Abstract
A L-valine-derived α-haloamide was synthesized and investigated as an initiator for the atom transfer radical polymerization of tert-butyl acrylate using commercially available CuBr, CuBr2 and N,N,N’,N”,N”-pentamethyldiethylenetriamine as the catalyst and ligand system. Kinetic studies and extension to a diblock copolymer with styrene, each indicated that the polymerizations were well controlled. Poly(tert-butyl acrylate) having MnNMR = 8.6 kDa, MnGPC = 8.3 kDa, PDI = 1.11 and a diblock of poly(tert-butyl acrylate)-b-polystyrene having MnNMR = 20.2 kDa, MnGPC = 22.5 kDa, PDI = 1.22 were prepared by the sequential polymerization of tert-butyl acrylate and styrene at 55 °C and 90 °C, respectively.
Introduction
The development of living radical polymerization (LRP) techniques constitutes one of the key developments in the field of synthetic polymer chemistry, allowing for the synthesis of a variety of polymers with low molecular weight distributions and well defined architectures, with precise control over the compositions and structures.1–5 Atom transfer radical polymerization (ATRP) has emerged as a versatile technique that has been applied broadly because of the robustness of the chemistry and the commercial availability of many initiators, catalysts and ligands. As with other LRP techniques, control of the α-chain terminus is accessed from the initiator design, which for ATRP most often involves an alkyl halide with an activating substituent on the α-carbon that undergoes homolytic cleavage in the presence of catalyst to initiate polymerization. Since ester groups are good activating groups, α-haloester-based compounds are commonly used as ATRP initiators, whereby the ester unit can also carry functionality. The functionality incorporated into the polymer via an ester linkage is prone to hydrolysis, however, this lability has been overcome by their replacement with amide linkages through the use of α-halo amide-based initiators.6,7
The presence of amides are often considered to present unique challenges for ATRP in the production of polymers of narrow molecular weight distribution and having molecular weights in agreement with theoretical values. A recent report from Haddleton’s laboratory thoroughly examined the conditions under which amide containing ATRP initiators gave well-controlled polymerization of various methacrylates and styrene.7 Prior work in Sawamoto’s laboratory demonstrated the preparation of poly(methyl methacrylate) with narrow polydispersity index (PDI) using N,N-dimethyl-2-bromopropanamide as the initiator.6 Matyjaszewski’s laboratory has studied the polymerization of methacrylamides using a model α-haloamide-based initiators, obtaining well defined block copolymers.8 Recently, Xia et al. have employed various chloropropionamides as initiators to polymerize N-isopropylacrylamide with narrow molecular weight distributions to study the influence of the end group composition resulting from the initiators on the thermal properties.9
Chain end composition in amphiphilic block copolymers can be utilized to prepare functional nanostructures with regiochemical control.10,11–13 In our laboratory, we have prepared nanostructures originating from a functional initiator, that upon polymerization and followed by post-polymerization reactions and self-assembly, will project amino acids and other biologically-active ligands from their surfaces.14–19 In one synthetic strategy, the growth of block copolymers of tert-butyl acrylate and styrene from α-bromo butyryl amido-terminated peptides loaded upon a solid support was followed by cleavage from the resin and deprotection to release an amphiphilic triblock of peptide, poly(acrylic acid) and poly(styrene).20 Determination of the polymerization behavior was complicated by the attachment of the polymer chain to the resin during growth and the unusual solubility and electrolyte character of the amphiphilic structure after cleavage. Herein, therefore, we discuss results from studies that investigated the employment of an amino acid-based ATRP initiator to polymerize tert-butyl acrylate and styrene in solution.
Experimental
Materials
All chemicals, solvents and reagents were purchased from Sigma Aldrich (Saint Louis, MO, USA) or Fisher Scientific (Pittsburgh, PA, USA). CH2Cl2 (99.9%), triethylamine (TEA) (99.9%), tert-butyl acrylate (tBA) (98%) and styrene (99 %) were distilled over CaH2 and stored under N2. SOCl2 (> 99%) was doubly distilled before use. CuBr (99.999 %), CuBr2 (99.999%), p-dimethoxy benzene (p-DMB) (99%), N,N-dimethyl formamide (DMF, 99.8%), PMDETA, (99 %), methanol (99.93 %), L-valine, and 2-bromopropionyl bromide (97 %) were used as received.
