Abstract
This investigation sought to develop methods that permit broad and tunable control over the erosion of multilayered polyelectrolyte assemblies and the release of anionic polymers in physiologically relevant media. We report the fabrication and characterization of multilayered films ~60 nm thick using sodium poly(styrene sulfonate) (SPS) and different combinations of three different hydrolytically degradable polyamines (1–3). We investigated two different approaches to film fabrication: 1) fabrication using solutions comprised of defined mixtures of two different polyamines, and 2) fabrication of films composed of different numbers of layers of two different polyamines. In general, films fabricated using polyamine solutions composed of defined mixtures of two different polyamines had erosion and release profiles that were dictated almost entirely by the most hydrophobic polyamine used to fabricate the films. In contrast, the fabrication of films having different numbers of layers of different polyamines permitted broad and tunable control over film erosion and the release of SPS. For example, films having the architecture (1/SPS)n(2/SPS)m released SPS with profiles that were intermediate to those of films fabricated exclusively from polymer 1 or polymer 2. Further, we demonstrated that it is possible to exert systematic control over the release of SPS by varying the relative numbers of layers of (1/SPS) or (2/SPS) incorporated into the films. The approaches reported here provide tunable control over the rate of the release of anionic polymers from surfaces coated with ultrathin multilayered films. This work could, with further development, contribute to the design of ultrathin films that permit tunable control over the release and delivery of therapeutically relevant macromolecules, such as proteins or DNA, from surfaces.
Introduction
The layer-by-layer deposition of oppositely charged polyelectrolytes on surfaces is a versatile method for the bottom-up assembly of multilayered thin films.1–4 This method of fabrication provides nanometer-scale control over the compositions and thicknesses of films assembled from a broad range of water-soluble polymers, and it permits the assembly of films on a wide variety of macroscopic and microscopic objects. This procedure is particularly attractive for the fabrication of films constructed from biologically-derived polyelectrolytes, and it has contributed significantly to the design of assemblies of interest in the contexts of biology, medicine, and biotechnology.3,5–13 Research motivated by potential biomedical applications of these materials has ignited recent interest in the application of multilayered assemblies as platforms for the controlled release of drugs3,14–24 and other therapeutically relevant agents.25–39 Here, we describe an approach to the design of ultrathin multilayered films that permits both broad and tunable control over the erosion of the films in aqueous media and the release of incorporated anionic polymers. The approach reported here could, with further development, contribute to the design of ultrathin assemblies that permit tunable control over the release of therapeutically relevant polyelectrolytes, such as proteins and DNA, from surfaces coated with multilayered films.
Many past studies describing the application of multilayered assemblies in the context of controlled release have focused on the use of these materials as membranes or as reservoirs that permit the diffusion-controlled release of small drug-like molecules.3,14–23 The application of these assemblies to the controlled release of macromolecular species25–39 introduces additional challenges, because polymeric molecules typically do not diffuse readily through these ionically crosslinked materials. Approaches that permit control over the rates and extents to which these assemblies erode, disintegrate, or dissolve in aqueous media would contribute to the development of films designed to release macromolecular payloads. Toward this goal, several groups have described approaches that permit the triggered disruption or gradual erosion of multilayered assemblies. Notable examples include films that erode or degrade in response to changes in environmental pH or ionic strength,40–45 the enzymatic degradation of film components,35,36,46–49 the addition of reducing agents,50,51 the application of electrical potentials,37,52,53 or the addition of small molecules that disrupt receptor-ligand interactions.54–56 Research in our group has focused on the design of multilayered assemblies fabricated from hydrolytically degradable polyamines.25–32 The incorporation of degradable polyamines into multilayered films provides a mechanism with which to promote erosion when these materials are exposed to aqueous environments. For example, we have demonstrated in several past studies that poly(ester-amine) 1 can be used to fabricate multilayered films that erode and sustain the release of synthetic anionic polymers,25–27,29 plasmid DNA,28–31 or therapeutically relevant polysaccharides33,34 when incubated under physiologically relevant conditions (e.g., PBS buffer, pH 7.4, 37 °C).32
Although a growing number of reports describe the incorporation of functionality that permits film disruption or erosion, the development of approaches that can be used to achieve broad and tunable control over erosion and release has remained a challenge. Picart et al.48 and, subsequently, Ren et. al.36 demonstrated recently that it is possible to tune the rates at which enzymatically-degradable multilayered films erode by chemically crosslinking the polysaccharide48 or poly(amino acid)36 components of these films. As an alternative approach, we reported that the rates of erosion of films fabricated using hydrolytically degradable polyamines could be extended by varying the hydrophobicity of the polyamines used to construct these films.26,27 For example, whereas films fabricated from alternating layers of polyamine 1 and sodium poly(styrene sulfonate) (SPS) erode over a period of two days, analogous films fabricated from polyamines 2 or 3, which are more hydrophobic and degrade more slowly than polyamine 1, erode over periods of 6 days and 14 days, respectively. The results of these past studies demonstrate that film erosion can be extended or prolonged by manipulating the structures of the polyamines used to fabricate multilayered films. Unfortunately, however, the range of erosion and release profiles that could be achieved using this approach was generally restricted by the number of degradable polyamine structures that were available or could be readily synthesized.
This investigation sought to determine whether it was possible to tune the erosion of multilayered films constructed from degradable polyamines by fabricating assemblies using blends or combinations of different polymers. In principle, such an approach could provide broad control over the erosion of films fabricated from a small pool of polyamine starting materials. Here, we report on the fabrication and characterization of multilayered films assembled using SPS and different combinations of polyamines 1, 2, and 3. We find that, in general, films fabricated using polyamine solutions composed of defined mixtures of two different polyamines have erosion and release profiles that are dictated almost entirely by the most hydrophobic polyamine used to fabricate the films. However, we demonstrate that it is possible to design films with broadly tunable release profiles by fabricating films composed of different numbers of layers of two different polyamines. For example, films composed of multiple layers of polymer 1/SPS and multiple layers of polymer 2/SPS erode and release SPS at rates that are intermediate to those of films fabricated exclusively from polymer 1/SPS or polymer 2/SPS. Using this approach, it is possible to tune systematically the erosion and release profiles of these materials over a broad range simply by varying the relative numbers of layers of each polyamine that are incorporated into a multilayered film.
Materials and Methods
General Considerations
Silicon substrates (e.g., 0.5 × 2.0 cm) used for the fabrication of multilayered films were cleaned with methylene chloride, ethanol, methanol, and deionized water, and dried under a stream of filtered compressed air. Surfaces were then activated by etching with an oxygen plasma for 5 minutes (Plasma Etch, Carson City, NV) prior to film deposition. The optical thicknesses of films deposited on silicon substrates were determined using a Gaertner LSE ellipsometer (632.8 nm, incident angle = 70°). Data were processed using the Gaertner Ellipsometer Measurement Program. Relative thicknesses were calculated assuming an average refractive index of 1.58 for the multilayered films. Thicknesses were determined in at least four different standardized locations on each substrate and are presented as an average (with standard deviation) for each film. All films were dried under a stream of filtered compressed air prior to measurement. UV-visible absorbance values for PBS solutions used to determine film release kinetics were recorded on a Beckman Coulter DU520 UV/vis Spectrophotometer (Fullerton, CA).
