Abstract
A novel bipartite vector system consisting of the Herpes Simplex Virus (HSV) amplicon and the Sleeping Beauty (SB) transposon was previously shown to efficiently deliver a “transgenon” (integrating transgene) in utero. This vector platform facilitated long-term transgenon expression specifically within neurons and neuronal precursor cells of the rodent brain. However, the mechanism underlying the neurospecificity of the HSV/SB amplicon in the setting of mouse embryogenesis is unknown. We find that embryonic cells expressing the Sox1 “neurocompetence” transcription factor represent the primary targets for HSV amplicon transduction in utero. These cells, which comprise the ependymal and subventricular zones, express significant levels of HMGB1, a co-factor shown to facilitate SB-mediated transposition. Using a conventional, non-integrating amplicon expressing Cre recombinase to embryonically “tag” transduced cells in ROSA26 Cre indicator mice in utero, we found transduced cells were exclusively of the neuronal lineage, but in comparison to HSV/SB-mediated in utero delivery, staining patterns were less widespread and “tagged” neuroprogenitor cells were absent. Our findings demonstrate in utero HSV/SB amplicon gene transfer is primarily neurospecific due to viral tropism and target cell populations present embryonically, where multi-potent cells of the developing embryo are supportive of SB-driven transposition.
Keywords: Gene transfer, HSV amplicon, helper virus-free packaging, stem cell, neuronal precursor, Sleeping Beauty
Introduction
The human brain is subject to a remarkable number of degenerative conditions, some of which present with symptoms at or prior to birth. A therapeutic strategy for treating or even preventing the neurological symptoms of early-onset diseases could be envisioned to involve a long lasting gene-based therapy most likely delivered in utero. Such a therapeutic approach could be undertaken before the brain has developed its post-mitotic character and the immune system is able to recognize transgene products as foreign antigens, and would provide a strategy to widely disseminate the expression of a therapeutic transgene by taking advantage of the proliferative cellular environment.
Potential gene-based therapeutics for degenerative conditions afflicting the central nervous system (CNS) are inherently required to safely and efficiently deliver their genetic payload and provide prolonged therapeutic benefit. While current CNS delivery vehicles have a number of advantages and disadvantages, the use of HSV helper virus-free amplicons offers many advantages over other platforms, including large gene capacity, sufficient to contain genes and regulatory elements, a broad tropism, reduced cellular toxicity, and low immunogenicity [1]. However, a conventional HSV amplicon cannot provide life-long gene expression necessary for CNS gene therapy due to the episomal nature of its genome and associated gene silencing within post-mitotic cell types [2]. The use of cell type-specific promoters has been shown to prolong amplicon-mediated transgene expression relative to employment of virus-based promoter elements, but these vectors too are progressively silenced [3]. Repeated administration of an amplicon, though potentially effective at extending gene expression, is not clinically feasible because this approach could increase the risk of brain injury and elicitation of a deleterious immune response (reviewed by [4]).
These barriers can be overcome by using a recently described two-component HSV amplicon system that employs the Sleeping Beauty transposon (SB) to efficiently integrate transgenes into the mammalian cell genome. This platform consists of two vectors: one containing a viral/cellular promoter-driven transgene flanked by the SB inverted/direct repeats (IR/DR) termed the “transgenon”, and a second harboring the SB transposase gene transcriptionally controlled by the HSV immediate-early 4/5 gene promoter (HSVsb). Co-delivery of these two vectors in vitro and in utero facilitates long-term transgenon expression and transmittance to dividing cell progeny following integration of the IR/DR-flanked transcription unit into the mouse cell genome [5].
Herein, we sought to elucidate the mechanisms responsible for the previously observed neuronal cell-specific transgenon expression pattern following in utero delivery of the bipartite HSV/SB amplicon vector platform. Stage-specific quantitative real-time RT-PCR assessments of cellular HSV receptor expression, immunocytochemical analyses of cell types and SB cellular co-factor expression present at the time of vector infusion, and a functional in vivo assay that marks cells undergoing HSV amplicon transduction were employed. Our findings demonstrate in utero HSV/SB amplicon gene transfer is exclusively localized to cells destined to differentiate into cells of the neuronal lineage due to viral tropism and the target cell populations present at the time of vector administration.
Results
In utero integration using the bipartite HSV/Sleeping Beauty vector platform
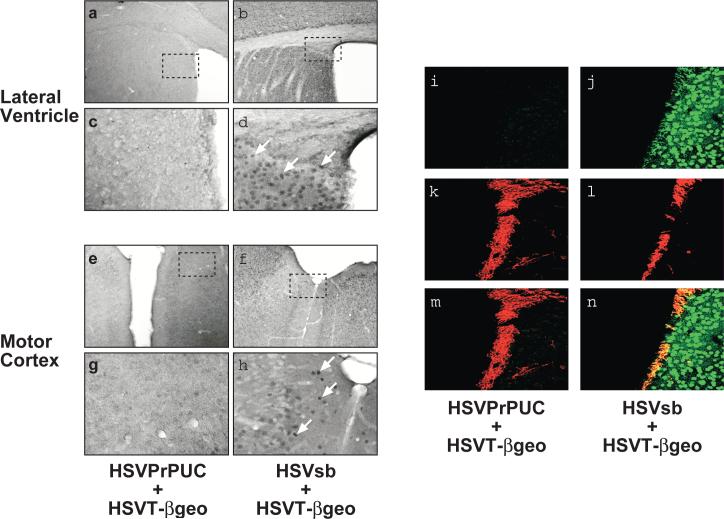
To firmly establish an experimental paradigm for assessing the role of vector tropism in the observed neurospecific phenotype of the bipartite amplicon platform, embryonic day (E) 14.5 C57BL/6 mouse embryos were intracerebroventricularly infused with 1:1 ratio of helper virus-free HSVsb or HSVPrPUC and HSVT-βgeo amplicon preparations (2 μl total volume: 2×104 total transducing units). HSVPrPUC served as a non-SB transposase-expressing vector control in this experiment. Brains transduced with HSVsb and HSVT-βgeo exhibited widespread transgenon expression with cell-associated staining most pronounced in the motor cortex and regions proximal to the lateral ventricle (Figure 1a-h), a finding similar to prior observations made at later experimental time points [5]. Mice receiving HSVPrPUC and HSVT-βgeo showed no evidence of βgeo expression, indicating that the βgeo expression observed in the HSVsb + HSVT-βgeo cohort is not due to episomal HSVT-βgeo amplicon genome-mediated expression, but requires SB for mobilization and expression of the T-βgeo transgenon at 29 days of age. Furthermore, we confirmed that βgeo expression specifically derived from co-infusion of HSVsb and HSVT-βgeo was robust in neuroprogenitor cells using co-immunohistochemistry to assess βgeo/doublecortin (DCX) double-positive cells (Figure 1i-n). Doublecortin is a marker of migratory neuroblasts arising from proliferative regions of the embryonic and adult brain [6]. Immunocytochemical analysis of other progenitor cell markers, including S100B for the astrocytic lineage and NG2 for oligodendrocyte precursors, did not reveal any co-localization between those non-neuronal lineage-specific markers and βgeo using confocal microscopy (data not shown).
