Abstract
Declines in immune function are well described in the elderly, and are considered to contribute significantly to disease burden in this population. Regulatory T cells (Tregs), a CD4+ T cell subset usually characterized by high CD25 expression, control the intensity of immune responses, both in rodents and humans. However, because CD25 expression does not define all Tregs, especially in aged hosts, we characterized Tregs by expression of FOXP3, a transcription factor crucial for Treg differentiation and function. The proportion of FOXP3+CD4+ Tregs increased in the blood of the elderly and the lymphoid tissues of aged mice. The expression of functional markers, such as CTLA-4 and GITR, was either preserved or increased on FOXP3+ Tregs from aged hosts, depending on the tissue analyzed. In vitro depletion of peripheral Tregs from elderly humans improves effector T cell responses in most subjects. Importantly, Tregs from old FoxP3-GFP knock-in mice were suppressive, exhibiting a higher level of suppression per cell than young Tregs. The increased proportion of Tregs in aged mice was associated with the spontaneous reactivation of chronic Leishmania major infection in old mice, likely because old Tregs efficiently suppressed the production of IFN-gamma by effector T cells. Finally, in vivo depletion of Tregs in old mice attenuated disease severity. Accumulation of functional Tregs in aged hosts could therefore play an important role in the frequent reactivation of chronic infections that occurs in aging. Manipulation of Treg numbers and/or activity may be envisioned to enhance control of infectious diseases in this fragile population.
Introduction
During aging, the integrity of the immune system progressively declines. In particular, the ability to fight off infections is decreased, as evidenced by increased numbers of infections, more severe symptoms, prolonged duration and poorer diagnosis (reviewed in (1-4)). Furthermore, reactivation of chronic infections occurs at a higher frequency in aged humans and mice (5, 6). These dysfunctions arise from alterations in every component of the immune system (7-10), but the most consistent and significant alterations are seen in the T lymphocyte compartment (11, 12), particularly within CD4+ T cells (8, 13-15).
CD4+ regulatory T cells (Tregs) maintain self-tolerance in the periphery (16-18) and play a role in the control of autoimmunity and tumor immunity (18-20). They have been shown to decrease the level of activation, proliferation and cytokine production of effector T cells (Teffs) in mice and humans (21-24), as well as control the immune function of dendritic cells (DCs) (25, 26). Tregs were first characterized by their expression of the IL-2Rα chain (CD25) (16). Additional molecules have been associated to Treg function, such as cytotoxic T lymphocyte associated antigen (CTLA)-4 (27) and the glucocorticoid-induced tumor necrosis factor receptor (GITR) (28). More recently, the transcriptional factor FoxP3 (Forkhead box P3) has been shown to play a crucial role in many aspects of murine Treg biology, namely their differentiation, function and maintenance (29-32). In humans, FOXP3 is also crucial for Treg function, as evidenced by the acquisition of Treg activity following de novo FOXP3 expression in non-Tregs (33).
Previous studies have shown increased numbers of CD25+CD4+ Tregs in the periphery of aged Balb/c (34, 35) or C57BL/6 mice (36). Similar increases were also reported in the peripheral blood of elderly people (37-40). Although, FOXP3 expression has recently been used to assess the proportion of Tregs in aged humans (41), it remains unclear whether Tregs maintain their suppressive activity in aged hosts. Indeed, some studies show maintenance of suppressive activity of Tregs in aged mice (34, 36) and elderly people (37, 39), whereas some studies reported decreased Treg-mediated suppression in aged mice (35) and humans (42).
During the acute phase of the infection by L. major, activation of Teffs leads to the development of a small cutaneous lesion that heals spontaneously after few weeks (43). We have previously shown that during the chronic phase of the infection a high number of both Teffs (CD4+CD25− T cells, producing IFN-γ) and Tregs accumulate at sites of infection (44). A tight equilibrium between the two populations is responsible for the parasite persistence at the site of inoculation (44). Importantly, changes in the Treg:Teff balance at the local site induces parasite multiplication and subsequently reappearance of the lesion (45)
In this report, we show in that: (i) FoxP3+ Tregs accumulate in aged mice and elderly humans; (ii) Tregs from aged mice and elderly humans are functional; and (iii) depletion of Tregs in vitro and/or in vivo increases Teff responses. Together, these data suggest that Treg accumulation in aged hosts contributes to the immune suppression associated with aging.
Materials and Methods
Human subjects
Healthy elderly individuals (≥ 70-year old) were recruited in a retirement community in the Cincinnati area. People in the upper third of functional status and with two or less comorbidities were eligible for enrollment. Enrolled individuals were not receiving immunosuppressive medication and had no chronic infection, known malignancy or cognitive impairment. Volunteers with mild chronic conditions not thought to affect immune function were not excluded. Young healthy donors (≤ 30-year old) were recruited at Cincinnati Children's Hospital Medical Center with the same exclusion criteria as those used in the recruitment of elderly subjects. All subjects provided written informed consent to protocols approved by the corresponding Institutional Review Boards.
Mice
6-8-week old C57BL/6 mice were purchased from Charles River (Wilmington, MA) or Taconic. 20-month old C57BL/6 mice were purchased from Harlan (Chicago, IL) through the National Institute on Aging contract. FoxP3-GFP knock-in C57BL/6 reporter mice were obtained from Dr. M. Oukka, Harvard Medical School, Cambridge, MA (46). Mice were maintained at Children's Hospital Research Foundation Animal Facility or NIH animal house facility under pathogen-free conditions. All experiments on mice were performed in accordance with institutional guidelines (Cincinnati Children's Hospital Medical Center and National Institute of Allergy and Infectious Diseases).
Cell preparation
For humans, blood samples (40 ml) were collected on sodium heparin. Peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation on Ficoll Paque™ Plus (GE Healthcare, Piscataway, NJ) within 4 hours of sample collection and frozen in fetal calf serum (FCS) with 10% dimethyl sulphoxide (DMSO; SIGMA, St. Louis, MO).
For mice, single cell suspensions were prepared from spleen, peripheral (retromaxillar and popliteal) lymph nodes (pLNs) and mesenteric LNs (mLNs). Blood was collected on heparin. Erythrocytes were lyzed by incubating the spleen or blood cells with 1 ml ACK (Cambrex, Walkersville, MD) for 2 min, on ice.
Phenotypic analysis of cells
Thawed human PBMCs were incubated on ice for 5 min with human IgG (SIGMA) to block Fc receptors and stained for 30 min for cell surface markers with a combination of the following antibodies: anti-CD3-PerCP-Cy5.5 (clone SK7), anti-CD4-Pacific Blue or PE-Cy7 (clone RPA-T4), anti-CD25-APC (clone M-A251), anti-CD69-APC-Cy7 (clone FN50), anti-CCR5-PE-Cy7 (clone 2D7/CCR5), anti-CCR7-PE-Cy7 (clone 3D12), anti-integrin α4-APC (clone 9F10), anti-integrin β7-PE (clone FIB504) from BD Pharmingen (San Diego, CA); anti-CD27-APC-Cy7 (clone O323) and anti-integrin β1-PE (clone MEM-101A) from eBioscience (San Diego, CA); anti-GITR-PE (clone 110416) and anti-TGFβRII-PE (clone 25508) from R&D System (Minneapolis, MN); anti-CD127-PE (clone IM1980) from Immunotech (Marseille, France); anti-CD45RA-Pacific Blue (clone MEM-56) and anti-CXCR4-APC (clone 44717) from Caltag (Burlingame, CA). For intracellular stainings, the eBioscience's protocol for FOXP3 staining and the following antibodies were used: anti-FOXP3-FITC (clone PCH101) and anti-CTLA-4-PE (clone 14D3) from eBioscience; anti-PD-1 (clone MIH4) and anti-Granzyme A-PE (clone CB9) from BD; anti-Granzyme B-APC (clone GB12) from Caltag. 200,000 events/sample were collected on a LSRII™ cytometer using the FACSDiva™ software (BD Biosciences). The CD25hi, FOXP3+, GITR+ and CTLA-4+ gates were determined in relation to the staining on non-CD4+ T cells. The gate for Granzyme B, CD127, CD45RA and CD27 expression was set up according to the biphasic distribution of this marker. For all the other markers, the appropriate isotype-matched control antibodies were used to define the positive gates within the CD4+CD3+ T cells.
Freshly isolated or cultured murine cells were incubated with 24G2 cell line culture supernatant to block Fc receptors. Cells were stained for 20 min on ice with the following fluorochrome-conjugated antibodies: anti-CD4-PE-Cy5 (clone RM4-5), anti-TCR-βchain-APC-Alexa Fluor 750 (clone H57-597), anti-CD25-APC (clone PC61.5), anti- CD69-PE (clone H1.2F3), anti-CD103-FITC (clone 2E7), anti-CD27-APC (clone LG.7F9), anti-CCR7-PE (clone 4B12), anti-GITR-APC (clone DTA-1) and anti-PD-1- FITC (clone J43). The isotype controls used were rat IgG1 (clone R3-34), rat IgG2a (clone eBR2a), rat IgG2b (clone KLH/G2b-1-2), and hamster IgG (clone Ha4/8). Cells were washed with PBS, 1% FCS, then stained with anti-FoxP3-Pacific Blue (clone FJK-16s) and anti-CTLA-4-PE (clone UC10-4F10-11) using the FoxP3 staining set reagents and protocol from eBioscience. All antibodies and controls were purchased from BD Pharmingen, eBioscience or BioLegend (San Diego, CA). Cell acquisition was performed on a FACSCalibur™ cytometer using the CellQuest Pro™ software, or a LSRII™ cytometer using the FACSDiva™ software (BD Biosciences). Analysis was performed after gating on CD4+TCR-β+ cells, using CellQuest Pro™ software or FlowJo™ software (Tree Star, Inc., Ashland, OR).
Human CD4+ T cell stimulation
Monocytes were isolated from PBMCs by positive selection using CD14 MicroBeads according to the manufacturer's instruction (Miltenyi Biotec, Auburn, CA), achieving a purity of > 87%, as determined by flow cytometry. CD4+ T cells (> 85% pure) were obtained by negative selection of CD14− cells using CD4+ T cell isolation kit II according to manufacturer's instruction (Miltenyi Biotec). CD4+ T cells were depleted of CD25+ cells using CD25 MicroBeads (Miltenyi Biotec). As depletion of CD25+ cells by MicroBeads induces non-specific cell loss in the magnetic column, we controlled for that loss in the total CD4+ T cell fraction by using irrelevant MicroBeads (CD14 MicroBeads) according to manufacturer's instructions. Passage of the CD4+ fraction through those CD14 MicroBeads did not change the percentage of CD25+ or FOXP3+CD4+ T cells (results not shown).
