Abstract
The mammalian target of rapamycin (mTOR) is a protein kinase that regulates protein translation, cell growth and apoptosis. Recently, there has been an enormous increase in our understanding on molecular mechanisms underlying the therapeutics of rapamycin in cancer. Alterations in the pathway regulating mTOR occur in many solid malignancies including prostate, bladder and kidney cancer; and in-vitro and in-vivo models of prostate and bladder cancer have established the importance of the mTOR pathway in control of cancer progression and metastasis. Temsirolimus (Torisel™) and everolimus (RAD-001), two ester analogues of rapamycin, as well as rapamycin itself have clear antitumor activity in in-vitro and in-vivo models, and are under clinical trial investigations for prostate and bladder cancer. Phase II and III trials have already established the clinical efficacy of temsirolimus in renal cancer, and current renal trials are evaluating the combined effects of VEGF and mTOR inhibition. Ongoing studies in prostate and bladder cancer will soon define the activity and safety profiles of everolimus and temsirolimus. Recent molecular advances have uncovered a startling complexity in the macromolecular function of mTOR complexes, with the identification of new mTOR partners (raptor, rictor, FKBP38, PRAS40 and mSIN1), putative cancer therapeutic/prognostic targets for future clinical trials.
Keywords: prostate, rapamycin, everolimus, RAD-001, temsirolimus, CCI-779, Kidney, neoadjuvant, TGF-β, Akt, IGF-I, mTOR, Smad
Introduction
Recent advances in cancer research have drawn increased interest in the use of mTOR inhibitors to treat a variety of cancers, consistent with the key roles of mTOR in cell survival, growth, protein synthesis, cellular metabolism and angiogenesis. mTOR, which is constitutively activated in many cancers by deregulated activation of oncogenes or loss of tumor suppressor genes, functions in macromolecular complexes that are either rapamycin sensitive or rapamycin insensitive. The rapamycin-sensitive complex of mTOR functions in a negative feedback loop to suppress mitogen activity through insulin receptor substrate-1 (IRS-1). Thus, inhibition of mTOR by rapamycin activates both cytostatic and cell survival responses. Combination therapeutics with agents that counteract this survival effect will likely synergize with mTOR inhibitors for tumor kill. Therefore, further understanding of the molecular mechanisms that regulate the function or mediate the activity of mTOR will undoubtedly impact on the therapeutics of numerous malignancies.
Two mTOR inhibitors (temsirolimus [Torisel™], everolimus [RAD-001]) have already undergone clinical testing in several hematological and solid malignancies. Recently in fact, temsirolimus was granted Food and Drug Administration (FDA) approval for the treatment of metastatic renal cell carcinoma (mRCC) patients. Preliminary phase II data demonstrating the antitumor activity of everolimus in mRCC has led to the design of a large multi-institutional phase III trial evaluating the activity of everolimus plus best supportive care (BSC) versus BSC in patients with previously treated mRCC. Similarly, studies on the molecular pathogenesis of prostate cancer (PCA) and transitional cell carcinoma of the urothelium (TCC) have also identified that the mTOR signaling pathway promotes tumor growth and proliferation in such cells. Therefore, several phase II trials evaluating these novel compounds in non-renal genitourinary (GU) malignancies are currently underway, and will further define the importance of this pathway as a therapeutic target in these solid tumors. In this review, we will discuss the biologic rationale behind mTOR inhibition in solid tumors as well as the current clinical data supporting their use in patients with GU tumors.
Discovery of Rapamycin, Rapamycin Analogues and mTOR
Rapamycin (also known as sirolimus or rapamune), which was first identified as a 914.2 kDa anti-fungal bacterial macrolide isolated from Streptomyces hygroscopicus (1), later became the preferred immunosuppressant for kidney transplantation since it was shown to be mildly immunosuppressive but did not enhance tumor incidence in contrast to cyclosporin A (2). Rapamycin was rather quickly recognized to have potent and broad anti-tumor activity, entering the scene for cancer therapy (2). More enthusiasm has been ignited by recent studies demonstrating that rapamycin can sensitize cancers to chemo- or radiation therapies (3). Rapamycin and a number of its structural derivatives (temirolimus [Wyeth pharmaceuticals], everolimus [Novartis pharmaceuticals], and AP-23573 [Ariad Pharmaceuticals]) are currently under robust and encouraging investigational use in Phase I and II Clinical Trials for a variety of cancers (4).
The biological responses of rapamycin appear to mostly depend on mTOR, which is a 290 kDa (2549 amino acid) serine/threonine kinase with many functional and protein-binding domains. mTOR controls protein synthesis in response to the sufficiency of certain cellular nutrients including amino acids such as leucine (5). The kinase domain of mTOR is located in its C-terminal region between an FATC domain and an FKBP-rapamycin binding (FRB) domain, and mTOR contains HEAT repeats its N-terminal region (6). Rapamycin binds to and suppresses mTOR only through first associating to the immunophilin FKBP12 (7). One of the down-stream targets of mTOR is the eukaryotic initiation factor 4E (eIF4E) binding protein (4E-BP1), whose phosphorylation by mTOR stimulates protein translation through releasing eIF4E from 4E-BP1 (8). Regulation of protein synthesis via mTOR also occurs through phosphorylation of p70S6K, a key regulator of cell growth, which phosphorylates the S6 40S ribosomal subunit. The mTOR associated small G protein Rheb (ras homolog enriched in brain) is an upstream regulator of mTOR-dependent phosphorylation of both 4E-BP1 and p70S6K (9).