Materials
Poly(sodium 4-styrenesulfonate) (SPS, MW = 70,000) and sodium acetate buffer were purchased from Aldrich Chemical Company (Milwaukee, WI). Test grade n-type silicon wafers were purchased from Si-Tech, Inc. (Topsfield, MA). Commercially available samples of linear poly(ethylene imine) (LPEI, MW = 25,000) were obtained from Polysciences, Inc. (Warrington, PA). Phosphate buffered saline was prepared by dilution of commercially available concentrate (EM science, Gibbstown, NJ). Poly(ester-amines) 1 (Mn = 7,700), 2 (Mn = 6,200), and 3 (Mn = 8,500) were synthesized as described previously.27 All materials were used as received without further purification unless noted otherwise. Deionized water (18 MΩ) was used for washing steps and to prepare all buffer and polymer solutions. All buffers and polymer solutions were filtered through a 0.2 µm membrane syringe filter prior to use unless noted otherwise. Compressed air used to dry films and coated substrates was filtered through a 0.4 µm membrane syringe filter.
Preparation of Polyelectrolyte Solutions
Solutions of LPEI and SPS used for the fabrication of LPEI/SPS precursor layers (20 mM with respect to the molecular weight of the polymer repeat unit) were prepared using a 50 mM NaCl solution in 18 MΩ water. LPEI solutions contained 5mM HCl to aid polymer solubility. SPS solutions used for the deposition of all other polyamine/SPS layers (20 mM with respect to the molecular weight of the polymer repeat unit) were prepared in water and the solution pH was adjusted to 4.9 using HCl. Solutions of polymers 1, 2, and 3 used for dipping (5 mM with respect to the molecular weight of polymer repeat units) were prepared in sodium acetate buffer (100 mM, pH = 5.1). Solutions of polycation blends used for dipping were prepared by mixing two individual polycation solutions (i.e., solutions of polymers 1, 2, or 3) at predetermined volumetric ratios to give the desired polymer/polymer ratios discussed in the text (total polymer concentration of these mixtures was 5 mM with respect to the molecular weight of polymer repeat units. For example, a 50/50 mixture of polymer 1 and polymer 2 was 2.5 mM in polymer 1 and 2.5 mM in polymer 2).
Fabrication of Multilayered Films
Films were deposited on planar silicon substrates pre-coated with a multilayered film composed of 10 bilayers of LPEI and SPS (terminated with a topmost layer of SPS) to ensure a suitably charged surface for the adsorption of polymers 1–3, as previously described.27,28 These precursor layers were fabricated using an automated dipping robot (Riegler & Kirstein GmbH, Potsdam, Germany). Multilayered films fabricated from polymers 1–3 and SPS were fabricated on these precursor layers manually using an alternating dipping procedure according to the following general protocol: 1) Substrates were submerged in a solution of polycation for 5 minutes, 2) substrates were removed and immersed in an initial water bath for 1 minute followed by a second water bath for 1 minute, 3) substrates were submerged in a solution of SPS for 5 minutes, and 4) substrates were rinsed in the manner described above. This cycle was repeated until the desired number of polycation/SPS bilayers (typically eight) had been deposited. For experiments aimed at fabricating films using solutions of polycation blends, this protocol was adopted with the exception that the polycation solution was composed of a mixture of two polycations, as described above. For experiments designed to evaluate the influence of polymer deposition sequence on film growth and erosion, films were first fabricated using solutions of polymers 1, 2 or 3 and SPS to yield a predetermined number of polyamine/SPS layer pairs (e.g., n = 2, 4, or 6). The polycation solution was then replaced with a solution containing a second, different polyamine and fabrication was continued until a total of eight polycation/SPS layers had been deposited. For experiments aimed at characterizing film growth profiles by ellipsometry, films were dried after every two cycles of the above procedure using filtered compressed air. Films to be used in erosion experiments were either used immediately or were dried under a stream of filtered compressed air and stored in a vacuum dessicator until use. All films were fabricated at ambient room temperature.
Erosion of Multilayered Films and Evaluation of Release Kinetics
Experiments designed to investigate the erosion profiles of multilayered polycation/SPS films were performed in the following general manner: Film-coated substrates were placed in a plastic UV-transparent cuvette and 1.0 mL of phosphate buffered saline (PBS, pH = 7.4, 137 mM NaCl) was added to cover completely the film-coated portion of the substrate. The samples were incubated at 37 °C and were removed at predetermined intervals for analysis by ellipsometry. For experiments designed to monitor a decrease in film thickness, optical thickness values were determined in at least four different predetermined locations on the substrate and the sample was returned immediately to the buffer solution. For experiments aimed at monitoring the release of SPS into solution, the UV absorbance of SPS in the solution was recorded directly on these buffers at a wavelength of 226 nm.
Results and Discussion
Investigation of Multilayered Films Fabricated from Blends of Degradable Polyamines
We reported previously that polymer 1 can be used to fabricate films that erode and sustain the release of anionic polyelectrolytes over a period of two days when incubated in physiologically relevant media.25,28,29 We also demonstrated recently that polymers 2 and 3, which are more hydrophobic and degrade more slowly than polymer 1, can be used to fabricate films that erode over periods of six days and 14 days, respectively.26,27 This past work revealed structure/property relationships that can be used to expand the range of erosion profiles of multilayered films fabricated from degradable polyamines. However, the number of erosion and release profiles that could be achieved using this approach (i.e., three) was limited to the number of degradable polyamine structures that were available.27 Using this approach, for example, the design of a film that erodes completely over a period between six and 14 days would require the synthesis of a new polymer with properties intermediate to those of polymers 2 and 3. While the synthesis of additional polymers is technically feasible,57–60 approaches that expand the usefulness of a limited pool of polyamines could permit more direct and tunable control over film erosion and release.
We hypothesized that multilayered films composed of combinations of polymers 1–3 would have erosion and release profiles intermediate to those fabricated exclusively from each individual polymer. Several past studies have demonstrated that it is possible to incorporate two structurally similar polycations61 or two structurally diverse polyanions62–64 into multilayered films by fabricating films using polyelectrolyte solutions comprised of blends of different polyelectrolytes. For example, Debreczeny et al. reported the fabrication of multicomponent multilayered films using a solution of poly(l-lysine) and a second solution containing a mixture of two polyanions, poly(l-glutamic acid) (PGA) and poly(l-aspartic acid) (PAA).62 These investigators demonstrated that it is possible to control the relative amounts of PGA and PAA incorporated into films by changing the proportion of these two polyelectrolytes in solution. Similarly, Caruso and coworkers reported multilayered films fabricated using a solution of poly(allyamine) (PAH) and a second solution containing either a mixture of SPS and poly(acrylic acid)63 or a mixture of SPS and single-stranded DNA.64 This work also demonstrated that the proportion of SPS in these films correlated with the relative concentrations of SPS in the solutions used to fabricate the films.