Methods
1H NMR spectra were recorded at 300 MHz on a Varian Mercury 300 spectrometer on solutions in CDCl3 or CD2Cl2 with the solvent proton signal as standard. 13C NMR spectra were recorded at 75.4 MHz in CDCl3 on a Varian Mercury 300 spectrometer with the solvent carbon signal as standard. IR spectra were obtained on a Perkin Elmer BX FT-IR system equipped with a diffuse reflectance accessory. Glass transition temperatures (Tg) were measured by differential scanning calorimetry (DSC) at a heating rate of 10 °C/min on a Mettler Toledo DSC822e using Mettler Toledo Star SW 7.01 software. Tg values were taken as the midpoint of the inflection tangent on the third heating scan. Tm values were taken as the onset of the transition.
Size exclusion chromatography (SEC) was conducted on a Waters 1515 HPLC (Waters Chromatography, Inc., Milford, MA, USA) equipped with a Waters 2414 differential refractometer, a PD2026 dual-angle (15° and 90°) light scattering detector (Precision Detectors, Inc., Franklin, MA, USA), and a three-column series PL gel 5µm Mixed C, 500 Å, and 104 Å, 300 × 7.5 mm columns (Polymer Laboratories Inc., Amherst, MA, USA). The system was equilibrated at 35 °C in THF, which served as the polymer solvent and eluent with a flow rate of 1.0 mL/min. Polymer solutions were prepared at a known concentration (ca. 3 mg/mL) and an injection volume of 200 µL was used. Data collection and analysis were performed, respectively, with Precision Acquire software and Discovery 32 software (Precision Detectors, Inc., Franklin, MA, USA). Interdetector delay volume and the light scattering detector calibration constant were determined by calibration using a nearly monodispersed polystyrene standard (Mp = 90 kDa, Mw/Mn < 1.04, Pressure Chemical Co., Pittsburg, PA, USA). The differential refractometer was calibrated with standard polystyrene reference material (SRM 706 NIST), of known S-5 specific refractive index increment dn/dc (0.184 mL/g). The dn/dc values of the analyzed polymers were then determined from the differential refractometer response.
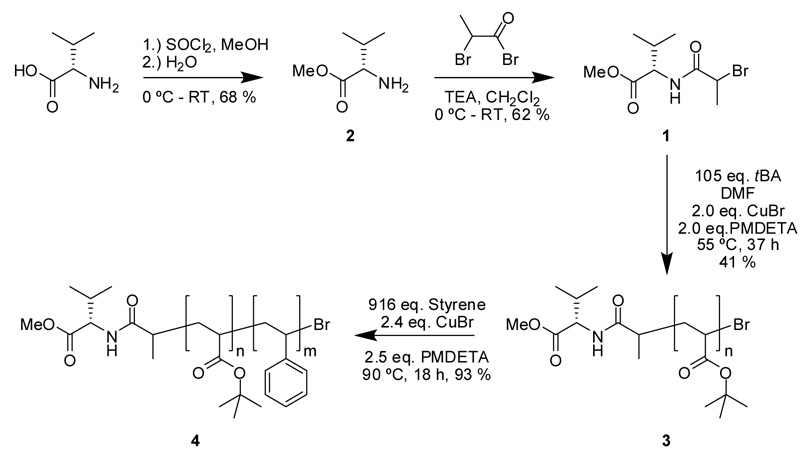
Synthesis of methyl 2-amino-3-methylbutanoate·HCl (2).21
L-valine (0.99 g, 8.5 mmol, 1.0 equiv.) was suspended in 40 mL methanol in a 100 mL 2-necked round bottom flask fitted with N2 inlet and rubber septa. The suspension was cooled in an ice bath and SOCl2 (4.7 mL, 7.7 g, 65 mmol, 7.6 equiv.) was added dropwise. The suspension became a homogeneous solution. De-ionized water (0.6 mL, 0.6 g, 30 mmol, 4 equiv.) was then added and the reaction mixture was allowed to stir for 24 h at room temperature. The solvent was removed in vacuo and the resultant solid was washed twice with methanol (2 mL) to result in a white solid, which was recrystallized from acetone to yield white needle like crystals. Yield: 0.96 g (68 %). Tm = 163 °C. IR: 3462, 3009, 2824, 2617, 1982, 1759, 1504, 1287, 1240 cm−1. 1H NMR (CDCl3 δ, ppm): 8.87 (br, s, NH2), 3.94 (m, CHCO), 3.83 (s, OCH3), 2.47 (m, CH(CH3)2), 1.15 (m, CH(CH3)2) 13C NMR (CDCl3 δ, ppm): 168.98, 58.65, 53.20, 30.17, 18.57.
