Abstract
ATP-sensitive K+ (KATP) channel mutations have been identified in individuals with dilated cardiomyopathy and overt heart failure. Here, a common E23K functional polymorphism in the Kir6.2 channel pore versus cardiac phenotype was studied in a cross-sectional community-based cohort (n = 2,031). The KK genotype was associated with greater left ventricular size among subjects with increased stress load due to hypertension. These findings implicate Kir6.2 K23 as a risk factor for adverse subclinical myocardial remodeling, and underscore the significance of cardiac KATP channels within the population.
Introduction
Susceptibility or resistance to heart failure, despite apparently similar risk load, is attributable to individual variation in homeostatic reserve (Bleumink et al. 2004). Ventricular cardiomyocytes are rich in stress-responsive KATP channels comprised pore-forming Kir6.2 and SUR2A regulatory subunits encoded by KCNJ11 and ABCC9, respectively (Zingman et al. 2002; Yamada et al. 2006). Their critical role in stress adaptation is exemplified by genetically defective channel complexes caused by ABCC9 mutations in human dilated cardiomyopathy (Bienengraeber et al. 2004) and Kcnj11−/− mice, vulnerable to hypertension-induced heart failure (Kane et al. 2006).
A common single nucleotide polymorphism (67G > A) in human KCNJ11 corresponds to glutamic acid or lysine at residue 23 of Kir6.2 (Riedel et al. 2005). Abnormal channel gating for the K23 variant has been reported, with altered activation and inhibition sensitivity profiles recorded in vitro for various ligands such as adenine nucleotides, long chain acyl CoA esters, and protons (Riedel et al. 2005; Li et al. 2005), Consequently, this functional polymorphism impairs the ability of KATP channels to respond properly to the cellular milieu. Case–control and case–cohort studies have demonstrated association between E23K and susceptibility to glucose intolerance and diabetes (Riedel et al. 2005; Fischer et al. 2008), as Kir6.2 also forms KATP channels in insulin-producing pancreatic beta-cells. Here, we explored the relationship between this KATP channel polymorphism and subclinical heart disease in the community.
Materials and methods
Subjects
A community-based cross-sectional cohort of 2,031 predominantly Caucasian adults residing in Olmsted County, Minnesota, was studied (Redfield et al. 2003), under a protocol approved by the Mayo Clinic Institutional Review Board. Individuals were age 45 years or older with detailed clinical and prospective cardiac structure/function data, and stored DNA samples. Hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg at the time of echocardiography, irrespective of clinical diagnosis or treatment of hypertension. Left ventricular (LV) measurements were determined by M-mode and 2D biplane echocardiography, and indexed to body surface area.
Genotyping
Genomic DNA comprising the 67A>G KCNJ11 variant was amplified by the polymerase chain reaction and digested with BanII. Resultant fragments, varying in size based on presence (67G) or absence (67A) of a BanII restriction site, were resolved on agarose gels to assign genotypes.
Statistical analyses
The association of genotype with echocardiographic parameters was analyzed using analysis of variance (ANOVA). The synergistic effects between genotype and LV mass in association with LV dimensions were evaluated by introducing an interaction term into the model.
Results
Clinical characteristics of the study cohort reflected the composition of Olmsted County, Minnesota, community members age 45 years or older (Redfield et al. 2003). In brief, 48% of participants were males, the mean age was 62.8 ± 10.6 years, and the mean body mass index was 28.4 ± 5.41 kg/m2. Prevalence of diagnosed disease was congestive heart failure 2.6%, coronary artery disease 12.2%, previous myocardial infarction 4.8%, hypertension 29%, and diabetes 4.5%. Genotype frequencies were in Hardy–Weinberg equilibrium (EE = 44%; EK = 47%; KK = 9%) and similar to previously reported control populations (Riedel et al. 2005). In the group at large, there was no significant association between genotypes and measures of cardiac structure/function (LV dimensions, mass, and ejection fraction), electrical instability (atrial and ventricular arrhythmias), or metabolism (fasting glucose, diabetes, and body mass index) at enrollment. Among individuals with documented hypertension at the time of echocardiography (n = 1,187), the KK genotype was significantly associated with greater LV dimension and volume in both diastole and systole (Table 1). A synergistic effect on LV size of KK genotype and LV mass, a marker of chronic cardiac stress load (Fig. 1), further validated the impact of Kir6.2 E23K on cardiac structure in hypertension.
Table 1.