Figure 1. In utero co-delivery of HSVT-βgeo and HSVsb to E14.5 mouse CNS results in extensive transgenon expression throughout the mouse brain.
At 29 days of age, inoculated animals were sacrificed, perfused with 4% paraformaldehyde, brain sections processed for LacZ/Diaminobenzidine (DAB) immunohistochemistry, and sections imaged using light microscopy (n=4 per treatment group). Representative photomicrographs of the lateral ventricle (a-d) and motor cortex (e-h) from mice receiving HSVPrPUC + HSVT-βgeo (a, c, e, and g) and HSVsb + HSVT-βgeo (b, d, f, and h) are depicted. Magnification = 10X in panels a, b, e, and f. Hatched boxes shown in a, b, e, and f represent regions magnified in c, d, g, and h, respectively (final magnification = 40X). White arrows highlight LacZ-immunopositive cells. Adjacent coronal brain sections were processed for dual LacZ/DCX fluorescent immunohistochemistry and imaged using confocal microscopy. Expression of LacZ in the mice was detected in the green channel (i, j). Neuronal progenitor marker DCX, expressed in migrating neuronal progenitors lining the sub-ventricular zone and associated with the rostal migratory pathway in adult mice, was detected in the red channel (k, l). Co-localization of LacZ with the DCX signal is depicted in yellow (m, n).
Cell type and SB co-factor expression in the embryonic mouse brain
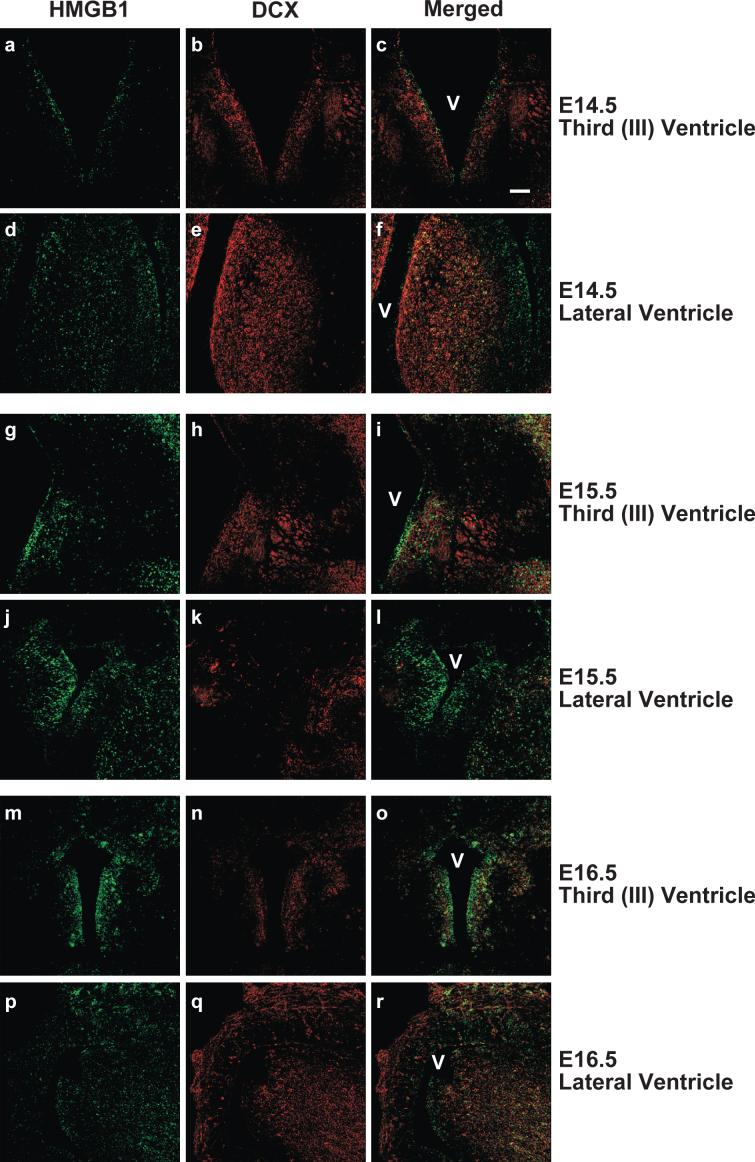
To determine the identity of cells available for transduction in the embryonic mouse brain and to assess the expression profile of the SB co-factor High Mobility Group protein B1 (HMGB1), brain sections from staged embryos were obtained for immunohistochemical analyses. HMGB1 is a non-histone DNA bending protein associated with eukaryotic chromatin that has been shown to be essential for efficient SB-mediated transposition and transient overexpression of HMGB1 increases transposition efficiency [7]. The embryonic tissue was subsequently processed for immunohistochemistry to specifically assess expression of the astrocyte progenitor marker S100B, oligodendrocyte progenitor marker NG2, and migratory neuroblast cell type marker DCX. Regions adjacent to the third (III) and lateral ventricles and respective subependymal zones were examined using confocal microscopy, as they represent the likely target field during intraventricular vector administration. NG2-positive cells were minimally observed at these embryonic time points (data not shown), suggesting that oligodendroglial precursor cells (OPCs) are in relatively low numbers at E14.5 – E16.5. Other studies have shown that mouse NG2 expression peaks within the period of postnatal day (P)8 – P12 and gradually diminishes thereafter (reviewed by [8]). The pre-astrocytic marker, S100B, was detected in distal regions of the embryonic brain, but S100B-positive cells were extremely rare in areas immediately encompassing the ventricles (data not shown). The pre-neuronal marker, DCX, was robustly expressed at all time points in the regions surrounding the ventricles (Figure 2). However, the majority of DCX-positive neuronal precursors were observed in the subventricular zone and deeper cellular layers, while very few DCX-positive cells were visible in the ependymal cell layers immediately adjacent to the ventricles. Co-immunocytochemical staining for DCX and the SB cellular co-factor HMGB1 revealed patterns of expression that were minimally overlapping (Figure 2). Interestingly, HMGB1 immunopositivity was readily detectable in DCX-negative cells comprising the ependymal layer of E14.5 – E16.5 mouse brains, suggesting that a more immature neuroprogenitor cell type is a primary target of HSV/SB vector transduction following intracerebroventricular infusion.