The Signaling Pathway
Macromolecular Complexes of mTOR
mTOR functions in macromolecular complexes with at least five other proteins. All complexes appear to have GβL (also called mLST8) (10) and either raptor (11) or rictor (12-14) (Fig. 1). Only the complex containing raptor (mTORC1) is directly suppressed by rapamycin. 4E-BP1 and p70 S6 kinase each associate to mTORC1 through their five amino acid TOS signal motif (15) (16) and are phosphorylated by the mTORC1 complex. The FKBP12/rapamycin complex binds to and then disrupts mTORC1 by preventing the interaction of mTOR with raptor. Raptor binds directly to proteins possessing a TOS motif, and thus serves as the docking site for both 4-EBP1 and p70 S6 kinase on mTORC1. mTOR complex containing rictor (TORC2) was shown to phosphorylate Akt at Ser473, as demonstrated in an elegant study where raptor, rictor, and mTOR were individually silenced by lentiviral-mediated shRNA in a variety of tumor cell lines (13). Silencing of either rictor or mTOR reduced the phosphorylation of Akt at Ser473, providing the first evidence of mTORC2 as the long-sought PDK2. Interesting, silencing raptor instead enhanced the phosphorylation of Akt at Ser473 in those cell lines, which may have resulted from increased levels of mTORC2 favored simply by the law of mass action. In that study, silencing of rictor likewise reduced the phosphorylation of Akt at Thr308, but led to the elevation of Akt levels.
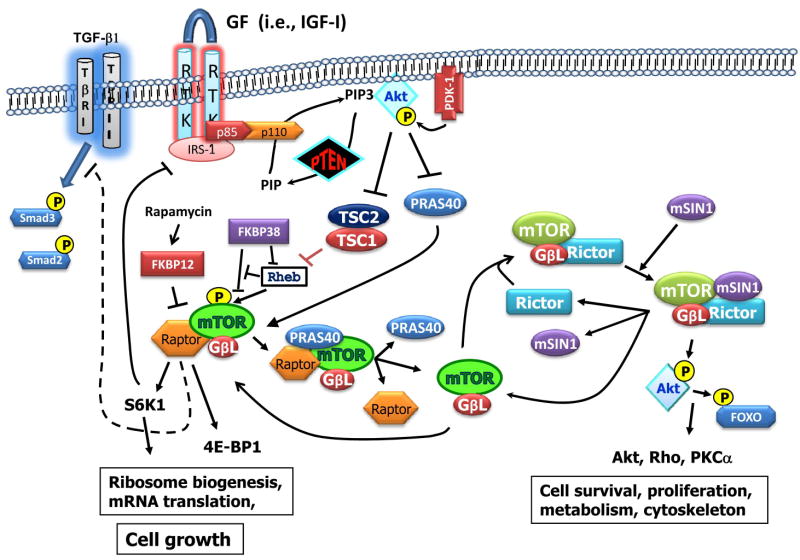
Figure 1. New partners in the mTOR signaling pathway.
mTOR signals through two functionally distinct macromolecular complexes, mTORC1 (raptor complex) and mTORC2 (rictor complex), which are rapamycin-dependent and rapamycin-independent, respectively. The activity of the mTORC1 complex is negatively regulated by the TSC1/TSC2 tumor suppressor complex and by PRAS40. TSC1/TSC2 inactivates Rheb, an enzyme critical to activation of mTORC1. Another protein that inactivates mTORC1 is FKBP38, which interacts with both Rheb and mTOR and interferes with Rheb's ability to interact with and activate mTORC1. FKBP38 is structurally similar to FKBP12, the protein that mediates the binding of rapamycin to mTORC1. Both TSC2 and PRAS40 are substrates of Akt kinase which are inactivated upon phosphorylation by Akt. Through this mechanism, the activation of PI3K and Akt (through growth factor signaling) or by loss of PTEN, leads to activation of mTORC1, which promotes cell growth. Rapamycin and rapamycin analogues through association with FKBP12 disrupt the mTORC1 complex, leading to growth suppression. However, disruption of may promote the assembly of mTORC2 following the interaction of mTOR with rictor and mSin1, thus counteracting the anti-tumor activity of current mTOR inhibitors. Moreover, disruption of mTORC1 by rapamycin may relieve negative feedback suppression on mitogen activation of Akt, leading to elevation of cell survival signals. The ratio of raptor to rictor and the relative levels of PRAS40, TSC2, mSIN1, FKBP38 and IRS-I in tumor cells may be useful cancer prognostic markers and therapeutic targets.
Although once thought to be necessary for full activation of Akt, phosphorylation of Akt at Ser 473 appears to dictate the substrate specificity of Akt. For example, phosphorylation of this site enables Akt to phosphorylate and thus inactivates the transcriptional factor/apoptosis-inducer proteins, FOXO (17). However, studies with rictor knockout mice suggest that Akt Ser473 phosphorylation site does not influence the ability of Akt to phosphorylate and inactivate TSC2 (17-19), a key negative regulator of mTORC1.
