Abstract
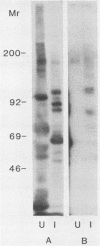
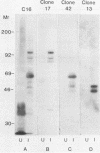
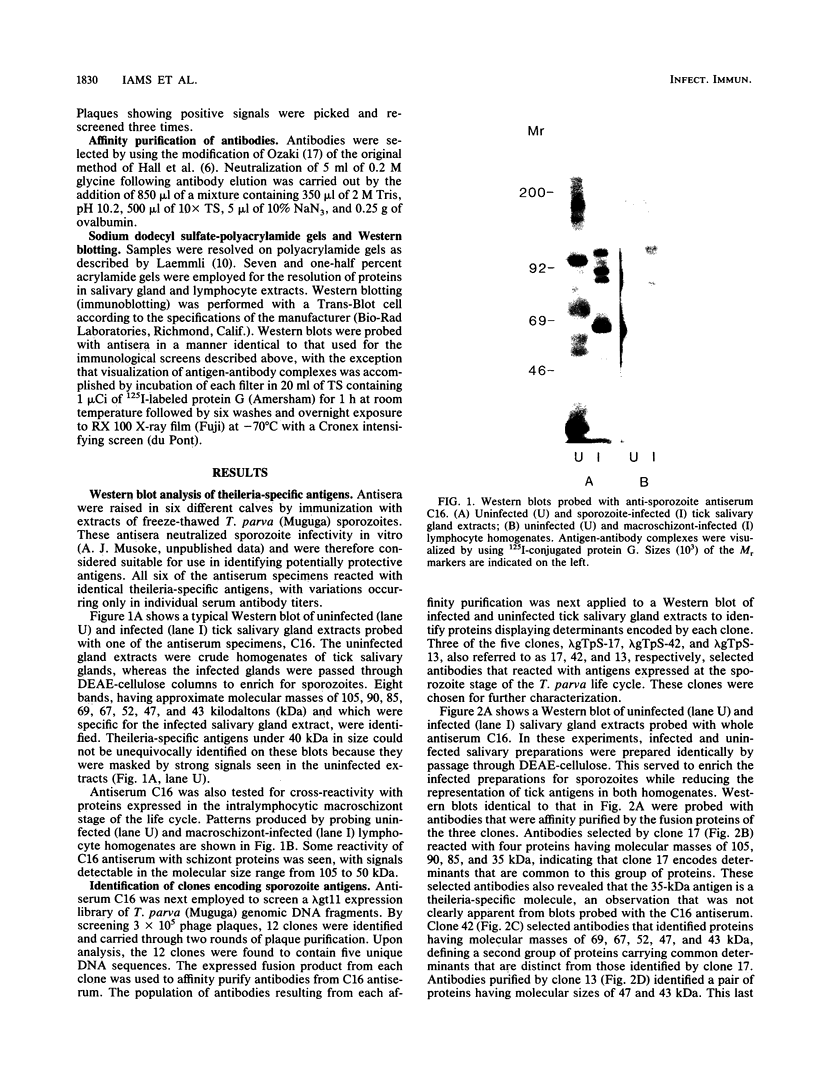
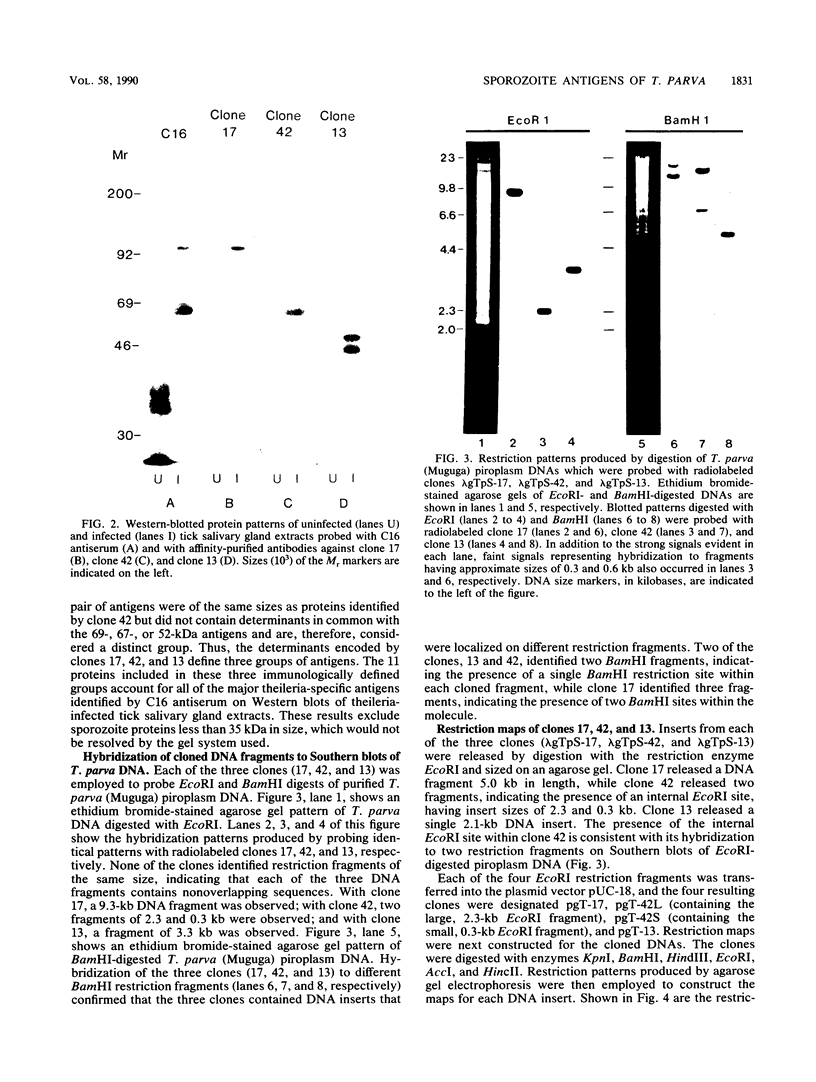
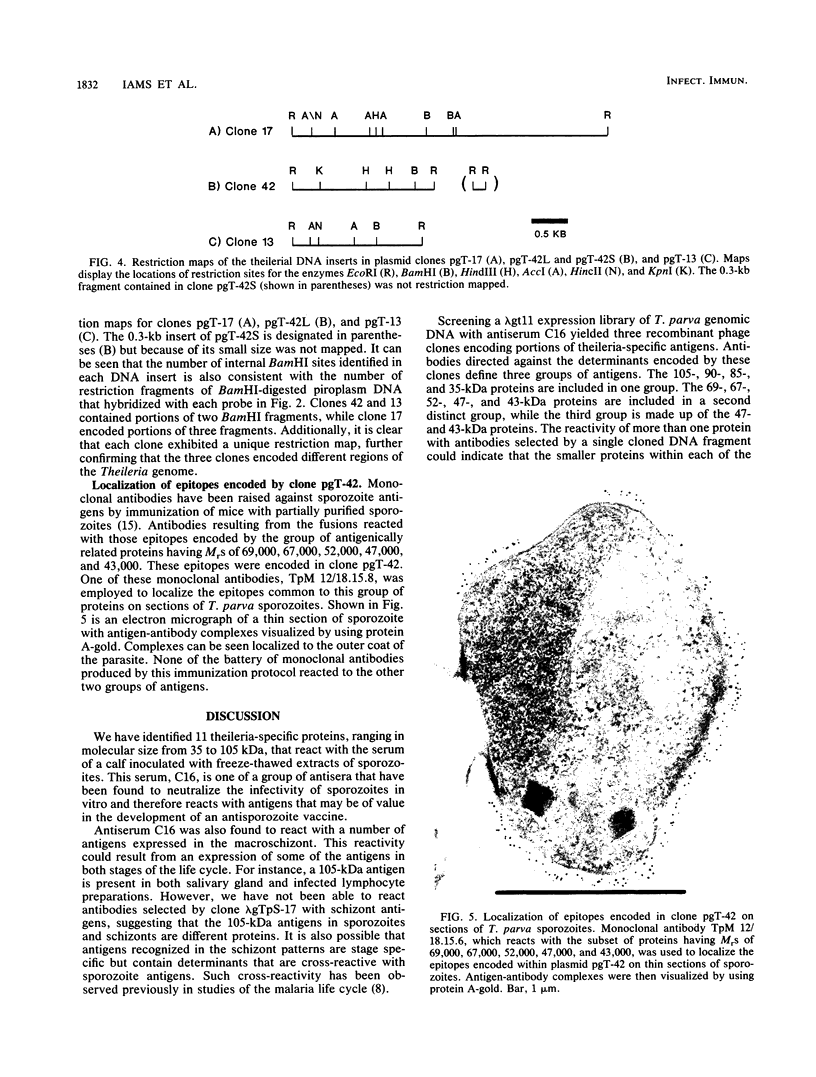
An antiserum, C16, was raised in cattle against freeze-thawed extracts of sporozoites of Theileria parva (Muguga). This antiserum, which neutralizes sporozoite infectivity in vitro, identified theileria-specific antigens having approximate molecular masses of 105, 90, 85, 69, 67, 52, 47, and 43 kilodaltons (kDa) on Western blots (immunoblots) of infected tick salivary gland extracts. The antiserum was used to screen an expression library of T. parva (Muguga) genomic DNA fragments. Three recombinant bacteriophage clones carrying different theileria DNA inserts were studied. The expressed gene product from each clone was used to affinity purify antibodies from C16 antiserum for use in probing Western blots of uninfected and infected tick salivary gland extracts. The population of antibodies selected by each clone specifically recognized a subset of the antigens identified by C16 antiserum. The antigens fell into three distinct groups as defined by their reactivity with each set of selected antibodies. One group included antigens of 105, 90, 85, and 35 kDa, a second group included antigens of 69, 67, 52, 47, and 43 kDa, and the third group included an apparently distinct pair of antigens of 47 and 43 kDa. Thus, antibodies that reacted with determinants encoded by the three recombinant phage clones recognized all of the major antigens seen on Western blots probed with whole C16 antiserum. These results suggest that there may be only three immunodominant antigens expressed in T. parva (Muguga) sporozoites. Additionally, monoclonal antibodies have been raised which neutralize sporozoite infectivity in vitro. These antibodies react with epitopes of the antigens with Mrs of 69,000, 67,000, 52,000, 47,000, and 43,000 which are encoded in clone pgT-42 and have been used to localize these epitopes on the sporozoite surface.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conrad P. A., Iams K., Brown W. C., Sohanpal B., ole-MoiYoi O. K. DNA probes detect genomic diversity in Theileria parva stocks. Mol Biochem Parasitol. 1987 Oct;25(3):213–226. doi: 10.1016/0166-6851(87)90085-5. [DOI] [PubMed] [Google Scholar]