Figure 2. HMGB1, a cellular co-factor of Sleeping Beauty transposase, is embryonically expressed in subventricular zone cells that do not co-express doublecortin (DCX).
C57BL/6 mouse embryos (E14.5, 15.5, and 16.5) were sacrificed, decapitated, and whole heads fixed and sectioned coronally (45 μm). Brain sections were processed for dual HMBG1/DCX fluorescent immunohistochemistry and imaged using confocal microscopy. Representative photomicrographs of areas proximal to the third (a-c, g-i, m-o) and lateral (d-f, j-l, p-r) ventricles for each embryonic time point are depicted. HMGB1 immunopositivity appears in the green channel. DCX, a marker for migratory neuroblasts, appears in the red channel, while an overlapping expression pattern is depicted in yellow. The ventricles are designated by the letter “V”. Magnification bar shown in Panel c = 100 μm (final magnification = 20X). N = 4 for each experimental group.
Sox1 and HMGB1 expression patterns overlap in the ependymal zone of E14.5 mouse embryos
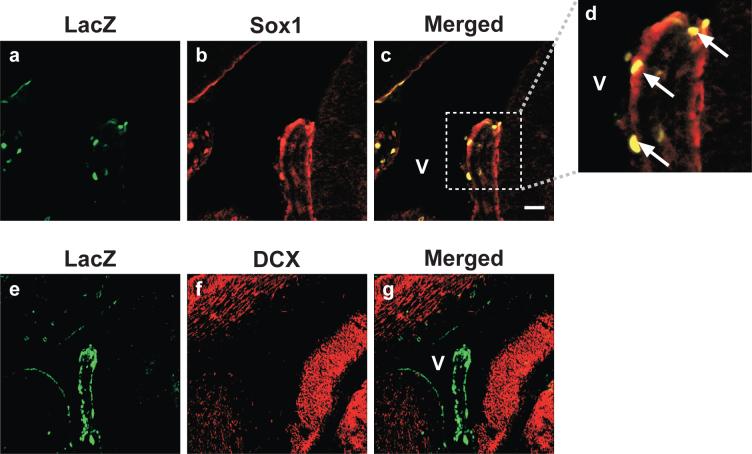
To identify the HMGB1-positive ependymal cell types present at the time of vector transduction, we performed co-immunohistochemistry for HMGB1 and Sox1 on E14.5 – E16.5 C57BL/6 mouse embryonic brain tissue. Sox1 is a transcription factor belonging to the SoxB family of high-mobility-group DNA-binding proteins that are expressed in early neuroectoderm and are believed to function as neural competence factors [9]. SoxB family members are expressed in overlapping patterns in the developing neuroectoderm and appear to function in a redundant manner to drive self-renewing progenitor cells into neuronally specified derivatives upon release from mitosis [10]. Immunocytochemical analysis revealed a high degree of cellular co-staining for HMGB1 and Sox1 in the embryonic ventricular and subventricular regions of E14.5 brain (Figure 3), as well as E15.5 and E16.5 brain (data not shown). These data strongly suggest Sox1-positive ependymal cells represent likely targets for HSV/SB transduction, and given that these cells also express the SB co-factor, HMGB1, transgenon integration in this self-renewing cell population may be responsible for the high numbers of transgenon-positive neuroprogenitor cells observed following in utero HSV/SB delivery.
Figure 3. Sox1-positive progenitor cells co-express the SB co-factor, HMGB1 within the ventricular and subventricular target field of in utero HSV amplicon transduction.
C57BL/6 mouse embryos (E14.5) were sacrificed, decapitated, and whole heads fixed and sectioned coronally (45 μm). Brain sections were processed for dual HMBG1/Sox1 fluorescent immunohistochemistry and imaged using confocal microscopy. Representative photomicrographs of areas proximal to the third (a-d) and lateral (e-h) ventricles for the E14.5 time point are depicted (analysis of E15.5 and E16.5 time points not shown). Panels d and h depict expanded views of the area outlined by the dotted box in Panels c and g, respectively. HMGB1 immunopositivity appears in the green channel. Sox1, a marker for proliferating progenitor cells, appears in the red channel, while an overlapping expression pattern is depicted in yellow. The ventricles are designated by the letter “V”. Magnification bar shown in Panel c = 100 μm (final magnification for Panels a-c and e-g= 20X). N = 4.
