Abstract
Background
Magnetic seizure therapy (MST) is under investigation as an alternative form of convulsive therapy that induces more focal seizures and spares cortical regions involved in memory. Using a newly expanded version of the Columbia University Primate Cognitive Profile, we compared the cognitive effects of high-dose MST delivered at 100 Hz (6X seizure threshold) with electroconvulsive shock (ECS) delivered at 2.5X seizure threshold.
Methods
Daily high-dose MST, ECS, and Sham (anesthesia-only) were administered for 4 weeks each in a within-subject cross-over design. Rhesus macaques (n = 3) were trained on five cognitive tasks assessing automatic memory, anterograde learning and memory, combined anterograde and retrograde simultaneous chaining, and spatial and serial working memory. Acutely following each intervention, monkeys were tested on the cognitive battery twice daily, separated by a 3-hour retention interval.
Results
Subjects were slower to complete criterion tasks (p’s<0.0001) following ECS, compared to sham and high-dose MST. Moreover, time to task-completion following high-dose MST did not differ from sham. Out of 6 measures of accuracy, treatment effects were found in 4; in all of these, ECS, but not MST, fared worse than Sham. On all accuracy and time to completion measurements, subjects performed as well as following high-dose MST as did subjects from a previous study on moderate-dose MST.
Conclusion
These findings provide evidence that high-dose MST results in benign acute cognitive side-effect profile relative to ECS, and are in line with our previous studies.
Keywords: Magnetic, Seizure, TMS, ECT, Cognitive, Impairment
INTRODUCTION
Electroconvulsive therapy (ECT) is a highly effective treatment for major affective disorders (1). Although its side effect profile has been substantially improved through modifications in electrode placement, stimulus dosage, and electrical parameters, ECT carries a risk of neurocognitive side effects (2; 3). The most prominent of these are retrograde amnesia (RA), which impairs retrieval of events prior to treatment, and anterograde amnesia (AA), which impairs the ability to encode new events into memory. Magnetic Seizure Therapy (MST) is under development as an alternative convulsive technique to minimize the neurocognitive adverse effects of convulsive therapy while maintaining antidepressant efficacy (4; 5). Preclinical testing suggests that MST may have several advantages over ECT, including increased precision of stimulation, greater control of intracerebral spatial distribution, lack of susceptibility to impedance from surface tissues, and the sparing of deep brain structures (6; 7).
The effects of MST on neurocognitive functioning have been examined in monkeys (8) and subsequently in humans (9). In a within-subject study design, two monkeys received MST, electroconvulsive shock (ECS), and sham treatment (anesthesia only) (8). Subjects were tested using a battery of cognitive tasks previously shown to be sensitive to the effects of ECS (10). Results showed that moderate-dose MST, administered at 2.5 times the seizure threshold, resulted in fewer cognitive adverse effects than ECS, also provided at 2.5 times seizure threshold. Specifically, subjects took significantly less time to complete cognitive tasks and showed greater accuracy following moderate-dose MST than ECS.
Similar results were found in depressed patients who received MST (9). In a within-subject design, ten patients received two MST treatments (one at seizure threshold, and the other at maximal stimulator output that was 1.5-2 x seizure threshold) during an acute course of ECT. Relative to performance on neurocognitive measures after ECT, patients performed significantly better on measures of category fluency, sentence recognition, face recognition, and visual cancellation after MST treatments. Moreover, patients showed faster orientation recovery after MST than after ECT (9). Longer reorientation time has been associated with longer-term retrograde amnesia (11).
Recently, we analyzed neurocognitive effects of a complete, acute course of MST in twenty depressed patients in a double-masked, randomized, controlled clinical trial comparing two forms of MST (both 1.5-2 x seizure threshold) (12; 13). After an average of 9.0 (+/− 2.8) MST treatments, no change was seen in global cognitive function or verbal and visual anterograde memory. Subjective improvement in cognitive functioning was seen on the Squire Self-Rating Scale of Memory Function and the Cognitive Failures Questionnaire. However, regarding retrograde amnesia, performance on the Autobiographical Memory Interview decreased minimally, and there was no difference on the Goldberg Remote Memory Questionnaire (14). Both this and the prior human studies used dosages of MST that were close to or slightly above seizure threshold.
While monkey and human studies have found the neurocognitive effects of MST to be benign relative to ECT, device limitations constrained those studies to low and moderate dosages of MST delivered at 50Hz. However, the efficacy and side effects of ECT are known to be highly dosage sensitive. This is especially true for right unilateral (RUL) ECT. RUL ECT administered at seizure threshold has a weak antidepressant effect (15; 16), but when the dosage is increased, it is more efficacious (17) and may also result in greater cognitive impairment (18–20). The viability of MST as a therapeutic alternative to ECT depends largely on whether it can retain cognitive advantages when administered at doses potent enough to approach the antidepressant effect of ECT. Thus, characterization of the cognitive effects of MST at higher doses is warranted to support subsequent human work.
Recently a more powerful MST device, capable of sustaining 100% of maximal stimulator output at 100 Hz for trains of ten-second duration (1000 pulses per train), became available (21). Here we present the first evaluation of the cognitive effects of chronic treatment with 100 Hz MST. A dosage of 6X seizure threshold duration was selected for MST stimulation in order to parallel studies that have found antidepressant efficacy with RUL ECT at a dosage of 6X seizure threshold (17). Rhesus monkeys received 4 weeks of daily high-dose MST (6X seizure threshold), ECS (2.5X seizure threshold), and Sham (anesthesia alone). We also compared high-dose MST to a prior experiment with moderate-dose MST in the same subjects to evaluate MST dosage effects. Further, we present an expanded cognitive battery for nonhuman primates to assess the impact of convulsive therapy on spatial working memory and serial probe recognition.
METHODS and MATERIALS
Subjects
This study was approved by the Institutional Animal Care and Use Committee (IACUC) of the New York State Psychiatric Institute and Columbia University. The subjects were three pathogen-free male rhesus macaca mulatta monkeys obtained from the same National Institutes of Health (NIH) breeding colony. Mean age upon entering the study was 83 (+/− 26) months, mean weight was 8 (+/− 1) kg, and all three were past sexual maturity. The approximate age equivalent in human years was 20.8(+/− 6.5) yrs (22; 23). They were individually housed in a colony where they maintained a 12 hour light-dark cycle. Subjects had ad-lib access to water and received daily feedings of standard monkey chow (LabDiet©, W.F. Fischer & Son, Inc., Somerville, NJ, USA), fruit, food pellets given as positive reinforcement during cognitive testing, and treats hidden in enrichment toys.