From PTEN loss to Akt Activation
Loss of function of the tumor suppressor gene PTEN (phosphatase and tensin homologue deleted on chromosome 10) is believed to play an important role in the etiology of numerous cancers including in about 50% of advanced prostate cancers. PTEN is a lipid phosphatase (20) that suppresses IGF-IR signaling by dephosphorylating phosphatidylinositol (PtdIns){3,4,5}P3 to PtdIns{4,5}P2, leading to inhibition of phosphatidylinositol-3-kinase (PI3K) and downstream Akt responses. Loss or reduced expression of PTEN appears to promote tumor growth and progression predominantly through elevating the levels of PtdIns{3,4,5}P3 and PtdIns{3,4}P2, which associate to the plasma membrane where they recruit Akt and 3-phosphoinositide-dependent protein kinase-1 (PDK-1), respectively, through binding to their PH domains. PDK-1, which a constitutively active kinase, then physically interacts with Akt and activates Akt by phosphorylating Thr308 (21). Akt and PDK-1 mediate most if not all the anti-apoptotic effects of IGF-I (21), a mitogen significantly implicated in the etiology of prostate cancer (22). Once activated, Akt is transported to cytosolic and nuclear compartments where it phosphorylates numerous proteins having the consensus motif RXRXXS/T, many of which are involved in cell growth and apoptosis (23). Although, most studies have focused on the role of a single Akt isoform (Akt1) in cancer, the other two isoforms (Akt2 and Akt3) have unique tissue expression patterns and are also likely to have unique roles in cancer progression (24-27).
The mTORC1 Complex
mTORC1 is principally activated through Akt kinase (21). However, the mechanism by which Akt activates mTORC1 appears to be controlled by at least two sets of Akt substrates, one involving the Tuberous Sclerosis Complex, TSC2/Tuberin (28, 29) (see Fig. 1). TSC2 and TSC1/ Harmatin form heterodimers that suppress mTORC1 kinase activity through inactivation of the small GTP-binding protein Rheb (30, 31), a protein shown to be critical to the activation of mTORC1. TSC2, which has GTPase activity, inactivates mTORC1 by converting the GTP to GDP on Rheb. The direct phosphorylation of TSC2 by Akt at multiple serines promotes the proteosomal degradation of TSC2, leading to elevation of the GTP-bound Rheb and the subsequent activation of mTORC1 kinase (30). A recent study showed that the FKBP12 homologue FKBP38 inhibits Rheb from activating mTORC1 by two independent mechanisms, one involving a direct association of Rheb to FKBP38 and the other involving the binding of FKBP38 to mTOR at the Rheb binding site (Fig. 1) (32). Similarly, overexpression of Rheb was shown to activate mTORC1 by reversing the inhibitory effect on FKBP38 on mTORC1, suggesting that Rheb activates mTORC1 through reversing the inhibition of mTORC1 by endogenous FKBP38. This study provides fresh insight on the mechanism by which rapamycin may inhibit mTORC1, as the rapamycin/FKBP12 complex may be mimicking the natural ligand FKBP38.
Recent evidence support that a proline-rich Akt substrate of 40 kDa (PRAS40), is also involved in Akt-dependent activation of mTOR (33, 34). Before getting phosphorylated by Akt, PRAS40 binds to raptor and sequesters raptor from mTORC1 (33, 35); through this mechanism PRAS40 disrupts the mTORC1 complex similar to the impact of rapamycin (Fig. 1). The interaction of PRAS40 with raptor competes with raptor's interaction with S6K1 and 4E-BP1 (35, 36). Moreover, this interaction of PRAS40 is very specific for the mTORC1 complex, as PRAS40 does not associate with or disrupts TORC2. Ironically, rapamycin has been shown to decrease the co-immunoprecipitation of PRAS40 with mTOR, suggesting that rapamycin/FKBP12 competes with PRAS40 for binding to mTOR (35). However, the inhibitory activity of PRAS40 on mTORC1 is lost following the activation of Akt, which promotes proteosomal degradation of PRAS40 following phosphorylation of Thr246 (33). The mechanism for such proteosomal dependent degradation is not clear, but may involve 14-3-3, as once phosphorylated by Akt, PRAS40 binds to 14-3-3. Thus, the constitutive activation of Akt occurring in many cancers favors the formation of mTORC1 over that of mTORC2, and hence therapeutic responses to rapamycin. Taken together, these studies indicate that, PRAS40 may play a pivotal role in the mechanism by which Akt activates mTORC1. Interestingly, PRAS40 has been recently reported to also be a substrate or downstream target of mTORC1, which phosphorylates PRAS40 at Ser183, and occurs through a mechanism that is blocked by rapamycin (36). Mutation of Ser183 to Asp, to mimic this phosphorylated form of PRAS40, was shown to disrupt its interaction with raptor, whereas mutation to Ala retains association to raptor, suggesting that phosphorylation of PRAS40 at Ser183 by mTORC1 disables PRAS40 from binding to raptor and disrupting mTORC1 (36). Although several groups have shown that PRAS40 disrupts mTORC1, one group recently reported that PRAS40 may function as a down-stream mediator of TOR signaling, since knockdown of PRAS40 by siRNA impaired insulin-induced phosphorylation S6 and 4E-BP1 (15). However, the in vivo role of PRAS40 in controlling or mediating mTOR responses awaits the development of knockout mice. The role of PRAS40 in controlling oncogenesis and tumor progression remains a relatively unexplored and promising area of future investigation (37, 38).
In line with a finely regulated homeostatic control mechanism, mTORC1 is under negative feedback control (39). One such negative feedback reported by a number of investigators occurs through S6K and IRS-1. Once activated by mTORC1, S6K phosphorylates and inactivates IRS-1(40, 41) (Fig. 1). Inactivation of IRS-1 quenches growth factors, particularly IGF-I, from activating the PI3K/Akt/mTORC1 pathway. Although adaptive in normal tissues, this negative feedback may account for resistance of tumors to killing by rapamycin, as inhibition of mTORC1 activates Akt which in turn inactivates apoptotic proteins. Thus, strategies that prevent the activation of IRS-1 or Akt by rapamycin are likely to have synergistic activity on tumor kill (40, 42-44).