Ventricular Sox1-positive cells in E14.5 mouse brain are the primary targets of HSV amplicon vector transduction following intraventricular infusion
To determine the identity of the cells initially targeted by the HSV amplicon within the ventricular regions of a developing embryo, E14.5 C57BL/6 mouse embryos were intracerebroventricularly infused with helper virus-free HSVlac or HSVPrPUC amplicon preparations (2 μl total volume: 2×104 total transducing units). HSVlac is a β-galactosidase expressing amplicon that harbors the E. coli lacZ gene under the transcriptional control of the same IE4/5 gene promoter as the HSVsb amplicon described above. HSVPrPUC served as a non-expressing vector control in this experiment (data not shown). Injected mice were sacrificed 24 h following vector infusion and their brains were processed for anti-β-galactosidase and Sox1 co-immunocytochemistry to assess the extent of amplicon-transduced Sox1-positive cells. Brains transduced with HSVlac exhibited high overlap of β-galactosidase and Sox1 expression, indicating that pluripotent Sox1-positive cells in the E14.5 ventricular zone were major targets of HSV amplicon gene transfer (Figure 4). Adjacent brain sections were analyzed by LacZ/DCX co-immunocytochemistry. At 24 h post-transduction, there were few, if any, β-galactosidase-expressing DCX-positive cells (Figure 4), further suggesting that the βgeo transgenon-expressing migratory neuroblast cells detected at Day 29 in HSVsb + HSVT-βgeo treated mice (Figure 1) arise from the originally transduced Sox1-positive progenitors and not from a primary transduction event of DCX-positive cells in utero.
Figure 4. HSV amplicon vectors initially target Sox1-positive cells when delivered intraventricularly to E14.5 mouse embryos.
C57Bl/6 mouse embryos intracerebroventricularly inoculated with HSVlac at E14.5 were sacrificed and post-fixed with 4% paraformaldehyde 24 h post-transduction. Coronal brain sections (45 μm) were processed for dual LacZ/Sox1 (a-d) or LacZ/DCX (e-g) fluorescent immunohistochemistry and imaged using confocal microscopy. Representative photomicrographs of the choroid plexus in the pineal recess of the lateral ventricle are depicted. Panel d depicts expanded views of the area outlined by the dotted box in Panel c. LacZ immunopositivity appears in the green channel (a and e). Sox1, a marker for proliferating progenitor cells (b), or DCX, a marker for migratory neuroblasts (f), appears in the red channel, while an overlapping expression pattern is depicted in yellow (c and g for Sox1 and DCX, respectively). Magnification bar shown in Panel c = 100 μm (final magnification for Panels a-c and e-g= 20X). N = 4.
In utero delivery of a conventional cre recombinase-expressing HSV amplicon to ROSA26 embryonic mouse brain
To determine whether the neurospecificity of the integration-competent HSV/SB platform is a general phenomenon of HSV-1 amplicon tropism or is a consequence unique to the inclusion of the SB transposase component, we employed an SB-independent means of examining HSV-1 amplicon-mediated transduction in the in utero setting. An HSV amplicon harboring the Cre recombinase gene under the transcriptional control of the HSV-1 IE4/5 gene promoter (HSVcre; [11]) was injected intracerebroventricularly into E14.5 ROSA26 mice as performed with the HSV/SB system in C57BL/6 mouse embryos described above (Figure 1). ROSA26 mice contain the LacZ reporter gene downstream of a transcriptional/translational stop cassette flanked by two loxP sites, and have been used previously to functionally tag cells of the neuronal lineage in vivo [12]. In a ROSA26 mouse, ectopic expression of Cre recombinase results in the juxtaposition of the ubiquitously expressed ROSA26 promoter and the LacZ gene, ultimately leading to the expression of the reporter gene product (Figure 5a). E14.5 embryos injected with HSVcre were sacrificed at 29 days of age and genomic DNA samples from tails were screened by PCR for the presence of the ROSA26 reporter locus sequence. Brain tissue was also harvested for co-immunohistochemistry for LacZ in combination with various cellular markers. There was no detectable expression of the reporter gene product in mice negative for the ROSA26 reporter locus, as expected (data not shown).
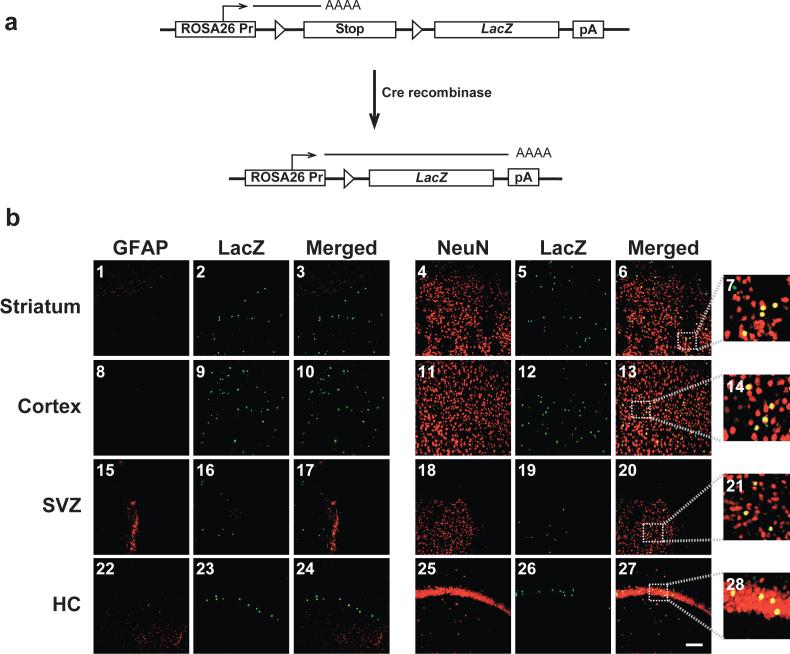
Figure 5. In utero intracerebroventricular delivery of HSVcre into E14.5 ROSA26 mouse embryos leads to Cre-mediated recombination and expression of β-galactosidase primarily in NeuN-positive neurons of the adult brain.
