | 24/48 weeks (2) 1.24 [1.07, 1.44] | 1.07 [0.91, 1.27] |
| Study population | Mixed NC- and D-IBS (2) 1.23 [1.13, 1.34] | D-IBS only (8) 1.35 [1.24, 1.47] | 0.91 [0.81, 1.03] |
| Publication Type | Full Paper (6) 1.23 [1.14, 1.32] | Abstract only (4) 1.41 [1.29, 1.54] | 0.87 [0.78, 0.98] |
| b) Endpoint: global improvement of IBS symptoms | |||
|---|---|---|---|
| Subgroups | Subgroup 1 (No of studies) RR [95%CI] | Subgroup 2 (No of studies) RR [95%CI] | Test of Interaction RR-Ratio[95%CI)] |
| Drugs | Alosetron (4) 1.55 [1.40, 1.72] | Cilansetron (3) 1.66 [1.44, 1.90] | 0.93 [0.79, 1.11] |
| Endpoint definition | Yes/No response (5) 1.61 [1.45, 1.78] | Responder cut-off: at least moderately improved (2) 1.58 [1.41, 1.78] | 1.02 [0.87, 1.19] |
| Endpoint assessment | Reported average (3) 1.66 [1.44, 1.90] | Calculated average (4) 1.55 [1.40, 1.72] | 1.07 [0.90, 1.27] |
| Gender | Only female (3) 1.56 [1.41, 1.74] | Mixed gender (4) 1.64 [1.46, 1.84] | 0.95 [0.81, 1.11] |
| Treatment duration | 12 weeks (6) 1.64 [1.51, 1.77] | 24 weeks (1) 1.33 [1.16, 1.52] | 1.23 [1.05, 1.44] |
| Study population | Mixed NC- and D-IBS (1) 1.46 [0.99,2.15] | D-IBS only (6) 1.60 [1.49, 1.73] | 0.91 [0.61, 1.35] |
| Publication Type | Full Paper (3) 1.57 [1.41, 1.76] | Abstract (4) 1.62[1.44, 1.82] | 0.97 [0.83, 1.14] |
CI = confidence interval
D-IBS = diarrhea-predominant irritable bowel syndrome
NC-IBS = non-constipated irritable bowel syndrome
RR = relative risk
Global improvement of IBS symptoms
Meta-analyses
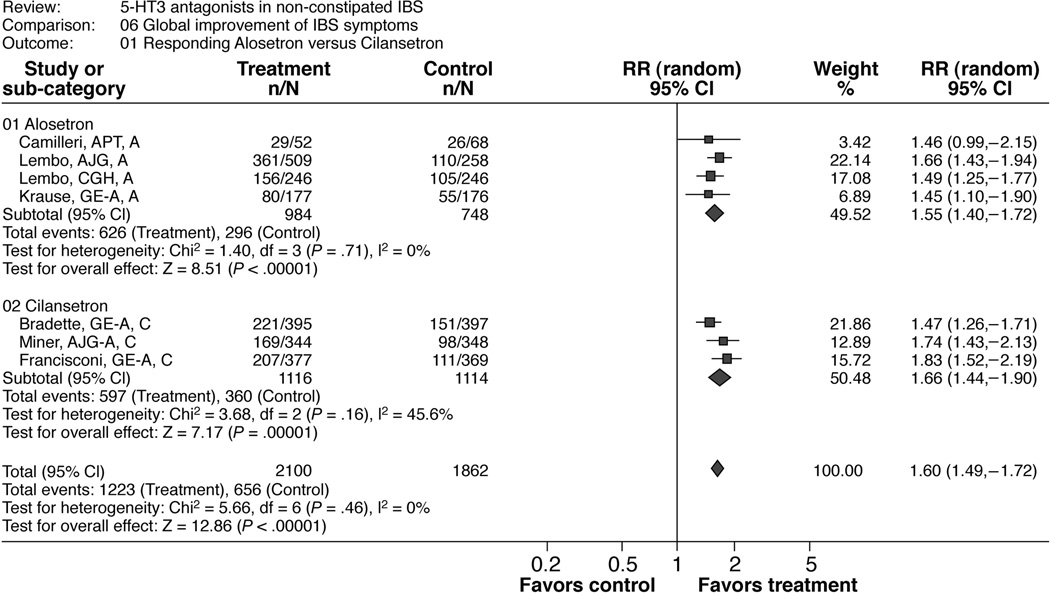
Table 3 and Figure 3 show the results of this meta-analysis and of the subgroup analyses by drugs (alosetron and cilansetron). The overall pooled estimated RR was 1.60 (1.49; 1.72) in favor of 5-HT3 antagonist treatment. The calculated NNT was 4.2 and the overall risk difference was 0.22 (0.18, 0.25). The results were consistent across studies (I2=0%).
Figure 3.
