Abstract
To eliminate overlap with monoclonal B-cell lymphocytosis (MBL), some have proposed basing the diagnosis of CLL on B lymphocyte count rather than absolute lymphocyte count (ALC). Such criteria should be based, in part, on patient outcomes. We evaluated the clinical implications of the proposed re-classification in 112 consecutive newly diagnosed Rai stage 0 patients. The new criteria would have changed the diagnosis from CLL to MBL in 47/112 (42%) patients. There was no difference in time to treatment (TTT) between those classified as MBL and CLL under the new criteria. In contrast, CD38 predicted TTT (p=0.02) regardless of the proposed new classification. Molecular characteristics of the leukemic clone are a better predictor of progression than an arbitrary ALC or B lymphocyte count threshold.
BACKGROUND
Chronic lymphocytic leukemia (CLL) is one of the most common lymphoid malignancies in the Western World. Criteria to formalize the diagnosis of CLL were developed by the National Cancer Institute (NCI) in 1988 and underwent revision in 19961. According to these criteria, individuals with a lymphocyte population that expresses B-cell markers (CD19, CD20, and CD23), the CD5 antigen, and dim surface immunoglobulin along with a peripheral blood absolute lymphocyte count (ALC) ≥5.0 × 109/L are classified as having CLL1. More recently, the advent of highly sensitive, multicolor flow cytometry techniques has revealed that approximately 3% of the population over the age of 40 has a circulating population of clonal lymphocytes with a CLL phenotype in the absence of lymphocytosis2. The frequency of this finding is even higher among first-degree relatives of patients with CLL3. Individuals with this combination of peripheral blood findings have been classified as having monoclonal B-cell lymphocytosis (MBL).
While the natural history of MBL continues to be defined, it is clear from data on the incidence of CLL that the majority of individuals with MBL will not develop CLL. To ensure uniformity of diagnosis and thereby facilitate studies of the biology and natural history of MBL, the International Familial CLL Consortium recently published formal diagnostic criteria for MBL4. These criteria classify individuals with a monoclonal B-cell population in the peripheral blood, a B lymphocyte count <5.0 × 109/L, and the absence of lymphadenopathy, organomegaly, or autoimmune/infectious disease as having MBL4. These criteria could cause confusion in patient classification as the diagnosis of CLL by the NCI 1996 criteria1 is based on the ALC (an aggregate lymphocyte measure comprised of B cells, T cells, and NK cells)1 whereas the diagnosis of MBL is based on the B lymphocyte count4. Since T-cells comprise the majority of normal peripheral blood lymphocytes, overlap is primarily due to variations in the normal T-cell count causing the ALC to exceed 5.0 × 109/L even though the B lymphocyte count remains <5.0 × 109/L. Relevant to this issue, the NCI criteria are now once again undergoing revision where the new criteria propose a B lymphocyte count >5.0 × 109/L rather than an ALC ≥5.0 × 109/L to establish the diagnosis of CLL5.
While this modification is intended to improve diagnostic precision, it was not based on objective evidence and involves re-classifying the diagnosis of patients from a leukemia diagnosis (CLL) to what is thought to be a pre-malignant condition (MBL) if their B lymphocyte count is <5.0 × 109/L. Ideally, such revisions to diagnostic criteria should be based on objective determination of the elements that best characterizes patient’s clinical outcome.
METHODS & RESULTS
To begin to acquire this type of information, we evaluated the clinical implications of re-classifying patients meeting the NCI 1996 criteria1 for CLL based on B lymphocyte count in accord with the proposed new criteria5. We identified 112 consecutive patients in the Mayo Clinic CLL database who were diagnosed with Rai stage 0 CLL between 2000 and 2002 and were evaluated with flow cytometry testing of peripheral blood at Mayo Clinic within 2 months of diagnosis. All patients had an ALC ≥5.0 × 109/L. All of the samples were evaluated on a FACSCalibur flow cytometer (Becton Dickinson). These data were used with the complete blood count and automated differential counts to determine the ALC and the absolute B-cell count for each case. The clinical features, prognostic characteristics, and clinical course of patients were abstracted. Approval for this study was obtained from the Mayo Clinic Institutional Review Board and was in accordance with US federal regulations and the Declaration of Helsinki.