Cognitive Testing
Details of the cognitive testing apparatus, stimuli presentation, and training procedures have been reported elsewhere (8; 10). We used the Columbia University Primate Cognitive Profile (CUPCP), which consists of three cognitive tasks that assess learning and memory. Tasks consist of stimuli presented on touchscreen monitors; stimuli consist of randomly chosen pictures, which the subject selects by touching. Task 1, an orientation task (long-term or automatic memory), requires the subject to select a single target stimulus from a field of distracter stimuli; this target stimulus does not change between trials or days. This task was given in the morning, immediately following treatment. The subject was required to get 4 of 5 trials correct to move on to Task 2 (figure 1). Task 2, a variable target task (anterograde learning and memory), required the subject to learn each day, by trial and error, which stimulus among a field of 9 stimuli was the target. The distracter stimuli, but not the target stimulus, changed from trial to trial, the subject was required to correctly select the target in 4 of 5 consecutive trials in order to move on to task three (figure 2). Task 2 was given in the morning session immediately following treatment and again in the afternoon session, three hours after treatment. Task 3, a serial learning and memory task (anterograde and retrograde memory), required the subject to learn the correct sequence in which to respond to a group of 3 stimuli. This task consisted of both new lists, which had to be learned each day through trial and error, and old lists, which had been learned between 1 and 3 years previously (figure 3). New lists were given in both the morning and afternoon sessions, while old lists were given only in the afternoon. For the present study, two new tasks assessing working memory were added to the CUPCP: Task 4, spatial working memory; and Task 5, serial probe recognition.
Figure 1.
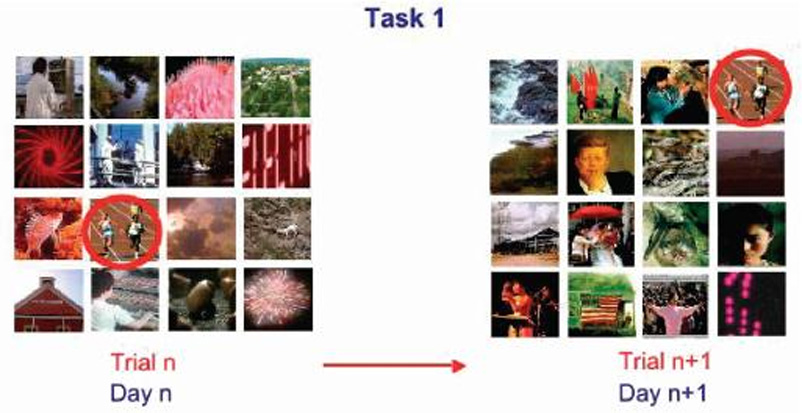
Task 1: Recall of an over-learned stimulus. The subject must correctly select which among a field of 16 stimuli is the target. The target remains the same on every trial of every day, while the distracters and their screen positions change randomly between trials and days. A food pellet is given for each correct trial. Trials are presented until the subject gets 4 out of 5 consecutive trials correct.
Figure 2.
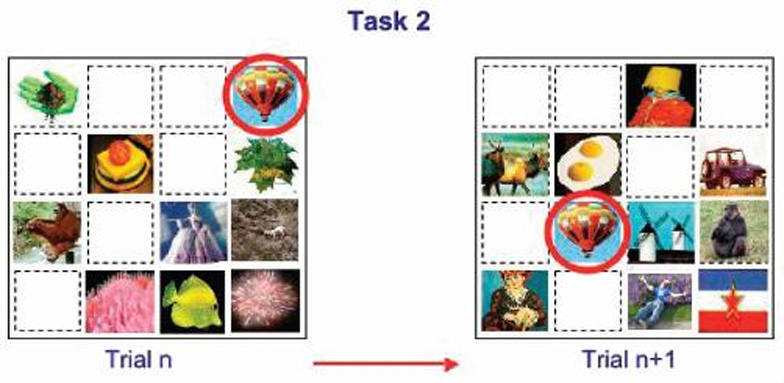
Task 2: New target learning. The subject must learn, by selecting stimuli through trial and error, which stimulus among a field of 8 distracters is the target, then correctly select the target on 4 of 5 consecutive trials. Distracters change between trials and days. The target stimulus remains the same between trials on a given day, but it changes between days.
Figure 3.
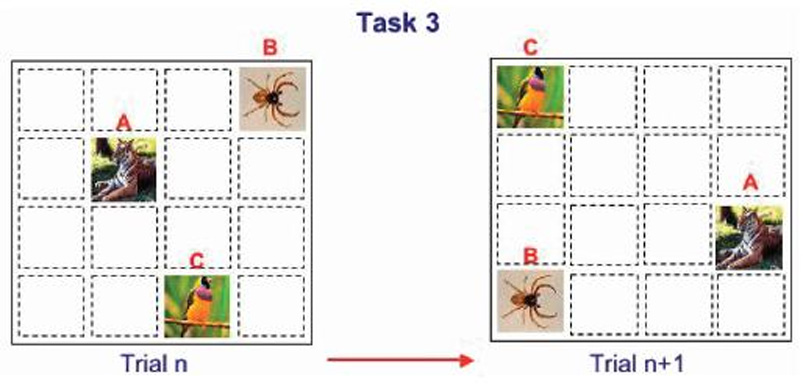
Task 3: Sequence learning and recall. The subject must learn, by trial and error, the correct sequence in which to select 3 simultaneously-presented. This task consisted of new lists, with a novel list presented each day on treatment days, and old lists, learned between 1 and 3 years previously.
Task 4 -- Spatial working memory
This task was modeled after a virtual radial arm maze task in which a subject must navigate through a maze to different locations, without returning to the same location twice (24). Rather than moving through 3-D space, the subject was required to move the cursor to different points on a 2-D touchscreen monitor. A list of 4, 5, or 6 targets was arranged in a spatial configuration that changed randomly from trial to trial. All stimuli in a given trial were identical, so that only their position on the screen differentiated them. In a given trial, subjects were required to select each stimulus exactly once, in whatever sequence they preferred. All stimuli remained on the screen until the end of the trial, and returning to a previously-selected stimulus would result in an incorrect trial (see figure 4). Subjects performed this task during the afternoon session.