The mTORC2 complex
Another recently identified mTOR binding protein is mSIN1, which selectively associates with the mTORC2 but does not interact with mTORC1 (Fig. 1) (45, 46). These studies revealed that mSIN1 is critical to the function of TORC2, particularly for the ability of the mTORC2 complex to phosphorylate Akt at Ser 473. In vivo and in vitro studies conducted with mSIN1 knockout mice showed that loss of mSIN1 rendered TORC2 unable to activate Akt at Ser473 (17, 19), indicating that mSIN1 is a key regulator of the substrate specificity of Akt. This effect may at least partly occurs through the activity of mSIN1 in stabilizing the mTORC2 complex (46). Moreover, those studies support mSIN1 is a potentially important therapeutic target of mTOR, as interfering with mSIN1's interaction with mTORC2 is likely to prevent the potentially deleterious activation of Akt that occurs with rapamycin and that may also occur with rapamycin analogues. Five splicing isoforms of mSIN1 have been identified, three of which bind to mTORC2 to form three distinct mTORC2s; however, only two of them mediate the activation of Akt by insulin (46). Those studies suggest the generation of three unique forms of mTORC2, each with different cellular functions. Although mTORC2 is considered to be the rapamycin-insensitive complex, long-term treatment with rapamycin has been shown to suppress the mTORC2 complex in certain cells, through a mechanism that is not well understood (47). It is thus likely that the levels of mSIN1 or the composition of its various isoforms may influence the sensitivity of mTORC2 to rapamycin.
Supressing a Tumor Suppresor Pathway
Transforming growth factor-β (TGF-β) is an important regulator of numerous cellular functions, including cell growth, differentiation, apoptosis, cell motility and cell adhesion. Deregulation of TGF-β expression and function is implicated in the pathogenesis of cancer (22), and is regarded as an important tumor suppressor in many early stage cancers. There is substantial cross-talk between insulin-like growth factor-I (IGF-I) and TGF-β receptor signaling pathways (21). Loss of the tumor suppressor function of TGF-β in prostatic carcinoma may occur through activation of the IGF-I signaling pathway (21, 22, 48, 49). Akt can block TGF-β responses through multiple mechanisms, not limited to the suppression of TGF-β-induced apoptosis. In the NRP-152 rat prostate epithelial cell line cell line, Akt has been shown to block TGF-β responses through suppressing phosphorylation of Smad3 (21). This occurs through an mTOR-dependent mechanism, as it is reversed by rapamycin and mTOR siRNA (48). These results support that mTOR interferes with the ability of TGF-β receptors to phosphorylate Smad3 (Fig. 1), molecular details of which are currently under investigation. We hypothesize that rapamycin can activate Smad3 by reversing the inhibitory activity of mTORC1 on TGF-β receptors. Through a different mechanism, involving the inhibitory association of FKBP12 to TGF-β type I receptor (TβRI), this macrolide was previously proposed to enhance TGF-β signaling (50). In that model, FKBP12 was reported to interact with TβRI and prevent ligand-independent activation of TβRI by TβRII (51-53). TGF-β first associates with TβRII, causing dimerization of TβRII with TβRI and release of FKBP12 from TβRI, allowing the TβRII kinase to activate TβRI. Activated TβRI then phosphorylates and activates the transcription factor/transcription co-regulators, Smads 2 and 3. However, in the absence of ligand, TβRII has low affinity for TβRI, and FKBP12 prevents the ligand-independent activation of TβRI by TβRII in that complex.
mTOR Inhibition in Prostate and Bladder Cancer
Genetic alterations, including loss of PTEN, mutation of the PI3K and amplification of AKT1 and AKT2 leading to activation of AKT kinase activity have been linked with the development of castrate progressive prostate cancer (CPPCA) (54). Transgenic mice with PTEN knockout confirmed a functional role of this pathway in acquisition of CPPCA (55). It is also known that alterations of the Akt signaling cascade (mTOR dependent), can lead to the development of prostate intraepithelial neoplasia (PIN) (54). Similarly, preliminary in vitro findings in PCA cell lines show a correlation between PTEN loss or Akt activation and sensitivity to growth inhibition by temsirolimus (56, 57). Although the in vitro data in TCC is less prominent, it is well established that PTEN mutations are present in a approximately 30% of TCC patients and that the PI3K pathway regulates TCC cell invasion (58). Thus, mTOR inhibition has been an attractive therapeutic strategy for this disease. Unfortunately, the development of clinical trials in this setting has been quite slow. To date, there are no active clinical trials evaluating mTOR inhibitors in TCC; however, with the availability of everolimus and temsirolimus, and the current published clinical and safety data, it is expected that these agents will be tested in patients with cisplatin-refractory TCC.
Preliminary data in PCA patients suggest that mTOR inhibition may have antitumor activity in these patients. A single phase II study led by Lerut and colleagues (59) evaluated everolimus in patients with newly-diagnosed, localized PCA. In this study, patients received 4 weeks of everolimus administered as either weekly (30mg, 50mg, or 70mg) or daily (5mg or 10mg) doses prior to undergoing radical prostatectomy (RP). Changes in immunohistochemical (IHC) scores for total and phosphorylated forms of S6, Akt and 4E-BP1 between pre-treatment and post-treatment tissue were evaluated. Fifteen patients completed treatment: 4 patients in each of the 30 and 50mg/week, 5mg/day cohorts, and 3 patients in the 10mg/day cohort. No dose-limiting toxicities (DLTs) were observed. Most frequently observed adverse events are CTC grade 1-2 stomatitis and rash. Initial IHC observations indicate highly heterogeneous phospho-S6 (p-S6) staining in hyperplasia, intraepithelial neoplasia and cancer cells, and no correlation between IHC score for p-S6 and Gleason score or proliferation index.