Staged embryonic mice were generated from mating C57BL/6 female mice with ROSA26 LacZ reporter heterozygous male mice. ROSA26 mice contain the LacZ reporter gene downstream of a transcriptional/translational stop cassette flanked by two loxP sites [12]. In a ROSA26 mouse, ectopic expression of Cre recombinase results in the juxtaposition of the ubiquitously expressed ROSA26 promoter and the LacZ gene, ultimately leading to the expression of the reporter gene product (a). ROSA26 mouse embryos intracerebroventricularly inoculated with HSVcre at E14.5 were sacrificed and perfused with 4% paraformaldehyde at 29 days of age. Coronal brain sections (30 μm) were processed for dual LacZ/NeuN or LacZ/GFAP fluorescent immunohistochemistry and imaged using confocal microscopy (b). Representative sections of the striatum (b1-b7), cortex (b8-b14), subventricular zone (SVZ; b15-b21), and hippocampus (HC; b22-b28) are shown. β-galactosidase immunopositivity, resulting from cre recombinase-mediated recombination and excision of the loxP-bound transcriptional/translational stop cassette, appears in the green channel (b2, b5, b9, b12, b16, b19, b23, and b26). Cell-type markers (GFAP and NeuN) were detected in the red channel (GFAP: b1, b8, b15, and b22; NeuN: b4, b11, b18, and b25), while co-localization is depicted in yellow (Merged: b3, b6, b10, b13, b17, b20, b24, and b27). Panels b7, b14, b21, and b28 depict 5X expanded views of the area outlined by the dotted box in Panels b6, b13, b20, and b27, respectively. Mice injected with the null vector, HSVPrPUC, did not exhibit any detectable β-galactosidase-positive cells (data not shown). Magnification bar shown in lower right panel = 50 μm (final magnification = 20X). N = 4 for each experimental group.
Transduction of PCR-confirmed ROSA26 mice (n = 5) with the HSVcre amplicon, however, resulted in detectable LacZ-expressing cells (green) in all four examined regions of the brain. Co-immunohistochemistry with antibodies specific for mature cell type markers for neurons and astrocytes revealed significant co-localization of LacZ with the mature neuronal marker NeuN, while very few, if any, LacZ-positive cells stained positively for the astrocyte marker GFAP (Figure 5b). It is important to note that LacZ-positive neurons in HSVcre-injected ROSA26 mice were qualitatively fewer in numbers than observed previously following HSVsb/HSVT-βgeo in utero infusion. These data indicate intraventricular delivery of HSV-1 amplicons in the embryonic context, regardless of platform iteration, leads to an overwhelmingly neurospecific expression pattern that is likely the result of cellular receptor expression and target cell population present at the time of vector delivery.
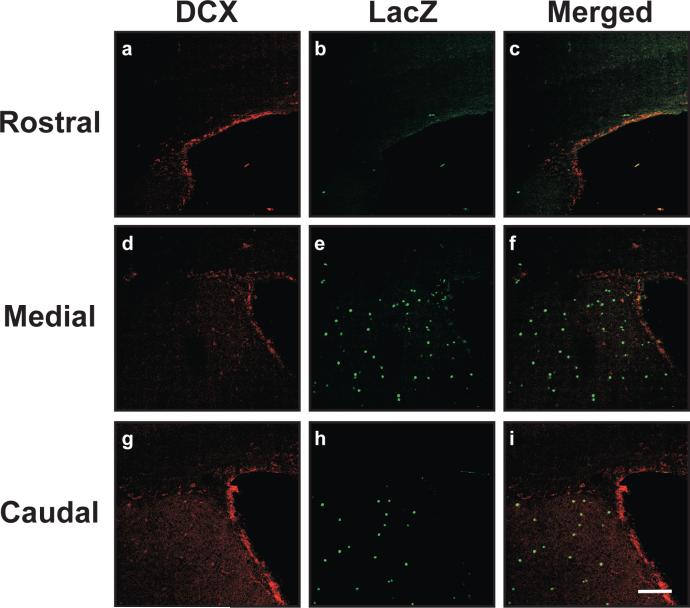
DCX/LacZ co-immunohistochemistry was also performed on these brain sections (Figure 6). Surprisingly, there were no detectable LacZ-positive cells that co-expressed the DCX migratory neuroblast marker in the subventricular zones of rostral, medial, or caudal areas of the adult mouse brains (N=5). These data may explain why the numbers of neurons expressing LacZ in HSVcre-transduced ROSA26 mice were significantly reduced, as renewable pools of transduced progenitor cells harboring Cre-modified ROSA26 loci were apparently absent in the subventricular zones of these in utero-manipulated animals.
Figure 6. Doublecortin (DCX)-positive neuronal precursor cells do not exhibit β-galactosidase expression following in utero intracerebroventricular delivery of HSVcre into E14.5 ROSA26 mouse embryos.
ROSA26 embryos inoculated with HSVcre at E14.5 were sacrificed and perfused with 4% paraformaldehyde at 29 days of age. Coronal brain sections (30 μm) were processed for dual LacZ/DCX immunohistochemistry and imaged using confocal microscopy. Representative images from the subventricular zone (SVZ) from rostral (a-c), medial (d-f), and caudal (g-i) brain sections are shown. Other regions of the brain distal to the ventricles did not harbor appreciable DCX-specific staining, though LacZ expression patterns were similar to those depicted in Figure 1 (data not shown). Mice injected with the null vector, HSVPrPUC, did not exhibit any detectable β-galactosidase-positive cells (data not shown). β-galactosidase staining appears in the green channel (a, d, g). DCX immunopositivity appears in the red channel (b, e, h), while co-localization of the markers is shown in yellow (c, f, i). Magnification bar shown in Panel i = 50 μm (final magnification = 20X). N = 4 for each experimental group.
Discussion
The observed neurospecificity of the bipartite HSV/SB gene transfer platform appears to be largely due to the nature of Sox1+ progenitor cell targets present at the time of intraventricular vector infusion. As Sox1 expression is extinguished, these progenitor cells exit the cell cycle and further differentiate into cell types that express more mature neuronal markers in a highly orchestrated manner (reviewed by [13]). The proliferative activity and the cell cycle status of Sox1+ cells may be important factors in influencing the efficiency of the HSV/SB bipartite platform in utero. Walisko and colleagues recently determined that SB-mediated transposition preferentially occurs in the G1 phase of the cell cycle, where transposition-induced DNA damage can be sufficiently repaired by nonhomologous end-joining [14]. The high correlation of lacZ/Sox1-positive cells in the transduction target field one day following intraventricular vector infusion of HSVlac into E14.5 embryos supports a role for these cells in contributing to the efficiency and neurospecificity of the HSV/SB amplicon vector platform in utero. Hence, gaining a detailed understanding of cell cycle status in these putative target cells and how that status impacts HSV/SB transposition represents an important focus of future experimentation.