Treg-depleted CD4+ and total CD4+ fractions were labeled for 5 min at room temperature with 0.625 μM of carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes, Eugene, OR) in PBS. 5 × 105 CFSE-labeled T cells were cultured with 2 × 105 CD14+ monocytes in RPMI supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 292 μg/ml L-glutamine (Invitrogen, Gibco®, Carlsbad, CA) and 10% FCS, and stimulated or not with phytohemagglutinin (PHA; 2 μg/ml; SIGMA) at 37°C in 5% CO2. After 3 days of stimulation, the recovered cells were incubated on ice for 5 min with human IgG to block Fc receptors and were stained 30 min with anti-CD4-PE (clone RPAT4; BD), anti-CD3-PerCP-Cy5.5, anti-CD69-APC (clone FN50; BD) and anti-CD95- Pacific Blue (clone DX2; Caltag). After washes and formaldehyde fixation, up to 50,000 events/sample were collected on a LSR-II™ cytometer with the FACSDiva™ software. Data were analyzed by using FlowJo™ software.
In vitro T cell suppression assay
GFP−CD4+ (Teffs) and GFP+CD4+ (Tregs) cells were sorted from spleens or pLNs of FoxP3-GFP knock-in C57BL/6 mice as described below for CD25hiCD4+ and CD25−CD4+ T cells. 5 × 104 Teffs were stimulated with 0.5 μg/ml anti-CD3 (clone 145-2C11, BD Biosciences) in the presence of 1 × 105 CD90+ cell-depleted and 3,000-rad γ-irradiated spleen cells. Cultures were set up in triplicate, in 96-well U-bottom plates, with different ratio of Tregs to Teffs. 48 hours later, 1 μCi [3H]-thymidine was added into each well for 22 hours. Plates were harvested and radioactivity measured on a Wallac Trilux MicroBeta scintillation counter.
L. major infection
Promastigotes (metacyclics) of L. major clone V1 (MHOM/IL/80/Friedlin) were isolated as previously described (47). 8-10-week old mice were infected in the ear dermis with 103 L. major metacyclic promastigotes using a 27½ G needle in a volume of 10 μl.
In vitro restimulation assay of lymphocytes from L. major-infected mice
A single-cell suspension was prepared from the retromaxillar LNs of L. major-infected mice as described (43). CD4+ T cells were pre-enriched by negative selection using magnetic beads (CD4+ T cell isolation kit; Miltenyi Biotec). CD25hiCD4+ and CD25−CD4+ T cells were then purified using a FACSVantage® cell sorter as previously described (21). The T cell subsets were > 98% pure as analyzed by flow cytometry. Isolated cells were labeled for 5 min at room temperature with 1.25 μM CFSE in PBS. 5 × 104 T cells were incubated with 1.4 × 105 bone marrow-derived dendritic cells (BMDCs) in 200 μl RPMI containing 100 U/ml penicillin, 100 μg/ml streptomycin, 100 μg/ml gentamicin, 55 μM 2-mercaptoethanol, 10% FCS, as previously described (48). BMDCs were previously incubated overnight with or without L. major metacyclics (parasites:BMDCs ratio = 5:1) and washed before culture with T cells. After 4 days of stimulation at 37°C in 5% CO2, cells were analyzed by flow cytometry (see above) and culture supernatants were collected for cytokine assays (see below).
CD25+ T cell depletion
Mice were injected with 1 mg anti-CD25 (clone PC6C1; American Type Culture Collection) or isotype control (clone A1101-1) antibodies, as described before (45). Antibodies were produced using serum-free medium (BD Biosciences) and a CELLine™ device (BD Biosciences) according to the manufacturer's instructions. Antibodies were purified by protein G affinity chromatography (Pierce Chemical Co.). The efficiency of depletion was shown to be > 80%.
Cytokine assays
Mouse IFN-γ, IL-2, IL-10, and GM-CSF were quantified in culture supernatants using the DuoSet Enzyme Linked Immunosorbent Assay (ELISA) system (R&D Systems). Alternatively, a multiplex assay (Linco Research, St. Charles, MO) was used following the manufacturer's protocol.
Statistical analysis
Statistical analysis was performed using Prism (GraphPad Software Inc., San Diego, CA). For mice, group comparisons were made using the two-group Student's t-test. For human, the distribution of the values in the different groups was tested by Shapiro-Wilk normality test. If the distribution was normal, group comparisons were made using the two-group Student's t-test. If the distribution was not normal, a Box-Cox transformation was made and transformed values were tested again for their normality. If the distribution became normal after transformation, a two-group Student's t-test was made; otherwise, group comparisons were made using a Mann-Whitney test. A p value < 0.05 was considered statistically significant. The Box-Cox transformation and the subsequent statistical analyses were conducted using SAS v9.
Results
The proportion of circulating Tregs is increased in healthy elderly subjects
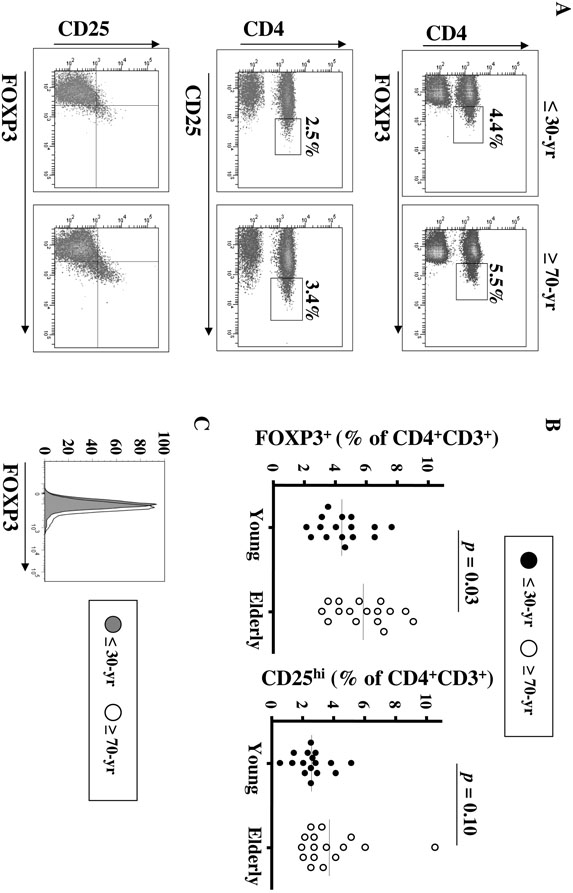
FOXP3 has been identified as the most specific marker of Tregs in humans (32). We therefore investigated whether the number of FOXP3+CD4+ T cells was altered in elderly individuals (≥ 70-year old) compared with young adults (≤ 30-year old). Healthy donors were enrolled, applying criteria detailed in the Materials and Methods. Mean age of the elderly and young subjects was 82.8 ± 7.2 and 25.8 ± 2.7-year old, respectively.
The proportion of FOXP3+ cells was significantly increased within CD4+ T cells from elderly compared to young subjects (5.8 ± 0.4% versus 4.4 ± 0.4%; p = 0.03, unpaired t-test; N = 16/group; Fig. 1A and B). Because the level of FOXP3 per cell is an important factor determining Treg activity (49), we evaluated the mean fluorescence intensities (MFI) of FOXP3 staining, which were similar in elderly and young donors (Fig. 1C). High level of CD25 expression is also described as a characteristic feature of Tregs. The number of CD25hiCD4+ T cells was determined using a stringent gate to define CD25hi expression (Fig. 1A). The proportion of CD25hi cells in the CD4+ T cell population was not significantly different in elderly and young subjects, although there was a trend towards increased proportion in the elderly (Fig. 1A and B; p = 0.1, Mann-Whitney test). FOXP3+CD4+ T cells were more frequent than CD25hiCD4+ T cells, in both young and elderly subjects (Fig. 1A and B; both p < 0.001). It has been recently described that Tregs expressed low levels of the IL-7 receptor, CD127, and that this expression pattern better defines Tregs in humans (50, 51). We therefore analyzed the percentage of CD25+CD127loCD4+ T cells and found an increased percentage of those cells in the elderly (6.5 ± 0.8% versus 3.9 ± 0.4% in elderly and young subjects, respectively; p = 0.01, t-test). Thus, whether assessed by FOXP3 or CD25, CD127 expression, the frequency of Tregs is significantly increased in elderly humans.
FIGURE 1. Treg frequency is increased in the blood of elderly individuals.
Treg frequency was analyzed in PBMCs from 16 young (≤ 30-year old) and 16 elderly (≥ 70- year old) subjects. For FOXP3 staining, CD4−CD3+ cells were used as negative control to determine the positivity threshold in CD4+CD3+ T cells. High expression of CD25 in CD4+ T cells was determined based on the absence of CD25hi cells within the CD3- cells. (A) Representative expression of FOXP3 and CD25 in young and elderly subjects. The percentages of FOXP3+CD4+ and CD25hiCD4+ cells in gated CD3+ T cells in a representative young and an elderly subject are shown in the upper and middle plot respectively. Expression of FOXP3 and CD25 in gated CD4+CD3+ T cells in a representative young and an elderly subject is shown in the lower plot. (B) Percentages of FOXP3+ and CD25hiCD4+CD3+ T cells in young and elderly subjects. Horizontal lines represent the mean values for each group. (C) Representative FOXP3 expression in gated CD4+CD3+ T cells from a 23-year old (filled line) and a 72-year old (unfilled line) donor.
Tregs from elderly and young subjects express the same functional markers
We then assessed the expression of markers on FOXP3+CD4+ T cells that have been previously associated with Treg phenotype and function (52), such as CD25, CTLA- 4 and GITR. Expression of CD25, CTLA-4 and GITR by FOXP3+CD4+ Tregs was similar in young and elderly subjects (Table I; all p > 0.05). In both groups of subjects, FOXP3+CD4+ Tregs expressed also higher levels of CTLA-4 than FOXP3-CD4+ T cells (~ 40% versus 4%; p < 0.0001) (Table I). Similar data were obtained when CD25hi expression was analyzed (Table I). GITR expression was very low in all groups (< 7%), but FOXP3+CD4+ Tregs expressed significantly higher levels than FOXP3−CD4+ T cells, in both groups of subjects (Table I). We also analyzed the proportion of naïve Tregs based on their expression of CD45RA. The proportion of naïve Tregs was lower in young individuals than the proportion of naïve Teffs, as previously reported (53). This proportion was further decreased in elderly individuals, although the difference did not reach statistical significance (p = 0.09; Table I). We also analyzed the expression of several molecules which have been reported to correlate with regulatory activity in human or mice Tregs, such as CD27 (54-56), PD-1 (57, 58), TGFβRII (59), Granzymes A and B (60-62). CD27 expression by FOXP3+CD4+ Tregs was similar in young and elderly subjects (Table I), in agreement with a recent publication (41). PD-1, TGFβRII and Granzyme A/B expression by Tregs was also similar between young and old Tregs (Table I).
Table 1.