Patients responding to alosetron or cilansetron regarding “global improvement of IBS symptoms”
Subgroup analyses
There was a significant subgroup-treatment interaction for the treatment duration with a higher RR for this efficacy endpoint in the 12-week subgroup (1.64 [1.29, 1.51]) compared to the 24-week subgroup (1.33 [1.16, 1.52]) and a RR-ratio of 1.23 [1.05, 1.44]) (Table 5b). The tests of interactions were not significant for all other subgroup analyses including the publication type (full text or abstract; Table 5b).
Safety Endpoints
Constipation
Most cases of self-reported constipation in these trials were considered mild to moderate in severity. Approximately 10 to 43% of the participants who developed constipation withdrew from the trials for this reason. In all of these cases, constipation reportedly resolved rapidly after stopping the treatment. None of the studies reported serious complications due to constipation.
Meta-analyses
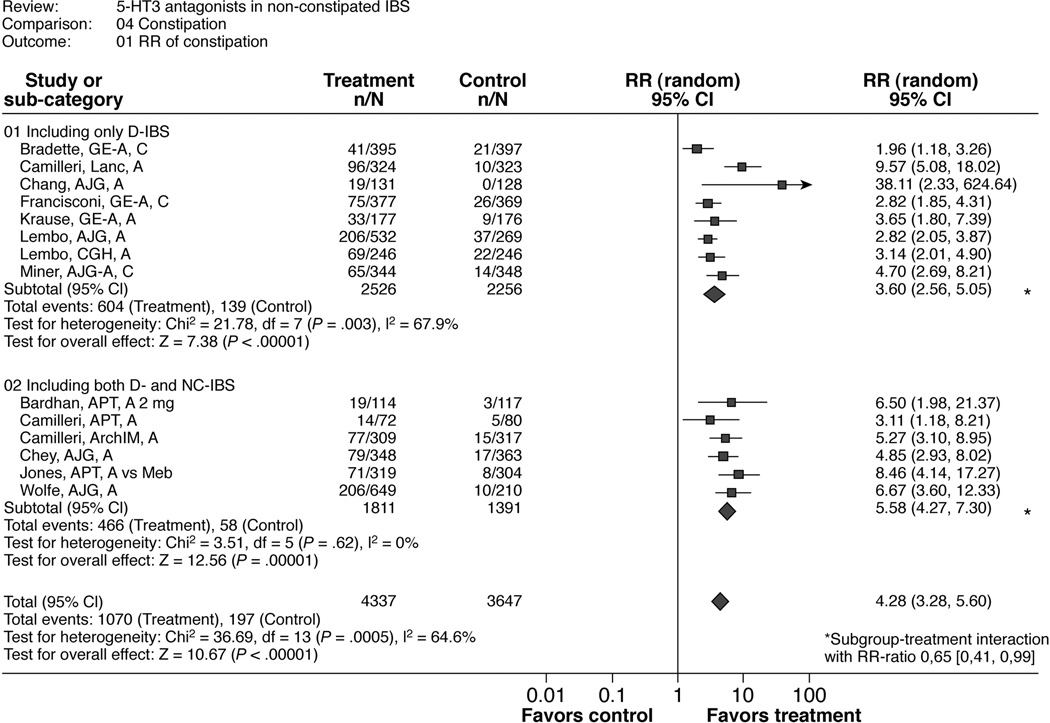
Table 4 and Figure 4 show the results for the RR of constipation and the RR-ratio of the subgroup analysis by study population (D-IBS only versus NC- and D-IBS). Participants were more likely to report constipation in the intervention group (pooled RR 4.28 [3.28; 5.60]) than in the placebo or mebeverine groups, although RR estimates were heterogeneous across trials (I2 = 65%). The calculated overall number needed to harm (NNH) was 4.7 and the overall risk difference was 0.17 (0.14, 0.21). The heterogeneity between trials may reflect differences in recording the occurrence of constipation. Constipation was typically reported as an adverse event based on the self-report of patients. However, this was not specified in all trials. In some studies, bowel diaries were also used to identify constipation.
Table 4.
Meta-analysis of the effect of alosetron and cilansetron on constipation, results by patient subgroup.