The robust HMGB1 signal in cells residing in the ependymal and sub-ependymal layers further supports the notion that these Sox1+ cells represent the primary targets for SB-mediated transposition. High Mobility Group protein B1 (HMGB1) is non-histone DNA bending protein associated with eukaryotic chromatin that preferentially binds AT-rich regions of the DNA in the minor groove [15]. HMGB1 is essential for efficient SB-mediated transposition and transient overexpression of HMGB1 increases transposition efficiency [7]. HMGB1 physically interacts with the transposase to stimulate binding of the transposase to one of the direct repeats within the IR/DR elements, while also inducing DNA helix bending at the inverted repeats of the transposon [7]. The expression of HMGB1 within Sox1+ ependymal cells strongly suggests, although correlatively, the SB transposase can efficiently mobilize and integrate an IR/DR-flanked transgene cassette in this cell population in utero. Our data also speak to the possibility that amplicon virions penetrating deeper sub-ependymal layers during vector infusion may be capable of integrating the transgenon cassette due to the high expression of HMGB1 co-factor in cells that do not necessarily co-express Sox1.
The correlative evidence of Sox1 and HMGB1 co-expression, however, cannot fully explain the neurospecificity of our HSV/SB bipartite vector system, since prior evidence has demonstrated Sox1+ cells manifest the ability to differentiate into cells comprising the neuronal and glial lineages. Penvy and colleagues have shown that overexpression of Sox1 in P19 cells, an embryonal carcinoma cell line, results in differentiation into both neurons and GFAP-positive glia [16], while expression of Sox1 from a lentiviral vector in progenitor cells cultured from the E17 mouse telencephalon resulted in neuronal differentiation in most, but not all, transduced cells [17]. Moreover, the SVZ in the developing mouse embryo has been shown to give rise to not only neurons, but also glia and astrocytes, suggesting that progenitors of these latter cell types, albeit relatively low in numbers, are theoretically available for vector transduction [18]. Thus, the inherent activity of the SB transposase and/or amplicon viral tropism may impart an effect on the ultimate cellular specificity of transgenon expression.
Our study revealed HSV amplicon-expressed Cre to be markedly less effective in catalyzing excision of the stop cassette in embryonic cells than transposition of the βgeo transgenon via the bipartite HSV/SB platform. It is possible that the expression of the SB co-factor HMGB1 in the embryonic ependymal and subventricular zones provides an advantage to Sleeping Beauty relative to Cre recombinase. Moreover, the DNA substrate for SB transposase is inherently different than that acted upon by Cre. In our bipartite HSV/SB system, SB must bind the IR/DR elements of the episomally maintained HSVT-βgeo genome and catalyze the transposition of the RSV promoter-driven βgeo transgene into many potential genomic sites. Cre recombinase, however, must act upon a single genomically disposed loxP-bound substrate at a specific site within the mouse genome and may be therefore more constrained in its ability to catalyze excision of the transcriptional/translational stop cassette to initiate LacZ expression. The chromatin status of the genomic integration site of the ROSA26 conditional transgene in neuroprogenitor cells is unknown, so it is possible that the region harboring the ROSA26 loxP/LacZ locus has a highly heterochromatic conformation at the time of in utero injection, thereby making the locus relatively inaccessible to Cre recombinase.
Herein, we have demonstrated that the HSV/SB bipartite vector platform can efficiently deliver a functional transgene to and facilitate expression in neuroprogenitors and adult neurons. However, more experimentation remains to be performed to optimize this system for clinically relevant therapeutic applications. It is imperative that the SB transposase be engineered to catalyze site-specific genomic integration. In its current iteration, the SB transposase can insert IR/DR-flanked sequences at relatively random TA dinucleotide motifs within the mammalian genome, suggesting that the potential exists for transposition to occur into a vital genetic locus or regulatory sequence. Due to the modular structure of SB, it appears feasible to engineer a modified transposase that preferentially catalyzes transposition into “safer” loci of the host chromosome. Such safety modifications will undoubtedly bring this approach to HSV-based gene delivery closer to clinical implementation for preventing and/or ameliorating early-onset pediatric neurological disorders.
Materials and Methods
Helper virus-free HSV amplicon packaging
Helper virus-free HSV amplicon vectors were packaged and titered as previously described [19, 20]. The detailed construction of HSVlac, HSVcre [11], HSVPrPUC [21], HSVsb, and HSVT-βgeo [5] have been described elsewhere. Amplicon stocks were stored at −80°C until use.
Mouse Lines
C57BL/6 mice and ROSA26 heterozygous mice were utilized in these experiments. C57BL/6 mice were originally obtained from Jackson Labs (Bar Harbor, ME) and a pathogen-free colony was established in house. The ROSA26 mouse line was kindly provided by Dr. D. Anderson (California Institute of Technology, Pasadena, CA). The ROSA26 mouse is a transgenic line that carries the ubiquitously active ROSA26 promoter upstream of a loxP-flanked transcriptional and translational stop cassette and LacZ reporter gene. Upon expression of Cre recombinase in a cell that harbors the ROSA26 cassette, Cre binds to the 34-bp loxP elements and catalyzes the excision of the transcriptional/translational stop cassette thereby facilitating ROSA26 promoter-driven expression of the now juxtaposed LacZ reporter gene. The ROSA26 mouse was previously backcrossed into the C57BL/6 genetic background and maintained in a hemizygous state. All mice were housed and bred in accordance with approved University of Rochester animal use guidelines.