On the basis of these past studies, we sought to determine whether it was possible to fabricate multilayered polyamine/SPS films using mixtures of polymers 1–3 and, subsequently, whether films fabricated in this manner would have erosion and release profiles intermediate to films fabricated exclusively from polymers 1, 2, or 3. To evaluate the feasibility of this approach, we fabricated multilayered films using an aqueous solution of SPS and a second solution consisting of a defined mixture of polymer 1 and polymer 3. All films in this study were fabricated on planar silicon substrates to facilitate the characterization of film growth and erosion using ellipsometry. In all cases, silicon substrates were pre-coated with a multilayered film ~20 nm thick composed of 10 alternating layers of LPEI and SPS (referred to hereafter as bilayers) to ensure a suitably charged surface for the adsorption of polymers 1 and 3, as described previously.27,28 These substrates were then dipped alternately into a solution of SPS and a second solution containing a mixture of polymer 1 and polymer 3 at one of four different solution compositions. To investigate a range of different solution compositions, we prepared four different polymer solutions comprised of 10, 25, 50, and 75 mole percent polymer 3 (with the remainder comprised of polymer 1; solutions were 5 mM with respect to total polymer repeat units). Hereafter, we describe films fabricated in this manner using the notation (X/SPS)n, where ‘X’ refers to the polyamine used and ‘n’ denotes the number of polyamine/polyanion bilayers deposited. For films fabricated from mixtures of polyamines, additional subscripts are used to denote the mole percentage of each polyamine in the solutions used to fabricate the films. For example, a film eight bilayers thick fabricated from a 50 mole percent mixture of polymer 1 and polymer 3 would be denoted (150350/SPS)8. As described below, this notation does not imply the relative amounts of polymer 1 or polymer 3 actually incorporated into a film during fabrication.
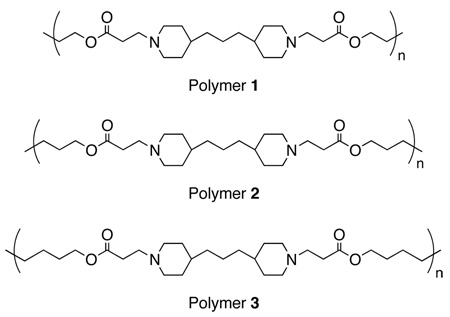
Figure 1 shows a plot of the optical thicknesses of a (1/SPS)8 film, a (3/SPS)8 film, and four different (1,3/SPS)8 films (fabricated using the four solution compositions noted above) as a function of the number of polyamine/SPS layers deposited. The growth of each film was linear, and uniform thicknesses ranging from 60 to 70 nm were achieved after the deposition of eight bilayers. These thickness and growth profiles suggest that the differences in the compositions of the polyamine solutions did not influence significantly the nature of the layer-by-layer assembly process (over the range of mole percentages investigated here). These six films were then incubated in phosphate buffered saline (PBS) at 37 °C to investigate the erosion profiles of these materials. Figure 2a shows a plot of the optical thicknesses of these materials as a function of incubation time. The decrease in film thicknesses observed for (1/SPS)8 and (3/SPS)8 films are consistent with the results of our previous study, in which films fabricated from polymer 3 were found to erode more slowly than films fabricated from polymer 1.26,27 Interestingly, however, the erosion profiles of the four films fabricated using solutions consisting of mixtures of polymer 1 and polymer 3 were almost identical to the erosion profiles observed for the control film fabricated from polymer 3 (Figure 2a).
Figure 1.
Plot of ellipsometric thickness versus the number of polyamine/SPS bilayers deposited for multilayered assemblies fabricated from polyamine solutions comprised of mixtures of polymer 1 and 3. Subscripts denote the mole percentage of each polyamine in the solution mixtures used to fabricate each film. Silicon substrates were pre-coated with a multilayered film ~20 nm thick composed of LPEI and SPS prior to fabrication (see text).
Figure 2.
A): Plot of ellipsometric thickness versus time for polycation/SPS films fabricated from mixtures of polymers 1 and 3 incubated in phosphate buffer (pH = 7.4) at 37 °C. Subscripts denote the mole percentage of each polyamine in the polycation solutions used to fabricate each film (see text). B): Plot of percentage of SPS released vs. time for films characterized in Figure 2a. Percent SPS released was calculated from the UV absorbance (at 226 nm) of the PBS solutions used to incubate these assemblies.
During the erosion experiments described above, we also characterized the release of SPS as a function of time by monitoring the UV absorbance (at 226 nm, the absorbance maximum of SPS) of the PBS solutions used to incubate these materials. Figure 2b shows SPS release profiles (based on normalized UV absorbance values) of the six films characterized in Figure 2a. These data demonstrate that the release of SPS from films fabricated using mixtures of polymer 1 and polymer 3 occurred with release profiles that were nearly identical to that of the (3/SPS)8 film. Taken together, the data in Figures 2a and 2b demonstrate that the fabrication of films using solution mixtures of polymer 1 and polymer 3 does not yield films that erode or release SPS with profiles that are intermediate to those of films fabricated exclusively from polymer 1 and 3 (over the range of mole percentages investigated here). We note here that we also investigated the fabrication and erosion of multilayered films assembled using SPS and polycation solutions consisting of mixtures of polymer 1 and polymer 2, and polymer 2 and polymer 3, over the same range of mole percentages described above. In each case, films fabricated using these solution mixtures also exhibited erosion and release profiles that were nearly identical to those of (2/SPS)8 or (3/SPS)8 films, respectively (data not shown).
The results above demonstrate that the fabrication of multilayered films using solution mixtures of two hydrolytically degradable polyamines yields films with erosion and release profiles that are dominated by the influence of the more hydrophobic polyamine. The reasons for this behavior are not entirely clear. However, we speculate that this behavior could reflect the competitive or preferential incorporation of the more hydrophobic polyamine used during the layer-by-layer assembly process. Although electrostatic interactions are considered to be the primary driving force for the assembly of these films, it is possible that polymer 3, which is more hydrophobic than polymer 1, may adsorb preferentially during fabrication from solutions comprised of mixtures of polymer 1 and polymer 3. If preferential adsorption does occur, the relative percentages of polymer 1 and polymer 3 incorporated into a film would not reflect the relative percentages of these polymers originally used to prepare dipping solutions. In the extreme, competitive adsorption of polymer 3 could result in films comprised almost entirely of polymer 3, and could explain the similarity of the erosion and release profiles of films fabricated from mixtures of polymer 1 and polymer 3 to that of films fabricated exclusively from polymer 3 (Figure 2). The possibly for such competitive incorporation to occur is supported by the results of a recent report by Quinn et al. on the fabrication of multilayered films using PAH and a second solution comprised of a mixture of DNA and SPS.64 These investigators used readily observed differences in the UV absorbance spectra of SPS and DNA to characterize the amounts of DNA in these films relative to amounts present in dipping solutions, and reported that, under certain conditions, these films contained predominately PAH and SPS.