Synthesis of methyl 2-[(2-bromopropanoyl)amino]-3-methylbutanoate (1)
Into a 250 mL 2-necked round bottom flask fitted with a N2 inlet and a rubber septum was placed 2 (1.0 g, 7.4 mmol, 1.0 equiv.), which was allowed to dissolve in CH2Cl2 (20 mL), and then was cooled in an ice bath. TEA (2.0 mL, 1.5 g, 15 mmol, 2 equiv.) and 2-bromopropionyl bromide (0.85 mL, 1.7 g, 8.1 mmol, 1.1 equiv.) were added dropwise via gas tight syringes and the reaction was allowed to proceed at room temperature overnight. The reaction mixture was then washed with de-ionized water (3 × 100 mL), dried over MgSO4, and concentrated in vacuo to afford the crude product as a dark colored oil. The product (Rf = 0.65) was purified by silica flash column chromatography using CH3OH / CH2Cl2 (1:9) as eluent to yield 1.22 g (62 %) as an orange colored oil. IR: 3312, 2960 - 2870, 1754, 1693, 1681, 1538, 1469, 1440, 1374, 1311, 1270, 1213, 1150, 988, 761 cm−1. 1H NMR (CDCl3, δ, ppm): 4.52 (m, COCHNH), 4.44 (m, CHBr), 3.76 (s, OCH3), 2.25 (m, CH(CH3)2), 1.88, 1.89 (m, CH(CH3)Br), 0.95 (m, CH(CH3)2). 13C NMR (CDCl3 δ, ppm): 172.16, 172.28, 169.49, 169.53, 57.70, 57.91, 52.56, 44.69, 45.21, 31.52, 31.74, 23.10, 23.34, 17.85, 17.95, 19.14. MS: theor. m/z = 288.0211 [M + Na]+, expt. m/z = 288.0221 [M + Na]+
Preparation of PtBA macroinitiator (3)
Into a 100 mL Schlenk flask, 1 ( 0.41g, 1.5 mmol, 1.0 equiv.), DMF ( 35.0 mL) and t-BA (25.0 mL, 20.8 g, 162 mmol, 105 equiv.) were placed and after a freeze-pump-thaw cycle, CuBr (0.45 g, 3.1 mmol, 2.0 equiv.) was added and again a freeze-pump-thaw cycle was performed. PMDETA (0.55 g, 3.2 mmol, 2.0 equiv.) was added and the reaction mixture was degassed by performing three freeze-pump-thaw cycles and allowing the reaction mixture to equilibrate to room temperature. The reaction mixture was immersed into an oil bath at 55 °C. Aliquots were removed via N2 washed gas tight syringes and the reaction was monitored by 1H NMR spectroscopy and SEC. The reaction was quenched at 37 h by immersing in a liquid N2 bath. The reaction mixture was dissolved in ~ 200 mL THF and the copper catalyst was removed by passing through a neutral alumina column. The solution was concentrated and precipitated thrice in an ice cold 60:40 methanol/water mixture to yield PtBA as a white powder. Yield = 3.14 g (41 %, based on conversion). MnNMR = 8.6 kDa, MnGPC = 8.3 kDa, PDI = 1.11. Tg = 41 °C. IR: 3462, 3433, 2977, 1716, 1446, 1366, 1251, 1142, 844, 751 cm−1. 1H NMR (CDCl3, δ, ppm): 4.50 (CHBr end group), 4.10 (COCHNH end group), 3.73 (OCH3, end group), 2.10-2.39 (br, CH of the polymer backbone), 1.68-2.00 (br, meso CH2 of the polymer backbone), 1.24-1.65 (br, meso and racemo CH2 of the polymer backbone), 1.20-1.50 (br, (CH3)3C), 0.95 (CH(CH3)2 end group) 13C NMR (CDCl3, δ, ppm): 174.4, 80.6, 42.6, 42.2, 37.7, 36.1, 28.3.