| Patient type Studies | Treatment group (No. with constipation / Total) | Control group (No. with constipation / Total) | RR (95% CI) | Risk Difference (95% CI) |
|---|---|---|---|---|
| Only D-IBS | ||||
| Bradette 22 | 41 / 395 | 21 / 397 | 1.96 (1.18, 3.26) | 0.05 (0.01, 0.09) |
| Camilleri 29 | 96 / 324 | 10 / 323 | 9.57 (5.08, 18.02) | 0.27 (0.21, 0.32) |
| Chang 38 | 19 / 131 | 0 / 128 | 38.1 (2.33, 624.6) | 0.15 (0.08, 0.21) |
| Francisconi 40 | 75 / 377 | 26 / 369 | 2.82 (1.85, 4.31) | 0.13 (0.08, 0.18) |
| Krause 35 | 33 / 177 | 9 / 176 | 3.65 (1.80, 7.39) | 0.14 (0.07, 0.2) |
| Lembo 17 | 206 / 532 | 37 / 269 | 2.82 (2.05, 3.87) | 0.25 (0.19, 0.31) |
| Lembo 32 | 69 / 246 | 22 / 246 | 3.14 (2.01, 4.90) | 0.19 (0.12, 0.26) |
| Miner 21 | 65 / 344 | 14 / 348 | 4.70 (2.69, 8.21) | 0.15 (0.10, 0.19) |
| Random effects RR (I2 = 68%) | 3.60 (2.56, 5.05) | 0.16 (0.11, 0.22) | ||
| D-IBS and NC-IBS | ||||
| Bardhan 31 | 19 / 114 | 3 / 117 | 6.50 (1.98, 21.4) | 0.14 (0.07, 0.22) |
| Camilleri 19 | 14 / 72 | 5 / 80 | 3.11 (1.18, 8.21) | 0.13 (0.03, 0.24) |
| Camilleri 30 | 77 / 309 | 15 / 317 | 5.27 (3.10, 8.95) | 0.2 (0.15, 0.26) |
| Chev 34 | 79 / 348 | 17 / 363 | 4.85 (2.93, 8.02) | 0.18 (0.13, 0.23) |
| Jones 20 | 71 / 319 | 8 / 304 | 8.46 (4.14, 17.3) | 0.2 (0.15, 0.25) |
| Wolfe 39 | 206 / 649 | 10 / 210 | 6.67 (3.60, 12.3) | 0.27 (0.22, 0.32) |
| Random effects RR (I2 = 0%) | 5.58 (4.27, 7.30) | 0.2 (0.16, 0.23) | ||
| Overall pooled effect (I2 = 65%) | 4.28 (3.28, 5.60) | 0.17 (0.14, 0.21) | ||
CI = confidence interval
D-IBS = diarrhea-predominant irritable bowel syndrom
NC-IBS = non-constipated irritable bowel syndrome
RR = relative risk
Figure 4.
Number of patients developing constipation
Subgroup analyses
Table 6 shows that the risk for constipation was lower in the studies including D-IBS only (RR 3.6 [2.56, 5.05]; risk difference 0.16 (0.11, 0.22); NNH 5.6) compared to the studies including both NC- and D-IBS (RR 5.58 [4.27, 7.3]; risk difference 0.2 (0.16, 0.23); NNH 4.5) with a significant RR-ratio of 0.65 [0.41, 0.99]. There was also a significant RR-ratio for abstracts versus full papers (0.60 [0.36, 0.98]). Since all abstracts included only D-IBS patients, there is an overlap with the subgroup analysis for the included study population. Post hoc analyses revealed that Chang et al 38, the only study exclusively enrolling men, reported no cases of constipation in the control group and a proportion of constipation in the treatment group similar to that observed in the other trials. Exclusion of this outlier trial yielded an RR for constipation of 4.19 [3.23; 5.45].
Table 6.
Subgroup analyses for constipation
| Subgroups | Subgroup 1 (No of studies) RR [95%CI] | Subgroup 2 (No of studies) RR [95%CI)] | Test of Interaction RR-Ration [95%CI)] |
|---|---|---|---|
| Drugs | Alosetron (11) 4.89 [3.6, 6.56] | Cilansetron (3) 2.92 [1.85, 4.63] | 1.68 [0.96, 2.91] |
| Gender | Only female (8) 4.85 [3.48, 6.76] | Mixed gender (6) 3.39 [2.19, 5.24] | 1.43 [0.85, 2.41] |
| Treatment duration | 12 weeks (11) 4.40 [3.27, 5.92] | 24/48 weeks (3) 3.94 [1.89, 8.23] | 1.12 [0.51, 2.47] |
| Study population | D-IBS only (8) 3.6 [2.56, 5.05] | Mixed NC- and D-IBS (6) 5.58 [4.27, 7.30] | 0.65 [0.41, 0.99] |
| Publication Type | Abstract only (4) 3.03 [2.11, 4.37] | Full Paper (10) 5.07 [3.62, 7.09] | 0.60 [0.36, 0.98] |
Ischemic colitis
There were 9 cases of at least possible ischemic colitis in the 5-HT3 antagonist treatment group (0.2%) and 0 in the control group (RR 16.01 [0.93, 275]; p=0.06).
DISCUSSION
Main Findings
This systematic review of large randomized controlled trials indicates that 5-HT3 antagonists, as a class, significantly improve abdominal pain and discomfort and global IBS symptoms in patients with NC- or D-IBS. Treatment response was consistent across a range of studies performed in different countries with an estimated pooled RR of 1.60 [1.49, 1.72], a NNT of 4.2 and a risk difference of 0.22 [0.18, 0.25] for the ‘improvement of global IBS symptoms’, and an estimated pooled RR of 1.30 [1.22, 1.39], a NNT of 7.7 and a risk difference of 0.13 [0.10, 0.16] for the ‘relief of abdominal pain and discomfort’. The effect of treatment appeared quite similar in men and women (RR of 1.39 for men or both genders vs. 1.23 for women only).