Although these results are encouraging, the evaluation and measurement of everolimus induced anti-prostate cancer activity and its correlation with biologic effects remains unclear. Clinical trials evaluating the activity of AP-23573 in refractory solid malignancies including metastatic CPPCA have been initiated. Similarly, trials evaluating the combination of bevacizumab and temsirolimus in chemotherapy-refractory CPPC have been initiated at our institution. Trials evaluating mTOR inhibitors early on in localized PCA are also underway. We hope the tissue and correlative endpoints of these trials will help to clarify the antitumor activity of mTOR inhibitors in PCA patients.
Activity of mTOR Inhibitors in Metastatic Renal Cell Cancer
Although the importance of this signaling cascade in RCC has not been well characterized, several in vitro studies have showed that the activation of the mTOR pathway leads to gene overexpression of hypoxia inducible factor-1α (HIF-1α), a crucial molecule in the pathogenesis of clear cell RCC (60). Other authors (61) have also reported that temsirolimus can reduce the expression of both HIF-1α and HIF-2α regardless of oxygen saturation. Another potential rationale for mTOR inhibition in RCC is through PTEN. In RCC, the gene expression of PTEN can be heterogeneous as PTEN mutations in RCC are not commonly seen. Nevertheless, PTEN deficient cells are more sensitive to the activity of mTOR inhibitors through an increased phosphorylation state in the Akt pathway. Further studies will help determine whether such enhanced sensitivity may be due to elevated ratio of mTORC1 to mTORC2, from loss of PRAS40 by Akt, from differences in the S6K/IRS-1 negative feedback control, or from differences in levels of mSIN1. Below, we will review the existing clinical data with the two mTOR inhibitors most commonly studied in mRCC, everolimus and temsirolimus.
Everolimus
Everolimus is an orally active derivative of rapamycin. In preclinical models, the administration of everolimus is associated with reduction of mTOR downstream p-S6 and p-4E-BP1, and occasionally with increase in upstream p-Akt. Several phase I and II studies evaluating dose, schedule and the pharmacodynamics of everolimus have been reported and are discussed elsewhere (62-65). Amato and colleagues (66) conducted a phase II trial where fourty-one previously treated mRCC patients received oral everolimus at a dose of 10 mg daily without an interruption (28-day cycle), with dose modifications for toxicity. The overall response rate (ORR) observed was 33% (12/37), 19 patients have stable disease (SD) 3+ months with a median duration of therapy of 8 months (range 1+ to 20+). Treatment related adverse events included mucositis, skin rash, pneumonitis, hypophosphatemia, hyperglycemia, thrombocytopenia, anemia and elevated liver function tests (LFTs).
Currently, a large phase III trial evaluating everolimus plus BSC versus placebo plus BSC in patients with previously treated mRCC is underway in Europe. This is an attractive trial evaluating the concept of sequential therapy in RCC that plans to accrue more than 300 patients with tyrosine kinase inhibitor-refractory mRCC.
Temsirolimus
Preliminary phase I data led to the design of a phase II trial in patients with treatment-refractory mRCC. In this study, 111 patients were randomized to one of multiple dose levels (25 mg, 75 mg or 250 mg IV weekly) (67). The overall response rate was 7%, with additional patients demonstrating minor responses. Given the high number of dose reductions and treatment discontinuations at the higher dose levels, the investigators advocated the 25 mg IV weekly dose for future temsirolimus studies. Retrospective assignment of risk criteria to patients in this study identified a poor-prognosis group (n=49). Temsirolimus-treated patients in this poor-prognosis group had a median overall survival (OS) of 8.2 months compared to 4.9 months for first-line interferon-alpha (IFN-α) treated patients (historical controls, n= 437). Loss of PTEN may be more common in poor risk patients and may account for this finding, since mutation of this tumor suppressor gene would activate mTOR and potentially increase the relevance of mTOR targeted therapy in this subgroup (68). A subsequent randomized phase III trial was conducted in patients with poor-risk metastatic RCC as defined by existing prognostic schema (69). Patients with mRCC and no prior systemic therapy were enrolled in this open-label study if they had ≥ 3 of 6 adverse risk factors [Karnofsky performance status (KPS) < 80%, time to metastatic disease < 1 year, hemoglobin < lower limit of normal, serum lactate dehydrogenase greater than 1.5× upper limit of normal, corrected serum calcium > 10 mg/dL and >1 metastatic disease site] (70). Patients were equally randomized to receive IFN-α up to 18 million units (MU) subcutaneous (SC) three times weekly (TIW); temsirolimus 25 mg IV once/weekly; or temsirolimus 15 mg IV once/weekly + IFN-α 6 MU SC TIW. The primary study endpoint was OS and the study was powered to compare each of the temsirolimus-containing arms to the IFN-α arm. The study demonstrated that patients treated with temsirolimus had a statistically longer survival than those treated with IFN-α alone (10.9 months vs. 7.3 months, p=0.0069). OS of patients treated with IFN-α or temsirolimus + IFN-α were not statistically different (7.3 months vs. 8.4 months, p=0.6912). Similarly, the ORR for each treatment arm was 7% IFN-α, 9% temsirolimus, and 11% for the combination arm. These results led to the recent FDA approval of temsirolimus in mRCC. Of importance, the lack of survival benefit in the combination arm is thought to be the result of a lower dose of temsirolimus coupled with an increased number of patients unable to receive temsirolimus secondary to IFN-α related toxicities. Similar to the everolimus toxicity profile, some of the most common toxicities observed in patients receiving weekly temsirolimus include hyperglycemia, hypertryglyceridemia, hypercholesterolemia and asthenia, edema and dyspnea.