Figure 4.
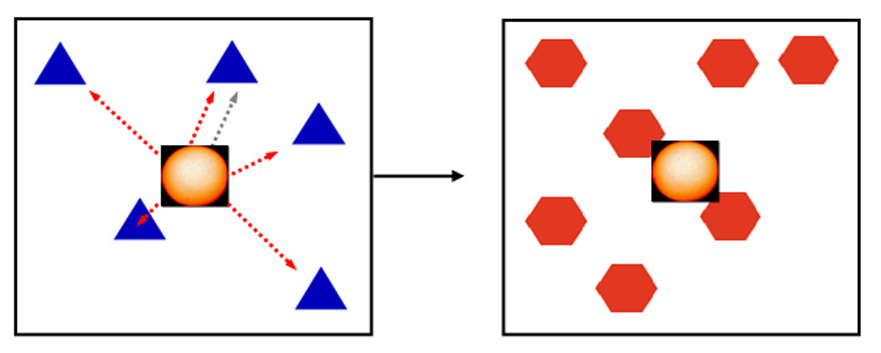
Task 4: Spatial working memory task. 4, 5, or 6 identical stimuli are presented on the screen simultaneously. The subject must select each stimulus in turn without repeating a selection. Because the stimuli are identical and remain on the screen until the trial is over, they can be differentiated only by their respective locations on the screen. Between selections on a given trial, the subject must return to the center and select a “reset” stimulus.
Task 5 -- Serial Probe Recognition (SPR)
The SPR task was designed to test working memory and was modeled after the Sternberg working memory task (25). In this task, a list of 4, 5, or 6 distinct items was presented, one item at a time (see figure 5). Following list presentation and a 2-sec delay, a sample stimulus was presented and subjects indicated, by responding to “yes” and “no” icons, whether or not the target had been in the list. This task was also administered during the afternoon session.
Figure 5.
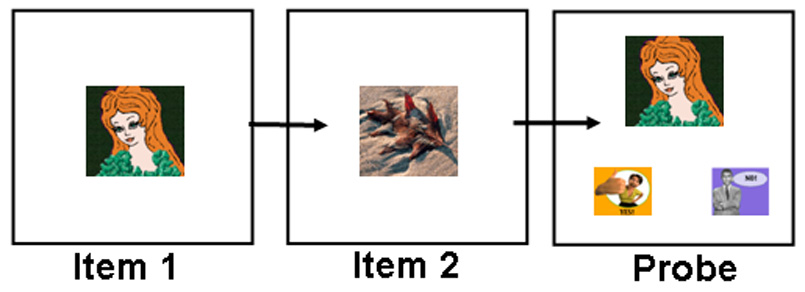
Task 5: Serial Probe Recognition. A list of 4, 5, or 6 stimuli is presented one by one, with no delay between stimuli. The last stimulus in a given trial is followed by a 2 second delay. A “probe” stimulus is then presented, and the subject must select either a “yes” or “no” stimulus to indicate whether the probe had been in the list.
Study design
The study utilized a within-subject design, allowing each monkey to serve as its own control. The final two weeks of data collection prior to each subject’s first treatment were designated as the baseline period. In randomized order, subjects received 20 days of ECS, high-dose MST, and sham treatment (anesthesia alone). A recovery phase, with a minimum of 20 days and a maximum of 30 days, immediately followed each treatment phase. The length of the recovery period was determined by a return to baseline performance. Titration for seizure threshold was performed on days 1 and 11 of both MST and ECS treatment phases. Treatment order was counterbalanced between subjects: subject 1 received ECS, then MST, then Sham; subject 2 received MST, then ECS, then Sham; subject 3 received Sham, then ECS, then MST.
ECS, MST, and Sham Interventions
Details of the interventions, including seizure threshold titration, anesthesia, seizure monitoring, and vital sign monitoring have been reported previously (Moscrip et al., 2004; Moscrip et al., 2006). Briefly, anesthesia consisted of methohexital (0.5 mg/kg i.v.) and succinylcholine (2.5 mg/kg i.v.). MST was administered via a custom modified, 100 Hz Magstim MST device (The Magstim Co Ltd., Wales, U.K.) with a pediatric-sized round coil (6.2 cm diameter) on the vertex. MST seizure threshold was defined by the number of pulses required to elicit a seizure. Seizure threshold titration was performed by starting with a 50Hz, 1s stimulation (50 pulses) and delivering stimulations approximately every 20 seconds, increasing the duration by 1 second with each stimulation until a seizure was induced. Subsequent daily dosage of MST was set at 100 Hz and either 6X seizure threshold, or the maximum output capacity of the device (1000 pulses at 100 Hz). ECS was given bilaterally with a human ECT device at 2.5X titrated seizure threshold at a frequency of 50 Hz. Seizure threshold titration for ECS was performed by increasing duration of stimulation by160ms with each stimulation (with current held at 800mA and pulse width at 0.5ms) until seizure was induced. An ECS frequency of 50 Hz is comprised of 50 pulse pairs (upward and downward going) per second, resulting in 100 total pulses per second. A MST frequency of 100 Hz is comprised of 100 pulses per second, thus the conditions were matched in total number of pulses per second. ECS electrode placement was conventional bilateral (bifrontotemporal). For sham, only anesthesia was given.
Immediately following each intervention, monkeys were transported to a test chamber where the neurocognitive battery was initiated following a response to a start stimulus on the touch screen.
Data Analysis
The analyses utilized mixed effects models (MEMs) which evaluated each cognitive task separately (26). Analyses were conducted using the PROC MIXED procedure of SAS® (27). For all tasks, fixed effects included “condition” (4 levels including baseline), and “session day” (a continuous variable). For tests administered in both morning and afternoon sessions (the T2 test and the Simchain new list test), the fixed effect “session” (2 levels) was included. For the Simchain old list test, in which the same list was shown on multiple consecutive days, the fixed effect “day-of-exposure” was included (3 levels). For the spatial working memory test and serial probe recognition test, the number of list items (3 levels) was included as a fixed effect. For Task 3, a software error midway through subject 3’s study participation caused old list and new list trials to be interspersed rather than separately blocked. Since this error persisted across multiple treatment conditions and was found to significantly reduce his Task 3 overall scores, that subject was dropped from that analysis. A separate analysis pooled data from the current study with data from a prior, moderate-dose MST (2.5X seizure threshold delivered at 50 Hz) study performed in the same subjects (8). Statistical significance was judged based on α = 0.05. The parameters were estimated with the iterative maximum-likelihood method. For all significant findings from cognitive test scores, effect size was calculated (Table 4).