First we evaluated the relationship between the ALC and the B lymphocyte count (Figure 1). Substantial variation in the size of the B lymphocyte population was observed in patients with ALC ≤10.0 × 109/L many of who had an ALC ≥5.0 × 109/L but a B lymphocyte count <5.0 × 109/L. In fact, 46/63 (73%) patients with an ALC ≤10.0 × 109/L had a B lymphocyte count <5.0 × 109/L and accordingly would be re-classified as having MBL rather than CLL under the new criteria. In contrast, the B lymphocyte count was consistently ≥5.0 × 109/L among patients whose ALC was >10.0 × 109 where only 1/49 (2%) patients had a B lymphocyte count <5.0 × 109/L. Overall, the new criteria would have changed the diagnosis from CLL to MBL in 47/112 (42%) patients.
Figure 1. Relationship absolute B-cell count to absolute lymphocyte count for patients with an absolute lymphocyte count < 20.0 × 109/L (n=98).
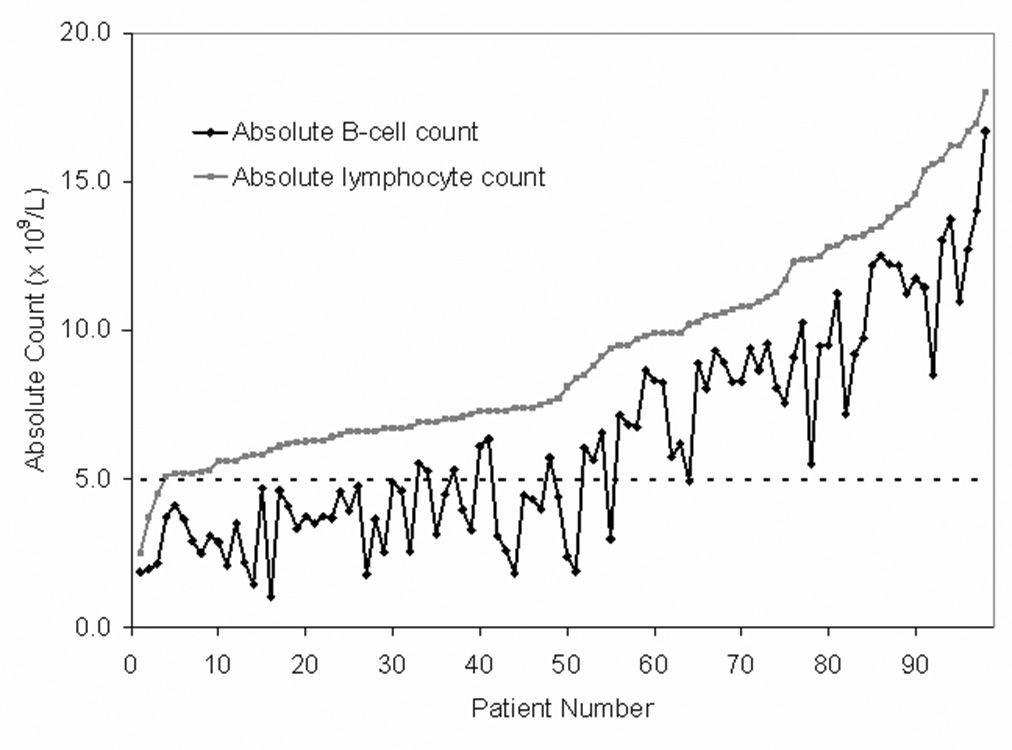
To evaluate the clinical outcome of these revisions to the diagnostic criteria, patients were classified as having MBL if their B lymphocyte count was <5.0 × 109/L. We then evaluated demographic characteristics and prognostic parameters based on whether patients fell into the proposed new MBL(n=47) and CLL (n=65) categories assessing IgVH mutation status, ZAP-70 status, CD38 status, and cytogenetic abnormalities by FISH testing using methods previously described by our group6. The baseline characteristics of patients are summarized in Table 1. Patients in the MBL category were slightly older with a trend toward being more likely to be female. No statistically significant differences in IgVH mutation status, ZAP-70 status, CD38 status, and cytogenetic abnormalities by FISH testing were observed between the two groups.
Table 1.