In Utero Injections
C57BL/6 female mice were mated to C57BL/6 male mice for the HSV/SB vector studies or ROSA26 hemizygous male mice for the HSVcre vector studies, plugs were checked the next day, and if present, the females were separated in accordance with approved University of Rochester animal use guidelines. Under Avertin (0.5 mg/g) anesthesia, the maternal abdomens of post-coitum (p.c.) 14.5 C57BL/6 mice were shaved and prepped with proviodine scrub (Operand, Bradford, CT). A laparotomy was performed and the uterus was gently exteriorized to a sterile disposable drape. Injection of fetuses was performed with a Borosil micropipette needle (FHC Inc. Bowdoinham ME), diameter: 1.0 mm, pulled with a Narishige PB-7 needle puller (Narishige International USA, Inc., New York, NY), then ground to a 30-degree angle with a Narishige EG-44 micro grinder (Narishige International USA, Inc, New York, NY). Two microliters of HSVsb or the control amplicon HSVPrPUC was mixed, at a ratio of 1:1 (2 μl total volume: 2×104 total transducing units), with HSVT-βgeo, and delivered to each E14.5 C57BL/6 embryo intracranially using an IM300 Programmable Microinjector (Narishige International USA, New York, NY). Two microliters of the amplicon HSVcre was delivered intracranially to each E14.5 C57BL/6 × ROSA26 mouse embryo. A subset of C57Bl/6 mouse embryos were injected with 2 μl of a β-galactosidase expressing control amplicon (HSVlac) or HSVPrPUC for assessment of transduced cell types 1 day following vector infusion. Embryos were visualized using an Olympus SZ60 dissection microscope (Olympus, Japan). The uterus was returned to the abdominal cavity and the abdominal wall was then closed with Coated VICRYL (polyglactin 910) sutures (Ethicon, Somerville, New Jersey), and the outer incision was closed with silk sutures (Tyco Healthcare, Ville St. Laurent, Quebec). A layer of triple antibiotic gel (Fougera Melville, NY) was applied over the incision site, and the mouse monitored for breathing and reflexive movements until it regained consciousness. Throughout the surgery, the mouse was maintained on a 97°F water pad circulated by a T-pump heat therapy system (Gaymar, Orchard Park, NY). Upon recovery, the mouse was administered 0.00325mg/kg Buprenorphine hydrochloride (Reckitt Benkisen Healthcare Ltd., Hull, England) subcutaneously, and returned to its cage. The female mouse was monitored for weight gain and movement, and 5% lidocaine (Fougera Melville, NY) was applied to the incision site twice a day until it gave birth. Once born, the pups were separated from the mother and placed in the care of a foster Swiss Webster time-pregnant mother, (Jackson Labs, Bar Harbor, ME), and pups allowed to develop to 29 days old. A subset of injected C57Bl/6 mouse embryos were sacrificed 1 day following vector infusion. Brains from all animals were removed, post-fixed overnight in 4% paraformaldehyde (PFA) in 0.1 M PB, transferred to a solution of 20% sucrose in PBS overnight, and finally to a solution of 30% sucrose in PBS. Brains were sectioned coronally (30 μm) using a sliding microtome, and stored in cryoprotectant at −20°C until used for immunohistochemical analyses.
Immunohistochemical analysis
Immunohistochemical (IHC) analysis was performed using the following antibodies: anti-β-galactosidase, rabbit IgG fraction (1:2000, Biodesign, Saco, ME, USA), anti-GFAP- Cy3 conjugate monoclonal antibody clone G-A-5 (1:2000, Sigma, St. Louis, MO, USA), anti-NeuN mouse monoclonal antibody (1:300 Chemicon International, Temecula, CA, USA), anti-DCX guinea pig polyclonal antibody (1:2000, Chemicon International, Temecula, CA, USA), anti-Sox1 chicken polyclonal antibody (1:500, Chemicon International, Temecula, CA, USA), and anti-HMGB1 rabbit polyclonal antibody (1:500, Abcam, Cambridge, MA). For embryonic tissue IHC, slides were washed twice for 10 min. each in 0.1 M PBS. Tissue was then permeabilized with two 15-min. washes for PBS + 0.1% Triton-X-100 (Sigma-Aldrich, St. Louis, MO). Non-specific interactions were blocked by incubation of the sections for 1 h at 22°C with PBS + 0.1% Triton-X 100 + 10% normal goat serum (GIBCO/Invitrogen, Carlsbad, CA). Sections were incubated overnight at 4°C with primary antibody diluted in PBS + 0.1% Triton-X 100 + 1% normal goat serum. Samples were washed six times for 10 min. each with PBS + 0.1% Triton- X 100 + 1% normal goat serum prior to addition of species-specific secondary antibody IgG (H+L) generated in goat (Invitrogen, Carlsbad, CA). Slides were incubated in secondary antibody for 90 min. and were then washed four times for 10 min. each with PBS + 0.1% Triton- X 100 + 1% normal goat serum, then twice in 0.1 M PBS. Slides were allowed to dry and coverslipped. For adult tissue IHC, the protocol was similar with the following exceptions: preliminary washing was performed three times for 5 min., then twice for 30 min.; tissue was permeabilized for 5 min.; and tissue was washed only twice following each antibody incubation step.
Determination of ROSA26 genotype
In utero HSVcre injections of E14.5 embryos derived from a mating between a C57BL/6 female mouse and hemizygous ROSA26 male mouse necessitated PCR-based screening of transduced mice upon sacrifice to ensure the presence of the ROSA26-driven LacZ transgene. At the time of tissue harvest, a section of tail was removed from each mouse, samples were proteinase K digested, and DNA was extracted using a phenol:chloroform/iso-amyl alcohol extraction. ROSA26 mouse genotype positivity was confirmed by standard PCR using the following primers specific to the LacZ transgene: The sense primer sequence was 5’-GTG GCA GCA TCA GGG GAA AAC CTT-3’, while the anti-sense primer sequence was 5’-GAA TTC CGC CGA TAC TGA CGG GCT-3’.
Statistical Analyses
Experiments were conducted using a minimal of N=4 per condition. Statistical analyses were carried out using Student T-test.