Unfortunately, the structural similarity of polymers 1, 2, and 3 does not permit the differentiation of these polymers on the basis of UV/visible spectrophotometry or methods of analysis such as infrared spectroscopy. As a result, we have thus far been unable to determine directly the relative amounts of polymers 1, 2, and 3 incorporated into these multilayered films after fabrication. However, we performed additional control experiments to determine whether solutions of polymer 3 prepared at low concentrations identical to those used in the mixtures above (but without the addition of polymer 1) could be used to support the growth of multilayered films. The growth profiles of films fabricated using solution concentrations of polymer 3 as low as 0.5 mM (equivalent to the amount present in the 10 mole percent solutions used above) were nearly identical to the growth profile of the (190310/SPS)8 film shown in Figure 1. This result suggests that the low concentration of polymer 3 present in the solution mixtures used to fabricate a (190310/SPS)8 film could be sufficient, in principle, to support the growth of a (3/SPS)8 film if competitive adsorption were to occur. We note here that the possibility of competitive adsorption resulting from differences in the molecular weights of these polymers (rather than differences in hydrophobicity) represents a less likely scenario, as the molecular weights of the polymers used in this study [polymer 1 (Mn = 7,700), polymer 2 (Mn = 6,200), and polymer 3 (Mn = 8,500)] are all similar.
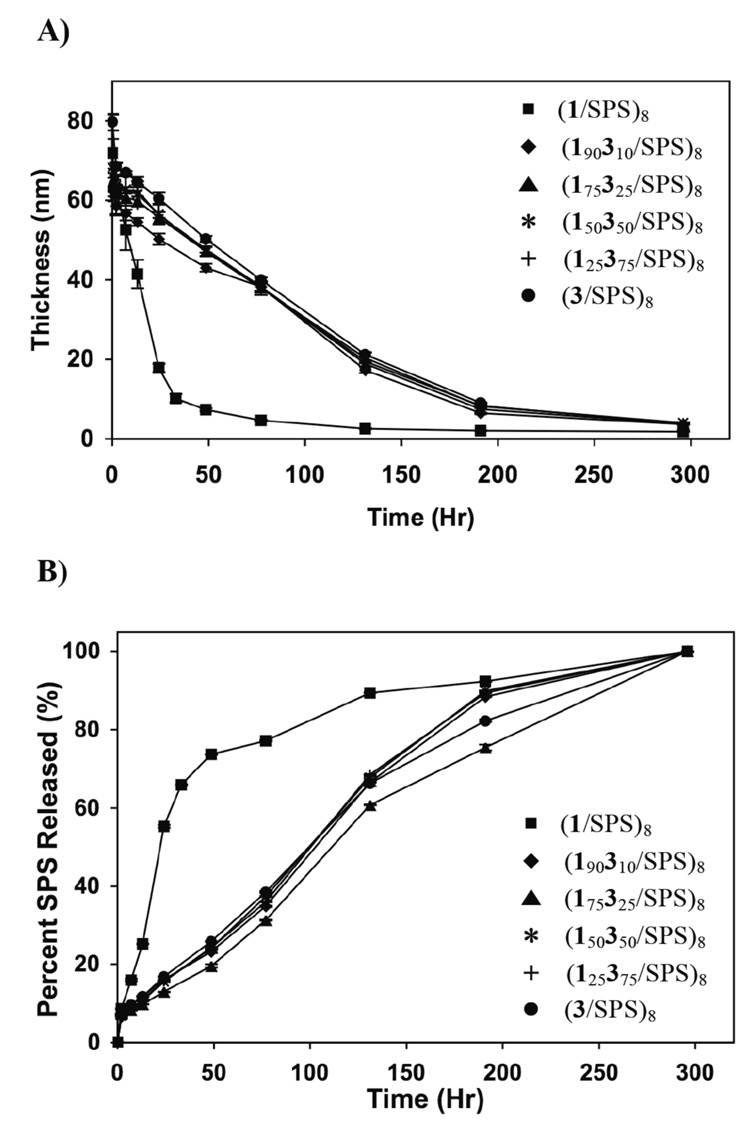
To investigate further the possible preferential adsorption of polymer 3 in the examples above, we prepared three additional (1,3/SPS)8 films using solutions composed of polymer 1 and extremely low mole percentages (e.g., 2.0, 1.0, and 0.5 mole percent) of polymer 3. The thicknesses of (1,3/SPS)8 films fabricated using polyamine solutions at these low mole percentages of polymer 3 increased linearly as a function of the number of bilayers incorporated. These films were ~60 nm thick, consistent with the results shown in Figure 1 for the fabrication of films using higher mole percentages of polymer 3. In contrast to the release profiles observed in Figure 2, however, we observed significant differences in the release of SPS when these films were incubated in PBS. Figure 3a shows normalized SPS release profiles for (19832/SPS)8, (19931/SPS)8, (199.530.5/SPS)8 films relative to the release profiles of films fabricated from polymer 1 or polymer 3. The release profiles of these films were observed to be intermediate to the release profiles of films fabricated exclusively from polymer 1 or polymer 3. Furthermore, the relative positions of these release profiles correlated systematically with the differences in the amounts of polymer 3 in the solution mixtures used to fabricate the films. For example, films fabricated using solutions containing 0.5 mole percent polymer 3 released SPS more rapidly than films fabricated using solutions containing higher concentrations of polymer 3 (e.g., either 1.0 or 2.0 mole percent polymer 3).
Figure 3.
A): Plot of percent SPS released versus time for polycation/SPS films fabricated from mixtures of polymers 1 and 3 incubated in phosphate buffer (pH = 7.4) at 37 °C. Percent SPS released is based on UV absorbance (at 226 nm) of the PBS solutions used to incubate these assemblies. Subscripts denote the mole percentage of each polyamine in the polycation solutions used to fabricate each film (see text). B): Plot of ellipsometric thickness versus the number of polyamine/SPS bilayers deposited for the fabrication of a 3/SPS film using an extremely low concentration of polymer 3 (0.025 mM) (◇). Also shown are thickness growth profiles of polyamine/SPS films fabricated from solutions containing only polymer 1 (●), only polymer 3 (■), or a mixture containing 99.5% of polymer 1 and 0.5% of polymer 3 (◆) (at a total polymer concentration of 5mM).