Left ventricular structure and function versus Kir6.2 E23K genotype in hypertensive individuals
| Phenotype | EE | EK | KK | P |
|---|---|---|---|---|
| LV diastolic dimension (cm/m2) | 2.93 ± 0.30 | 2.94 ± 0.28 | 3.06 ± 0.41 | 0.0394 |
| LV systolic dimension (cm/m2) | 1.76 ± 0.34 | 1.74 ± 0.27 | 1.89 ± 0.48 | 0.0149 |
| LV diastolic volume (ml/m2) | 57.91 ± 16.99 | 57.47 ± 14.60 | 62.89 ± 20.24 | 0.0410 |
| LV systolic volume (ml/m2) | 21.52 ± 10.19 | 20.77 ± 8.01 | 24.56 ± 14.68 | 0.0302 |
| LV mass (gm/m2) | 101.36 ± 23.84 | 100.80 ± 23.56 | 106.31 ± 27.7 | 0.4031 |
| LV ejection fraction (%) | 63.0 ± 7.6 | 63.5 ± 6.7 | 62.2 ± 9.1 | 0.5296 |
| Age (years) | 65.4 ± 10.6 | 65.0 ± 10.7 | 65.4 ± 9.9 | 0.8407 |
| Male gender (%) | 51.1 | 49.5 | 52.4 | 0.7885 |
| Systolic BP (mmHg) | 146.17 ± 14.89 | 146.24 ± 15.28 | 147.41 ± 15.12 | 0.6938 |
| Diastolic BP (mmHg) | 81.17 ± 9.68 | 80.50 ± 9.92 | 81.31 ± 9.59 | 0.4592 |
| Mean BP (mmHg) | 102.84 ± 9.36 | 102.42 ± 9.25 | 103.34 ± 9.47 | 0.4284 |
Mean ± standard deviation LV left ventricle, BP blood pressure
Fig. 1.
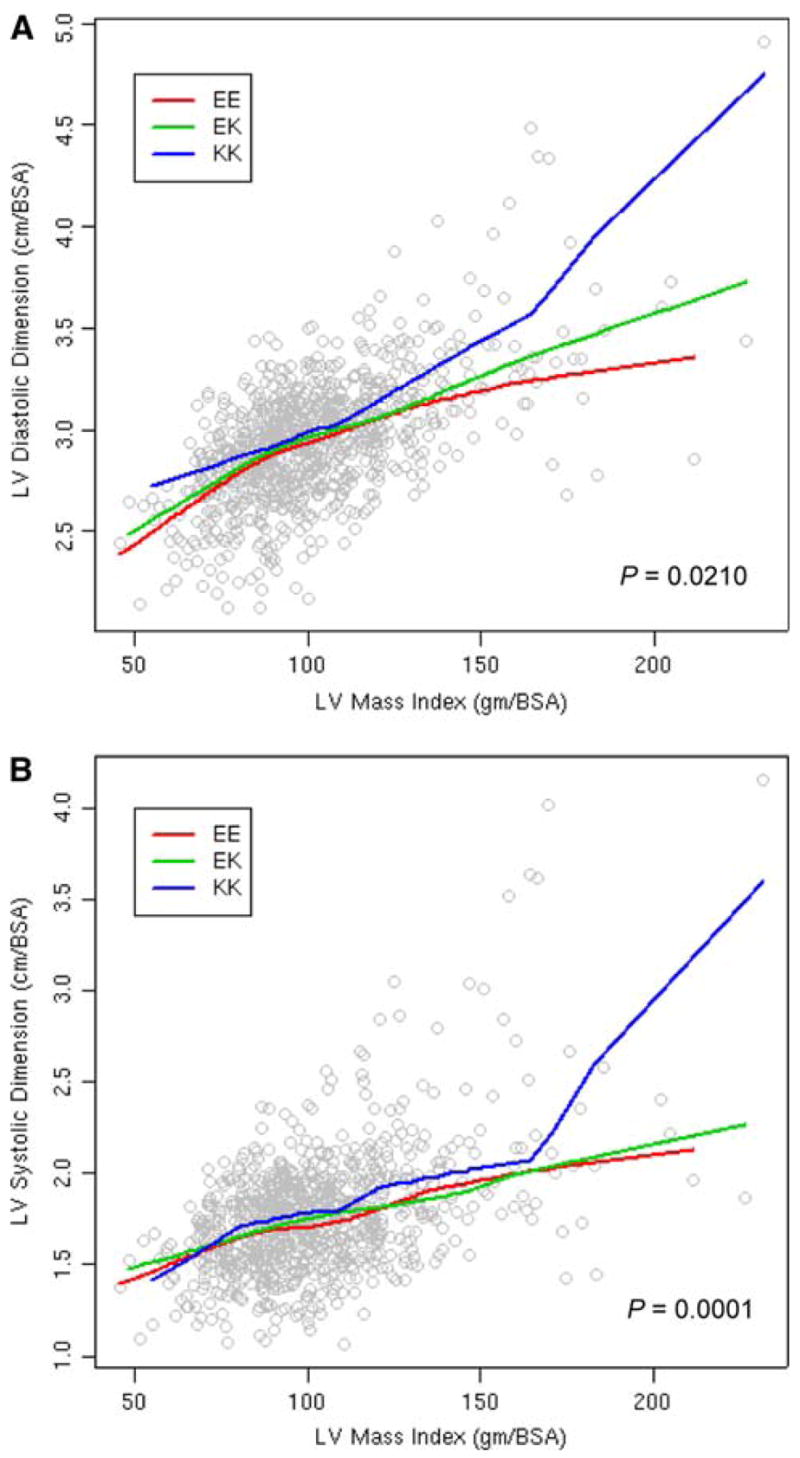
Interaction between left ventricular (LV) mass and Kir6.2 E23K genotype versus LV dimensions in diastole (a) and systole (b). BSA body surface area
Discussion
Hypertension is the most common risk factor for congestive heart failure, and LV enlargement is an established precursor of symptomatic ventricular dysfunction (Kannel et al. 1972; Vasan et al. 1997). The Kir6.2 K23 allele, present in over half the population, is here implicated as a risk factor for transition from hypertensive stress load to subclinical maladaptive cardiac remodeling. Intact KATP channels function as high-fidelity homeostatic rheostats that adjust membrane potential-dependent functions to match cellular energetic demand. Genetic or pharmacologic alterations predicted to abnormally increase or decrease the channel open probability have been reported to uncouple this metabolic signal decoding function, and thereby compromise cardiac stress responsiveness and increase susceptibility to heart disease (Bienengraeber et al. 2004; Kane et al. 2006; Yamada et al. 2006; Lee et al. 2007). Our findings with the K23 allele of the Kir6.2 pore, consistent with previous work with the ABCC9-encoded regulatory subunit (Olson et al. 2007), uncover an interactive KATP channel gene-environment substrate that confers cardiac disease risk in a predominantly Caucasian population. Determining the overall impact of Kir6.2 E23K across ethnic groups and on long-term clinical outcome, i.e., progression to LV enlargement and clinical heart failure, will require further study.
Acknowledgments
This work was supported by the National Institutes of Health (HL071225, HL064822, and HL55502), Marriott Heart Disease Research Program, and Mayo Graduate School.