For the endpoint “relief of abdominal pain and discomfort”, there were significant subgroup-treatment interactions for the different drugs (alosetron vs. cilansetron), the included gender (female only vs. male or mixed gender) and the publication type (full paper vs. abstract). However, since all cilansetron studies included both men and women and were only available as abstracts, subgroup inferences are confounded. The only significant subgroup-treatment interaction influencing the RR for the other efficacy outcome “global improvement of IBS symptoms” was treatment duration, with a lower RR in the long-term group. This could suggest that the treatment effect might wear off over time. However, the fact that there was only one study with treatment for 24 weeks compared to 6 studies with treatment for 12 weeks does not allow a definite conclusion as to whether the treatment efficacy wanes with time.
Eligible trials reported constipation rates of 20–30% in the treatment group. The risk for constipation was lower in trials including only patients with D-IBS compared to the studies including patients with both NC- and D-IBS with a significant subgroup-treatment interaction, indicating that patients with D-IBS may have a more favorable benefit/risk ratio of 5-HT3-antagonist treatment.
Limitations and Strengths
Limitations and strengths of this systematic review pertain to the primary data and the review itself. It is a limitation of the primary data that four studies (including all three studies with cilansetron) were only available as abstracts at the time of this analysis and their methodological quality could not be fully evaluated. The majority of included trials (10 of 14), however, were high quality trials published in full. Another strength of the primary data is that all studies used comparable, standardized endpoints and similar trial designs.
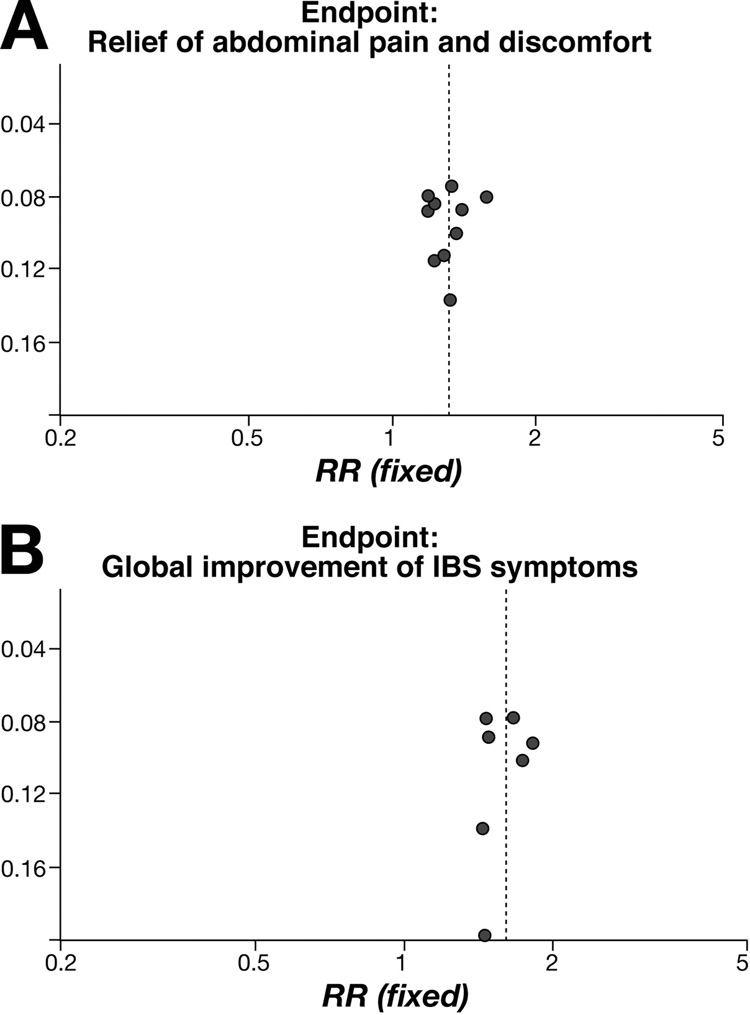
Regarding limitations of the review, we cannot exclude publication bias and reporting bias. To some extent, the involvement of one of the authors (M.C.) with the alosetron program allows us to be more confident that we included all conducted trials with this compound. Moreover, the funnel plot analysis did not indicate publication bias (Figure 5), although this type of analysis may sometimes be misleading 41. We still may have missed small trials and trials that were only published in abstract form. In terms of reporting bias, all eligible trials informed the safety outcomes, but one of which did not inform the efficacy outcomes 42. Another limitation is that the estimated relative risk is calculated on the basis of published papers and abstracts, particularly those on cilansetron. It is also unclear whether the greater efficacy in men than women is due, in part, to the typically smaller number of males in IBS studies that included both genders (even though this is not reported in the abstracts on cilansetron) or the fact that several studies with alosetron excluded males.
Figure 5.