Control experiments demonstrated that it was possible to fabricate (3/SPS)8 films using solutions of polymer 3 (alone, in the absence of polymer 1) at the extremely low concentrations used in the above experiments. For example, Figure 3b (open diamonds) shows the growth profile of a (3/SPS)8 film fabricated using a solution of polymer 3 at 0.025 mM [equivalent to the concentration of polymer 3 in a solution mixture used to fabricate a (199.530.5/SPS)8 film]. Although thickness was observed to increase linearly as a function of the number of bilayers under these conditions, the final thicknesses of films fabricated at this polymer concentration were significantly lower than those of films fabricated using solutions of polymer 1 alone, polymer 3 alone, or mixtures of polymer 1 and 3 [at a total polymer concentration of 5 mM (Figure 3b)]. These results suggest that films fabricated from mixtures of polymer 1 and polymer 3 at very low percentages of polymer 3 [e.g., a (199.530.5/SPS)8 film] likely contain significant amounts of polymer 1. As noted above, additional analytical work will be required to determine quantitatively the extent to which the compositions of polymer 1 and 3 in these films reflects the defined compositions of these polymers used to prepare the dipping solutions. We are currently investigating the synthesis of fluorescently-labeled derivatives of these polyamines that could permit more direct characterization of the molecular compositions of these films. In the context of this current study, however, we do conclude that it is possible to manipulate the erosion and release profiles of these assemblies by using defined mixtures of polyamines that contain very low mole percentages of a more hydrophobic polyamine. Below, we describe an alternate approach to the fabrication of erodible films that permits broader and more systematic control over the tuning of film erosion and release profiles.
Investigation of Multilayered Films Fabricated From Multiple Layers of Different Polyamines
The layer-by-layer fabrication procedure described above also provides a straightforward method for the assembly of multilayered films composed of multiple different layers of multiple different polyelectrolytes.33,38,42,44,65,66 For example, it is possible to fabricate assemblies having the general architecture (X/SPS)n(Y/SPS)m by depositing ‘n’ bilayers of polyamine X and SPS (deposited first to form the bottommost layers of the film) followed by the deposition of ‘m’ bilayers of polyamine Y and SPS (deposited last to form the topmost layers of the film). Films fabricated in this manner provide, in principle, opportunities to engineer the release of incorporated macromolecules with complex release profiles. Ongoing investigations in our laboratory are focused on controlling the architectures of these films with a view toward fabricating assemblies that permit the staged release of multiple film components.33,38 We demonstrate here, however, that the incorporation of multiple different layers of polyamines 1–3 into multilayered films can also provide a straightforward and highly tunable approach to exerting control over film erosion and the release of SPS.
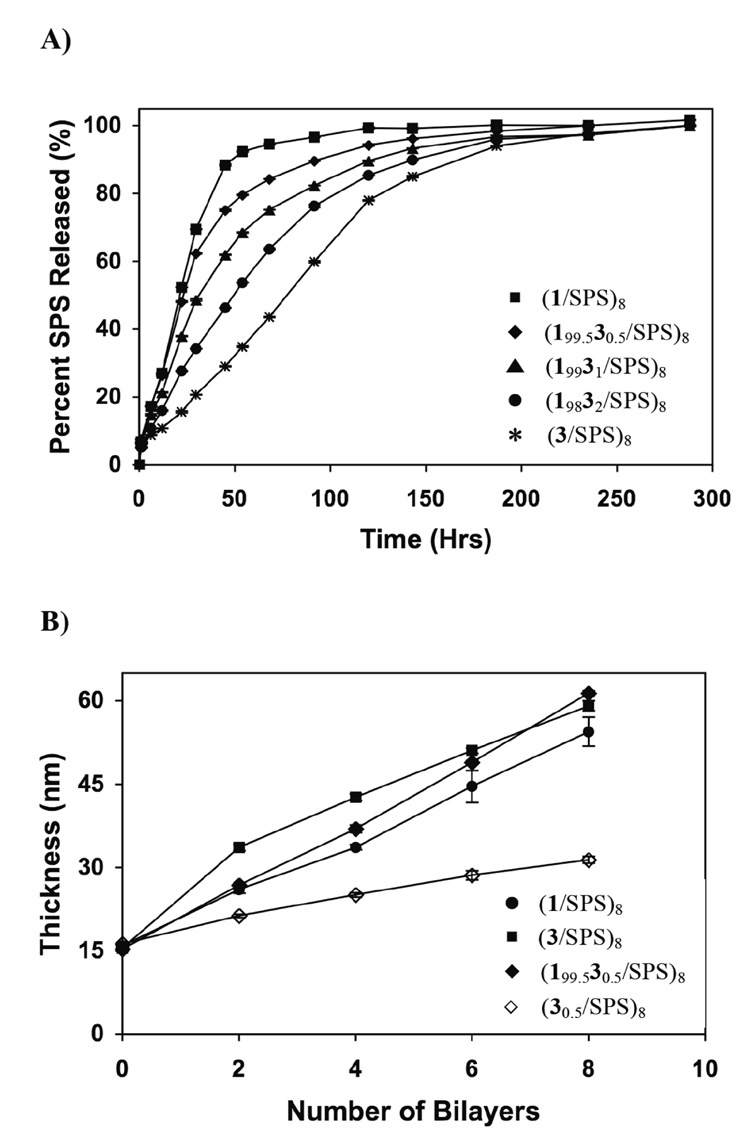
Figure 4 shows a plot of ellipsometric thickness versus the number of bilayers deposited during the fabrication of a (1/SPS)4(2/SPS)4 film and a (2/SPS)4(1/SPS)4 film (fabricated using the general approach described above). For the (1/SPS)4(2/SPS)4 film, thickness increased linearly for the deposition of the first four 1/SPS bilayers and continued to increase with the same slope (6.4 nm/layer) during the deposition of the final four 2/SPS layers to give a final film thickness of 70 nm. In contrast, the slope of the growth profile for the (2/SPS)4(1/SPS)4 film changed during the deposition of the final four 1/SPS layers (from 6.4 nm/layer to 4.0 nm/layer), resulting in a final film thickness that was lower (10 nm less) than the (1/SPS)4(2/SPS)4 film. In addition, visual inspection of (2/SPS)4(1/SPS)4 films indicated the presence of non-uniform optical patterns and irregular film coverage over large areas of the silicon substrate. By contrast, (1/SPS)4(2/SPS)4 films were smooth and uniform (RMS roughness was 3.3 nm as determined by atomic force microscopy). On the basis of these results, which suggested non-uniform growth of (2/SPS)4(1/SPS)4 films, all subsequent studies were directed toward the characterization of films having the structure (1/SPS)4(2/SPS)4, for which the least hydrophobic polyamine was deposited first.
Figure 4.
Plot of ellipsometric thickness versus the number of polyamine/SPS bilayers for multilayered films fabricated by depositing 4 bilayers of 1/SPS followed by the deposition of 4 bilayers of 2/SPS [◆ (1/SPS)4(2/SPS)4] as well as films fabricated in the reverse sequence [▲ (2/SPS)4(1/SPS)4].