Patient Characteristics
| Characteristic | MBL (B-cells <5.0 × 109/L; N=47) | CLL (B-cells≥5.0 × 109/L; N=65) | P value* |
|---|---|---|---|
| Age at diagnosis | 0.02 | ||
| [median (range), years] | 70 (48, 87) | 65 (43, 87) | |
| Age (years) | 0.25 | ||
| <50 | 2 (4%) | 5 (8%) | |
| 50–60 | 8 (17%) | 17 (26%) | |
| 61–70 | 13 (28%) | 22 (34%) | |
| >70 | 24 (51%) | 21 (32%) | |
| Gender | 0.09 | ||
| Male | 20 (43%) | 39 (60%) | |
| Female | 27 (57%) | 26 (40%) | |
| Rai Stage | |||
| 0 | 47 (100%) | 65 (100%) | |
| CD38 | 1.00 | ||
| Positive | 10 (22%) | 15 (23%) | |
| Negative | 35 (78%) | 49 (77%) | |
| Missing | 2 | 1 | |
| ZAP-70 | 0.64 | ||
| Positive | 1 (11%) | 6 (24%) | |
| Negative | 8 (89%) | 19 (76%) | |
| Missing | 38 | 40 | |
| IgVH gene mutation status | 0.28 | ||
| Unmutated | 2 (13%) | 9 (31%) | |
| Mutated | 13 (87%) | 20 (69%) | |
| Missing | 32 | 36 | |
| FISH result | 0.12 | ||
| 13q- | 7 (58%) | 15 (44%) | |
| Normal | 2 (17%) | 11 (32%) | |
| +12 | 1 (8%) | 4 (12%) | |
| 11q- | 0 | 4 (12%) | |
| 17p- | 2 (17%) | 0 | |
| Missing | 35 | 31 | |
Fisher’s exact test (categorical factors) or Wilcoxon rank sum test (continuous factors)
We next evaluated time to treatment (TTT) and overall survival (OS) for patients in the MBL and CLL categories using clinical outcomes obtained from the Mayo Clinic CLL database. The decision to treat patients was based on physician discretion in accordance with the NCI 96 CLL Working Group Criteria1. TTT was defined as time from diagnosis to time of first therapy and OS was defined as the time from diagnosis to death of any cause. Differences between groups were evaluated using standard Kaplan-Meier methods and log rank statistics. A trend toward longer TTT was observed for patients in the MBL category however this difference did not reach statistical significance (Figure 2A; p=0.16) and no difference in overall survival was observed (Figure 2B).
Figure 2. Clinical Outcomes by B-cell Count.
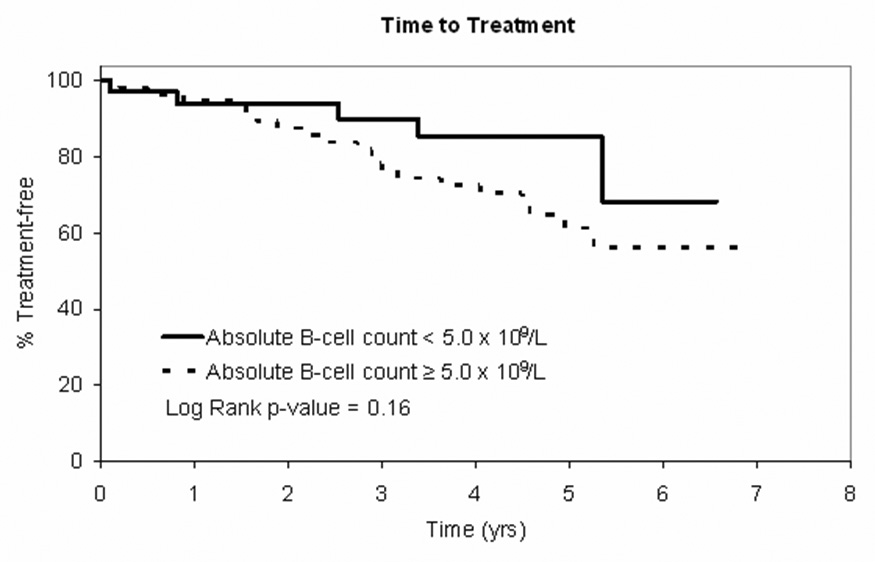
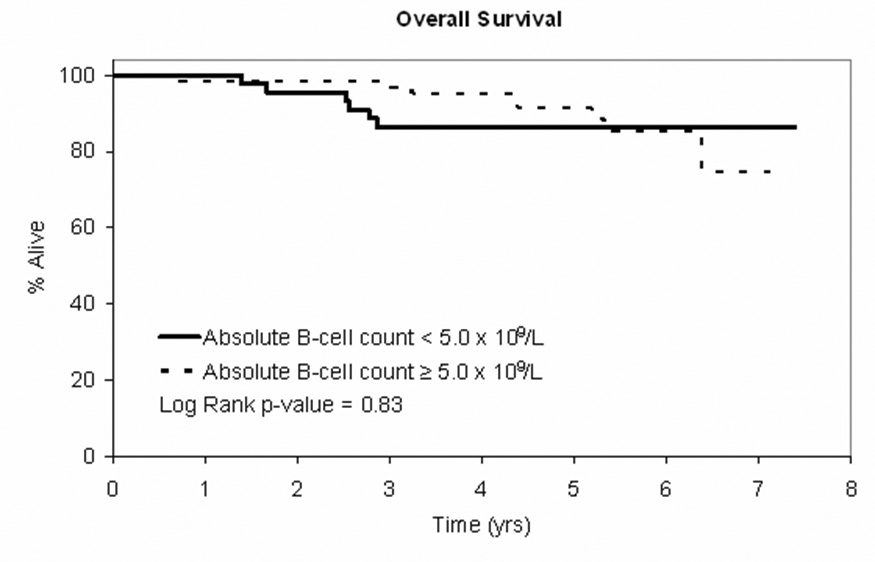
Figure 2A: Time to treatment curve for patients with Rai stage 0 CLL at diagnosis (NCI 1996 criteria) based on absolute B-cell count <5.0 × 109/L (n=47) vs. B-cell count ≥5.0 × 109/L (n=65)