Phenotypic characterization of Tregs and Teffs in young and elderly subjectsa
| Young | Elderly | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nb | Treg | Teff | Pc | ratiod | Nb | Treg | Teff | Pc | ratiod | pTrege | pTefff | |
| CD25hi | 16 | 42.5 ± 2.4 | 0.6 ± 0.1 | ** | 16 | 49.1 ± 2.9 | 1.0 ± 0.3 | ** | 0.10 | 0.32 | ||
| CD25+ | 16 | 75.4 ± 1.8 | 8.5 ± 0.8 | ** | 16 | 78.8 ± 1.9 | 11.8 ± 1.8 | ** | 0.20 | 0.45 | ||
|
CD25+ CD127lo |
9 | 58.8 ± 4.3 | 2.2 ± 0.2 | ** | 11 | 69.0 ± 3.1 | 3.4 ± 0.6 | ** | 0.07 | 0.21 | ||
| CTLA-4+ | 16 | 44.6 ± 3.9 | 3.9 ± 0.6 | ** | 13 | 39.8 ± 3.9 | 3.7 ± 0.4 | ** | 0.41 | 0.96 | ||
| GITR+ | 16 | 5.2 ± 0.4 | 1.3 ± 0.1 | ** | 16 | 6.4 ± 0.7 | 1.7 ± 0.3 | ** | 0.16 | 0.24 | ||
| CD45RA+ | 10 | 14.7 ± 2.5 | 39.0 ± 4.3 | ** | 2.65 | 10 | 9.5 ± 1.4 | 21.5 ± 3.0 | * | 2.26 | 0.09 | < 0.01 |
| CD27+ | 16 | 92.1 ± 0.9 | 88.5 ± 1.5 | * | 13 | 93.3 ± 0.9 | 81.2 ± 4.6 | * | 0.38 | 0.17 | ||
| PD-1+ | 9 | 22.9 ± 7.9 | 21.9 ± 9.2 | ns | 11 | 22.8 ± 6.8 | 25.1 ± 7.6 | ns | 0.82 | 0.59 | ||
| TGFβRII+ | 8 | 6.8 ± 2.6 | 2.2 ± 0.6 | ns | 10 | 4.9 ± 1.1 | 2.0 ± 0.4 | * | 1.00 | 0.82 | ||
| GranzA+ | 9 | 3.7 ± 1.1 | 4.1 ± 1.0 | ns | 9 | 4.6 ± 2.0 | 7.2 ± 3.0 | ns | 1.00 | 1.00 | ||
| GranzB+ | 9 | 4.0 ± 1.5 | 4.1 ± 1.3 | ns | 9 | 4.2 ± 1.6 | 12.6 ± 4.5 | * | 0.55 | 0.05 | ||
| CD69+ | 15 | 4.3 ± 0.9 | 2.7 ± 0.4 | ns | 15 | 4.4 ± 0.9 | 2.4 ± 0.3 | ns | 0.98 | 0.80 | ||
| CCR5+ | 9 | 27.5 ± 4.3 | 9.4 ± 1.2 | ** | 0.3 | 11 | 28.6 ± 3.2 | 15.1 ± 1.9 | * | 0.5 | 0.85 | 0.02 |
| CCR7+ | 18 | 29.4 ± 3.6 | 41.0 ± 5.2 | ns | 1.3 | 19 | 38.4 ± 3.9 | 49.8 ± 5.2 | ns | 1.3 | 0.10 | 0.23 |
| CXCR4+ | 9 | 28.2 ± 3.7 | 37.4 ± 3.6 | ns | 1.3 | 11 | 15.8 ± 2.0 | 33.2 ± 2.1 | ** | 2.1 | < 0.01 | 0.31 |
| α4+β1+ | 18 | 44.2 ± 2.0 | 65.2 ± 2.6 | ** | 1.4 | 19 | 38.5 ± 2.1 | 63.1 ± 2.2 | ** | 1.6 | 0.06 | 0.54 |
| α4+β7+ | 18 | 12.0 ± 1.2 | 41.5 ± 2.4 | ** | 3.5 | 19 | 7.6 ± 0.6 | 29.0 ± 2.5 | ** | 3.8 | 0.02 | 0.001 |
PBMCs were obtained from healthy elderly individuals (≥ 70-year old) and young donors (≤ 30-year old). Tregs were defined as FOXP3+CD4+, Teffs were defined as FOXP3- CD4+ T cells. Values represent the mean (± SEM) percentage of positive cells for each marker.
N is the number of tested samples.
p values compare the percentage of Treg and Teff expressing each marker. ** p<0.0001; * p<0.05; ns: not significant (p>0.05).
ratio was calculated as the proportion of Teffs expressing each marker divided by the proportion of Tregs expressing it.
pTreg values compare the percentage of Tregs expressing each marker in young versus elderly subjects.
pTeff values compare the percentage of Teffs expressing each marker in young versus elderly subjects.
FOXP3, CD25, GITR and CTLA-4 are not only markers for Tregs, but also are transiently upregulated on human non-Tregs upon activation (22, 63, 64). Therefore, we investigated whether the increased percentage of FOXP3+CD4+ T cells in elderly subjects reflects increased numbers of activated T cells. To do that, we looked at the expression of CD69 by FOXP3+ cells (65). Low CD69 expression (< 5%) was observed in FOXP3+CD4+ Tregs from both young and elderly, and was similar between the 2 groups (Table I). Together, these data indicate that the phenotype of circulating FOXP3+CD4+ Tregs is similar between young and elderly subjects, as assessed by multiple markers. Moreover, it appears unlikely that the increased proportion of FOXP3+CD4+ T cells in elderly individuals reflect recent activation, because it was not associated with the expression of a classical activation marker.
The ratio of expression (Teffs versus Tregs) of homing markers is similar between old and young subjects
The increased proportion of Tregs in the blood from elderly subjects could be the consequence of their altered homing to tissues. To address this issue, we analyzed the expression by Tregs and Teffs of several T cell homing markers, namely CCR7, which mediates T cell entry into secondary lymphoid organs (66), α4β1 integrin, CXCR4 and CCR5, which allows T cell migration to inflamed tissues, and the gut-associated α4β7 integrin (67-72).
FOXP3+CD4+ Tregs expressed similar levels of CCR5, CCR7 and α4β1 in young and old subjects (Table I), as recently reported for CCR7 (41). In contrast, FOXP3+CD4+ Tregs expressed lower levels of α4β7 in aged subjects compared to those in young subjects (Table I). However, the same change in expression pattern was observed in Teffs from old subjects compared with young Teffs (Table I). Indeed, when the ratio of expression (Teffs versus Tregs) was calculated for homing markers, no difference was found between old and young subjects (Table I). Only CXCR4 expression was different, with a specific decrease on old Tregs. Interestingly, CXCR4 has been associated with Treg migration and maintenance in the bone marrow (71). All together, these data suggest that the increased proportion of circulating Tregs in the elderly is not likely due to a selective dysregulation of Treg homing to tissues, although we cannot rule out a role for decreased CXCR4 in the retention of Tregs in the blood.
The proportion of Tregs is increased in aged mice
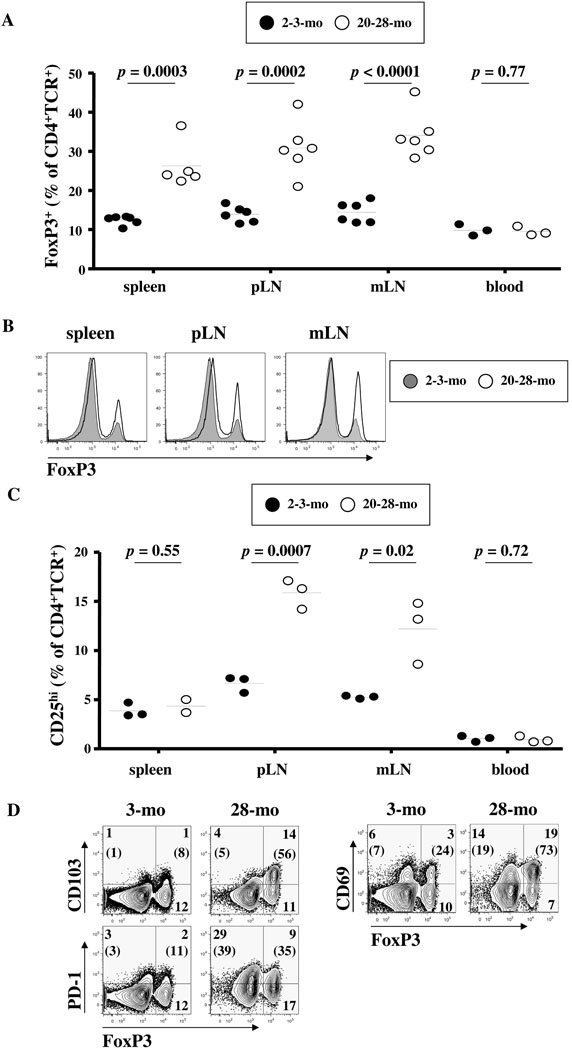
Further determination of Treg dynamics in humans being difficult due to the obvious restricted access to tissues, we pursued our analysis through the characterization of Treg markers in multiple lymphoid organs from aged (≥ 20-month old) and young adult C57BL/6 mice (≤ 3-month old). In all tissues, the proportion of FoxP3+CD4+TCR+ cells was significantly higher in aged mice compared to young mice (Fig. 2A). FoxP3 level per cell was identical in cells from aged and young mice (Fig. 2B). In contrast, the proportion of circulating FoxP3+CD4+TCR+ cell was the same in the blood of aged and young mice (Fig. 2A). When the proportion of CD25hiCD4+TCR+ cells was analyzed, we found a significant accumulation of these cells in peripheral (pLNs) and mesenteric lymph nodes (mLNs), but not in the spleen or the blood, of aged mice compared to young mice (Fig. 2C). The proportion of CD25hiCD4+TCR+ cells was always lower than the proportion of FoxP3+CD4+TCR+ cells in all tissues, in young and in aged mice (all p < 0.05). Furthermore, the proportion of Tregs expressing CD103, a specific marker for natural Tregs (73, 74), was increased in aged mice (Fig. 2D).
FIGURE 2. Increased proportion of Tregs in aged mice.
Single cell suspensions from spleens, peripheral (pLNs), mesenteric (mLNs) lymph nodes and blood were first stained for the surface markers CD4, TCR, CD25, CD69, CD103 and PD-1, followed by staining for the intracellular markers FoxP3. Flow cytometry analysis on gated CD4+TCR+ cells is shown. (A) Percentages of FoxP3+ cells in the CD4+TCR+ cell populations from 2-3- month old (closed circles) or 20-28-month old (open circles) mice. Horizontal lines represent the mean values for each group. (B) Representative overlay of Foxp3 expression in spleen, pLNs and mLNs cells from a 3-month (filled line) and a 28-month (unfilled line) old mouse. (C) Percentages of CD25hi cells in the CD4+TCR+ cell populations from 2-3-month old (closed circles) or 20-28-month old (open circles) mice. Horizontal lines represent the mean values for each group. (D) Expression of FoxP3 with CD69, CD103 and PD-1 is shown for splenic cells of a 3-month (left panel) or 28-month (right panel) old mouse, representative of 6 mice each. Values represent the percentages of each population in the indicated quadrant. Values in parenthesis are the percentages of gated FoxP3- (left) or FoxP3+ (right) cells that are positive for the Y axis marker.