Table 4.
Summary of Main Finding Comparing ECS, High-Dose MST, and Sham
| Task | Treatment Condition | Cohen’s Standard (effect size) |
|---|---|---|
| Task 1 | ||
| Time to Wake/Begin | No Difference | -- |
| Time to Completion | ECS>(MST=Sham)>Baseline | Large (0.7, 0.6, 0.9) |
| Accuracy | No Difference | -- |
| Task 2 | ||
| Time to Completion | ECS>(MST=Sham=Baseline) | Small/Medium (0.2, 0.2, 0.5) |
| Accuracy | ECS<(MST=Sham=Baseline) | Medium (0.4, 0.4, 0.4) |
| Task 3 | ||
| Old List Accuracy | ECS<(MST=Sham=Baseline) | Medium (0.4, 0.4, 0.5) |
| New List Accuracy | No Difference | -- |
| Task 4 | ||
| Accuracy | ECS<Sham | Small (0.2) |
| Task 5 | ||
| Accuracy | ECS<(MST=Sham) | Small (0.2, 0.2) |
RESULTS
Feasibility of High-Dose MST Seizure Induction
MST seizure was induced in all 3 subjects, and there were no adverse events. The observed MST seizure thresholds were 225 pulses for subject 1, 100 pulses for subject 2, and 300 pulses for subject 3. In the MST condition, there was a mean seizure duration of 24.2 ± 5.3 (SD) seconds. This was significantly longer (F=0.51, df=48, p=0.015) than MST seizure duration in the moderate-dose study, where the mean seizure was 19.8 ± 7.4 (SD) seconds. However, there was no significant difference between MST seizure duration and ECS seizure duration, which had a mean of 20.2 ± 5.7 (SD) seconds. There was no cross-study difference in the ECS group for seizure duration.
Time to Recover Consciousness
Time from final anesthesia injection until the subject pressed the “start” button in the cognitive testing chamber was used to gauge time to recovery of consciousness (Table 1). This measure showed no significant main effects for condition, session day, or order.
Table 1.
Effects of High Dose-MST, ECS, and Sham on Recovery of Orientation and Task 1 Performance 1
| Baseline | HD-MST | ECS | Sham | P-value | |
|---|---|---|---|---|---|
| Time to Wake (min) | -- | 14 (11) | 12 (9) | 12 (10) | NS |
| Time to Task 1 Completion (min) | 0.5 (0.4) | 3 (2) | 8 (3) | 4 (2) | 0.005 |
| Task 1 Accuracy (%) | 95.5% (8.6) | 76.0% (17.9) | 72.9% (19.6) | 73.0% (17.0) | NS |
all values are reported in mean (standard deviation) unless otherwise noted
HD-MST=High Dose MST
ECS=Electroconvulsive Shock
Sham=Anesthesia only
Recall Time and Accuracy of an Over-Learned Stimulus (Task 1)
Task-Completion Time
Analysis of the time required to complete the task (i.e., task time) once it was started, yielded a main effect of order (F=5.87, df=52, p=0.0050, with the task taking the most time in earlier treatments, indicating a decrease in overall treatment effects over time), and a main effect of condition (F =25.14, df=52, p<0.0001). Task time was significantly longer for ECS than for MST, sham, or baseline (t=5.23, df=170, p<0.0001; t=4.40, df=170, p<0.0001; t=−6.83, df=170, p<0.0001, respectively). Baseline times were significantly shorter than both MST and Sham times (t=−2.61, df=170, p=0.0100; t=−3.31, df=170, p=0.0012, respectively) and there was no difference between the MST and sham condition task times (Table 1).
Accuracy
Accuracy scores for Task 1 showed no significant main effects in the high-dose study (Table 1).
Completion Time and Accuracy of Learning New Targets (Task 2)
Task-Completion Time
There was a significant order effect in times to task completion (F=13.86, df=2, p<0.0001), with the task taking longer in the first phase than in the third (Table 2). There was also a main effect of condition (F=12.10, df=2, p<0.0001), with ECS taking longer than MST, sham, or baseline (t=5.15, df=331, p<0.0001; t=5.35, df=331, p<0.0001; t=−4.33, df=331, p<0.0001, respectively), and no difference was found for task time among the MST, sham, and baseline conditions.
Table 2.
Task 2 Completion Time and Accuracy of New Target Learning between Treatment Conditions
| Baseline | HD-MST | ECS | Sham | P-value | |
|---|---|---|---|---|---|
| Completion Time (min)1 | 1.4 (1.4) | 5.2 (7.9) | 15.2 (28.3) | 4.3 (3.2) | <0.0001 |
| Accuracy (%) | 85.9% (20.9) | 80.2% (22.7) | 56.4% (28.5) | 80.3% (23.9) | <0.0001 |
mean(standard deviation)
HD-MST=High Dose MST
ECS=Electroconvulsive Shock
Sham=Anesthesia only
Accuracy
Accuracy scores yielded a significant main effect of order (F=5.43, df=144, p=0.0054, with performance improving from the first to the last phase of the study), and a significant main effect of condition (F=286.13, df=4, p<0.0001). Task 2 scores in the ECS condition were significantly lower than in the Baseline, MST and sham conditions (F=8.78, df=391, p<0.0001; F=−7.00, df=391, p<0.0001; F=−7.09, df=391, p<0.0001, respectively). Among the baseline, MST, and Sham conditions, there were no significant differences between any pair.
Accuracy of Serial List Learning and Retention (Task 3)
Old List Accuracy
Main effects of exposure were found (F=17.59, df=190, p<0.0001) with scores higher on the third exposure than on the first. Significant differences between conditions (F=3.56, df=190, p=0.0153) showed that ECS scores were significantly lower than baseline, MST, or sham scores (for baseline, t=2.54, df=114, p=0.0125; for MST, t=−2.36, df=114, p=0.0200; for sham, t=−2.45, df=114, p=0.0158), and there was no difference between scores from any pair among baseline, MST, and sham (figure 6).