Acknowledgements
We would like to thank Ann Casey, Clark Burris, and Louis Lotta (University of Rochester) for helper virus-free amplicon packaging; and Landa Prifti (University of Rochester) for animal husbandry. We also thank John Wolfe and Brian Karolewski (University of Pennsylvania) for instruction and advice they kindly provided for the in utero surgical procedures. We also thank Pushkar Joshi for advice on embryonic tissue immunohistochemistry. Finally, we thank Dr. Gary H. Cohen and Dr. Roselyn J. Eisenberg (University of Pennsylvania) for kindly providing anti-nectin-1 polyclonal rabbit sera. A de Kiewiet Summer Research Fellowship (EBP), AFAR Research Grant (WJB), NIH U54-NS045309 (HJF/WJB), and NIH R01-NS36420A (HJF) supported this work.
References
- 1.Oehmig A, Fraefel C, Breakefield XO. Update on herpesvirus amplicon vectors. Mol Ther. 2004;10:630–643. doi: 10.1016/j.ymthe.2004.06.641. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki M, Kasai K, Saeki Y. Plasmid DNA sequences present in conventional herpes simplex virus amplicon vectors cause rapid transgene silencing by forming inactive chromatin. J Virol. 2006;80:3293–3300. doi: 10.1128/JVI.80.7.3293-3300.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin BK, Belloni M, Conti B, Federoff HJ, Starr R, Son JH, et al. Prolonged in vivo gene expression driven by a tyrosine hydroxylase promoter in a defective herpes simplex virus amplicon vector. Hum Gene Ther. 1996;7:2015–2024. doi: 10.1089/hum.1996.7.16-2015. [DOI] [PubMed] [Google Scholar]
- 4.Bowers WJ, Olschowka JA, Federoff HJ. Immune responses to replication-defective HSV-1 type vectors within the CNS: implications for gene therapy. Gene Ther. 2003;10:941–945. doi: 10.1038/sj.gt.3302047. [DOI] [PubMed] [Google Scholar]
- 5.Bowers WJ, Mastrangelo MA, Howard DF, Southerland HA, Maguire-Zeiss KA, Federoff HJ. Neuronal precursor-restricted transduction via in utero CNS gene delivery of a novel bipartite HSV amplicon/transposase hybrid vector. Mol Ther. 2006;13:580–588. doi: 10.1016/j.ymthe.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Koizumi H, Higginbotham H, Poon T, Tanaka T, Brinkman BC, Gleeson JG. Doublecortin maintains bipolar shape and nuclear translocation during migration in the adult forebrain. Nat Neurosci. 2006;9:779–786. doi: 10.1038/nn1704. [DOI] [PubMed] [Google Scholar]
- 7.Zayed H, Izsvak Z, Khare D, Heinemann U, Ivics Z. The DNA-bending protein HMGB1 is a cellular cofactor of Sleeping Beauty transposition. Nucleic Acids Res. 2003;31:2313–2322. doi: 10.1093/nar/gkg341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karram K, Chatterjee N, Trotter J. NG2-expressing cells in the nervous system: role of the proteoglycan in migration and glial-neuron interaction. J Anat. 2005;207:735–744. doi: 10.1111/j.1469-7580.2005.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishi M, Mizuseki K, Sasai N, Yamazaki H, Shiota K, Nakanishi S, et al. Requirement of Sox2-mediated signaling for differentiation of early Xenopus neuroectoderm. Development. 2000;127:791–800. doi: 10.1242/dev.127.4.791. [DOI] [PubMed] [Google Scholar]
- 10.Zhao S, Nichols J, Smith AG, Li M. SoxB transcription factors specify neuroectodermal lineage choice in ES cells. Mol Cell Neurosci. 2004;27:332–342. doi: 10.1016/j.mcn.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Brooks AI, Muhkerjee B, Panahian N, Cory-Slechta D, Federoff HJ. Nerve growth factor somatic mosaicism produced by herpes virus-directed expression of cre recombinase. Nat Biotechnol. 1997;15:57–62. doi: 10.1038/nbt0197-57. [DOI] [PubMed] [Google Scholar]
- 12.Stein CS, Martins I, Davidson BL. The lymphocytic choriomeningitis virus envelope glycoprotein targets lentiviral gene transfer vector to neural progenitors in the murine brain. Mol Ther. 2005;11:382–389. doi: 10.1016/j.ymthe.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist's view of neural development. Trends Neurosci. 2005;28:583–588. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Walisko O, Izsvak Z, Szabo K, Kaufman CD, Herold S, Ivics Z. Sleeping Beauty transposase modulates cell-cycle progression through interaction with Miz-1. Proc Natl Acad Sci U S A. 2006;103:4062–4067. doi: 10.1073/pnas.0507683103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol Cell Biol. 1999;19:5237–5246. doi: 10.1128/mcb.19.8.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R. A role for SOX1 in neural determination. Development. 1998;125:1967–1978. doi: 10.1242/dev.125.10.1967. [DOI] [PubMed] [Google Scholar]
- 17.Kan L, Israsena N, Zhang Z, Hu M, Zhao LR, Jalali A, et al. Sox1 acts through multiple independent pathways to promote neurogenesis. Dev Biol. 2004;269:580–594. doi: 10.1016/j.ydbio.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Hatten ME. Central nervous system neuronal migration. Annu Rev Neurosci. 1999;22:511–539. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- 19.Bowers WJ, Howard DF, Brooks AI, Halterman MW, Federoff HJ. Expression of vhs and VP16 during HSV-1 helper virus-free amplicon packaging enhances titers. Gene Ther. 2001;8:111–120. doi: 10.1038/sj.gt.3301340. [DOI] [PubMed] [Google Scholar]
- 20.Bowers WJ, Howard DF, Federoff HJ. Discordance between expression and genome transfer titering of HSV amplicon vectors: recommendation for standardized enumeration. Mol Ther. 2000;1:294–299. doi: 10.1006/mthe.2000.0039. [DOI] [PubMed] [Google Scholar]
- 21.Geller AI, Breakefield XO. A defective HSV-1 vector expresses Escherichia coli β-galactosidase in cultured peripheral neurons. Science. 1988;241:1667–1669. doi: 10.1126/science.241.4873.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]