We also analyzed the expression by FoxP3+CD4+ T cells of markers associated with Treg function. FoxP3+CD4+TCR+ Tregs express high levels of GITR, CTLA-4 and PD-1 in both groups of animals, with a trend towards higher expression in aged mice, depending on the tissue analyzed (Table II and Fig. 2D). The activation marker CD69 was more expressed by old FoxP3+ cells than young FoxP3+CD4+TCR+ cells, and that in all analyzed tissues (Table II and Fig. 2D). However, there was a trend towards decreased CD69 expression on circulating Tregs from aged mice (p = 0.07, Table II). The trend towards decreased proportion of CD27 and CCR7 expression in old Tregs (Table II) also suggests increased Treg differentiation in aged mice.
Table 2.
Expression of markers in FoxP3+CD4+TCR+ cells from young and aged micea
| CD25+ | GITR+ | CTLA-4+ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tissue | Young | Aged | pd | Young | Aged | pd | Young | Aged | pd |
| Spleenb | 62.6 ± 1.8 | 35.6 ± 4.4 | < 0.01 | 93 ± 1 | 97.5 ± 0.7 | < 0.01 | 51.5 ± 5.1 | 65.6 ± 6.3 | 0.11 |
| pLNb | 72.6 ± 1.1 | 65.3 ± 5.2 | 0.19 | ND | ND | ND | 55.0 ± 5.2 | 71.8 ± 5.7 | 0.05 |
| mLNb | 72.0 ± 1.1 | 58.6 ± 4.5 | 0.02 | 93.1 ± 1.5 | 95.5 ± 1.3 | 0.24 | 55.6 ± 5.7 | 76.6 ± 6.2 | 0.03 |
| Bloodc | 37.2 ± 2.7 | 33.2 ± 2.3 | 0.33 | 83.9 ± 5.2 | 94.0 ± 1.2 | 0.13 | 26.7 ± 5.0 | 26.1 ± 1.2 | 0.92 |
| CD27+ | CCR7+ | CD69+ | |||||||
| Tissue | Young | Aged | pd | Young | Aged | pd | Young | Aged | pd |
| Spleenc | 87.0 ± 1.9 | 70.1 ± 3.7 | 0.02 | 5.2 ± 0.6 | 1.5 ± 0.2 | 0.02 | 14.8 ± 0.5 | 50.2 ± 1.6 | < 0.01 |
| pLNc | 85.4 ± 1.6 | 75.5 ± 3.5 | 0.06 | 3.7 ± 0.8 | 1.5 ± 0.5 | 0.08 | 20.1 ± 0.9 | 38.4 ± 1.7 | < 0.01 |
| mLNc | 86.3 ± 0.8 | 73.0 ± 1.3 | < 0.01 | 4.4 ± 0.6 | 2.0 ± 0.5 | 0.03 | 25.8 ± 0.0 | 59.2 ± 5.4 | < 0.01 |
| Bloodc | 27.0 ± 6.6 | 11.2 ± 1.8 | 0.08 | 15.4 ± 7.1 | 3.5 ± 1.2 | 0.18 | 19.2 ± 5.7 | 5.2 ± 1.0 | 0.07 |
Single cell suspensions from spleens, pLNs, mLNs and blood were stained for surface expression of CD4, TCR, CD25, CD27, CD69, CCR7 and GITR, followed by intracellular staining for FoxP3 and CTLA-4. Results are expressed as mean (± SEM) percentages of FoxP3+CD4+TCR+ cells expressing each marker. Young mice were 2-3 month old and aged mice were 20-28 month old.
6 mice/group were analyzed. ND: not determined.
3 mice/group were analyzed.
p values (t-test) compare proportions in young and old mice.
Treg depletion increases in vitro Teff function in the elderly
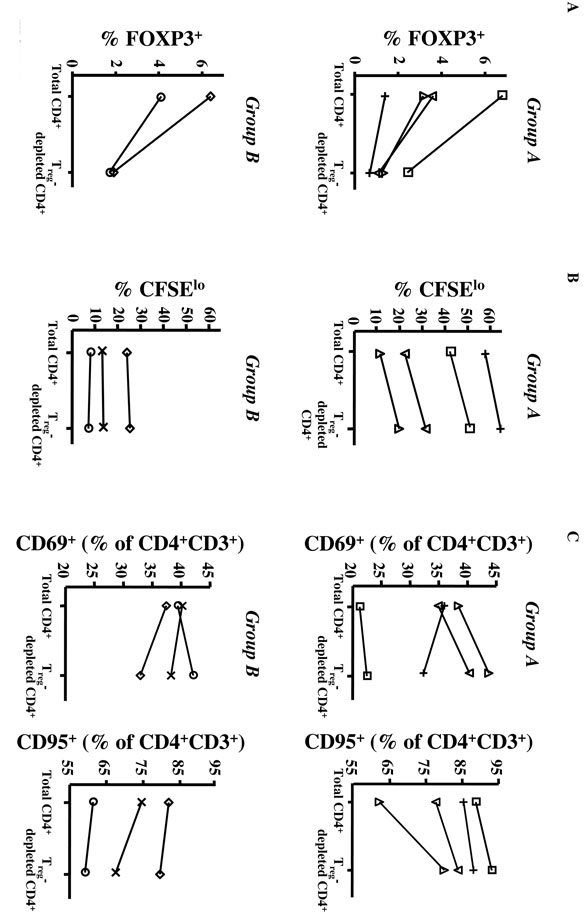
Our phenotypic data show increased proportion of cells with Treg characteristics in aged humans and mice. However, because FOXP3+ or CD25hi cells are not always functionally suppressive, we further characterized Treg function in elderly humans by analyzing the effect of Treg depletion on CD4 function. Because of its intracellular localization, FOXP3 expression cannot be used to deplete cells. However, the number of FOXP3+ cells was significantly reduced following the depletion of CD25+ cells from total CD4+ T cells and no significant difference was observed between individuals (Fig. 3A). In absence of stimulation, CD4+ T cells, with or without Tregs, did not proliferate or express activation markers (data not shown). After 3 days of PHA stimulation, Treg- depleted cells proliferated better than total CD4+ T cells (not depleted of Tregs) in 4 of the 7 tested individuals (group A: subjects ; Fig. 3B). In contrast, in 3 individuals, Treg depletion did not result in increased proliferation after PHA stimulation (group B: subjects ◊, ○, ×; Fig. 3B).
FIGURE 3. Depletion of CD25hiCD4+ T cells from the blood of elderly individuals increased CD4 T cell function.
5 × 105 CFSE-labeled total CD4+ or Treg-depleted CD4+ (CD25-CD4+) T cells from 7 elderly individuals (≥ 70-year old) were cultured with 2 × 105 autologous CD14+ monocytes and 2 μg/ml PHA for 3 days. (A) Percentage of FOXP3+ cells before CD25hi depletion (Total CD4+, left) and after CD25hi depletion (Treg-depleted CD4+, right). The values from the same subject are linked by a line. Group A is composed of the 4 individuals in whom Treg depletion led to increased proliferation, whereas group B comprised the 3 individuals who exhibited no increase in proliferation after PHA stimulation. The percentage of FoxP3+ cells following CD25 depletion was determined in all subjects, except subject X. (B) CFSE dilution and (C) the expression of CD69 and CD95 markers were analyzed by flow cytometry on gated CD4+CD3+ T cells. Percentages of dividing cells (CFSElow, corresponding to cells that have divided at least once), in total or Treg-depleted CD4+ T cells, are shown in (B). Percentages of CD69+ and CD95+ cells, in total or Treg-depleted CD4+ T cells, are shown in (C). Each individual is represented by the same symbol in panels (A), (B) and (C).
Because proliferation does not recapitulate overall CD4 T cell function, particularly for memory cells, we also analyzed other read-outs of T cell activation, such as expression of the activation markers CD69 and CD95 (Fig. 3C). PHA stimulation induced CD69 and CD95 up-regulation and cytokine production in all PHA-stimulated cultures, compared with unstimulated cultures (data not shown). In 3/4 samples of the group A, CD69 and CD95 expression both increased following Treg depletion (Fig. 3C). In the 4th subject from that group (subject +), Treg depletion led to decreased CD69 expression, but stable and high CD95 expression (Fig. 3C). Of note, this individual exhibited the highest level of proliferation, before and after Treg depletion. In group B individuals, depletion did not change CD95 expression and increased CD69 expression in only one individual (subject ○) (Fig. 3C). Depletion of Tregs therefore increased activation and/or proliferation of CD4+ Teffs in 5/7 elderly after 3 days of culture with PHA. Of note, this percentage of responders is similar to that reported in young individuals, in whom Treg depletion led to increased proliferation in response to PHA in 4/6 individuals (75). These data suggest that Tregs in elderly humans are functional in most individuals and may be able to inhibit Teff responses in vitro.
Tregs from old mice suppress anti-CD3-induced proliferation of Teffs
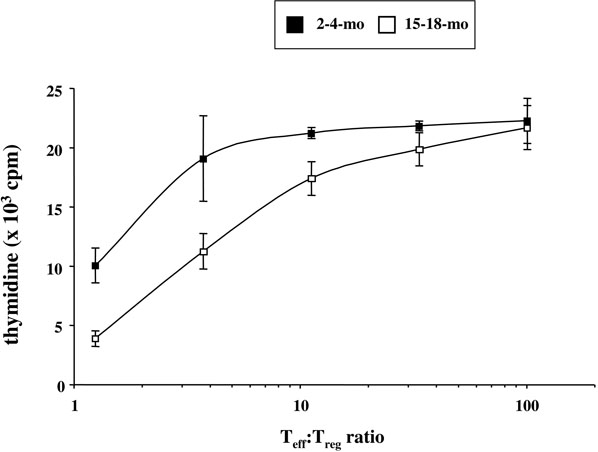
To more clearly delineate the functional activity of FoxP3+ cells during aging, we sorted FoxP3+ T cells from pLNs of aged (15- to 18-month old) or young (2- to 4-month old) FoxP3-GFP knock-in C57BL/6 mice. Teffs (CD4+GFP− T cells) were sorted from pLNs of 2-month old mice, to eliminate the confounding effect of decreased responsiveness of aged Teffs (reviewed by (76, 77)). Tregs from pLNs of aged mice were more suppressive on a per cell basis than those from young mice, suppressing 80% of anti-CD3-stimulated Teff proliferation at a Teff:Treg ratio of 1:1 in comparison to the 54% suppression achieved by young Tregs (Fig. 4). At a Teff:Treg ratio of 10:1, 20% suppression was observed with old Tregs, compared to the 3% induced by young Tregs (Fig. 4). Interestingly, Teffs from aged mice could be inhibited equally by Tregs from old and young mice (data not shown).
FIGURE 4. FoxP3+CD4+ T cell suppressive function is intact in aged mice.