Figure 6.
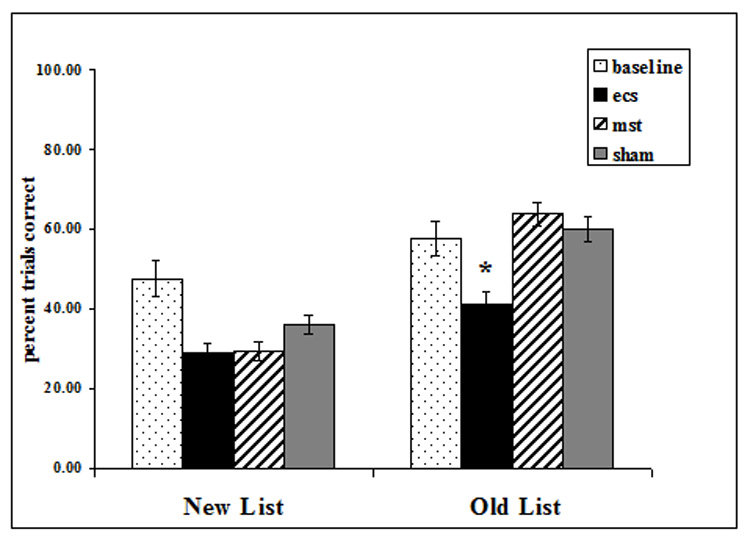
Task 3: Sequence memory. For previously learned (old) lists, ECS resulted in significantly poorer performance than in Baseline, MST, or Sham (* p ≤0.02). For new lists, ECS and MST did not differ from sham.
New List Accuracy
Analyses of new list accuracy and reaction time scores yielded no significant main effects or interactions.
Spatial Working Memory (Task 4) and Serial Probe Recognition (Task 5) Accuracy
Spatial Working Memory
Spatial accuracy scores yielded significant effects for the number of list items (F=25.96, df=2, p<0.0001), with higher scores found for the 4-item than 6-item lists. There was a main effect of condition (F=3.89, df=2, p=0.0389). ECS scores were significantly lower than sham (t=−2.46, df=654, p=0.0140). While MST scores were higher than ECS, and sham higher than MST, these differences were not significant. Scores from the 3 treatment conditions did not differ significantly from Baseline (figure 7).
Figure 7.
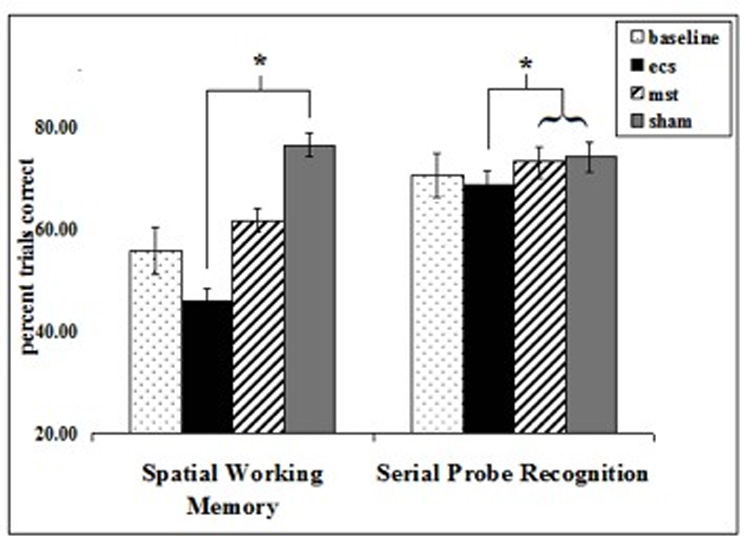
In Task 4, the spatial working memory task, ECS resulted in significantly lower scores than Sham (* p=0.0140). In Task 5, the serial probe recognition task, ECS resulted in lower scores than MST or Sham (* p<0.05).
Serial Probe Recognition
Accuracy scores for the serial probe recognition task yielded a main effect of condition (F=3.73, df=2, p<0.0248). ECS scores were significantly lower than sham (t=−2.59, df=567, p=0.0099) and MST (t=−2.03, df=567, p=0.0429), and no difference was found between MST and sham scores. Scores from the 3 treatment conditions did not differ significantly from Baseline.
Comparison of Moderate- and High-Dose MST Effects on Cognitive Tasks
As seen in Table 3, the only significant differences between the MST dosage conditions were increased start time to begin Task 1, and increased accuracy on Task 2 and Task 3 old list accuracy in the high-dose MST relative to moderate-dose MST.
Table 3.
Comparison of High- and Moderate-Dose MST on Cognitive Effects
| Task1 | High-Dose MST | Mod-Dose MST | Statistic | p-value | Effect Size (Cohen’s d) |
|---|---|---|---|---|---|
| Task 1 | (3 subjects) | (2 subjects) | |||
| Time to Wake/Begin (min)2 | 14 (11) | 6 (2) | T=−3.56, df=260 | P=0.0004 | Medium (0.45) |
| Time to Completion (min) | 3 (2) | 3 (2) | NS | -- | |
| Accuracy (%) | 76.0% (18.0) | 85.4% (15.3) | NS | -- | |
| Task 2 | (3 subjects) | (2 subjects) | |||
| Time to Completion (min) | 5.2 (7.9) | 8.2 (8.8) | NS | -- | |
| Accuracy (%) | 80.2% (22.7) | 54.2% (29.1) | F=−3.86, df=600 | P=0.0001 | Medium (0.45) |
| Task 3 | (2 subjects) | (2 subjects) | |||
| Old List Accuracy (%) | 63.8% (20.0) | 43.6% (24.7) | T=1.98, df=293 | P=0.0488 | Medium (0.41) |
| New List Accuracy (%) | 39.1% (25.9) | 29.5% (19.8) | NS | -- |
All values are expressed as mean (SD) unless otherwise noted
The time to wake/begin showed a study-by-condition interaction in which both the MST and Sham conditions showed lengthier times in the new study relative to the old study.
