Abstract
Thy-1 is an abundant neuronal glycoprotein of poorly defined function. We recently provided evidence indicating that Thy-1 clusters a β3-containing integrin in astrocytes to induce tyrosine phosphorylation, RhoA activation and the formation of focal adhesions and stress fibers. To date, the α subunit partner of β3 integrin in DI TNC1 astrocytes is unknown. Similarly, the ability of neuronal, membrane-bound Thy-1 to trigger astrocyte signaling via integrin engagement remains speculation. Here, evidence that αv forms an αvβ3 heterodimer in DI TNC1 astrocytes was obtained. In neuron-astrocyte association assays, the presence of either anti-αv or anti-β3 integrin antibodies reduced cell-cell interaction demonstrating the requirement of both integrin subunits for this association. Moreover, anti-Thy-1 antibodies blocked stimulation of astrocytes by neurons but not the binding of these two cell types. Thus, neuron-astrocyte association involved binding between molecular components in addition to the Thy-1-integrin; however, the signaling events leading to focal adhesion formation in astrocytes depended exclusively on the latter interaction. Additionally, wild-type (RLD) but not mutated (RLE) Thy-1 was shown to directly interact with αvβ3 integrin by Surface Plasmon Resonance analysis. This interaction was promoted by divalent cations and was species-independent. Together, these results demonstrate that the αvβ3 integrin heterodimer interacts directly with Thy-1 present on neuronal cells to stimulate astrocytes.
Keywords: Thy-1, integrins, brain cells, cell-cell interaction, adhesion molecules
1. Introduction
Thy-1 is a 25–37 kDa glycosyl phosphatidyl inositol (GPI)-anchored protein of the immunoglobulin superfamily expressed in various cell types, including those of the T cell lineage, neurons, a subset of CD34+ blood stem cells, fibroblasts and activated endothelial cells [1–4]. Thy-1 modulates cell death/proliferation, signaling, motility and adhesion. Specific examples in the latter case include Thy-1-induced adhesion of thymocytes to thymic epithelium, binding of monocytes and polymorphonuclear leukocytes to Thy-1+ activated endothelial cells and fibroblasts, activation of lymphocytes and adhesion of Thy-1+ cells to astrocytes [5–11].
Studies completed by 1992 had shown the GPI-anchored Thy-1 to be the first lymphocyte surface antigen restricted to a functional subset of lymphocytes, that signalled across the membrane despite lacking a transmembrane spanning domain. However, Thy-1 function was not known [12]. A number of functions have been attributed to Thy-1 and possible mechanisms by which this molecule may function are beginning to emerge [10, 13–17], in part due to our discovery identifying a β3-containing integrin on astrocytes as a Thy-1 receptor that, upon ligation, promotes cell adhesion and spreading of astrocytes [8]. Following this initial finding, Thy-1 expressed on activated endothelial cells was reported to bind in vitro to the integrins αvβ3 on melanoma cells [16], as well as αXβ2 and αMβ2 on leukocytes [15, 18]. Taken together, these results identify Thy-1-integrin interactions as potentially relevant to cell-cell adhesion events, although the integrin receptor varies depending on the cells involved.
Our reported data show that β3 integrin engagement by Thy-1 leads to integrin clustering, tyrosine phosphorylation of focal adhesion kinase (FAK) and p130Cas, as well as the activation of RhoA and p160ROCK. This interaction also induces recruitment of paxillin, vinculin, and FAK to focal contacts, thereby promoting focal adhesion and stress fiber formation in rat astrocytes [8, 19, 20]. These data were obtained by stimulating astrocytes with either recombinant soluble Thy-1-Fc proteins or EL-4 thymoma cells which abundantly express Thy-1 at their surface. Therefore, the relevance of Thy-1/integrin interaction in neuron-astrocyte communication has never been addressed.
Integrins are heterodimeric transmembrane proteins that mediate cell-matrix, as well as cell-cell interactions, essential for adhesion, spreading, migration, and proliferation. Integrins are formed by the non-covalent association of α and β subunits and only two heterodimers have been identified containing a β3 chain, αvβ3 and αIIbβ3. While, αIIbβ3 presence is restricted to platelets and monocytes, αvβ3 is more ubiquitously expressed [21]. Based on this pattern of expression, astrocyte αvβ3 integrin was considered the likely candidate integrin involved in interacting with Thy-1. However, experiments demonstrating that the αv subunit is associated with β3 were required‥
Although, the subfamily of RGD-binding integrins (αIIb, α5, αv, α8, β3, β5, β6 and β8) is promiscuous with respect to ligand binding, each integrin appears to have specific and non-redundant functions [22]. In the case of the leukocyte-specific receptors, even promiscuity between species has been reported. For instance, pig ICAM-1 and ICAM-2 bind to human αL β2 integrin [23]. Additionally, αXβ2 integrin binds equally well to both human and mouse Thy-1 [18].
To unravel remaining open questions concerning Thy-1-integrin interaction, we characterized in detail the interaction between Thy-1 and its astrocytic integrin binding partner. Immunoprecipitation experiments revealed that the αvβ3 integrin heterodimer is indeed expressed in DI TNC1 astrocytes and both integrin subunits are required in Thy-1-induced focal adhesion formation in astrocytes. Importantly, Thy-1 on the surface of cathecolaminergic CAD cells specifically associated with αvβ3 integrin and stimulated astrocytes via a ROCK-dependent signaling pathway. Although antibodies to Thy-1 blocked stimulation, binding between neurons and astrocytes was only partially reduced. Additionally, surface plasmon resonance analysis using murine and human Thy-1-Fc recombinant proteins indicated that Thy-1 binds directly to human αVβ3 integrin. Furthermore, this study shows that the Thy-1/integrin interaction depended on a) the integrin-binding RLD motif present in Thy-1 molecule, b) the divalent cations Mg2+ or Mn2+ but not Ca2+ or the Ca2+/Mg2+ mix. and c) that it was species independent, at least in vitro.