- Cunningham M. P., Brown C. G., Burridge M. J., Purnell R. E. Cryopreservation of infective particles of Theileria parva. Int J Parasitol. 1973 Sep;3(5):583–587. doi: 10.1016/0020-7519(73)90082-9. [DOI] [PubMed] [Google Scholar]
- Dobbelaere D. A., Shapiro S. Z., Webster P. Identification of a surface antigen on Theileria parva sporozoites by monoclonal antibody. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1771–1775. doi: 10.1073/pnas.82.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere D. A., Spooner P. R., Barry W. C., Irvin A. D. Monoclonal antibody neutralizes the sporozoite stage of different Theileria parva stocks. Parasite Immunol. 1984 Jul;6(4):361–370. doi: 10.1111/j.1365-3024.1984.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Eugui E. M., Emery D. L. Genetically restricted cell-mediated cytotoxicity in cattle immune to Theileria parva. Nature. 1981 Mar 19;290(5803):251–254. doi: 10.1038/290251a0. [DOI] [PubMed] [Google Scholar]
- Hall R., Hyde J. E., Goman M., Simmons D. L., Hope I. A., Mackay M., Scaife J., Merkli B., Richle R., Stocker J. Major surface antigen gene of a human malaria parasite cloned and expressed in bacteria. 1984 Sep 27-Oct 3Nature. 311(5984):379–382. doi: 10.1038/311379a0. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981 Nov 26;294(5839):361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- Hope I. A., Hall R., Simmons D. L., Hyde J. E., Scaife J. G. Evidence for immunological cross-reaction between sporozoites and blood stages of a human malaria parasite. Nature. 1984 Mar 8;308(5955):191–194. doi: 10.1038/308191a0. [DOI] [PubMed] [Google Scholar]