Funnel plots
The main strength of this review is its comprehensive approach. First, we included four studies only available as abstracts. Exclusion of these abstracts would have led to increases in random error and in publication bias since these were large multicenter trials including more than 2500 patients and were of high quality regarding randomization and blinding and sufficiently defined the study populations and outcomes. Moreover, abstracts provided the only available data for the one of the two 5-HT3 antagonist drugs, cilansetron, evaluated for efficacy and safety in large multicenter IBS trials. Second, by including studies of both alosetron and cilansetron, this review has combined all available randomized controlled trials on 5-HT3 antagonists in the treatment of NC-IBS regarding the main clinical endpoints. This strengthens the validity of the results with regard to the effects of this class of drugs in the treatment of NC-IBS.
Comparison with Other Studies
The observation of similar efficacy of these agents in men and women in the meta-analysis is in contrast to earlier suggestions that this drug class might be ineffective in men, which were based on an early phase IIB trial in the drug development of alosetron that failed to show significant treatment effect in men 19. However, there were only few men included in that study and the negative results could be explained by a type II error, as openly acknowledged by the authors of that study. Subsequent phase II and III trials with alosetron were restricted to women and the evidence of efficacy in men had been missing until the post-approval study conducted by Chang et al 38. It is worth noting that this therapeutic efficacy in men had been predicted by a pharmacodynamic study in which men with IBS had colonic transit responses to alosetron consistent with the efficacy in women 26. Only recent trials with both alosetron and cilansetron have specifically included men. In summary, both the individual trials, as well as the pooled RR from this meta-analysis indicate effectiveness of 5-HT3 antagonists in men. It has been suggested that the beneficial effects of 5-HT3 antagonists on the global IBS symptom complex and IBS related abdominal pain may reflect the beneficial effects on decreasing diarrhea in these patients. However, other effects of this drug class, e.g. on visceral sensation43 and compliance,44 indicate additional positive effects responsible for the improvement of IBS symptoms. Moreover, other agents with pure antidiarrheal effects such as loperamide have failed to show beneficial effects on improving IBS related abdominal pain.45
In this review, we found a higher risk of constipation in patients receiving 5-HT3 antagonists, particularly in studies including patients with NC-IBS. Approximately 10 to 43% of the participants who developed constipation withdrew from the trials for this reason. In all of these cases, constipation reportedly resolved rapidly after stopping the treatment. After initial market introduction of alosetron in 2000, there have been reports of serious complications due to constipation in association with alosetron. A recent meta-analysis of clinical trials and post-marketing surveillance data focusing on serious adverse events showed no significant difference in the rate of serious complications of constipation between alosetron- and placebo-using patients46.
In our meta-analysis, eligible trials have reported 9 cases of at least suspected ischemic colitis in drug-treated patients (estimated incidence 0.2 %) versus none in the comparator group. All cases resolved without sequelae. The recent meta-analysis of clinical trials and post-marketing surveillance data of alosetron reported an incidence of 0.15% with a total of 19 cases of ischemic colitis that all resolved without sequelae. The etiology, pathophysiology and experimental basis for the development of ischemic colitis with this class of compounds remain unclear 47.
Implications for Research and Clinical Policy
This systematic review and meta-analyses aimed to comprehensively assess clinically relevant effects of 5-HT3 antagonists in NC- and D-IBS and was able to bring to the fore data that up to this point had only been partially reported. For instance, thanks to our inclusion of full text and abstracts, we can infer with some confidence that there is a class effect despite the reporting delays associated with the publication of the 3 cilansetron trials in full (their abstracts were published 2 to 3 years ago). Furthermore, we provide more precise estimates of the risk of constipation and ischemic colitis from these trials. In all, these data, alongside the relative merits of lifestyle behavioral interventions and treatment costs, can help patients and clinicians make informed treatment decisions about the use of these agents for NC- and D-IBS.
CONCLUSIONS
This systematic review and meta-analysis finds that 5-HT3 antagonists improve abdominal pain and discomfort, and global IBS symptoms in men and women with non-constipated and diarrhea-predominant IBS. This evidence is consistent across agents within this class and across a broad range of participants in clinical trials. Constipation is a common, but usually mild to moderate side effect of the treatment with 5-HT3 antagonists. The risk for constipation is lower in patients with predominance of diarrhea and this emphasizes the importance of assessing the individual benefit/risk ratio before starting treatment. Ischemic colitis is a rare adverse event with an incidence of approximately 0.2%. Ischemic colitis and the complications of constipation are still of concern to the regulatory agencies and have led to restriction of this drug class to patients with severe, refractory D-IBS who have failed to respond to conventional treatment.
Acknowledgements
Dr. Andresen is supported by the Gustav und Catharina Schuerfeld Foundation, Hamburg, Germany. Dr. Camilleri is funded in part by grants RO1- DK54681 and K24-DK02638 from National Institutes of Health. The excellent secretarial support of Mrs. Cindy Stanislav is gratefully acknowledged.