Figure 5a shows normalized SPS release profiles measured during the incubation of a (1/SPS)4(2/SPS)4 film relative to the release profiles of a (1/SPS)8 film and a (2/SPS)8 film. These data demonstrate that the release profile of the (1/SPS)4(2/SPS)4 film was intermediate to the release profiles of the films fabricated entirely from polymer 1 or polymer 2. Further, the relative position of the release profile of this film was approximately midway between those of the (1/SPS)8 and (2/SPS)8 films. We next sought to determine whether it was possible to tune the release profiles of (1/SPS)n(2/SPS)m films by varying the relative numbers of layers of 1/SPS and 2/SPS deposited during fabrication. To investigate the potential of this approach, we fabricated two additional films to give a series of three multilayered films [(1/SPS)2(2/SPS)6, (1/SPS)4(2/SPS)4, and (1/SPS)6(2/SPS)2] in which the relative number of layers of 1/SPS and 2/SPS was varied systematically. Figure 5a shows the normalized release profiles of these three films relative to those of the (1/SPS)8 and (2/SPS)8 films. Inspection of these data reveals that the relative positions of these release curves vary systematically according to the relative numbers of 2/SPS layers incorporated into a film. For example, films fabricated from six bilayers of 1/SPS and two bilayers of 2/SPS [i.e., (1/SPS)6(2/SPS)2)] released SPS faster than films fabricated from four bilayers of 1/SPS and four bilayers of 2/SPS. Likewise, the (1/SPS)2(2/SPS)6 film released SPS more slowly than these two films, but faster than the (2/SPS)8 film. These results demonstrate that varying the relative numbers of 2/SPS layers incorporated into these films provides a rational and straightforward method for tuning the release of SPS from erodible multilayered films.
Figure 5.
A): Plot of percent SPS released versus time for (1/SPS)n(2/SPS)m films incubated in phosphate buffer (pH = 7.4) at 37 °C. B): Plot of percent SPS released versus time for (2/SPS)n(3/SPS)m films incubated in phosphate buffer (pH = 7.4) at 37 °C. In each plot, release profiles of (1/SPS)8, (2/SPS)8, and (3/SPS)8 films are shown for comparison.
To evaluate the generality of this approach, we also investigated the fabrication and erosion of films fabricated from multiple different layers of polymer 2 and polymer 3. Figure 5b shows normalized release profiles of three films having the structures (2/SPS)6(3/SPS)2, (2/SPS)4(3/SPS)4, and (2/SPS)2(3/SPS)6 relative to the release profiles of two films fabricated entirely from polymer 2 or polymer 3. Consistent with results observed in Figure 5a, the relative release profiles of the (2/SPS)n(3/SPS)m films correlated directly with the relative numbers of 2/SPS or 3/SPS layers deposited during the fabrication of these materials.
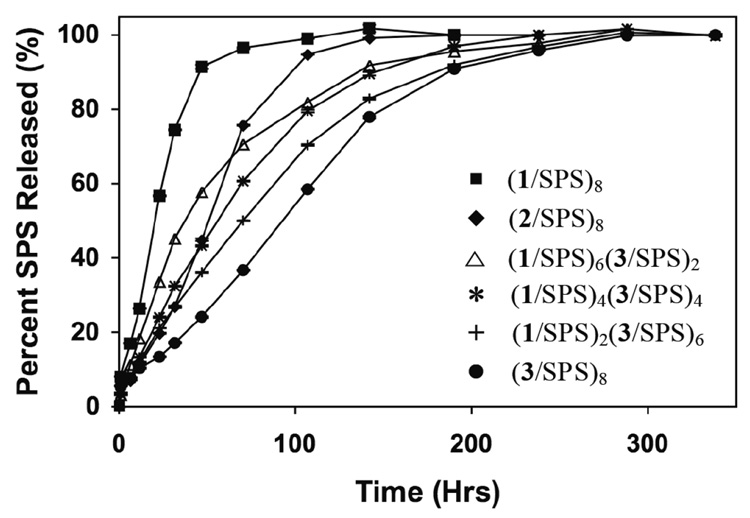
When combined, the data in Figures 5a and 5b demonstrate that it is possible to tune systematically the release of SPS over a period ranging from two days to ten days by fabricating films containing different numbers of layers of polymers 1, 2, or 3. We also sought to determine whether it was possible to cover this range of release profiles by fabricating films using only different combinations of (1/SPS) or (3/SPS) (i.e., without making use of layers fabricated using polymer 2). Figure 6 shows normalized release profiles of three films having the general structures (1/SPS)6(3/SPS)2, (1/SPS)4(3/SPS)4, and (1/SPS)2(3/SPS)6 relative to the release profiles of two films fabricated using only polymer 1, polymer 2, or polymer 3. These data demonstrate that it is possible to fabricate films with release profiles that are intermediate to the release profiles of (1/SPS)8 or (3/SPS)8 films and, further, that it is possible to reproduce qualitatively some features of the release profile of a (2/SPS)8 film (included in Figure 6 for comparison) using this approach. We note, however, that in contrast to the release profiles in Figure 5a and 5b (for films fabricated using polymers 1 and 2, or polymers 2 and 3, respectively) the relative positions of the release profiles of these (1/SPS)n(3/SPS)m films are weighted more heavily toward the profile of the (3/SPS)8 film, suggesting that the more hydrophobic polymer 3 may dominate the behavior of these assemblies.
Figure 6.
Plot of percent SPS released versus time for (1/SPS)n(3/SPS)m films incubated in phosphate buffer (pH = 7.4) at 37 °C. Release profiles of (1/SPS)8, (2/SPS)8, and (3/SPS)8 films are shown for comparison.
The data in Figure 5 and Figure 6 demonstrate that varying the number of layers of different degradable polyamines in films having the general structure (X/SPS)n(Y/SPS)m provides a straightforward and systematic method for control over the release of SPS from the surfaces of coated objects. As noted above, the layer-by-layer methods used to fabricate these films have been used in past studies to fabricate multilayered films having stratified or ‘tiered’ architectures.33,38,42,44,65,66 Although it is possible to fabricate films having stratified, two tiered architectures under certain conditions, the interpenetration of adjacent polyelectrolyte layers1 and interlayer diffusion during film assembly33,67 can also contribute to film architectures having a more homogeneous distribution of individual film components. A more complete understanding of the molecular architectures of these films and the relative locations of the different polyamines within these assemblies could permit more sophisticated levels of control over the erosion of these films33,38 and the extension of the approach reported here to tunable control over the release of therapeutic macromolecules, such as DNA. Studies to this end are currently underway.