More recently, Dutcher and colleagues (71) performed a subgroup analysis of the IFN-α and temsirolimus-treated patients on the phase III study. In their retrospective analysis, OS and progression-free survival (PFS) correlated with type of histology, age and Memorial Sloan-Kettering Cancer Center (MSKCC) prognostic risk group (70, 72). Caution should be used when interpreting subgroup analyses as their sample size and lack of statistical power to detect meaningful differences between groups may not reflect the entire population studied. Despite its limitations, it is provocative that patients with histologies other than clear cell carcinoma (n=37 – 18%) receiving temsirolimus appeared to have a superior median PFS and OS (7 versus 1.8 months and 11.6 versus 4.3 months respectively). Similarly, when poor-risk and intermediate-risk patients were stratified using the standard prognostic criteria, intermediate-risk patients did not have any benefit by receiving temsirolimus when compared with IFN-α, treated patients. Other variables that appeared to predict a better outcome in temsirolimus treated patients include age <65 and prior nephrectomy, although the patients with primary tumors in place also received benefit from this agent.
Based upon the current available data, temsirolimus is a standard of care for patients with poor-risk features as well as for patients with non-clear cell RCC. Further investigation of this agent is planned in patients with fewer adverse risk features and in combination with other VEGF-targeting strategies.
Future of mTOR Inhibitors in GU Malignancies
Current in-vitro and in-vivo data clearly demonstrates the importance of the mTOR signaling pathway in the pathogenesis of GU malignancies. Although current existing data in prostate and bladder is not as robust as it is in renal cancer, ongoing studies will further define the clinical efficacy and safety profile of these agents in this subset of patients. Once their activity as single agents has been defined, the endpoint of future studies should be aimed to identify biologic markers of response and potential combination strategies that could lead to improvement in anti-tumor activity. Clinical studies evaluating dual inhibition (mTOR and VEGF inhibitor) have initiated. Preliminary data using bevacizumab and temsirolimus in advanced RCC has demonstrated clinical efficacy and no new safety signal of concerns. This type of combinations will soon be tested in patients with CPPCA.
The mTOR field has witnessed an explosion of information in the past 5 years, with major advances in the basic biology of mTOR signaling, particularly the composition and function of macromolecular components of mTOR that confer responses to mTOR inhibitors. These studies have provided new tissue markers (i.e., raptor, rictor, PRAS40, mSin1, FKBP38, IRS-1) that are likely to not only predict therapeutic response but also serve as new therapeutic targets for intervention of cancer.
Acknowledgments
NIH grant support: R01CA092102 and R01CA102074 (to David Danielpour)
List of Abbreviations
- mTOR
mammalian target of rapamycin
- IRS-1
insulin receptor substrate-1
- PDK-1
3-phosphoinositide-dependent protein kinase-1
- PI3K
phosphatidylinositol-3-kinase
- mRCC
metastatic renal cell carcinoma
- BSC
best supportive care
- PCA
prostate cancer
- TCC
transitional cell carcinoma of the urothelium
- GU
genitourinary
- FKBP12
FK506-binding protein 12
- FKBP38
FK506-binding protein 38
- FRB
FKBP-rapamycin binding
- eIF4E
eukaryotic initiation factor 4E
- 4E-BP1
eIF4E binding protein
- Rheb
Ras homolog enriched in brain
- PRAS40
proline-rich Akt substrate of 40 kDa
- PH
pleckstrin homology
- PTEN
phosphatase and tensin homologue deleted on chromosome 10
- PtdIns
phosphatidylinositol
- TSC1
Tuberous Sclerosis Complex-1
- TSC2
Tuberous Sclerosis Complex-2
- TGF-β
transforming growth factor-β
- CPPCA
castrate progressive prostate cancer
- PIN
prostate intraepithelial neoplasia
- RP
radical prostatectomy
- IHC
immunohistochemical
- DLT
dose-limiting toxicities
- CTC
common toxicity criteria
- HIF-1
hypoxia inducible factor-1
- OS
overall survival
- ORR
overall response rate
- PFS
progression-free survival
References
- 1.Sehgal SN, Baker H, Vezina C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo) 1975;28:727–32. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- 2.Sehgal SN, Molnar-Kimber K, Ocain TD, Weichman BM. Rapamycin: a novel immunosuppressive macrolide. Med Res Rev. 1994;14:1–22. doi: 10.1002/med.2610140102. [DOI] [PubMed] [Google Scholar]
- 3.Easton JB, Houghton PJ. mTOR and cancer therapy. Oncogene. 2006;25:6436–46. doi: 10.1038/sj.onc.1209886. [DOI] [PubMed] [Google Scholar]
- 4.Dancey JE. Inhibitors of the mammalian target of rapamycin. Expert Opin Investig Drugs. 2005;14:313–28. doi: 10.1517/13543784.14.3.313. [DOI] [PubMed] [Google Scholar]
- 5.Hara K, Yonezawa K, Weng QP, et al. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273:14484–94. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 6.Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–62. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 7.Heitman J, Movva NR, Hiestand PC, Hall MN. FK 506-binding protein proline rotamase is a target for the immunosuppressive agent FK 506 in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991;88:1948–52. doi: 10.1073/pnas.88.5.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hara K, Yonezawa K, Kozlowski MT, et al. Regulation of eIF-4E BP1 phosphorylation by mTOR. J Biol Chem. 1997;272:26457–63. doi: 10.1074/jbc.272.42.26457. [DOI] [PubMed] [Google Scholar]