Figure 2B: OS curve for patients with Rai stage 0 CLL at diagnosis (NCI 1996 criteria) based on absolute B-cell count <5.0 × 109/L (n=47) vs B-cell count ≥5.0 × 109/L (n=65)
Although insufficient numbers of patients had ZAP-70, IgVH gene mutation status, or FISH results to analyze whether these characteristics predicted TTT and OS, nearly all patients (n=109, 97%) had CD38 status available. CD38 predicted TTT (p=0.02) regardless of the proposed new MBL vs. CLL designation (Figure 3A). No differences in OS were observed by CD38 status (p=0.20; Figure 3B).
Figure 3. Clinical Outcomes by CD38 Status.
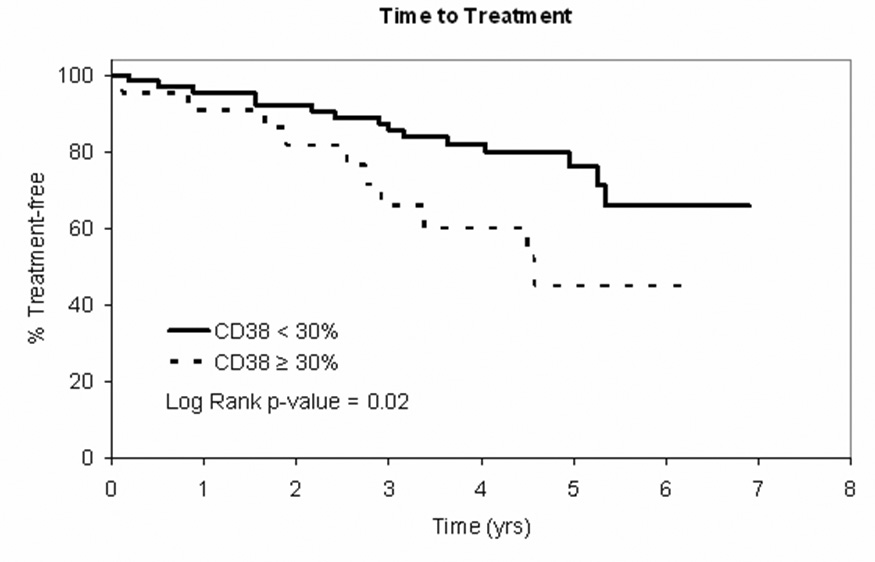
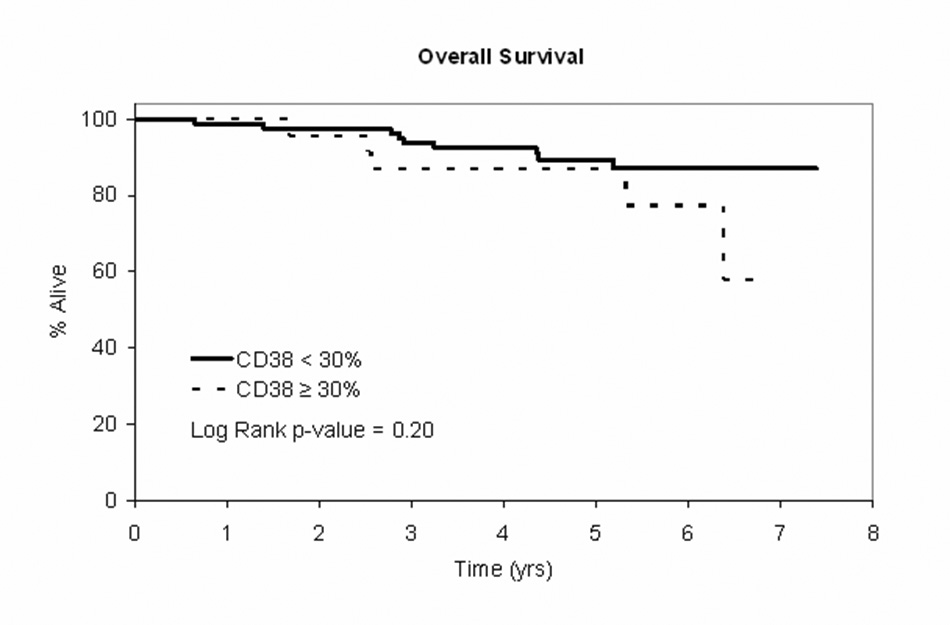
Figure 3A: TTT curve for patients with Rai stage 0 CLL at diagnosis (NCI 1996 criteria) by CD38 < 30% (n=84) vs CD38 ≥ 30% (n=25)
Figure 3B: OS curve for patients with Rai stage 0 CLL at diagnosis (NCI 1996 criteria) by CD38 < 30% (n=84) vs CD38 ≥ 30% (n=25)
CONCLUSION
We conclude that the ALC and B-lymphocyte count are not interchangeable terms and that the B-lymphocyte count is a poor surrogate of the ALC when the ALC is ≤10.0 × 109/L. Due to this fact, a large number of patients (42% in the present series) who historically would be classified as having Rai stage 0 CLL1 will be classified as having MBL4 using the proposed new diagnostic criteria 5. Although the diagnosis of leukemia carries potential psychologic distress, failing to identify patients who will need appropriate follow-up care and support is potentially even more problematic. Labeling patients as having CLL should be based in part on their likelihood of progressing and requiring chemotherapeutic treatment7. Consistent with other reports8, our analysis suggests that the biologic characteristics of the leukemic clone (e.g. CD38 status) are a better predictor of an individual’s likelihood of progression than an arbitrary ALC or B lymphocyte count threshold. Further studies evaluating how best to draw the boundary between MBL and CLL in patients with borderline lymphocyte count elevations are clearly needed and will add to our clinical and biological understanding of these commonly encountered disorders. Finally, we wish to emphasize that if absolute B lymphocyte counts become a part of any diagnostic disease criteria, a standard laboratory approach to making that quantitative determination needs to be acknowledged and uniformly adopted before patient’s can be clinical classified based on such results.