Preparation of PtBA-b-PS block copolymer (4)
In a 50 mL Schlenk flask, PtBA macroinitiator, (Mn GPC = 8.3 kDa, PDI = 1.11, 1.01 g, 0.11 mmol, 1.0 equiv.), CuBr (38 mg, 0.26 mmol, 2.4 equiv.) and styrene (11.6 mL, 10.5 g, 101 mmol, 916 equiv.) were mixed. A freeze-pump-thaw cycle was conducted followed by the addition of PMDETA (60 µL, 50 mg, 0.28 mmol, 2.5 equiv.). The reaction mixture was degassed by performing three freeze-pump-thaw cycles and then was immersed into an oil bath maintained at 90 °C. The reaction was allowed to proceed for 18 h and quenched by immersing in a liquid N2 bath. The reaction mixture was dissolved in THF (50 mL) and the copper catalyst was removed by passing the solution through a neutral alumina column. The polymer solution was concentrated and was precipitated thrice in 50:50 methanol/water mixtures to result in the block copolymer. Yield = 1.8 g (93 %, based on conversion). Mn NMR = 20.2 kDa, Mn GPC = 22.5 kDa, PDI = 1.22. (Tg)PtBA = 51 °C, (Tg)PS= 97 °C. IR: 3434, 3024, 1977, 2922, 1943, 1871, 1803, 1724, 1601, 1494, 1452, 1367, 1254, 1144, 847, 752, 697 cm−1. 1H NMR (CD2Cl2, δ, ppm): 6.90-7.45 (br, ortho-and para-ArH), 6.30-6.90 (br, meta-ArH), 4.40 (CHBr end group), 4.10 (COCHNH end group), 3.72 (OCH3, end group), 1.72-2.07 (br, meso CH2 of the polymer backbone) 2.04-2.40 (br, CH of the polymer backbone), 1.20-1.50 (br, (CH3)3C), 1.24-1.65 (br, meso and racemo CH2 of the polymer backbone), 0.94 (CH(CH3)2 end group). 13C NMR (CD2Cl2, δ, ppm): 174.6, 146.0, 128.6, 126.2, 80.9, 40.1-46.5, 38.0, 36.5, 30.7, 28.5.
Kinetics of t-BA polymerization using (1) as initiator
Into an oven-dried 10 mL Schlenk flask was placed 1 (106.2 mg, 0.40 mmol, 1.0 equiv.), CuBr (56.2 mg, 0.40 mmol, 1.0 equiv.), CuBr2 (2.5 mg, ca. 5% relative to CuBr), t-BA (4.98 g 39 mmol, 97 equiv.) and p-DMB (100.0 mg ca. 2% relative to monomer as an internal 1H NMR standard to estimate monomer conversion) and the reaction mixture was degassed by performing a freeze-pump-thaw cycle. PMDETA (0.8 µL, 0.4 mmol, 1.0 equiv.) was added via gas tight syringe and the reaction mixture was degassed by performing three freeze-pump-thaw cycles and upon equilibration to room temperature after the third cycle, the flask was immersed into an oil bath pre-maintained at 60 °C. Aliquots (ca. 0.2 µL) were removed via N2 washed gas tight syringe. 1H NMR spectroscopy was used to determine the monomer conversion. SEC samples were prepared by dissolving the crude mixture into THF and the resultant solution was passed through a short column of neutral alumina to remove the copper catalyst.
Results and Discussion
Amphiphilic polymers originating from a functional initiator offer, upon self-assembly in water, regiochemical control of the surface and/or core compositions of the resultant nanostructures by the design of the polymer sequence. In our study, we chose amino acids as the precursor for the preparation of functional initiators due to their biological relevance, commercial availability, amphiphilic nature and simplicity.