There was a main effect of study on start time to begin Task 1, with wake/start time significantly longer in the high-dose than in the moderate-dose study (F=9.33, df=1, p=0.0026), and a condition-by-study interaction (F=34.35, df=6, p<0.0001). There was no study effect for the ECS group (which was provided at the identical dosage between the 2 studies), but both the MST and sham groups took significantly longer time to recover in the high-dose study than in the moderate-dose study (t=−3.56, df=260, p=0.0004; t=−2.31, df=260, p=0.0217, respectively).
For Task 2 accuracy, there was a significant main effect of study, with accuracy scores in the current study higher than those in the moderate-dose study (F=25.05, df=1, p<0.0001), and a condition-by-study interaction (F=180.45, df=8, p<0.0001). This increase in scores between studies was not found in the baseline or ECS conditions, but it was significant in the MST and sham conditions (F=−3.86, df=600, p=0.0001; F=−4.50, df=600, p<0.0001, respectively). For time to complete Task 2, there was no main effect of study, and no study-by-condition interaction.
On Task 3 old list recall, there was a significant condition-by-study interaction (F=3.19, df=3, p=0.0250). Specifically, high-dose MST showed higher accuracy than moderate-dose MST (t=1.98, df=293, p=0.0488). There were no differences between high- and moderate-dose MST on Task 3 new list learning.
DISCUSSION
We have shown for the first time that chronic treatment with high-dose MST resulted in less cognitive impairment than ECS (see Table 4 for summary) and that high-dose MST did not significantly differ from the effects of anesthesia alone. Moreover, increasing MST dosage from 2.5X seizure threshold to 6X seizure threshold did not impair cognitive performance on most measures. These results support the feasibility and safety of high-dose MST for subsequent work in humans to assess its efficacy in the treatment of depression.
Replicating our prior report, we found that ECS impaired performance on anterograde amnesia (Task 2) and retrograde amnesia (Task 3 old list), while high-dose MST did not differ from Sham on these measures (8; 10). This result supports the conclusion that high-dose MST dosage at 6X seizure threshold retains cognitive advantages relative to ECS on these measures. Although we failed to replicate our previously reported finding of a difference between ECS and moderate-dose MST on the new list-learning component of Task 3, neither ECS nor high-dose MST differed from Sham on this task in the current study, likely due to a high degree of interday variance (mean = 42% +/− 27%).
As an additional test of the cognitive impact of increasing MST dosage, we compared the current results with 100 Hz high-dose MST with results seen in our prior study with 50 Hz moderate-dose MST (8). On the measure of reorientation time (the time required to complete Task 1 upon waking), high-dose MST did not differ from moderate-dose MST, suggesting that MST dosage within the range tested is unrelated to reorientation time. Further evidence for rapid reorientation post MST has been seen in human studies, which found ECT to result in significantly longer reorientation time than acute (9) and chronic (12; 13) low-dose MST. This difference may be clinically meaningful, since prolonged time to orientation is correlated with retrograde amnesia (11). More recently, a report on the first 11 depressed patients to receive 100 Hz MST likewise found high-dose MST to result in faster orientation recovery than ECT (28).
Interestingly, we found improved accuracy on Task 2 and Task 3 old list recall in the high-dose compared to the moderate-dose study for the MST and Sham groups, but not for ECS condition. Task 3 old list accuracy also improved significantly in the second study for the MST group alone. A possible explanation is a practice effect that allowed subjects to adapt to testing after recovery from anesthesia. The two subjects in the moderate-dose study were experimentally naïve, whereas the three subjects in the current high-dose study had collectively received numerous treatment days involving methohexitol anesthesia and subsequent cognitive testing. The fact that the ECS condition failed to show this practice effect provides further evidence for ECS-related impairment not seen in the MST conditions.
The apparent lack of MST dose dependence, within the range tested here, on learning and memory functions assessed by Tasks 1, 2, and 3, warrants further discussion. Both MST conditions were given at the same intensity (e.g., strength of the magnetic field), and they differed only in frequency (50 versus 100 Hz) and the duration of each stimulation train (in seconds). Since the induced electric field generated by individual pulses within the two conditions was identical, the depth of penetration and regions of the brain directly stimulated by each pulse should be identical. We have previously reported the depth of penetration of MST to be superficial compared to ECS (6). Thus, repeatedly stimulating superficial cortex at higher frequencies and longer train durations would not be expected to adversely affect cognitive functions subserved by brain regions remote from the site of stimulation, except by transsynaptic action. The learning and memory functions involved in Tasks 1, 2, and 3 may be expected to involve hippocampal regions, which would not be directly stimulated even in the high-dose MST condition. However, our dosage comparison was retrospective and confounded by subject age, thus a definitive test of the dose-response relationships will require random assignment to different MST dosage levels.
In this study we introduce an expanded version of the CUCPC that includes two new measures of working memory: (i) spatial working memory (Task 4), and (ii) serial probe recognition (Task 5). ECS significantly impaired working memory on both tasks relative to sham, while high-dose MST did not. The spatial working memory task was based upon the radial arm maze, a task which has been reported to result in increased activation of the hippocampus (29). Because previous research has shown that MST results in less marked physiological and anatomical changes in the dentate gyrus than ECS (6; 30), we predicted high-dose MST would have less of an effect on this task than ECS. Further work is needed to establish the extent to which this task, as implemented here, provides a measure of hippocampal functioning in monkeys. The serial probe recognition task was modeled after the Sternberg delayed match-to-sample task, which has been reported to activate frontal, parietal, occipital and other cortical regions in humans, as well as deeper structures such as cingulate cortex, insula, and hypothalamus, and to be associated with sustained firing in parahippocampal neurons (31–33). We expected that high-dose MST might impair performance on this task by virtue of its action on superficial cortex. However, high-dose MST did not impair function on this task relative to sham, and fared better than ECS, perhaps again due to their differential impact on areas that are active during the task but that fall outside the area most focally stimulated by MST. However, definitive interpretation of these differences awaits further study of the neurobiological underpinnings of these tasks in primates, e.g., via functional imaging.
Limitations of this study include the small sample size, the limited number of cognitive domains assessed, the risk of carry-over effects, practice effects, the fact that all 3 subjects were male, and the aforementioned confound between age and MST dosage in the retrospective dosage comparisons. A larger sample size would be needed before these results could be generalized. Additionally, because this is the first report of the two new working memory tasks added to the CUPCP, further work will be needed to characterize and validate their psychometric properties. A further limitation is the use of bilateral ECS, rather than unilateral, which may be expected to have fewer cognitive side effects. However, bilateral ECS was selected to match the MST placement, which was also bilateral. It should also be noted that the dosage relative to seizure threshold was imbalanced between the two conditions. Specifically, MST was given at 6X whereas ECS was given at 2.5X seizure threshold. This dosage imbalance could be expected to bias the study in favor of seeing an advantage of ECS. Our results were the opposite, further strengthening the support for the relative safety of MST.