5 × 104 GFP−CD4+ T cells (Teffs) were sorted from LNs of 2-4-month old FoxP3-GFP knock-in C57BL/6 mice, and stimulated in triplicate with 0.5 μg/ml anti-CD3 and 1 × 105 irradiated T cell-depleted spleen cells from the same mice. GFP+CD4+ T cells (Tregs) were sorted from LNs of 2-4-month (closed squares) or 15-18-month (open squares) old mice and co-cultured with Teffs at different Teff:Treg ratios, ranging from 1.3:1 to 101:1. Proliferation was measured by thymidine incorporation in the last 22 hours of a 3-day culture. In the absence of Tregs, 21,800 ± 2,075 cpm were counted.
L. major infection spontaneously reactivates in aged mice
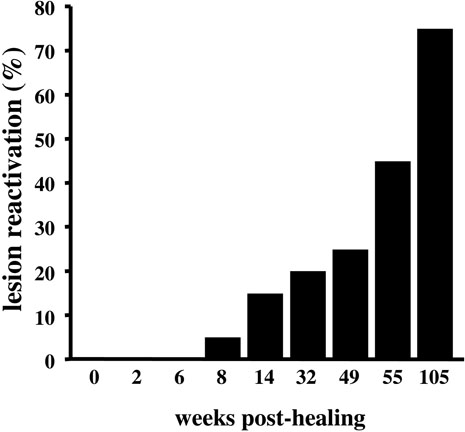
We next reasoned that, if Treg function and proportion were increased with age, it would interfere with the ability of the aged hosts to control chronic infections. To test this hypothesis, we used the L. major model, in which Tregs have been shown to play a major role in lesion reactivation (44, 45). After inoculation of 103 metacyclic promastigotes of L. major into the ear dermis, young C57BL/6 mice develop a small lesion that resolves spontaneously within 12 weeks post-inoculation, although a few viable parasites persist in the site of the former lesion and in the draining LNs (44). 8 weeks post-healing, 5% of the infected mice exhibited clinical signs of lesion reactivation (Fig. 5). Importantly, spontaneous reactivation increased with aging, until 75% of the mice had reactivated at 24 months post-healing (Fig. 5), suggesting increased Treg activity in aged L. major-infected mice.
FIGURE 5. L. major spontaneously reactivates in aged mice.
8-week old C57BL/6 mice (n = 20) were inoculated in the ear dermis with 103 L. major metacyclic promastigotes. After the lesions were resolved 12 weeks later, mice were monitored for clinical signs of ear swelling and inflammation indicating a reactivation of the lesions.
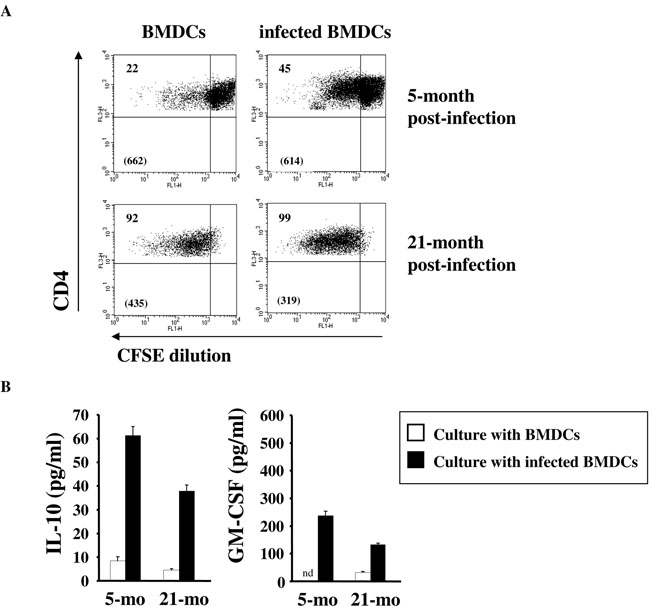
We previously reported that Tregs from L. major-infected young mice are able to respond specifically to L. major (78). Therefore, we analyzed whether L. major specific Treg activity was detectable at the time when spontaneous disease reactivation occurs. CD25+CD4+ Tregs were purified from draining LNs of aged mice or young mice, which had all been infected when they were 2-month old, and were restimulated in vitro with bone marrow-derived dendritic cells (BMDCs), infected or not with L. major metacyclic promastigotes. In aged mice, extensive proliferation of Tregs was detected in response to both uninfected and infected BMDCs (Fig. 6A). The fact that old Tregs from infected mice proliferate in presence of uninfected BMDCs may reflect their higher state of in vivo activation, a finding in agreement with their higher expression of several activation markers on Tregs from non-infected mice (Table II and Fig. 2). However, the lower MFI observed in the culture with infected BMDCs suggests a more sustained proliferation of Tregs in this condition. Tregs from aged mice also produced cytokines in response to infected BMDCs, although at a lower level than young Tregs (Fig. 6B). Taken together, these results suggest that the Treg activation level is higher in aged mice, potentially through antigen-independent pathways, but the proportion of L. major specific Tregs and/or their per cell basis capacity to respond is lower. There are several potential explanations for these results. First, as antigen load increases, more Tregs are stimulated by L. major antigen in vivo and continue to proliferate when they are removed from the animal, seemingly non-specifically. Second, Tregs that are not specific for L. major are expanded in vivo, recognize and respond to endogenous antigens displayed on BM-DCs. Third, the proliferation of aged Tregs in response to uninfected BM-DCs has nothing to do with antigen recognition by Tregs but could be due to soluble factors released from BMDCs that cause Tregs to proliferate. Without L. major specific MHC class II tetramer, it is difficult to address the frequency of L. major specific Tregs, how this frequency changes with age, and whether exogenous/endogenous antigens versus soluble factors drive Treg proliferation.
FIGURE 6. Tregs from aged mice respond to L. major.
8- to 10-week old C57BL/6 mice were inoculated in the ear dermis with L. major. 5 or 21 months later, CD25+CD4+ Tregs were purified by FACS from the draining LNs. 5 × 104 CFSE-labeled T cells were restimulated with 1.4 × 105 uninfected or L. major-infected BMDCs for 4 days. (A) CFSE dilution was analyzed by flow cytometry on gated CD4+TCR+ cells. Values in top left quadrants are the percentages of CFSElow cells. Values in parenthesis are the CFSE mean fluorescence intensities (MFI) within the CFSElow cells. (B) Cytokines were quantified in Treg cultures with uninfected BMDCs (open bars) or L. major-infected BMDCs (black bars). Results are representative of 6 independent experiments. nd, not detected.
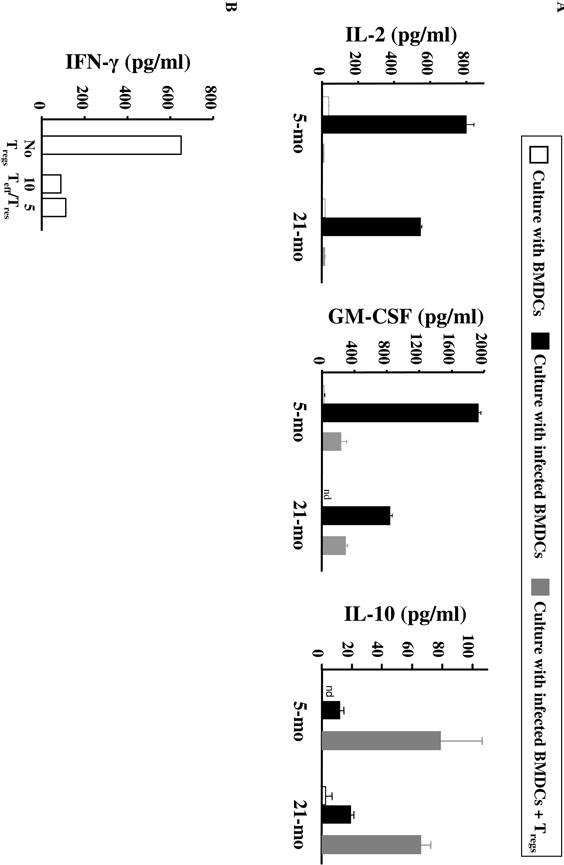
CD25−CD4+ Teffs from aged mice produced IL-2 and GM-CSF after stimulation with infected BMDCs, albeit at a lower level than those from young mice, and a similar amount of IL-10 (Fig. 7A). Importantly, the co-culture of Teffs and Tregs clearly decreased the production of both IL-2 and GM-CSF in aged mice, as well as in young mice (Fig. 7A), confirming the maintained suppressive capacity of Tregs in aged mice and their potential to suppress protective immune responses in aged L. major-infected mice. Increased IL-10 production was observed in the Treg:Teff co-cultures, likely due to the IL- 10 production by Tregs, but levels were similar between aged and young T cells (Fig. 7A). Because Treg purification on the basis of CD25 expression may lead to their contamination by Teffs, we confirmed those data by purifying Tregs from old FoxP3-GFP knock-in mice that had been infected by L. major when they were young. As shown in Fig. 7B, significant suppression of IFN-γ production by Teffs was achieved when increasing numbers of Tregs were added to Teff culture stimulated with L. major-infected BMDCs. No major effect was observed for IL-10 production (data not shown).
FIGURE 7. Cytokine production by Teffs from L. major-infected mice in response to L. major antigens is blocked by Tregs.
(A) 8- to 10-week old C57BL/6 mice were inoculated in the ear dermis with L. major. 5 or 21 months later, CD25+CD4+ (Tregs) and CD25-CD4+ (Teffs) were purified from the draining LNs (see Fig. 6). Teffs were cultured with uninfected BMDCs (open bars), L. major-infected BMDCs (black bars), or cocultured with Tregs (Teff:Treg ratio of 5:4) and L. major-infected BMDCs (grey bars). Cytokines were measured by ELISA. Results are representative of 6 independent experiments. nd, not detected. (B) FoxP3+ CD4+ T cells were sorted from FoxP3-GFP knock-in animals of 57-week old L. major-infected mice that were infected when young (9-10-week old). FoxP3+ cells were then mixed at different Teff:Treg ratios with Teffs (CD4+CD25-CD62L-) purified from a 22-week old infected mice in presence of L. major-infected BMDCs. IFN-_ was measured by ELISA in 4 day-supernatant.
Tregs play a critical role in L. major reactivation in aged mice
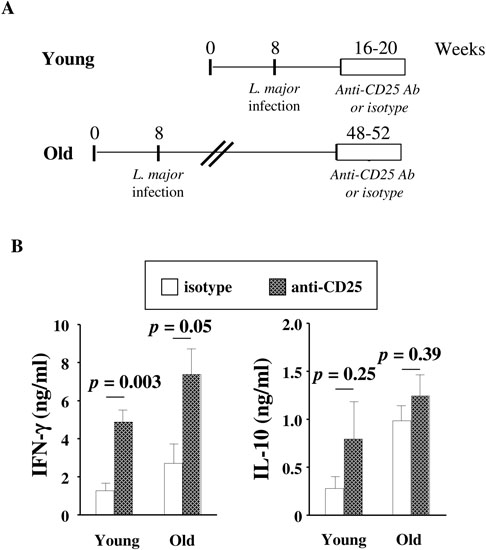
To directly address the role played by Tregs in L. major reactivation in old mice, we treated old L. major-infected mice (> 48-week old, which had been infected when they were young) with anti-CD25 Ab or isotype control, following the experimental protocol that we had previously used (45). As shown in Fig. 8, in vivo depletion of Tregs in the anti-CD25-treated group significantly increased IFN-γ production by the T cells purified from the infection site and draining LNs. As expected, anti-CD25 treatment significantly increased IFN-γ production in young infected mice (Fig. 8B). However, IL-10 production did not change.