Valine was chosen as a model amino acid and it was readily converted into an ATRP initiator, 1. To avoid potential problems with the carboxylic acid functionality, such as poor control over the polymerization due to the interference of the acid with the catalyst system, as reported previously,22 the free carboxylic acid was esterified using methanol in the presence of thionyl chloride.21 Amidation of the amino group of the methyl ester of L-valine, 2, with 2-bromopropionyl bromide in the presence of TEA afforded the desired initiator 1 (Scheme 1), which was purified by silica flash column chromatography eluting with CH3OH/CH2Cl2 (1:9).
Scheme 1.
Synthesis of PtBA-b-PS (4) diblock copolymer from L-valine-based ATRP initiator (1)
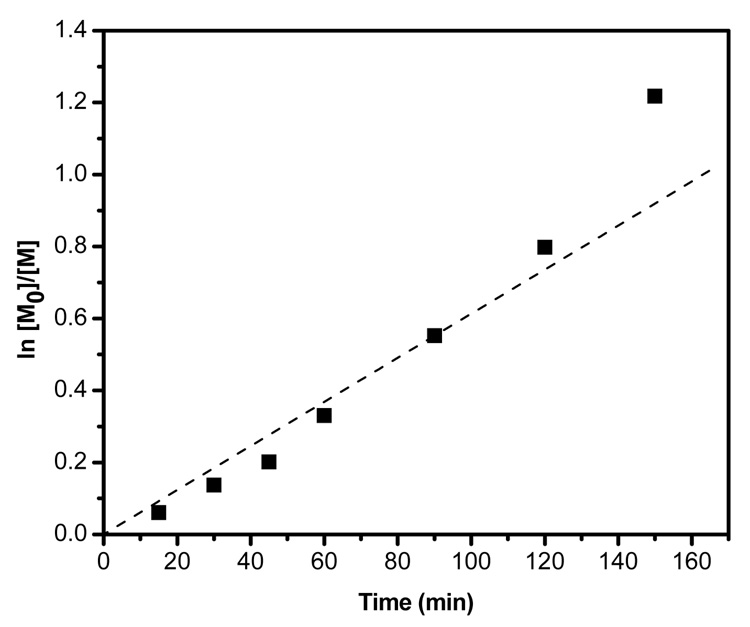
Kinetic studies of the polymerization of tBA, initiated by 1, were conducted at 60 °C in the bulk, employing CuBr and PMDETA as the catalyst system. In order to impart better control over the polymerization, 5 % CuBr2, relative to CuBr, was added.23 The first-order kinetic plot of polymerization of tBA illustrates that the polymerization occurred in a controlled manner and the monomer consumption increased linearly with time (Figure 1). The linearity also suggests that there is a constant radical concentration throughout the polymerization.
Figure 1.
First-order kinetic plot for the bulk polymerization of tBA at 60 °C, [1]0:[CuBr]0:[CuBr2]0:[PMDETA]0:[tBA]0 = 1:1:0.05:1:100.
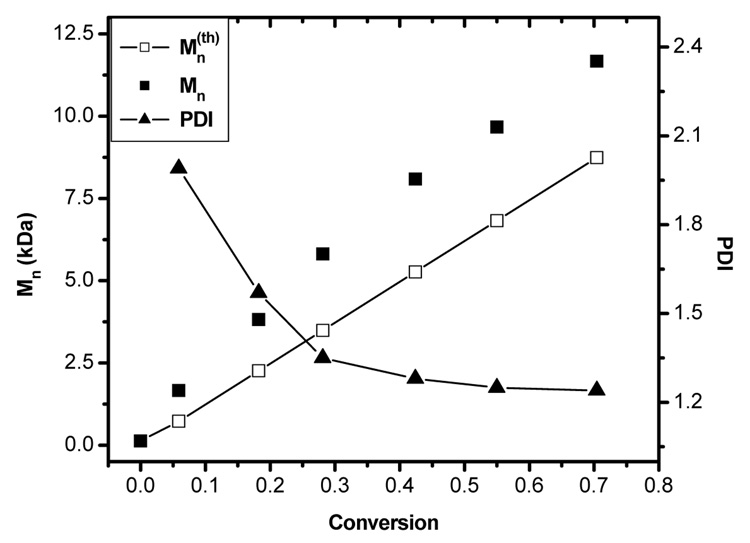
Evolution of Mn with conversion was found to be linear as shown in Figure 2 (squares). As observed previously7, experimental Mn values were found to be higher than the theoretical values throughout the polymerization. This phenomenon could be partially attributed to the fact that the experimental Mn values are obtained from PS calibration, which does not account for the difference in the polymer-column interaction for PtBA. The PDI was high (~ 2) initially and decreased with conversion, a behavior observed commonly under living radical polymerization conditions. The final PDI at 70 % conversion was found to be 1.25 (Figure 2 (triangles)).