Summary and Conclusions
We have reported the fabrication and characterization of multilayered polyelectrolyte assemblies fabricated from sodium poly(styrene sulfonate) (SPS) and combinations of three different hydrolytically degradable polyamines (1–3). In general, the erosion and release profiles of films fabricated using solution mixtures of two different polyamines were dictated almost entirely by the most hydrophobic polyamine used to fabricate the films. However, it was possible to fabricate films with intermediate and tunable release profiles using solution mixtures containing extremely low concentrations of the more hydrophobic polyamine (e.g., less than 2 mole%). The fabrication of films having different numbers of layers of different polyamines permitted broader and more tunable control over film erosion and the release of SPS. For example, films having the architecture (1/SPS)n(2/SPS)m released SPS with profiles that were intermediate to those of films fabricated exclusively from polymer 1 or polymer 2. Further, we demonstrated that this approach makes possible systematic control over the release of SPS by varying the relative numbers of layers of (1/SPS) or (2/SPS) incorporated into the films. The approaches reported here provide tunable control over the rate of the release of anionic polymers from surfaces coated with ultrathin multilayered films. This work could, with further development, contribute to the design of ultrathin films that permit tunable control over the release and delivery of therapeutically relevant macromolecules, such as proteins or DNA, from surfaces.
Acknowledgment
Financial support was provided by the National Institutes of Health (R21 EB02746) and the Arnold and Mabel Beckman Foundation. The authors acknowledge Brent Lemberg and John Janz for technical assistance, and Nat Fredin and Chris Jewell for helpful discussions.
Contributor Information
Jingtao Zhang, Email: jingtaozhang@wisc.edu.
David M. Lynn, Email: dlynn@engr.wisc.edu.
References
- 1.Decher G. Science. 1997;277:1232–1237. [Google Scholar]
- 2.Bertrand P, Jonas A, Laschewsky A, Legras R. Macromol Rapid Comm. 2000;21:319–348. [Google Scholar]
- 3.Peyratout CS, Dahne L. Angew Chem Int Ed Engl. 2004;43:3762–3783. doi: 10.1002/anie.200300568. [DOI] [PubMed] [Google Scholar]
- 4.Hammond PT. Adv Mater. 2004;16:1271–1293. [Google Scholar]
- 5.Ai H, Jones SA, Lvov YM. Cell Biochem and Biophys. 2003;39:23–43. doi: 10.1385/CBB:39:1:23. [DOI] [PubMed] [Google Scholar]
- 6.Jin W, Shi XY, Caruso F. J Am Chem Soc. 2001;123:8121–8122. doi: 10.1021/ja015807l. [DOI] [PubMed] [Google Scholar]
- 7.Lvov Y, Antipov AA, Mamedov A, Mohwald H, Sukhorukov GB. Nano Lett. 2001;1:125–128. [Google Scholar]
- 8.Groth T, Lendlein A. Angew Chem Int Ed Engl. 2004;43:926–928. doi: 10.1002/anie.200301708. [DOI] [PubMed] [Google Scholar]
- 9.Tryoen-Toth P, Vautier D, Haikel Y, Voegel JC, Schaaf P, Chluba J, Ogier J. J Biomed Mater Res. 2002;60:657–667. doi: 10.1002/jbm.10110. [DOI] [PubMed] [Google Scholar]
- 10.Yang SY, Mendelsohn JD, Rubner MF. Biomacromolecules. 2003;4:987–994. doi: 10.1021/bm034035d. [DOI] [PubMed] [Google Scholar]
- 11.Salloum DS, Olenych SG, Keller TC, Schlenoff JB. Biomacromolecules. 2005;6:161–167. doi: 10.1021/bm0497015. [DOI] [PubMed] [Google Scholar]
- 12.Kidambi S, Lee I, Chan C. J Am Chem Soc. 2004;126:16286–16287. doi: 10.1021/ja046188u. [DOI] [PubMed] [Google Scholar]
- 13.Grunlan JC, Choi JK, Lin A. Biomacromolecules. 2005;6:1149–1153. doi: 10.1021/bm049528c. [DOI] [PubMed] [Google Scholar]
- 14.Thierry B, Winnik FM, Merhi Y, Silver J, Tabrizian M. Biomacromolecules. 2003;4:1564–1571. doi: 10.1021/bm0341834. [DOI] [PubMed] [Google Scholar]
- 15.Thierry B, Kujawa P, Tkaczyk C, Winnik FM, Bilodeau L, Tabrizian M. J Am Chem Soc. 2005;127:1626–1627. doi: 10.1021/ja045077s. [DOI] [PubMed] [Google Scholar]
- 16.Dai ZF, Heilig A, Zastrow H, Donath E, Mohwald H. Chem-Eur J. 2004;10:6369–6374. doi: 10.1002/chem.200400579. [DOI] [PubMed] [Google Scholar]
- 17.Qiu XP, Leporatti S, Donath E, Mohwald H. Langmuir. 2001;17:5375–5380. [Google Scholar]
- 18.Zahr AS, de Villiers M, Pishko MV. Langmuir. 2005;21:403–410. doi: 10.1021/la0478595. [DOI] [PubMed] [Google Scholar]
- 19.Chung AJ, Rubner MF. Langmuir. 2002;18:1176–1183. [Google Scholar]
- 20.Serizawa T, Matsukuma D, Akashi M. Langmuir. 2005;21:7739–7742. doi: 10.1021/la0505263. [DOI] [PubMed] [Google Scholar]
- 21.Serpe MJ, Yarmey KA, Nolan CM, Lyon LA. Biomacromolecules. 2005;6:408–413. doi: 10.1021/bm049455x. [DOI] [PubMed] [Google Scholar]
- 22.Guyomard A, Nysten B, Muller G, Glinel K. Langmuir. 2006;22:2281–2287. doi: 10.1021/la052871y. [DOI] [PubMed] [Google Scholar]
- 23.Berg MC, Zhai L, Cohen RE, Rubner MF. Biomacromolecules. 2006;7:357–364. doi: 10.1021/bm050174e. [DOI] [PubMed] [Google Scholar]
- 24.Vodouhe C, Le Guen E, Garza JM, Francius G, Dejugnat C, Ogier J, Schaaf P, Voegel JC, Lavalle P. Biomaterials. 2006;27:4149–4156. doi: 10.1016/j.biomaterials.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Vazquez E, Dewitt DM, Hammond PT, Lynn DM. J Am Chem Soc. 2002;124:13992–13993. doi: 10.1021/ja026405w. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Fredin NJ, Lynn DM. J Polym Sci Pol Chem. 2006;44:5161–5173. [Google Scholar]