- 9.Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–68. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 10.Kim DH, Sarbassov DD, Ali SM, et al. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 11.Kim DH, Sarbassov DD, Ali SM, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 12.Ali SM, Sabatini DM. Structure of S6 kinase 1 determines whether raptor-mTOR or Rictor-mTOR phosphorylates its hydrophobic motif site. J Biol Chem. 2005;280:19445–8. doi: 10.1074/jbc.C500125200. [DOI] [PubMed] [Google Scholar]
- 13.Sarbassov dos D, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 14.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 15.Fonseca BD, Smith EM, Lee VH, MacKintosh C, Proud CG. PRAS40 Is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J Biol Chem. 2007;282:24514–24. doi: 10.1074/jbc.M704406200. [DOI] [PubMed] [Google Scholar]
- 16.Nojima H, Tokunaga C, Eguchi S, et al. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem. 2003;278:15461–4. doi: 10.1074/jbc.C200665200. [DOI] [PubMed] [Google Scholar]
- 17.Jacinto E, Facchinetti V, Liu D, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–37. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 18.Polak P, Hall MN. mTORC2 Caught in a SINful Akt. Dev Cell. 2006;11:433–4. doi: 10.1016/j.devcel.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Shiota C, Woo JT, Lindner J, Shelton KD, Magnuson MA. Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev Cell. 2006;11:583–9. doi: 10.1016/j.devcel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–7. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 21.Danielpour D, Song K. Cross-talk between IGF-I and TGF-beta signaling pathways. Cytokine Growth Factor Rev. 2006;17:59–74. doi: 10.1016/j.cytogfr.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Danielpour D. Functions and regulation of transforming growth factor-beta (TGF-beta) in the prostate. Eur J Cancer. 2005;41:846–57. doi: 10.1016/j.ejca.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–65. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 24.Nakatani K, Thompson DA, Barthel A, et al. Up-regulation of Akt3 in estrogen receptor-deficient breast cancers and androgen-independent prostate cancer lines. J Biol Chem. 1999;274:21528–32. doi: 10.1074/jbc.274.31.21528. [DOI] [PubMed] [Google Scholar]
- 25.Stahl JM, Sharma A, Cheung M, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–10. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Sakon M, Nagano H, et al. Akt2 expression correlates with prognosis of human hepatocellular carcinoma. Oncol Rep. 2004;11:25–32. [PubMed] [Google Scholar]
- 27.Cheng GZ, Chan J, Wang Q, et al. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–87. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- 28.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–62. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 29.Tee AR, Fingar DC, Manning BD, et al. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci U S A. 2002;99:13571–6. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manning BD, Cantley LC. United at last: the tuberous sclerosis complex gene products connect the phosphoinositide 3-kinase/Akt pathway to mammalian target of rapamycin (mTOR) signalling. Biochem Soc Trans. 2003;31:573–8. doi: 10.1042/bst0310573. [DOI] [PubMed] [Google Scholar]
- 31.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–34. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai X, Ma D, Liu A, et al. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007;318:977–80. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- 33.Sancak Y, Thoreen CC, Peterson TR, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–15. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–23. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Harris TE, Roth RA, Lawrence JC., Jr PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem. 2007;282:20036–44. doi: 10.1074/jbc.M702376200. [DOI] [PubMed] [Google Scholar]
- 36.Oshiro N, Takahashi R, Yoshino K, et al. The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J Biol Chem. 2007;282:20329–39. doi: 10.1074/jbc.M702636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang B, Porter G. Expression of proline-rich Akt-substrate PRAS40 in cell survival pathway and carcinogenesis. Acta Pharmacol Sin. 2005;26:1253–8. doi: 10.1111/j.1745-7254.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- 38.Madhunapantula SV, Sharma A, Robertson GP. PRAS40 deregulates apoptosis in malignant melanoma. Cancer Res. 2007;67:3626–36. doi: 10.1158/0008-5472.CAN-06-4234. [DOI] [PubMed] [Google Scholar]
- 39.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Hartman ME, Villela-Bach M, Chen J, Freund GG. Frap-dependent serine phosphorylation of IRS-1 inhibits IRS-1 tyrosine phosphorylation. Biochem Biophys Res Commun. 2001;280:776–81. doi: 10.1006/bbrc.2000.4214. [DOI] [PubMed] [Google Scholar]
- 41.Zick Y. Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci STKE. 2005;2005:pe4. doi: 10.1126/stke.2682005pe4. [DOI] [PubMed] [Google Scholar]