References
- Bienengraeber M, Olson TM, Selivanov VA, Kathmann EC, O’Cochlain F, Gao F, Karger AB, Ballew JD, Hodgson DM, Zingman LV, Pang YP, Alekseev AE, Terzic A. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat Genet. 2004;36:382–387. doi: 10.1038/ng1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleumink GS, Schut AF, Sturkenboom MC, Deckers JW, van Duijn CM, Stricker BH. Genetic polymorphisms and heart failure. Genet Med. 2004;6:465–474. doi: 10.1097/01.gim.0000144061.70494.95. [DOI] [PubMed] [Google Scholar]
- Fischer A, Fisher E, Möhlig M, Schulze M, Hoffmann K, Weickert MO, Schueler R, Osterhoff M, Pfeiffer AF, Boeing H, Spranger J. KCNJ11 E23K affects diabetes risk and is associated with the disposition index: results of two independent German cohorts. Diabetes Care. 2008;31:87–89. doi: 10.2337/dc07-1157. [DOI] [PubMed] [Google Scholar]
- Kane GC, Behfar A, Dyer RB, O’Cochlain DF, Liu X-K, Hodgson DM, Reyes S, Miki T, Seino S, Terzic A. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum Mol Genet. 2006;15:2285–2297. doi: 10.1093/hmg/ddl154. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Castelli WP, McNamara PM, McKee PA, Feinleib M. Role of blood pressure in the development of congestive heart failure: the Framingham study. N Engl J Med. 1972;287:781–787. doi: 10.1056/NEJM197210192871601. [DOI] [PubMed] [Google Scholar]
- Lee TM, Lin MS, Tsai CH, Huang CL, Chang NC. Effects of sulfonylureas on left ventricular mass in type 2 diabetic patients. Am J Physiol Heart Circ Physiol. 2007;292:H608–H613. doi: 10.1152/ajpheart.00516.2006. [DOI] [PubMed] [Google Scholar]
- Li L, Shi Y, Wang X, Shi W, Jiang C. Single nucleotide polymorphisms in K(ATP) channels: muscular impact on type 2 diabetes. Diabetes. 2005;54:1592–1597. doi: 10.2337/diabetes.54.5.1592. [DOI] [PubMed] [Google Scholar]
- Olson TM, Alekseev AE, Moreau C, Liu XK, Zingman LV, Miki T, Seino S, Asirvatham SJ, Jahangir A, Terzic A. KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2007;4:110–116. doi: 10.1038/ncpcardio0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- Riedel MJ, Steckley DC, Light PE. Current status of the E23K Kir6.2 polymorphism: implications for type-2 diabetes. Hum Genet. 2005;116:133–145. doi: 10.1007/s00439-004-1216-5. [DOI] [PubMed] [Google Scholar]
- Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left ventricular dilation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med. 1997;336:1350–1355. doi: 10.1056/NEJM199705083361903. [DOI] [PubMed] [Google Scholar]
- Yamada S, Kane GC, Behfar A, Liu XK, Dyer RB, Faustino RS, Miki T, Seino S, Terzic A. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. J Physiol. 2006;577:1053–1065. doi: 10.1113/jphysiol.2006.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci USA. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]