- Irvin A. D., Dobbelaere D. A., Mwamachi D. M., Minami T., Spooner P. R., Ocama J. G. Immunisation against East Coast fever: correlation between monoclonal antibody profiles of Theileria parva stocks and cross immunity in vivo. Res Vet Sci. 1983 Nov;35(3):341–346. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mack S. R., Vanderberg J. P., Nawrot R. Column separation of Plasmodium berghei sporozoites. J Parasitol. 1978 Feb;64(1):166–168. [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Musoke A. J., Nantulya V. M., Buscher G., Masake R. A., Otim B. Bovine immune response to Theileria parva: neutralizing antibodies to sporozoites. Immunology. 1982 Apr;45(4):663–668. [PMC free article] [PubMed] [Google Scholar]
- Musoke A. J., Nantulya V. M., Rurangirwa F. R., Buscher G. Evidence for a common protective antigenic determinant on sporozoites of several Theileria parva strains. Immunology. 1984 Jun;52(2):231–238. [PMC free article] [PubMed] [Google Scholar]
- Ozaki L. S., Mattei D., Jendoubi M., Druihle P., Blisnick T., Guillotte M., Puijalon O., Da Silva L. P. Plaque antibody selection: rapid immunological analysis of a large number of recombinant phage clones positive to sera raised against Plasmodium falciparum antigens. J Immunol Methods. 1986 May 22;89(2):213–219. doi: 10.1016/0022-1759(86)90360-1. [DOI] [PubMed] [Google Scholar]
- Schofield L., Bushell G. R., Cooper J. A., Saul A. J., Upcroft J. A., Kidson C. A rhoptry antigen of Plasmodium falciparum contains conserved and variable epitopes recognized by inhibitory monoclonal antibodies. Mol Biochem Parasitol. 1986 Feb;18(2):183–195. doi: 10.1016/0166-6851(86)90037-x. [DOI] [PubMed] [Google Scholar]
- Schwartzman J. D. Inhibition of a penetration-enhancing factor of Toxoplasma gondii by monoclonal antibodies specific for rhoptries. Infect Immun. 1986 Mar;51(3):760–764. doi: 10.1128/iai.51.3.760-764.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster P., Dobbelaere D. A., Fawcett D. W. The entry of sporozoites of Theileria parva into bovine lymphocytes in vitro. Immunoelectron microscopic observations. Eur J Cell Biol. 1985 Mar;36(2):157–162. [PubMed] [Google Scholar]
- Young R. A., Mehra V., Sweetser D., Buchanan T., Clark-Curtiss J., Davis R. W., Bloom B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985 Aug 1;316(6027):450–452. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]