FIGURE 8. In vivo depletion of Tregs in old L. major-infected mice increases the production of IFN-γ by Teffs at the infection site.
(A) Old L. major-infected mice (> 48 week old, which had been infected when they were 8-week old) or young L. major-infected mice (16-week old, which had been infected when they were 8-week old) were treated with anti-CD25 Ab or isotype control (N = 4/group) (1 mg for 3 weeks, twice a week). After Ab treatment, mice were sacrificed. T cells were purified from the infection site and draining LNs and restimulated in vitro with L. major-infected BMDCs. (B) IFN-γ and IL-10 were measured by ELISA in 4-day supernatants. P values correspond to the comparisons between mice treated with isotype control (open symbols) or anti-CD25 Ab (hatched symbols) using t-tests.
Tregs play a critical role in the exacerbation of L. major infection in aged mice
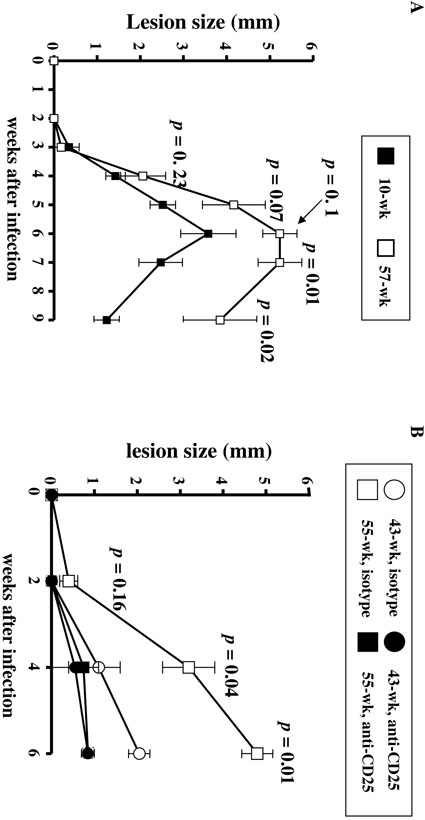
Another important and related question is whether the increased proportion of functionally suppressive Tregs in aged hosts plays a role in the increased disease severity observed for multiple infections in such aged hosts. We have previously shown that lesion size is a direct indication of parasite growth. As shown in Fig. 9A, primary infection with L. major induces a more severe course of disease in old mice, compared with young mice. Indeed, lesions appeared 3 weeks post-infection in both groups, but tended to be larger in old animals already at 4 weeks post-infection. Disease severity was clearly apparent at all the following time points. Moreover, 1 out of the 3 old infected animals lost the infected ear at 7 weeks post-infection, through an acute necrotic process, at a time when lesions were starting to heal in young animals. To determine whether the age-related increased proportion of Tregs had played a major role in such an exacerbated pathological process, we treated old mice (> 43-week old) with anti-CD25 Ab or isotypematched Ab control at the time they were infected with L. major parasites, and then twice a week. Infection outcome was then monitored by measuring the lesion size. As shown in Fig. 9B, treatment with anti-CD25 Ab significantly reduced the lesion size at 4 and 6 weeks, suggesting the importance of Tregs in the increased severity of L. major infection in aged animals.
FIGURE 9. L. major causes exacerbated disease in old mice, and Tregs contribute to such increased disease severity.
(A) Primary infection with L. major parasites: 4 young mice (10-week old) and 3 old C57BL/6 mice (57-week old) were inoculated in the ear dermis with 103 L. major metacyclic promastigotes. Lesion size was measured weekly in all animals. Mean (and SD) lesion sizes (mm) are shown for young (black symbols) and old (white symbols) mice. P values indicate differences at each time point (t-test). At 7 weeks post-infection, one of the old mice lost an ear through an acute necrotic process. (B) Effect of anti-CD25 Ab on primary L. major infection in old mice. Two 43-week old mice (black circles) and two 55-week old mice (black squares) received anti-CD25 treatment (1 mg for 3 weeks, twice a week) at the time of L. major infection. Three 43- week old mice (white circles) and three 55-week old mice (white squares) received the isotype control Ab following the same regimen. Lesion size (mm) was measured weekly in all animals. P values correspond to the differences in lesion size between anti-CD25- treated and isotype-treated mice, at the indicated time points.
Discussion
In this study, we investigated the proportion, phenotype and suppressive function of Tregs in aged mice and humans. Our study shows increased proportion of FoxP3+CD4+ T cells in tissues of aged C57BL/6 mice as well as in the blood of healthy elderly humans. Importantly, our in vitro and in vivo data strongly support our hypothesis that Tregs inhibit immune responses in aged hosts, contributing to reactivation of chronic infectious diseases.
Our data show a significantly increased proportion of FoxP3+CD4+ T cells in multiple lymphoid tissues from aged C57BL/6 mice, compared to young mice, as already reported in aged Balb/c mice (34). Increased proportion of CD25hiCD4+ T cells in the lymphoid tissues of aged animals was also found in these animals, in agreement with other studies (35, 36). Interestingly, the proportion of CD25hiCD4+ T cells is always lower than that of FoxP3+CD4+ T cells in both aged and young hosts, supporting the notion that CD25 expression defines only a portion of Tregs (79). FoxP3 expression clearly plays a crucial role in the maintenance of Treg activity, its ablation in adult mice leading to catastrophic autoimmune diseases (80). In contrast, CD25−/− mice exhibit reduced numbers of FoxP3+ cells but those cells are fully able to suppress in vitro (81, 82).
Increased proportion of FOXP3+CD4+ T cells was also found in the blood of healthy elderly humans. There was a trend towards increased proportion of CD25hiCD4+ T cells in the blood of elderly donors, although the difference was not significant. It has recently been shown that low level of the IL-7 receptor, CD127, increases the specificity of Treg characterization for human cells (50, 51). Significantly increased proportion of CD25+CD127loCD4+ T cells was also found in elderly humans. Previous studies using CD25hi expression to characterize Tregs have reported similar (53, 83) or increased Treg proportions (37-40) in elderly individuals. Discordances between studies may arise from differences in the phenotyping techniques and/or the characteristics of the studied populations (e.g. mean age, health status, or criteria of exclusion). In mice, the proportion of Tregs in the blood was not increased; in contrast, we observed an increased proportion of circulating FOXP3+CD4+ Tregs in elderly humans compared to young subjects. Differences between mice and humans could reflect the fact that FoxP3 is less specific of the Treg lineage in humans than in mice, because its expression is transitorily induced in Teffs following TCR activation (64). However, the low level of CD69 expression on FoxP3+ cells argues against that hypothesis. Alternatively, increased triggering of the Treg compartment in humans may come from the higher level of stimulation of the immune system exerted by constant exposure to pathogens. Of note, we have found that, in young adults, the proportion of FoxP3+CD4+ T cells was higher in tonsils than in blood (approximately 10% versus 4%, unpublished data), similar to the murine data. Study of tissue Tregs in elderly humans has not yet been undertaken and will be essential to clarify this issue.
An important question raised by our data is how Tregs accumulate with age. Circulating Tregs in elderly humans do not express specifically altered patterns of homing receptors, suggesting that defective tissue homing is not likely to explain their increased proportion, which is confirmed by the fact that increased Treg proportion was found in all lymphoid tissues in aged mice. Peripheral Tregs could derive either from Tregs that have developed in the thymus or converted non-Tregs. In aged mice, the total number of CD25+CD4+ single positive (SP) thymocytes decreased following the reduction in thymocyte numbers, although the percentage of CD25+ cells increased in CD4+ SP thymocytes (35), suggesting a reduced thymic Treg input in those mice. Tregs in aged hosts are mostly memory cells with a highly differentiated phenotype as shown in this study and by others (35, 41, 79, 84, 85). These data suggest that Tregs in elderly might come from thymic-derived Tregs that have proliferated in the periphery. Few data are available on Treg in vivo turnover, particularly in aging. In young mice, the CD25+CD4+ Treg population is composed of two subsets with distinct homeostasis (86), one subset exhibiting a rapid proliferation rate, whereas the other subset did not divide, but was long-lived. These findings suggest that, although Tregs can proliferate in vivo, resting thymic-derived Tregs may also persist for long time in vivo and this population may participate in the maintenance of peripheral Treg numbers. In healthy humans, young and elderly alike, CD45RO+ Tregs had a rapid doubling time compared with those of memory or naïve CD4+ T cells (40). However, human CD45RO+ Tregs exhibited short telomere length -in both young and elderly individuals-, and did not upregulate telomerase after activation (40, 87), raising the question of whether peripheral proliferation of thymic-derived Tregs is sufficient to maintain the increased proportion of Tregs seen in aged hosts.
Tregs might also be generated from CD25-CD4+ T cells in the periphery. In both mice and humans, transforming growth factor-β (TGF-β) induces CD25−FOXP3−CD4+ T cells to become FOXP3+ Tregs (reviewed by (88)), although the stability of such conversion, as well as the requirement for other molecules than TGF-β, is still debated (89-93). The state of activation of DCs, as well as the proportion of different DC subsets (mDC versus pDC), also plays a role in both Treg conversion and peripheral expansion (25, 26). Interestingly, DC subsets and maturation levels are changed during aging (94, 95), and this could play a role in Treg accumulation in elderly individuals. However, the contribution of such converted cells to the pool of circulating Tregs remains an open question, since no marker(s) have been found that distinguish between natural FoxP3+ Tregs and induced FoxP3+ Tregs. Of note, our published data support the idea that no neo-generation of Tregs occur during L. major infection (78).
An extensive phenotypic characterization of Tregs (defined as FoxP3+CD4+ T cells) was performed in the old mice. Expression of CD25 by Tregs from aged animals was lower than in their young counterparts, a result in agreement with previous studies (34, 36). The underlying mechanisms have not been completely elucidated, and could include the loss of CD25 expression by FoxP3+CD4+ Tregs. In support of that argument, in vivo expanded Tregs become CD25low while maintaining similar levels of other Treg-associated molecules (96). Similarly, CD25 turnover may be increased in aging, as suggested by increased soluble CD25 levels in the serum of aged humans (97, 98). In contrast to decreased CD25 expression, expression of other markers associated with Treg function such as GITR, CTLA-4 or PD-1, was maintained or increased on FoxP3+CD4+ Tregs from aged mice, depending on the tissue analyzed. The activation marker CD69 was also more expressed by old FoxP3+CD4+ Tregs than young Tregs, and that in all analyzed tissues except in the blood in which the frequency of CD69+ Tregs tended to decrease, suggesting an accumulation of activated Tregs in tissues in aged mice. Similarly, we found a trend towards decreased proportion of CD27 and CCR7 expression in old Tregs (Table II), suggesting increased Treg differentiation. Taken together, those data suggest that Tregs are more activated in tissues from aged mice than in young mice.