- 27.Zhang J, Fredin NJ, Janz JF, Sun B, Lynn DM. Langmuir. 2006;22:239–245. doi: 10.1021/la052360b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Chua LS, Lynn DM. Langmuir. 2004;20:8015–8021. doi: 10.1021/la048888i. [DOI] [PubMed] [Google Scholar]
- 29.Fredin NJ, Zhang J, Lynn DM. Langmuir. 2005;21:5803–5811. doi: 10.1021/la050596+. [DOI] [PubMed] [Google Scholar]
- 30.Jewell CM, Zhang J, Fredin NJ, Wolff MR, Hacker TA, Lynn DM. Biomacromolecules. 2006;7:2483–2491. doi: 10.1021/bm0604808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jewell CM, Zhang J, Fredin NJ, Lynn DM. J Control Release. 2005;106:214–223. doi: 10.1016/j.jconrel.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Lynn DM. Soft Matter. 2006;2:269–273. doi: 10.1039/b517860f. [DOI] [PubMed] [Google Scholar]
- 33.Wood KC, Chuang HF, Batten RD, Lynn DM, Hammond PT. Proc Natl Acad Sci U S A. 2006;103:10207–10212. doi: 10.1073/pnas.0602884103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood KC, Boedicker JQ, Lynn DM, Hammond PT. Langmuir. 2005;21:1603–1609. doi: 10.1021/la0476480. [DOI] [PubMed] [Google Scholar]
- 35.Ren KF, Ji J, Shen JC. Biomaterials. 2006;27:1152–1159. doi: 10.1016/j.biomaterials.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 36.Ren KF, Ji J, Shen JC. Bioconjugate Chem. 2006;17:77–83. doi: 10.1021/bc050264g. [DOI] [PubMed] [Google Scholar]
- 37.Recksiedler CL, Deore BA, Freund MS. Langmuir. 2006;22:2811–2815. doi: 10.1021/la053031m. [DOI] [PubMed] [Google Scholar]
- 38.Jessel N, Oulad-Abdelghani M, Meyer F, Lavalle P, Haikel Y, Schaaf P, Voegel JC. Proc Natl Acad Sci U S A. 2006;103:8618–8621. doi: 10.1073/pnas.0508246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer F, Ball V, Schaaf P, Voegel JC, Ogier J. BBA-Biomembranes. 2006;1758:419–422. doi: 10.1016/j.bbamem.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Sukhishvili SA, Granick S. J Am Chem Soc. 2000;122:9550–9551. [Google Scholar]
- 41.Sukhishvili SA, Granick S. Macromolecules. 2002;35:301–310. [Google Scholar]
- 42.Dubas ST, Farhat TR, Schlenoff JB. J Am Chem Soc. 2001;123:5368–5369. doi: 10.1021/ja015774+. [DOI] [PubMed] [Google Scholar]
- 43.Dubas ST, Schlenoff JB. Macromolecules. 2001;34:3736–3740. [Google Scholar]
- 44.Cho J, Caruso F. Macromolecules. 2003;36:2845–2851. [Google Scholar]
- 45.Schuler C, Caruso F. Biomacromolecules. 2001;2:921–926. doi: 10.1021/bm010052w. [DOI] [PubMed] [Google Scholar]
- 46.Serizawa T, Yamaguchi M, Akashi M. Angew Chem Int Ed Engl. 2003;42:1115–1118. doi: 10.1002/anie.200390293. [DOI] [PubMed] [Google Scholar]
- 47.Serizawa T, Yamaguchi M, Akashi M. Macromolecules. 2002;35:8656–8658. [Google Scholar]
- 48.Picart C, Schneider A, Etienne O, Mutterer J, Schaaf P, Egles C, Jessel N, Voegel JC. Adv Funct Mater. 2005;15:1771–1780. [Google Scholar]
- 49.Etienne O, Schneider A, Taddei C, Richert L, Schaaf P, Voegel JC, Egles C, Picart C. Biomacromolecules. 2005;6:726–733. doi: 10.1021/bm049425u. [DOI] [PubMed] [Google Scholar]
- 50.Li B, Haynie DT. Biomacromolecules. 2004;5:1667–1670. doi: 10.1021/bm0496155. [DOI] [PubMed] [Google Scholar]
- 51.Zelikin AN, Quinn JF, Caruso F. Biomacromolecules. 2006;7:27–30. doi: 10.1021/bm050832v. [DOI] [PubMed] [Google Scholar]
- 52.Boulmedais F, Tang CS, Keller B, Voros J. Adv Funct Mater. 2006;16:63–70. [Google Scholar]
- 53.Yamauchi F, Kato K, Iwata H. Langmuir. 2005;21:8360–8367. doi: 10.1021/la0505059. [DOI] [PubMed] [Google Scholar]
- 54.Sato K, Imoto Y, Sugama J, Seki S, Inoue H, Odagiri T, Hoshi T, Anzai J. Langmuir. 2005;21:797–799. doi: 10.1021/la048059x. [DOI] [PubMed] [Google Scholar]
- 55.Inoue H, Sato K, Anzai J. Biomacromolecules. 2005;6:27–29. doi: 10.1021/bm0495856. [DOI] [PubMed] [Google Scholar]
- 56.Inoue H, Anzai J. Langmuir. 2005;21:8354–8359. doi: 10.1021/la0508341. [DOI] [PubMed] [Google Scholar]
- 57.Lynn DM, Langer R. J Am Chem Soc. 2000;122:10761–10768. [Google Scholar]
- 58.Lynn DM, Anderson DG, Putnam D, Langer R. J Am Chem Soc. 2001;123:8155–8156. doi: 10.1021/ja016288p. [DOI] [PubMed] [Google Scholar]
- 59.Akinc A, Lynn DM, Anderson DG, Langer R. J Am Chem Soc. 2003;125:5316–5323. doi: 10.1021/ja034429c. [DOI] [PubMed] [Google Scholar]
- 60.Anderson DG, Lynn DM, Langer R. Angew Chem Int Ed Engl. 2003;42:3153–3158. doi: 10.1002/anie.200351244. [DOI] [PubMed] [Google Scholar]
- 61.Sui Z, Schlenoff JB. Langmuir. 2004;20:6026–6031. doi: 10.1021/la0495985. [DOI] [PubMed] [Google Scholar]
- 62.Debreczeny M, Ball V, Boulmedais F, Szalontai B, Voegel JC, Schaaf P. J Phys Chem B. 2003;107:12734–12739. [Google Scholar]
- 63.Cho JH, Quinn JF, Caruso F. J Am Chem Soc. 2004;126:2270–2271. doi: 10.1021/ja039830d. [DOI] [PubMed] [Google Scholar]
- 64.Quinn JF, Yeo JCC, Caruso F. Macromolecules. 2004;37:6537–6543. [Google Scholar]
- 65.Nolte AJ, Rubner MF, Cohen RE. Langmuir. 2004;20:3304–3310. doi: 10.1021/la0363229. [DOI] [PubMed] [Google Scholar]
- 66.Garza JM, Schaaf P, Muller S, Ball V, Stoltz JF, Voegel JC, Lavalle P. Langmuir. 2004;20:7298–7302. doi: 10.1021/la049106o. [DOI] [PubMed] [Google Scholar]
- 67.Picart C, Mutterer J, Richert L, Luo Y, Prestwich GD, Schaaf P, Voegel JC, Lavalle P. Proc Natl Acad Sci U S A. 2002;99:12531–12535. doi: 10.1073/pnas.202486099. [DOI] [PMC free article] [PubMed] [Google Scholar]