Figure 2.
Evolution of Mn and PDI with conversion for the polymerization of tBA in the bulk at 60 °C, [1]0:[CuBr]0:[CuBr2]0:[PMDETA]0:[tBA]0 = 1:1:0.05:1:100; open squares with line, theoretical Mn; closed squares, experimental Mn; closed triangles, PDI.
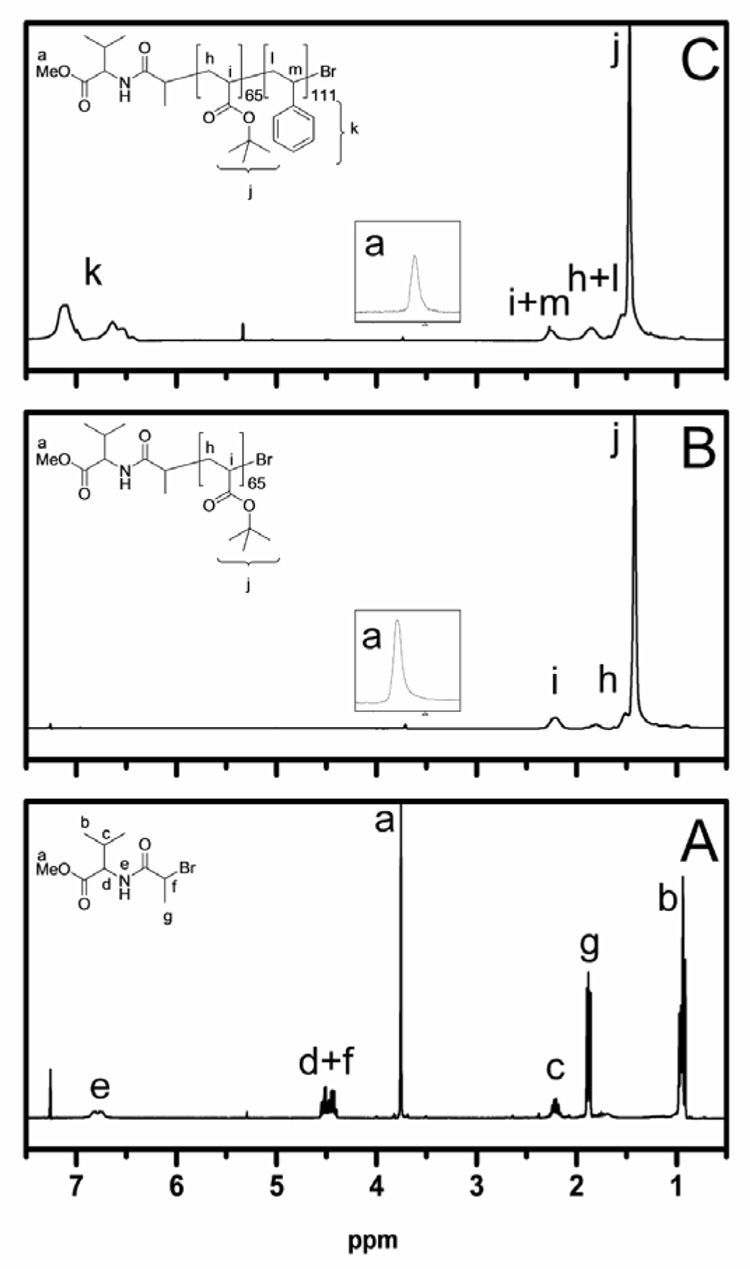
In order to demonstrate the living characteristic of this polymerization system, a PtBA macroinitiator prepared from 1 was used for the preparation of a block copolymer. The PtBA employed was found have Mn GPC = 8300 Da (PDI=1.11) and this was in good agreement with the Mn NMR = 8600 Da, which was determined by comparing the resonances of methoxy proton from the chain end and the backbone CH resonances. This PtBA was chain extended with styrene and the resultant block copolymer, PtBA-b-PS was found to have Mn GPC = 22500 Da and PDI = 1.22. Figure 3B and 3C illustrate the 1H NMR spectra of the macroinitiator and the block copolymer, respectively. The aromatic resonances from 6.5 – 7.2 ppm show that the polymerization of styrene occurred. The inset shows the resonances corresponding to the methoxy protons from the initiator, demonstrating that the initiator functionalities are retained for both the PtBA macroinitiator and the block copolymer.