For animal models of cognitive function to meaningfully inform the development of safer neurostimulation interventions for patient populations, it will be important to develop systematic translational techniques that allow for cross-species comparison of neurocognitive function. A potentially useful approach to such translational research would be the development of parallel animal and human models of specific neurocognitive domains (34). Such an approach allows one to leverage invasive measures of anatomy and physiology available in the animal to elucidate mechanisms of action and the specific neurocircuitry affected by neurostimulation techniques (35). Moreover, the parallel examination of human and nonhuman responses to neurostimulation can shed light on similar and divergent cognitive processes across species. As we learn more about the effects of various types of neurostimulation modalities, controlling for variables such as stimulation focality, current density, depth, strength and duration of seizure propagation, the neurocognitive effects of these types of stimulation can provide insight into the roles of anatomy and cross-species divergence in cognition.
ACKNOWLEDGEMENTS
This study was supported by NIMH R01 MH60884. Support for the development of Magnetic Seizure Therapy has also come from the Stanley Medical Research Foundation, American Federation for Aging Research / Beeson Scholars Program, and the National Alliance for Research on Schizophrenia and Depression. The authors thank Dr. Tammy Moscrip, Dr. Mohammed Osman, Niko Reyes, Nick DeWind, and the members of the Columbia Primate Cognition Laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
For work unrelated to the present study, Dr. Lisanby has received research support from Magstim Company, Neuronetics, Cyberonics, NIH, AFAR, NARSAD, Stanley Medical Research Foundation, DARPA, and NYSTAR. Dr. McClintock has received funding from NIH. Dr. Husain: Dr. Husain has received research support from the National Institute of Mental Health, Stanley Medical Research Institute, Cyberonics, Inc., Pfizer, Inc. (in process), Neuronetics, Inc., Magstim, and Medtronics, Inc. (potential research sponsor). He has served on Advisory Boards for AstraZeneka, VersusMed, Avinar, Boston Scientific, MEASURE, Bristol-Meyer-Squibb, and Clinical Advisors and on speakers bureaus for Cyberonics, Inc., Avinar, Inc., Cerebrio, Inc., AstraZeneka, Bristol-Meyers-Squibb, Optima/Forrest Pharmaceuticals, Glaxo-Smith-Kline, Forrest Pharmaceuticals, and Janssen. Timothy Spellman, Herb Terrace, Shawn McClintock, and Bruce Luber have no conflicts or potential conflicts to report.
REFERENCES
- 1.American Psychiatric Association. Task Force on Electroconvulsive Therapy. The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging. 2nd ed. Washington, DC: American Psychiatric Association; 2001. [Google Scholar]
- 2.Sackeim HA, Prudic J, Fuller R, Keilp J, Lavori PW, Olfson M. The Cognitive Effects of Electroconvulsive Therapy in Community Settings. Neuropsychopharmacology. 2007;32:244–254. doi: 10.1038/sj.npp.1301180. [DOI] [PubMed] [Google Scholar]
- 3.Lisanby S, Maddox J, Prudic J, Devanand D, Sackeim H. The effects of electroconvulsive therapy on memory of autobiographical and public events. Arch Gen Psychiatry. 2000;57:581–590. doi: 10.1001/archpsyc.57.6.581. [DOI] [PubMed] [Google Scholar]
- 4.Lisanby SH, Schlaepfer TE, Fisch HU, Sackeim HA. Magnetic seizure therapy of major depression. Archives of General Psychiatry. 2001;58:303–305. doi: 10.1001/archpsyc.58.3.303. [DOI] [PubMed] [Google Scholar]
- 5.Lisanby SH, Luber B, Finck AD, Schroeder C, Sackeim HA. Deliberate seizure induction with repetitive transcranial magnetic stimulation in nonhuman primates. Archives of General Psychiatry. 2001;58:199–200. doi: 10.1001/archpsyc.58.2.199. [DOI] [PubMed] [Google Scholar]
- 6.Lisanby SH, Moscrip TD, Morales O, Luber B, Schroeder C, Sackeim HA. Neurophysiological characterization of magnetic seizure therapy (MST) in nonhuman primates. Supplements to Clinical Neurophysiology. 2003;56:81–89. doi: 10.1016/s1567-424x(09)70212-0. [DOI] [PubMed] [Google Scholar]
- 7.Lisanby SH, Peterchev AV. Magnetic Seizure Therapy for the Treatment of Depression. In: Marcolin MA, Padberg F, editors. Transcranial Brain Stimulation for Treatment of Psychiatric Disorders. Karger Publishers; 2007. pp. 155–171. [Google Scholar]
- 8.Moscrip TD, Terrace HS, Sackeim HA, Lisanby SH. Randomized, controlled trial of the cognitive side-effects of magnetic seizure therapy (MST) and electroconvulsive shock (ECS) International Journal of Neuropsychopharmacology. 2006;8:1–11. doi: 10.1017/S146114570500578X. [DOI] [PubMed] [Google Scholar]
- 9.Lisanby SH, Luber B, Schlaepfer TE, Sackeim HA. Safety and Feasibility of Magnetic Seizure Therapy (MST) in Major Depression: Randomized Within-Subject Comparison with Electroconvulsive Therapy. Neuropsychopharmacology. 2003;28:1852–1865. doi: 10.1038/sj.npp.1300229. [DOI] [PubMed] [Google Scholar]
- 10.Moscrip T, Terrace HS, Sackeim HA, Lisanby SH. A Primate Model of Anterograde and Retrograde Amnesia Produced by Convulsive Treatment. Journal of ECT. 2004;20:26–36. doi: 10.1097/00124509-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Sobin C, Sackeim HA, Prudic J, Devanand DP, Moody BJ, McElhiney MC. Predictors of retrograde amnesia following ECT. American Journal of Psychiatry. 1995;152:995–1001. doi: 10.1176/ajp.152.7.995. [DOI] [PubMed] [Google Scholar]