Abbreviations used
- CI
confidence interval
- GI
gastrointestinal
- 5-HT
5-hydroxytryptamine (serotonin)
- IBS
irritable bowel syndrome
- NC
non-constipated
- RR
relative risk
- RD
Risk difference
- NNT
number needed to treat
- NNH
number needed to harm
Appendix I
Search strategy
The key words were: serotonin, 5-HT, 5-HT3 antagonist, alosetron, cilansetron, irritable bowel syndrome, therapy, clinical trial, diarrhea-predominant, and functional bowel disease.
1 Step: single keywords: alosetron, cilansetron
2. Step: combinations of 2 key words:
5-HT3 antagonist AND clinical trial, 5-HT3 antagonist AND irritable bowel syndrome, 5-HT3 antagonist AND functional bowel disease, 5-HT3 antagonist AND therapy,
alosetron AND clinical trial, alosetron AND irritable bowel syndrome, alosetron AND functional bowel disease, alosetron AND therapy
cilansetron AND clinical trial, cilansetron AND irritable bowel syndrome, cilansetron AND functional bowel disease, cilansetron AND therapy
3. Step: combination of 3 key words:
5-HT AND irritable bowel syndrome AND clinical trial, 5-HT AND irritable bowel syndrome AND therapy
5-HT AND functional bowel disease AND clinical trial, 5-HT AND functional bowel disease AND therapy
serotonin AND irritable bowel syndrome AND clinical trial, serotonin AND irritable bowel syndrome AND therapy
serotonin AND functional bowel disease AND clinical trial, serotonin AND functional bowel disease AND therapy
diarrhea-predominant AND irritable bowel syndrome AND clinical trial, diarrhea-predominant AND irritable bowel syndrome AND therapy
diarrhea-predominant AND functional bowel disease AND clinical trial, diarrhea-predominant AND functional bowel disease AND therapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. V. Andresen has served as a consultant for Solvay, the manufacturer of cilansetron, from 2004 to 2005 and was employed in the medical department of the German affiliate of GlaxoSmithKline, the manufacturer of alosetron, from 2000 to 2001. Dr. M. Camilleri received research support in 2006 to 2007 for a single-center pharmacodynamic study with a drug not in the 5-HT3 antagonist class from GlaxoSmithKline, manufacturer of alosetron, and has served as a consultant in 2006, receiving annually less than the federal threshold for significant financial conflict of interest.
Dr. J Keller has served as a consultant for GlaxoSmithKline from 2000 to 2001.
Dr. P. Layer has served as a consultant for GlaxoSmithKline from 2000 to 2001 and for Solvay from 2004 to 2005.
REFERENCES
- 1.Drossman DA, Sandler RS, McKee DC, Lovitz AJ. Bowel patterns among subjects not seeking health care. Use of a questionnaire to identify a population with bowel dysfunction. Gastroenterology. 1982;83:529–534. [PubMed] [Google Scholar]
- 2.Talley NJ, Zinsmeister AR, Van Dyke C, Melton LJ., 3rd Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology. 1991;101:927–934. doi: 10.1016/0016-5085(91)90717-y. [DOI] [PubMed] [Google Scholar]
- 3.Jones R, Lydeard S. Irritable bowel syndrome in the general population. Bmj. 1992;304:87–90. doi: 10.1136/bmj.304.6819.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, Whitehead WE, Janssens J, Funch-Jensen P, Corazziari E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 5.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45 Suppl 2:II43–II47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foxx-Orenstein AE, Kuemmerle JF, Grider JR. Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and guinea pig intestine. Gastroenterology. 1996;111:1281–1290. doi: 10.1053/gast.1996.v111.pm8898642. [DOI] [PubMed] [Google Scholar]
- 7.Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000;95:2698–2709. doi: 10.1111/j.1572-0241.2000.03177.x. [DOI] [PubMed] [Google Scholar]
- 8.Moss HE, Sanger GJ. The effects of granisetron, ICS 205-930 and ondansetron on the visceral pain reflex induced by duodenal distension. Br J Pharmacol. 1990;100:497–501. doi: 10.1111/j.1476-5381.1990.tb15836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banner SE, Sanger GJ. Differences between 5-HT3 receptor antagonists in modulation of visceral hypersensitivity. Br J Pharmacol. 1995;114:558–562. doi: 10.1111/j.1476-5381.1995.tb13263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miura M, Lawson DC, Clary EM, Mangel AW, Pappas TN. Central modulation of rectal distension-induced blood pressure changes by alosetron, a 5-HT3 receptor antagonist. Dig Dis Sci. 1999;44:20–24. doi: 10.1023/a:1026633629141. [DOI] [PubMed] [Google Scholar]