Figure 3.
1H NMR spectra (300 MHz): A) initiator 2 (in CDCl3); B) PtBA macroinitiator 3 (in CDCl3), with inset (3.8 – 3.65 ppm) showing the methoxy chain end of the polymer; C) PtBA-b-PS diblock copolymer 4 (in CD2Cl2), with inset (3.8 – 3.65 ppm) showing the methoxy chain end of the polymer.
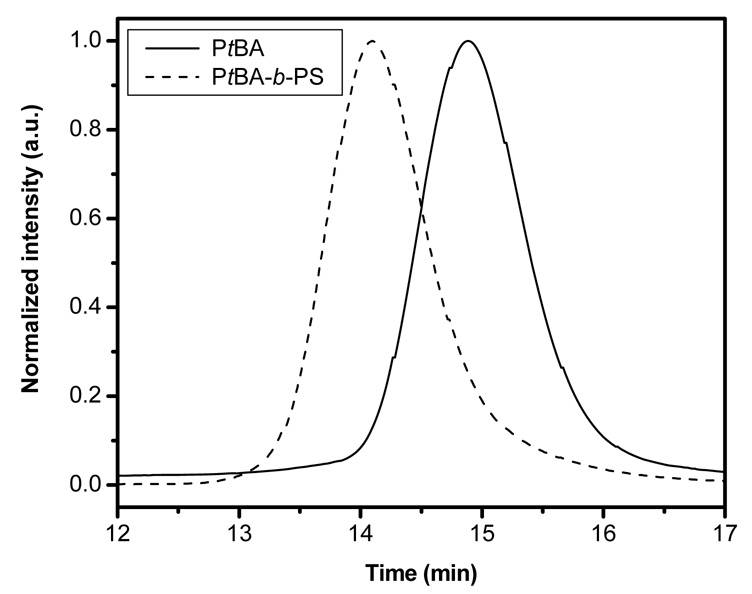
Overlaid SEC traces corresponding to the macroinitiator and PtBA-b-PS diblock copolymer are shown in Figure 4. The shift in peak elution time to the left indicates that the chain extension has occurred.
Figure 4.
SEC traces of PtBA macroinitiator and PtBA-b-PS block copolymer prepared from 2
Conclusions
A methyl ester-protected ATRP initiator from L-valine was demonstrated to provide for the initiation and controlled polymerization of tBA, using commercially available ligands, to afford well-defined polymers that retained the valine functionality at the α-chain terminus. Control during the polymerizations was confirmed by kinetic studies of the tBA polymerization and by extension of the resulting macroinitiator into a diblock copolymer with the polymerization of styrene. Each macroinitiator and block copolymer had a narrow PDI, but still experienced the problem of the experimental molecular weight being in excess of the theoretical value. Nonetheless, the ability to prepare well defined block copolymers will allow for their conversion into amino-acid labeled nanostructures, for which this study can be extended potentially to other amino acids and peptides. This work also complements the highly interesting recent examples of protein-polymer conjugates or soft hybrid materials, involving the growth of polymers directly from proteins in a controlled fashion.12,24,25
Acknowledgements
This material is based upon work supported by the National Science Foundation under Grant numbers DMR-0210247 and DMR-0451490. Mass spectrometry was provided by the Washington University Mass Spectrometry Resource with support from the NIH National Center for Research Resources Grant Number P41RR0954.