We also extensively characterized circulating Tregs in healthy elderly humans, in comparison to young Tregs, and did not find major age-related differences in the expression of molecules that have previously been associated with Treg function, such as CD27, PD-1, TGFβRII or Granzymes (A and B). Similar to our data in old mice, there was a trend towards decreased expression of CD25 on FoxP3+CD4+ Tregs in elderly humans. Low and constant expression of CD69 was found on both young and old human blood Tregs, in contrast to its decreased expression in circulating Tregs of aged mice, suggesting a difference in activation patterns between species.
Because phenotype does not recapitulate functional activity, we analyzed Treg-mediated suppression during aging. Interpretation of suppression assays in aging mice can be confounded by the fact that CD25 expression is not accurate to recapitulate FoxP3 expression (and Treg activity) in aged mice (35, 36, 79). Therefore, to circumvent this caveat, we used FoxP3-GFP knock-in mice, and clearly show that FoxP3+ cells from aged mice have a greater in vitro suppressive activity on a per cell basis than their young counterparts. Old Tregs were also able to suppress Teffs from aged mice. The use of FoxP3-GFP cells in our study may explain the difference between our results and previous studies that showed similar or decreased suppressive activity of old CD25hi Tregs compared to young Tregs. As we show that many of the FoxP3+ cells in aged mice are CD25lo, the previous studies sorting old Tregs based on CD25 expression may have been by contamination of the effector population with FoxP3+CD25− Tregs. In addition, CD25+ Tregs from aged chronically L. major-infected mice also maintained their responsiveness to L. major antigens and their ability to suppress IFN-γproduction by Teffs in response to L. major-infected DCs. In elderly humans, Tregs appear functional, their in vitro depletion leading to increased CD4+ T cell function in most individuals, although the functional consequence of such depletion was modest. Several factors could have contributed to such a result, including the expected heterogeneity between human subjects, the use of old autologous Teffs and the fact that human Tregs could not be depleted on the basis of their FOXP3 expression.
Increased proportion of functional Tregs in aging may translate into dampened immune responses in aged hosts. Strongly supporting this hypothesis, spontaneous reactivation of L. major lesions, which we have previously shown to result from Treg accumulation in the chronic infectious site (44, 45), occurred in the majority of aged infected mice. We also demonstrate a direct role of Tregs in such reactivation, because in vivo depletion of Tregs in old mice, infected young, significantly increased the production of the effector cytokine IFN-γ by the Teffs purified from the infection site and draining LNs. Furthermore, our experiments show that Tregs play a critical role in the increased disease severity of L. major infection in old mice. Taken together, our data strongly suggest that the age-associated Treg accumulation is likely to play a major role in the increased severity of chronic infections, as well as the reactivation of chronic infections, in aged mice and humans. Our data in an infectious model are thus in agreement with the recent study showing that Treg accumulation in aged mice plays a crucial role in inhibiting the activation of anti-tumor responses (34).
How Tregs act is still unclear and we have not formally ruled out that the mechanisms mediating such suppression may be different in old Tregs. Expression of activation markers by old tissue Tregs was increased, whereas, on a per a cell basis, old Tregs produced less IL-10, which has been shown to play a role in Treg-mediated suppression in the L. major model (44). Tregs from old murine lymphoid tissues were clearly suppressive both in vivo and in vitro, although the mechanisms by which old Tregs suppress will need to be further investigated in the future.
An alternative explanation of increased severity and frequency of infectious diseases in aged hosts could be the decreased capacity of aged Teffs to proliferate and produce cytokines. Indeed, profound, diverse alterations in TCR-mediated activation have been described in T cells from aged mice and humans (99, 100). Accordingly, decreased cytokine production by old Teffs, including in response to L. major antigens, was observed in both our study (Fig. 6) and previous studies (36, 79). Similarly, in 3/7 old individuals, depletion of CD25 resulted in partial or absent improvement of CD4 T cell function, suggesting intrinsic decreased responsiveness of the Teff subset in these individuals. However, when old memory TCR Tg CD4+ T cells were transferred into young animals, they function as well as their young counterparts (101) suggesting that antigenic-specific protective effector mechanisms are retained in old hosts. In support of that hypothesis, purified CD4+ Teffs from old animals produced large amounts of the effector cytokine IFN-γ after restimulation with L. major-antigens, which was blocked by co-culture with Tregs. Furthermore, Treg depletion in vivo allowed for better control of L. major, suggesting that aged Teffs had retained some function in such experimental conditions. Of note, in the model of infection with high doses of L. major, which is less sensitive to Treg regulation than our model, old mice controlled infection as well as young mice (102), further suggesting that Teff function is not completely abolished in old animals.
Taken together, our findings suggest that decreased T cell responsiveness in aged hosts results from both intrinsic defects and an altered balance between stimulatory/regulatory mechanisms, the exact contribution of each mechanism likely to be variable depending on the context (infectious diseases/auto-immunity/cancer). Manipulation of Treg numbers and/or activity may therefore be critical to enhance immune responses in the aged, and may be envisioned to enhance control of chronic infectious diseases, as well as vaccine efficiency, in this fragile population.
Acknowledgements
We would like to thank all the study participants and Dr. M. Oukka, Harvard Medical School, Cambridge, MA for the kind gift of FoxP3-GFP knock-in C57BL/6 reporter mice. We also thank Dan Marmer (Cincinnati Children's Hospital Research Foundation Sorting Core), Kevin Holmes and Carol Henry (NIAID Flow Cytometry Unit) for cell sorting, as well as Dr. Keller (Cincinnati Children's Hospital Research Foundation) and Kim Beacht (NIAID) for help with mouse care, and Kris Orsborn (Cincinnati Children's Hospital Research Foundation) for help with cell stainings.
Abbreviations
- Tregs
regulatory T cells
- Teffs
effector T cells
- L. major
Leishmania major
- FoxP3
Forkhead box P3
- CTLA-4
Cytotoxic T Lymphocyte Associated antigen 4
- GITR
Glucocorticoid-Induced Tumor necrosis factor Receptor
- MFI
Mean Fluorescence Intensity
- PHA
phytohemagglutinin
- LNs
lymph nodes
- pLNs
peripheral LNs (retromaxillar and popliteal)
- mLNs
mesenteric LNs
- PBMCs
Peripheral Blood Mononuclear Cells
- FCS
Fetal Calf Serum
- DMSO
Dimethyl sulphoxide
- CFSE
Carboxyfluorescein diacetate, succinimidyl ester
- ELISA
Enzyme Linked Immunosorbent Assay
- DCs
Dendritic cells
- BMDCs
Bone Marrowderived DCs
- pDCs
plasmacytoid DCs
- mDCs
myeloid DCs
- TGF-β
Transforming Growth Factor-β
Footnotes
This study was supported by NIH AG025149 (to C.C.), the Division of Intramural Research, NIAID, National Institutes of Health (to Y.B.), and a Colciencias fellowship (to P.A.V.).
References
- 1.Effros RB. Long-term immunological memory against viruses. Mech Ageing Dev. 2000;121:161–171. doi: 10.1016/s0047-6374(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 2.Murasko DM, Bernstein ED, Gardner EM, Gross P, Munk G, Dran S, Abrutyn E. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol. 2002;37:427–439. doi: 10.1016/s0531-5565(01)00210-8. [DOI] [PubMed] [Google Scholar]
- 3.Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis. 2002;2:659–666. doi: 10.1016/s1473-3099(02)00437-1. [DOI] [PubMed] [Google Scholar]
- 4.Effros RB. Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine. 2007;25:599–604. doi: 10.1016/j.vaccine.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 5.Rytel MW. Effect of age on viral infections: possible role of interferon. J Am Geriatr Soc. 1987;35:1092–1099. doi: 10.1111/j.1532-5415.1987.tb04926.x. [DOI] [PubMed] [Google Scholar]
- 6.Arvin AM. Varicella-zoster virus: overview and clinical manifestations. Semin Dermatol. 1996;15:4–7. [PubMed] [Google Scholar]
- 7.Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J Leukoc Biol. 2004;76:291–299. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- 8.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 9.Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005;17:457–462. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner EM, Murasko DM. Age-related changes in Type 1 and Type 2 cytokine production in humans. Biogerontology. 2002;3:271–290. doi: 10.1023/a:1020151401826. [DOI] [PubMed] [Google Scholar]
- 12.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 13.Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Agerelated defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. Newly generated CD4 T cells in aged animals do not exhibit age-related defects in response to antigen. J Exp Med. 2005;201:845–851. doi: 10.1084/jem.20041933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovaiou RD, Grubeck-Loebenstein B. Age-associated changes within CD4+ T cells. Immunol Lett. 2006;107:8–14. doi: 10.1016/j.imlet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25) Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 17.Sakaguchi S. Regulatory T cells: key controllers of immunologic selftolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 18.Sakaguchi S. Policing the regulators. Nat Immunol. 2001;2:283–284. doi: 10.1038/86283. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 20.Shevach EM, McHugh RS, Piccirillo CA, Thornton AM. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol Rev. 2001;182:58–67. doi: 10.1034/j.1600-065x.2001.1820104.x. [DOI] [PubMed] [Google Scholar]
- 21.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–1140. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 25.Tang Q, Bluestone JA. Plasmacytoid DCs and T(reg) cells: casual acquaintance or monogamous relationship? Nat Immunol. 2006;7:551–553. doi: 10.1038/ni0606-551. [DOI] [PubMed] [Google Scholar]
- 26.Mahnke K, Johnson TS, Ring S, Enk AH. Tolerogenic dendritic cells and regulatory T cells: a two-way relationship. J Dermatol Sci. 2007;46:159–167. doi: 10.1016/j.jdermsci.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyteassociated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 29.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 30.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 31.Hori S, Sakaguchi S. Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes Infect. 2004;6:745–751. doi: 10.1016/j.micinf.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 32.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 33.Smith EL, Finney HM, Nesbitt AM, Ramsdell F, Robinson MK. Splice variants of human FOXP3 are functional inhibitors of human CD4+ T-cell activation. Immunology. 2006;119:203–211. doi: 10.1111/j.1365-2567.2006.02425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma S, Dominguez AL, Lustgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol. 2006;177:8348–8355. doi: 10.4049/jimmunol.177.12.8348. [DOI] [PubMed] [Google Scholar]
- 35.Zhao L, Sun L, Wang H, Ma H, Liu G, Zhao Y. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. J Leukoc Biol. 2007;81:1386–1394. doi: 10.1189/jlb.0506364. [DOI] [PubMed] [Google Scholar]