- 11.Kozlowski CM, Green A, Grundy D, Boissonade FM, Bountra C. The 5-HT(3) receptor antagonist alosetron inhibits the colorectal distention induced depressor response and spinal c-fos expression in the anaesthetised rat. Gut. 2000;46:474–480. doi: 10.1136/gut.46.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delvaux M, Louvel D, Mamet JP, Campos-Oriola R, Frexinos J. Effect of alosetron on responses to colonic distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1998;12:849–855. doi: 10.1046/j.1365-2036.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuo B, Camilleri M, Burton D, Viramontes B, McKinzie S, Thomforde G, O'Connor MK, Brinkmann BH. Effects of 5-HT(3) antagonism on postprandial gastric volume and symptoms in humans. Aliment Pharmacol Ther. 2002;16:225–233. doi: 10.1046/j.1365-2036.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- 14.Houghton LA, Foster JM, Whorwell PJ. Alosetron, a 5-HT3 receptor antagonist, delays colonic transit in patients with irritable bowel syndrome and healthy volunteers. Aliment Pharmacol Ther. 2000;14:775–782. doi: 10.1046/j.1365-2036.2000.00762.x. [DOI] [PubMed] [Google Scholar]
- 15.Stacher G, Weber U, Stacher-Janotta G, Bauer P, Huber K, Holzapfel A, Krause G, Steinborn C. Effects of the 5-HT3 antagonist cilansetron vs placebo on phasic sigmoid colonic motility in healthy man: a double-blind crossover trial. Br J Clin Pharmacol. 2000;49:429–436. doi: 10.1046/j.1365-2125.2000.00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camilleri M. Pharmacology and clinical experience with alosetron. Expert Opin Investig Drugs. 2000;9:147–159. doi: 10.1517/13543784.9.1.147. [DOI] [PubMed] [Google Scholar]
- 17.Lembo T, Wright RA, Bagby B, Decker C, Gordon S, Jhingran P, Carter E. Alosetron controls bowel urgency and provides global symptom improvement in women with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2001;96:2662–2670. doi: 10.1111/j.1572-0241.2001.04128.x. [DOI] [PubMed] [Google Scholar]
- 18.Watson ME, Lacey L, Kong S, Northcutt AR, McSorley D, Hahn B, Mangel AW. Alosetron improves quality of life in women with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2001;96:455–459. doi: 10.1111/j.1572-0241.2001.03525.x. [DOI] [PubMed] [Google Scholar]
- 19.Camilleri M, Mayer EA, Drossman DA, Heath A, Dukes GE, McSorley D, Kong S, Mangel AW, Northcutt AR. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Aliment Pharmacol Ther. 1999;13:1149–1159. doi: 10.1046/j.1365-2036.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 20.Jones RH, Holtmann G, Rodrigo L, Ehsanullah RS, Crompton PM, Jacques LA, Mills JG. Alosetron relieves pain and improves bowel function compared with mebeverine in female nonconstipated irritable bowel syndrome patients. Aliment Pharmacol Ther. 1999;13:1419–1427. doi: 10.1046/j.1365-2036.1999.00678.x. [DOI] [PubMed] [Google Scholar]
- 21.Miner P, Stanton DB, Carter F, Caras S, Krause G, Steinborn C. Cilansetron in irritable bowel syndrome with diarrhea predominance (IBS-D): efficacy and safety in a 3-month US study. Am J Gastroenterol. 2004:99. [Google Scholar]
- 22.Bradette M, Moennikes H, Carter F, Krause G, Caras S, Steinborn C. Cilansetron in Irritable Bowel Syndrome with Diarrhea Predominance (IBS-D): Efficacy and Safety in a 6 months global study. Gastroenterology. 2004;126:A-42. [Google Scholar]
- 23.Veldhuyzen van Zanten SJ, Talley NJ, Bytzer P, Klein KB, Whorwell PJ, Zinsmeister AR. Design of treatment trials for functional gastrointestinal disorders. Gut. 1999;45 Suppl 2:II69–II77. doi: 10.1136/gut.45.2008.ii69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camilleri M, Mangel AW, Fehnel SE, Drossman DA, Mayer EA, Talley NJ. Primary endpoints for irritable bowel syndrome trials: a review of performance of endpoints. Clin Gastroenterol Hepatol. 2007;5:534–540. doi: 10.1016/j.cgh.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Olden K, DeGarmo RG, Jhingran P, Bagby B, Decker C, Markowitz M, Carter E, Bobbitt W, Dahdul A, DeCastro E, Gringeri L, Johanson J, Levinson L, Mula G, Poleynard G, Stoltz RR, Truesdale R, Young D. Patient satisfaction with alosetron for the treatment of women with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2002;97:3139–3146. doi: 10.1111/j.1572-0241.2002.07111.x. [DOI] [PubMed] [Google Scholar]
- 26.Viramontes BE, Camilleri M, McKinzie S, Pardi DS, Burton D, Thomforde GM. Gender-related differences in slowing colonic transit by a 5-HT3 antagonist in subjects with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2001;96:2671–2676. doi: 10.1111/j.1572-0241.2001.04138.x. [DOI] [PubMed] [Google Scholar]