References
- 1.Matyjaszewski K, Xia J. Chem. Rev. 2001;101:2921–2990. doi: 10.1021/cr940534g. [DOI] [PubMed] [Google Scholar]
- 2.Kamigaito M, Ando T, Sawamoto M. Chem. Rev. 2001;101:3689–3746. doi: 10.1021/cr9901182. [DOI] [PubMed] [Google Scholar]
- 3.Hawker CJ, Bosman AW, Harth E. Chem. Rev. 2001;101:3661–3688. doi: 10.1021/cr990119u. [DOI] [PubMed] [Google Scholar]
- 4.Chiefari J, Chong YK, Ercole F, Krstina J, Jeffery J, Le TPT, Mayadunne RTA, Meijs GF, Moad CL, Moad G, Rizzardo E, Thang SH. Macromolecules. 1998;31:5559–5562. [Google Scholar]
- 5.Perrier S, Takolpuckdee P. J. Polym. Sci. Part A: Polym. Chem. 2005;43:5347–5393. [Google Scholar]
- 6.Baek K-Y, Kamigaito M, Sawamoto M. J. Polym. Sci. Part A: Polym. Chem. 2002;40:1937–1944. [Google Scholar]
- 7.Limer A, Haddleton DM. Macromolecules. 2006;39:1353–1358. [Google Scholar]
- 8.Teodorescu M, Matyjaszewski K. Macromolecules. 1999;32:4826–4831. [Google Scholar]
- 9.Xia Y, Burke NAD, Stover HDH. Macromolecules. 2006;39:2275–2283. [Google Scholar]
- 10.Hawker CJ, Wooley KL. Science. 2005;309:1200–1205. doi: 10.1126/science.1109778. [DOI] [PubMed] [Google Scholar]
- 11.Klok H-A, Becker S, Schuch F, Pakula T, Müllen K. Macromolecular Chemistry and Physics. 2002;203:1106–1113. [Google Scholar]
- 12.Bontempo D, Maynard HD. J. Am. Chem. Soc. 2005;127:6508–6509. doi: 10.1021/ja042230+. [DOI] [PubMed] [Google Scholar]
- 13.Reynhout IC, Löwik DWPM, van Hest JCM, Cornelissen JJLM, Nolte RJM. Chem. Commun. 2005:602–604. doi: 10.1039/b413973a. [DOI] [PubMed] [Google Scholar]
- 14.Joralemon MJ, Smith NL, Holowka D, Baird B, Wooley KL. Bioconjugate Chem. 2005;16:1246–1256. doi: 10.1021/bc0501505. [DOI] [PubMed] [Google Scholar]
- 15.Cheng C, Khoshdel E, Wooley KL. Macromolecules. 2005;38:9455–9465. [Google Scholar]
- 16.Qi K, Ma Q, Remsen EE, Clark CG, Wooley KL. J. Am. Chem. Soc. 2004;126:6599–6607. doi: 10.1021/ja039647k. [DOI] [PubMed] [Google Scholar]
- 17.Joralemon MJ, Murthy KS, Remsen EE, Becker ML, Wooley KL. Biomacromolecules. 2004;5:903–913. doi: 10.1021/bm0344710. [DOI] [PubMed] [Google Scholar]
- 18.Becker ML, Remsen EE, Pan D, Wooley KL. Bioconjugate Chem. 2004;15:699–709. doi: 10.1021/bc049946e. [DOI] [PubMed] [Google Scholar]
- 19.Wooley KL. J. Polym. Sci. Part A: Polym. Chem. 2000;38:1397–1407. [Google Scholar]
- 20.Becker ML, Liu J, Wooley KL. Biomacromolecules. 2005;6:220–228. doi: 10.1021/bm049551y. [DOI] [PubMed] [Google Scholar]
- 21.Mustafi D, Sachleben JR, Wells GB, Makinen MW. J. Am. Chem. Soc. 1990;112:2558–2566. [Google Scholar]
- 22.Zhang X, Matyjaszewski K. Macromolecules. 1999;32:7349–7353. [Google Scholar]
- 23.Matyjaszewski K, Patten TE, Xia J. J. Am. Chem. Soc. 1997;119:674–680. [Google Scholar]
- 24.Klok H-A. J. Polym. Sci. Part A: Polym. Chem. 2005;43:1–17. [Google Scholar]
- 25.Lele BS, Murata H, Matyjaszewski K, Russell AJ. Biomacromolecules. 2005;6:3380–3387. doi: 10.1021/bm050428w. [DOI] [PubMed] [Google Scholar]