Acknowledgements
Support through grants from the National Cancer Institute (NCI CA 113408) gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-Sponsored Working Group Guidelines fo Chronic Lymphocytic Leukemia: Reised Guidelines for Diagnosis and Treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 2.Rawstron A, Green M, Kuzmicki A, et al. Monoclonal B lymphocytes with the characteristics of "indolent" chronic lymphocytic leukemia are present in 3.5% of adults with normal blood counts. Blood. 2002;100:635–639. doi: 10.1182/blood.v100.2.635. [DOI] [PubMed] [Google Scholar]
- 3.Rawstron A, Yuille M, Fuller J, et al. Inherited predispoition to CLL is detectable as subclinical monoclonal B-lymphocyte expansion. Blood. 2002;100:2289–2290. doi: 10.1182/blood-2002-03-0892. [DOI] [PubMed] [Google Scholar]
- 4.Marti GE, Rawstron AC, Ghia P, et al. Diagnostic criteria for monoclonal B-cell lymphocytosis. Br J Haematol. 2005;130:325–332. doi: 10.1111/j.1365-2141.2005.05550.x. [DOI] [PubMed] [Google Scholar]
- 5.Eichhorst B, Hallek M. Revision of the guidelines for diagnosis and therapy of chronic lymphocytic leukemia (CLL) Best Pract Res Clin Haematol. 2007;20:469–477. doi: 10.1016/j.beha.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Shanafelt TD, Witzig TE, Fink SR, et al. Prospective evaluation of clonal evolution during long-term follow-up of patients with untreated early-stage chronic lymphocytic leukemia. J Clin Oncol. 2006;24:4634–4641. doi: 10.1200/JCO.2006.06.9492. [DOI] [PubMed] [Google Scholar]
- 7.Victor Hoffbrand A, Hamblin TJ. Is "leukemia" an appropriate label for all patients who meet the diagnostic criteria of chronic lymphocytic leukemia? Leuk Res. 2007;31:273–275. doi: 10.1016/j.leukres.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Hamblin TJ, Gardiner AC, Mould SJ, et al. Can we predict which patients with early stage CLL will progress and require treatment. Leukemia Lymphoma. 2005;46:S46 P24. [Google Scholar]