- 27.Cremonini F, Delgado-Aros S, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Neurogastroenterol Motil. 2003;15:79–86. doi: 10.1046/j.1365-2982.2003.00389.x. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 29.Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035–1040. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- 30.Camilleri M, Chey WY, Mayer EA, Northcutt AR, Heath A, Dukes GE, McSorley D, Mangel AM. A randomized controlled clinical trial of the serotonin type 3 receptor antagonist alosetron in women with diarrhea-predominant irritable bowel syndrome. Arch Intern Med. 2001;161:1733–1740. doi: 10.1001/archinte.161.14.1733. [DOI] [PubMed] [Google Scholar]
- 31.Bardhan KD, Bodemar G, Geldof H, Schutz E, Heath A, Mills JG, Jacques LA. A double-blind, randomized, placebo-controlled dose-ranging study to evaluate the efficacy of alosetron in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2000;14:23–34. doi: 10.1046/j.1365-2036.2000.00684.x. [DOI] [PubMed] [Google Scholar]
- 32.Lembo AJ, Olden KW, Ameen VZ, Gordon SL, Heath AT, Carter EG. Effect of alosetron on bowel urgency and global symptoms in women with severe, diarrhea-predominant irritable bowel syndrome: analysis of two controlled trials. Clin Gastroenterol Hepatol. 2004;2:675–682. doi: 10.1016/s1542-3565(04)00284-8. [DOI] [PubMed] [Google Scholar]
- 33.Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. Bmj. 2001;323:42–46. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chey WD, Chey WY, Heath AT, Dukes GE, Carter EG, Northcutt A, Ameen VZ. Long-term safety and efficacy of alosetron in women with severe diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2004;99:2195–2203. doi: 10.1111/j.1572-0241.2004.30509.x. [DOI] [PubMed] [Google Scholar]
- 35.Krause R, Ameen V, Gordon S, West M, Heath A, Perschy T, Carter E. Safety and Efficacy of 0.5 mg Qd, 1 mg Qd, and 1 mg Bid Alosetron Hydrochloride in Women with Chronic, Severe Diarrhea-Predominant IBS (IBS-D) Gastroenterology. 2006;130:A-320. [Google Scholar]
- 36.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. Bmj. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang L, Ameen VZ, Dukes GE, McSorley DJ, Carter EG, Mayer EA. A dose-ranging, phase II study of the efficacy and safety of alosetron in men with diarrhea-predominant IBS. Am J Gastroenterol. 2005;100:115–123. doi: 10.1111/j.1572-0241.2005.40365.x. [DOI] [PubMed] [Google Scholar]
- 39.Wolfe SG, Chey WY, Washington MK, Harding J, Heath AT, McSorley DJ, Dukes GE, Hunt CM. Tolerability and safety of alosetron during long-term administration in female and male irritable bowel syndrome patients. Am J Gastroenterol. 2001;96:803–811. doi: 10.1111/j.1572-0241.2001.03626.x. [DOI] [PubMed] [Google Scholar]
- 40.Francisconi CF, Drossman D, Mayer EA, Carter F, Caras S, Krause G, Steinborn C. Relief of Symptoms in Iritable Bowel Syndrome with Diarrhea Predominance (IBS-D) Patients Decreases after Blinded Withdrawal of Cilansetron Treatment. Gastroenterology. 2005:128. [Google Scholar]
- 41.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. Bmj. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furukawa TA, Watanabe N, Omori IM, Montori VM, Guyatt GH. Association between unreported outcomes and effect size estimates in Cochrane meta-analyses. Jama. 2007;297:468–470. doi: 10.1001/jama.297.5.468-b. [DOI] [PubMed] [Google Scholar]
- 43.Mayer EA, Berman S, Derbyshire SW, Suyenobu B, Chang L, Fitzgerald L, Mandelkern M, Hamm L, Vogt B, Naliboff BD. The effect of the 5-HT3 receptor antagonist, alosetron, on brain responses to visceral stimulation in irritable bowel syndrome patients. Aliment Pharmacol Ther. 2002;16:1357–1366. doi: 10.1046/j.1365-2036.2002.01287.x. [DOI] [PubMed] [Google Scholar]
- 44.Delvaux M, Louvel D, Mamet JP, Campos-Oriola R, Frexinos J. Effect of alosetron on responses to colonic distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1998;12:849–855. doi: 10.1046/j.1365-2036.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- 45.Efskind PS, Bernklev T, Vatn MH. A double-blind placebo-controlled trial with loperamide in irritable bowel syndrome. Scand J Gastroenterol. 1996;31:463–468. doi: 10.3109/00365529609006766. [DOI] [PubMed] [Google Scholar]
- 46.Chang L, Chey WD, Harris L, Olden K, Surawicz C, Schoenfeld P. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol. 2006;101:1069–1079. doi: 10.1111/j.1572-0241.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- 47.Camilleri M. Is there an experimental basis for the development of ischaemic colitis as a result of 5-HT(3) antagonist treatment? Neurogastroenterol Motil. 2007;19:77–84. doi: 10.1111/j.1365-2982.2006.00861.x. [DOI] [PubMed] [Google Scholar]