- 42.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliveira JC, Souza KK, Dias MM, et al. Antineoplastic effect of rapamycin is potentiated by inhibition of IRS-1 signaling in prostate cancer cells xenografts. J Cancer Res Clin Oncol. 2008 doi: 10.1007/s00432-008-0359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Easton JB, Kurmasheva RT, Houghton PJ. IRS-1: auditing the effectiveness of mTOR inhibitors. Cancer Cell. 2006;9:153–5. doi: 10.1016/j.ccr.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 45.Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–32. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frias MA, Thoreen CC, Jaffe JD, et al. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–70. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–34. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 48.Song K, Wang H, Krebs TL, Danielpour D. Novel roles of Akt and mTOR in suppressing TGF-beta/ALK5-mediated Smad3 activation. Embo J. 2006;25:58–69. doi: 10.1038/sj.emboj.7600917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song K, Cornelius SC, Reiss M, Danielpour D. Insulin-like growth factor-I inhibits transcriptional responses of transforming growth factor-beta by phosphatidylinositol 3-kinase/Akt-dependent suppression of the activation of Smad3 but not Smad2. J Biol Chem. 2003;278:38342–51. doi: 10.1074/jbc.M304583200. [DOI] [PubMed] [Google Scholar]
- 50.Wang T, Donahoe PK. The immunophilin FKBP12: a molecular guardian of the TGF-beta family type I receptors. Front Biosci. 2004;9:619–31. doi: 10.2741/1095. [DOI] [PubMed] [Google Scholar]
- 51.Okadome T, Oeda E, Saitoh M, et al. Characterization of the interaction of FKBP12 with the transforming growth factor-beta type I receptor in vivo. J Biol Chem. 1996;271:21687–90. doi: 10.1074/jbc.271.36.21687. [DOI] [PubMed] [Google Scholar]
- 52.Bassing CH, Shou W, Muir S, et al. FKBP12 is not required for the modulation of transforming growth factor beta receptor I signaling activity in embryonic fibroblasts and thymocytes. Cell Growth Differ. 1998;9:223–8. [PubMed] [Google Scholar]
- 53.Stockwell BR, Schreiber SL. TGF-beta-signaling with small molecule FKBP12 antagonists that bind myristoylated FKBP12-TGF-beta type I receptor fusion proteins. Chem Biol. 1998;5:385–95. doi: 10.1016/s1074-5521(98)90072-2. [DOI] [PubMed] [Google Scholar]
- 54.Majumder PK, Febbo PG, Bikoff R, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 55.Jiao J, Wang S, Qiao R, et al. Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res. 2007;67:6083–91. doi: 10.1158/0008-5472.CAN-06-4202. [DOI] [PubMed] [Google Scholar]
- 56.Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci U S A. 1998;95:15587–91. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi Y, Gera J, Hu L, et al. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 2002;62:5027–34. [PubMed] [Google Scholar]
- 58.Wu X, Obata T, Khan Q, et al. The phosphatidylinositol-3 kinase pathway regulates bladder cancer cell invasion. BJU Int. 2004;93:143–50. doi: 10.1111/j.1464-410x.2004.04574.x. [DOI] [PubMed] [Google Scholar]
- 59.Lerut E, Roskams T, Goossens E, et al. Molecular pharmacodynamic (MPD) evaluation of dose and schedule of RAD001 (everolimus) in patients with operable prostate carcinoma. J Clin Oncol (ASCO Annual Meeting Proceedings) 2005;23:3071. [Google Scholar]
- 60.Hudson CC, Liu M, Chiang GG, et al. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–14. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Del Bufalo D, Ciuffreda L, Trisciuoglio D, et al. Antiangiogenic potential of the Mammalian target of rapamycin inhibitor temsirolimus. Cancer Res. 2006;66:5549–54. doi: 10.1158/0008-5472.CAN-05-2825. [DOI] [PubMed] [Google Scholar]
- 62.Wanner K, Hipp S, Oelsner M, et al. Mammalian target of rapamycin inhibition induces cell cycle arrest in diffuse large B cell lymphoma (DLBCL) cells and sensitises DLBCL cells to rituximab. Br J Haematol. 2006;134:475–84. doi: 10.1111/j.1365-2141.2006.06210.x. [DOI] [PubMed] [Google Scholar]
- 63.Yee KW, Zeng Z, Konopleva M, et al. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2006;12:5165–73. doi: 10.1158/1078-0432.CCR-06-0764. [DOI] [PubMed] [Google Scholar]
- 64.Tabernero J, Rojo F, Calvo E, et al. Dose- and Schedule-Dependent Inhibition of the Mammalian Target of Rapamycin Pathway With Everolimus: A Phase I Tumor Pharmacodynamic Study in Patients With Advanced Solid Tumors. J Clin Oncol. 2008 doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 65.van Oosterom A, Reichardt P, Baly J, et al. A phase I/II trial of the oral mTOR-inhibitor Everolimus (E) and Imatinib Mesylate (IM) in patients (pts) with gastrointestinal stromal tumor GIST refractory to IM. J Clin Oncol (ASCO Annual Meetings Proceedings) 2005;23:9033. [Google Scholar]
- 66.Abid MR, Guo S, Minami T, et al. Vascular endothelial growth factor activates PI3K/Akt/forkhead signaling in endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:294–300. doi: 10.1161/01.ATV.0000110502.10593.06. [DOI] [PubMed] [Google Scholar]
- 67.Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–18. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 68.Neshat MS, Mellinghoff IK, Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci U S A. 2001;98:10314–9. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 70.Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol. 2005;23:832–41. doi: 10.1200/JCO.2005.05.179. [DOI] [PubMed] [Google Scholar]
- 71.Dutcher JP, Szczylik C, Tannir N, et al. Correlation of survival with tumor histology, age, and prognostic risk group for previously untreated patients with advanced renal cell carcinoma (adv RCC) receiving temsirolimus (TEMSR) or interferon-alpha (IFN) J Clin Oncol (ASCO Annual Meeting Proceedings) 2007;25:5033. [Google Scholar]
- 72.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–96. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]