- 12.Lisanby SH, Husain M, Morales O, Thornton L, White PF, Payne N, et al. Controlled Clinical Trial of the Antidepressant Efficacy of Magnetic Seizure Therapy in the Treatment of Major Depression; ACNP Annual Meeting Abstracts; 2003. [Google Scholar]
- 13.White PF, Amos Q, Zhang Y, Stool L, Husain MM, Thornton L, et al. Anesthetic Considerations for Magnetic Seizure Therapy: A Novel Therapy for Severe Depression. Current Research in Anesthesia and Analgesia. 2006;103:76–80. doi: 10.1213/01.ane.0000221182.71648.a3. [DOI] [PubMed] [Google Scholar]
- 14.McClintock SM, Husain MM, Cullum CM, Luber B, Lisanby SH. Neurocognitive Associated Effects of Magnetic Seizure Therapy. The Clinical Neuropsychologist. 2007;21:399. [Google Scholar]
- 15.Sackeim HA, Prudic J, Devanand D, Kiersky JE, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. New England Journal of Medicine. 1993;328:839–846. doi: 10.1056/NEJM199303253281204. [DOI] [PubMed] [Google Scholar]
- 16.Ng C, Schweitzer I, Alexopoulos P, Celi E, Wong L, Tuckwell V, et al. Efficacy and cognitive effects of right unilateral electroconvulsive therapy. Journal of ECT. 2000;16:370–379. doi: 10.1097/00124509-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Ward WK, Lush P, Kelly M, Frost AD. A naturalistic comparison of two right unilateral electroconvulsive therapy dosing protocols: 2-3X seizure threshold versus fixed high-dose. Psychiatry & Clinical Neurosciences. 2006;60:429–433. doi: 10.1111/j.1440-1819.2006.01527.x. [DOI] [PubMed] [Google Scholar]
- 18.Sackeim HA, Prudic J, Devanand D, Nobler MS, Lisanby SH, Peyser S, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Archives of General Psychiatry. 2000;57:425–434. doi: 10.1001/archpsyc.57.5.425. [DOI] [PubMed] [Google Scholar]
- 19.McCall WV, Dunn A, Rosenquist PB, Hughes D. Markedly suprathreshold right unilateral ECT versus minimally suprathreshold bilateral ECT: antidepressant and memory effects.[see comment] Journal of ECT. 2002;18:126–129. doi: 10.1097/00124509-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 20.McCall WV, Reboussin DM, Weiner RD, Sackeim HA. Titrated moderately suprathreshold vs fixed high-dose right unilateral electroconvulsive therapy: acute antidepressant and cognitive effects. [see comment] Archives of General Psychiatry. 2000;57:438–444. doi: 10.1001/archpsyc.57.5.438. [DOI] [PubMed] [Google Scholar]
- 21.Peterchev A, Kirov G, Ebmeier K, Scott A, Husain M, Lisanby S. Frontiers in TMS Technology Development: Controllable Pulse Shape TMS (cTMS) and Magnetic Seizure Therapy (MST) at 100 Hz. Biological Psychiatry. 2007;61:107S. [Google Scholar]
- 22.Gavan JA, Swindler DR. Growth rates and phylogeny in primates. American Journal of Physical Anthropology. 1996;24:181–190. doi: 10.1002/ajpa.1330240206. [DOI] [PubMed] [Google Scholar]
- 23.Tigges J, Gordon T, McClure H, Hall E, Peters A. Survival rate and life span of rhesus monkeys at the Yerkes Regional Primate Research Center. American Journal of Primatology. 1988;15:263–273. doi: 10.1002/ajp.1350150308. [DOI] [PubMed] [Google Scholar]
- 24.Hackers S, Zalesak M. Hippocampal activation during transitive inference in humans. Hippocampus. 2004;14:53–162. doi: 10.1002/hipo.10189. [DOI] [PubMed] [Google Scholar]
- 25.Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- 26.Diggle PJHP, Liang KY, Zeger SL. Analysis of Longitudinal Data. 2nd ed. Oxford, UK: Oxford University Press; 2002. [Google Scholar]
- 27.Littell RMG, Stroup W, Wolfinger R. SAS System for Mixed Models. Cary, NC: SAS Institute, Inc; 1996. [Google Scholar]
- 28.Kirov G, Ebmeier KP, Scott A, Atkins M, Khalid N, Carrick L, et al. Quick recovery of orientation after 100 Hz magnetic seizure therapy (MST) for major depressive disorder. British Journal of Psychiatry. doi: 10.1192/bjp.bp.107.044362. (Submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Astur RS, Germain SA, Baker EK, Calhoun V, Pearlson GD, Constable T. fMRI Hippocampal Activity During a Virtual Radial Arm Maze. Applied Psychophysiology and Biofeedback. 2005;30:307–317. doi: 10.1007/s10484-005-6385-z. [DOI] [PubMed] [Google Scholar]
- 30.Scalia J, Lisanby S, Underwood M, Sackeim H, Dwork A, Morales O, et al. The spatial distribution of mossy fiber sprouting in a non-human primate model for electroconvulsive therapy and magnetic seizure therapy. Biological Psychiatry. 2004:207S. [Google Scholar]
- 31.Walter H, Wolf RC, Spitzer M, Vasic N. Increased left prefrontal activation in patients with unipolar depression: an event-related, parametric, performance-controlled fMRI study. Journal of Affective Disorders. 2007;101:175–185. doi: 10.1016/j.jad.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 32.Schon K, Atri A, Hasselmo ME, Tricarico MD, LoPresti ML, Stern CE. Scopolamine reduces persistent activity related to long-term encoding in the parahippocampal gyrus during delayed matching in humans. Journal of Neuroscience. 2005;25:9112–9123. doi: 10.1523/JNEUROSCI.1982-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jansma JM, Ramsey NF, de Zwart JA, van Gelderen P, Duyn JH. fMRI study of effort and information processing in a working memory task. Human Brain Mapping. 2007;28:431–440. doi: 10.1002/hbm.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosen JB. Translational research: Parallels of human and animal research in biological psychology. Biological Psychology. 2006;73:1–2. doi: 10.1016/j.biopsycho.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Strauman TJ, Merrill KA. The basic science/clinical science interface and treatment development. Clinical Psychology: Science and Practice. 2004;11:263–266. [Google Scholar]