- 36.Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25-Foxp3+ T cells in aged mice. J Immunol. 2006;176:6586–6593. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 37.Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss PA. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005;140:540–546. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gottenberg JE, Lavie F, Abbed K, Gasnault J, Le Nevot E, Delfraissy JF, Taoufik Y, Mariette X. CD4 CD25high regulatory T cells are not impaired in patients with primary Sjogren's syndrome. J Autoimmun. 2005;24:235–242. doi: 10.1016/j.jaut.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Trzonkowski P, Szmit E, Mysliwska J, Mysliwski A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of CTL and NK cells in humansimpact of immunosenescence. Clin Immunol. 2006;119:307–316. doi: 10.1016/j.clim.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, Rustin MH, Taams LS, Beverley PC, Macallan DC, Akbar AN. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116:2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santner-Nanan B, Seddiki N, Zhu E, Quent V, Kelleher A, de St Groth BF, Nanan R. Accelerated age-dependent transition of human regulatory T cells to effector memory phenotype. Int Immunol. 2008;20:375–383. doi: 10.1093/intimm/dxm151. [DOI] [PubMed] [Google Scholar]
- 42.Tsaknaridis L, Spencer L, Culbertson N, Hicks K, LaTocha D, Chou YK, Whitham RH, Bakke A, Jones RE, Offner H, Bourdette DN, Vandenbark AA. Functional assay for human CD4+CD25+ Treg cells reveals an age-dependent loss of suppressive activity. J Neurosci Res. 2003;74:296–308. doi: 10.1002/jnr.10766. [DOI] [PubMed] [Google Scholar]
- 43.Belkaid Y, Butcher B, Sacks DL. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: selective impairment of IL-12 induction in Leishmania-infected cells. Eur J Immunol. 1998;28:1389–1400. doi: 10.1002/(SICI)1521-4141(199804)28:04<1389::AID-IMMU1389>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 44.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 45.Mendez S, Reckling SK, Piccirillo CA, Sacks D, Belkaid Y. Role for CD4(+) CD25(+) regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity. J Exp Med. 2004;200:201–210. doi: 10.1084/jem.20040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 47.Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 48.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 49.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 50.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yi H, Zhen Y, Jiang L, Zheng J, Zhao Y. The phenotypic characterization of naturally occurring regulatory CD4+CD25+ T cells. Cell Mol Immunol. 2006;3:189–195. [PubMed] [Google Scholar]
- 53.Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest. 115:1953–1962. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koenen HJ, Fasse E, Joosten I. CD27/CFSE-based ex vivo selection of highly suppressive alloantigen-specific human regulatory T cells. J Immunol. 2005;174:7573–7583. doi: 10.4049/jimmunol.174.12.7573. [DOI] [PubMed] [Google Scholar]
- 55.Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, Sallusto F. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med. 2005;201:1793–1803. doi: 10.1084/jem.20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duggleby RC, Shaw TN, Jarvis LB, Kaur G, Gaston JS. CD27 expression discriminates between regulatory and non-regulatory cells after expansion of human peripheral blood CD4+ CD25+ cells. Immunology. 2007;121:129–139. doi: 10.1111/j.1365-2567.2006.02550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kitazawa Y, Fujino M, Wang Q, Kimura H, Azuma M, Kubo M, Abe R, Li XK. Involvement of the programmed death-1/programmed death-1 ligand pathway in CD4+CD25+ regulatory T-cell activity to suppress alloimmune responses. Transplantation. 2007;83:774–782. doi: 10.1097/01.tp.0000256293.90270.e8. [DOI] [PubMed] [Google Scholar]
- 58.Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Treg suppressive activity involves estrogen-dependent expression of programmed death-1 (PD-1) Int Immunol. 2007;19:337–343. doi: 10.1093/intimm/dxl151. [DOI] [PubMed] [Google Scholar]
- 59.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGFbeta- TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci U S A. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 61.Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104:2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- 62.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 63.Kwon B, Yu KY, Ni J, Yu GL, Jang IK, Kim YJ, Xing L, Liu D, Wang SX, Kwon BS. Identification of a novel activation-inducible protein of the tumor necrosis factor receptor superfamily and its ligand. J Biol Chem. 1999;274:6056–6061. doi: 10.1074/jbc.274.10.6056. [DOI] [PubMed] [Google Scholar]
- 64.Pillai V, Ortega SB, Wang CK, Karandikar NJ. Transient regulatory T-cells: a state attained by all activated human T-cells. Clin Immunol. 2007;123:18–29. doi: 10.1016/j.clim.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Werfel T, Boeker M, Kapp A. Rapid expression of the CD69 antigen on T cells and natural killer cells upon antigenic stimulation of peripheral blood mononuclear cell suspensions. Allergy. 1997;52:465–469. doi: 10.1111/j.1398-9995.1997.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 66.Sallusto F, Kremmer E, Palermo B, Hoy A, Ponath P, Qin S, Forster R, Lipp M, Lanzavecchia A. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur J Immunol. 1999;29:2037–2045. doi: 10.1002/(SICI)1521-4141(199906)29:06<2037::AID-IMMU2037>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 67.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 68.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994;152:3282–3293. [PubMed] [Google Scholar]
- 69.Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 70.Williams MB, Butcher EC. Homing of naive and memory T lymphocyte subsets to Peyer's patches, lymph nodes, and spleen. J Immunol. 1997;159:1746–1752. [PubMed] [Google Scholar]
- 71.Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, Wei S, David O, Curiel TJ, Zou W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer research. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 72.Yurchenko E, Tritt M, Hay V, Shevach EM, Belkaid Y, Piccirillo CA. CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J Exp Med. 2006;203:2451–2460. doi: 10.1084/jem.20060956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lehmann J, Huehn J, de la Rosa M, Maszyna F, Kretschmer U, Krenn V, Brunner M, Scheffold A, Hamann A. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25- regulatory T cells. Proc Natl Acad Sci U S A. 2002;99:13031–13036. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, Niesner U, de la Rosa M, Schmidt CA, Brauer R, Buer J, Scheffold A, Hamann A. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow's milk allergy. J Exp Med. 2004;199:1679–1688. doi: 10.1084/jem.20032121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chakravarti B, Abraham GN. Aging and T-cell-mediated immunity. Mech Ageing Dev. 1999;108:183–206. doi: 10.1016/s0047-6374(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 77.Fulop T, Larbi A, Wikby A, Mocchegiani E, Hirokawa K, Pawelec G. Dysregulation of T-cell function in the elderly : scientific basis and clinical implications. Drugs Aging. 22:589–603. doi: 10.2165/00002512-200522070-00005. [DOI] [PubMed] [Google Scholar]
- 78.Suffia IJ, Reckling SK, Piccirillo CA, Goldszmid RS, Belkaid Y. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J Exp Med. 203:777–788. doi: 10.1084/jem.20052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shimizu J, Moriizumi E. CD4+CD25- T cells in aged mice are hyporesponsive and exhibit suppressive activity. J Immunol. 2003;170:1675–1682. doi: 10.4049/jimmunol.170.4.1675. [DOI] [PubMed] [Google Scholar]
- 80.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 81.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 82.Soper DM, Kasprowicz DJ, Ziegler SF. IL-2Rbeta links IL-2R signaling with Foxp3 expression. Eur J Immunol. 2007;37:1817–1826. doi: 10.1002/eji.200737101. [DOI] [PubMed] [Google Scholar]
- 83.Luther C, Poeschel S, Varga M, Melms A, Tolosa E. Decreased frequency of intrathymic regulatory T cells in patients with myasthenia-associated thymoma. J Neuroimmunol. 2005;164:124–128. doi: 10.1016/j.jneuroim.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 84.Akbar AN, Taams LS, Salmon M, Vukmanovic-Stejic M. The peripheral generation of CD4+ CD25+ regulatory T cells. Immunology. 2003;109:319–325. doi: 10.1046/j.1365-2567.2003.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimizu J, Moriizumi E. Aging-dependent generation of suppressive CD4+CD25-R123loCD103+ T cells in mice. Eur J Immunol. 2003;33:2449–2458. doi: 10.1002/eji.200324040. [DOI] [PubMed] [Google Scholar]
- 86.Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taams LS, Vukmanovic-Stejic M, Smith J, Dunne PJ, Fletcher JM, Plunkett FJ, Ebeling SB, Lombardi G, Rustin MH, Bijlsma JW, Lafeber FP, Salmon M, Akbar AN. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur J Immunol. 2002;32:1621–1630. doi: 10.1002/1521-4141(200206)32:6<1621::AID-IMMU1621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 88.Wan YY, Flavell RA. The roles for cytokines in the generation and maintenance of regulatory T cells. Immunol Rev. 2006;212:114–130. doi: 10.1111/j.0105-2896.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 89.Selvaraj RK, Geiger TL. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-beta. J Immunol. 2007;178:7667–7677. doi: 10.4049/jimmunol.178.12.7667. [DOI] [PubMed] [Google Scholar]
- 90.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 91.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shodell M, Siegal FP. Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand J Immunol. 2002;56:518–521. doi: 10.1046/j.1365-3083.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- 95.Della Bella S, Bierti L, Presicce P, Arienti R, Valenti M, Saresella M, Vergani C, Villa ML. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin Immunol. 2007;122:220–228. doi: 10.1016/j.clim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 96.Nishimura E, Sakihama T, Setoguchi R, Tanaka K, Sakaguchi S. Induction of antigen-specific immunologic tolerance by in vivo and in vitro antigen-specific expansion of naturally arising Foxp3+CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1189–1201. doi: 10.1093/intimm/dxh122. [DOI] [PubMed] [Google Scholar]
- 97.Rea IM, McNerlan SE, Alexander HD. CD69, CD25, and HLADR activation antigen expression on CD3+ lymphocytes and relationship to serum TNF-alpha, IFN-gamma, and sIL-2R levels in aging. Exp Gerontol. 1999;34:79–93. doi: 10.1016/s0531-5565(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 98.Luz C, Dornelles F, Preissler T, Collaziol D, da Cruz IM, Bauer ME. Impact of psychological and endocrine factors on cytokine production of healthy elderly people. Mech Ageing Dev. 2003;124:887–895. doi: 10.1016/s0047-6374(03)00148-9. [DOI] [PubMed] [Google Scholar]
- 99.Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Agedependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 2000;165:1243–1251. doi: 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
- 100.Garcia GG, Miller RA. Age-dependent defects in TCR-triggered cytoskeletal rearrangement in CD4+ T cells. J Immunol. 2002;169:5021–5027. doi: 10.4049/jimmunol.169.9.5021. [DOI] [PubMed] [Google Scholar]
- 101.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc Natl Acad Sci U S A. 2003;100:15053–15058. doi: 10.1073/pnas.2433717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ehrchen J, Sindrilaru A, Grabbe S, Schonlau F, Schlesiger C, Sorg C, Scharffetter-Kochanek K, Sunderkotter C. Senescent BALB/c mice are able to develop resistance to Leishmania major infection. Infection and immunity. 2004;72:5106–5114. doi: 10.1128/IAI.72.9.5106-5114.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]