2. Materials and methods
2.1. Cells, antibodies and reagents
The rat astrocytic cell line DI TNC1 (ATCC CRL-2005) was maintained in RPMI medium 1640 (GIBCO) containing 5% serum (FBS, HyClone), 0.1mM 2-mercaptoethanol (GIBCO) and 100U/ml penicillin/ 100µg/ml streptomycin (PS mixture, GIBCO). The mouse catecholaminergic neuronal cell line CAD [24] was maintained in DMEM-F12 (GIBCO) containing 8% FBS and PS mixture. Other cells used were rat neuron-like cell line PC12 (ATCC CRL-1721), human embryonic kidney 293 and 293T cells (HEK293, ATCC CRL-1573; HEK293T, ATCC CRL-11268) grown according to ATCC guidelines. All cells were maintained in a humidified atmosphere of 5% CO2 at 37°C.
The mouse recombinant proteins Thy-1(RLD)-Fc, Thy-1(RLE)-Fc and human TRAIL-R2-Fc were obtained as previously reported [8] and coupled to Protein-A Sepharose beads (Sigma) for cell stimulation. The RGD-containing proteins (Kis RGD-GFP, Kis RGE-GFP, Kis peptide, described elsewhere [8]) and the cDNAs for αV and β3 integrin were provided by Dr. Curzio Rüegg (ISREC, Switzerland). RLD peptide was obtained as described [8]. Polystyrene microspheres (polybeads) were from PolyScience, Inc (Pensylvania, PA). The Rho-kinase (ROCK) inhibitor Y-27632 was from Calbiochem (Merck).
Rhodamine-conjugated phalloidin (Sigma) and the antibodies mouse anti-paxillin mAb (Transduction Laboratories), mouse anti-vinculin mAb (Sigma) and goat anti-mouse IgG-Alexa Fluor 488 pAb (Molecular Probes) were used in immunofluorescence experiments. The antibodies used in Western blotting experiments were horseradish peroxidase (HRP)-coupled goat anti-human IgG (Sigma), rabbit anti-Thy-1 (Serum 2881, made by ProScience against mouse Thy-1-Fc recombinant protein and characterized by Western blot analysis using extractsfrom different Thy-1+ cell lines, Figure 1S, see Supplementary Material), anti-β3 pAb (Santa Cruz) and mouse anti-αV mAb (Transduction Laboratories), goat anti-rabbit IgG-HRP pAb (Bio-Rad) and goat anti-mouse IgG-HRP pAb (Sigma). Secondary antibody binding was visualized using the ChemiLucent Detection System Kit (Chemicon International). The mouse anti-rat β3 integrin mAb (clone F11, Beckton & Dickinson) was used in immunoprecipitation experiments. Non-permeable Sulfo-NHS-biotin employed to biotinylate cells and the BCA reagent used to determine protein concentrations were obtained from Pierce Chemical Co. All other reagents used were from Sigma or of the highest grade available. For inhibition of cell-cell interaction or astrocyte stimulation, antibodies used were: mouse anti-rat β3 integrin mAb (clone F11, Beckton & Dickinson), mouse anti-αV mAb (Transduction Laboratories), hamster anti-β1 integrin mAb (clone Ha2/5, Beckton & Dickinson), rat anti-Thy-1 mAb (clone V8 [25]). Anti-thymic-shared antigen-1 (TSA-1/Sca2) mAb MTS-35 was employed as a control antibody [26].
2.2. Preparation of recombinant proteins
The human Thy-1 cDNA, obtained by PCR amplification of EST clones flanked by HindIII and SalI sites, was subcloned into the Fc expression vector as reported [27]. Recombinant RLD (wild type) and RLE (mutant) human Thy-1-Fc proteins were purified from culture supernatants of stably transfected HEK293 cells using high-trap Protein-A-Sepharose columns (GE Healthcare) as previously reported for mouse Thy-1-Fc proteins [8]. Characterization of these proteins by Western blotting revealed that all these Thy-1-Fc fusion proteins were recognized as a single band at the expected molecular mass by anti-human IgG and anti-Thy-1 antibodies (Figure 2S, see Supplementary Material).
The cDNA encoding for the extracellular domain of human β3 integrin was amplified by PCR and cloned into the pCR3 expression vector for Fc fusion proteins using BamHI and SalI sites available in the primers. The construct for β3-Fc integrin contains the signal peptide of the Ig heavy chain (MNFGFSLIFLVLVLKGVQCEV), the sequence KLVPRGS, amino acids 27–718 of human β3 integrin, amino acids VD and the hinge, CH2 and CH3 domains of human IgG1. The cDNA encoding for the extracellular domain of αV integrin (amino acids 1–994) preceded by a consensus Kozak sequence (GCCACC) was amplified by PCR and cloned into the Fc expression vector using the BamHI and SalI sites as described for the β3 integrin. The αVβ3-Fc fusion protein was expressed in HEK293T cells by transient co-transfection with equal amounts of both integrin-Fc expression plasmids. Transfected cells were grown in serum-free medium (Opti-MEM) for 5 days and the culture supernatant, containing the secreted heterodimer was concentrated 20-fold in a Centricon 30 (Amicon). Control supernatants were obtained growing non-transfected HEK293T cells in Opti-MEM for 5 days and concentrating them as indicated. Recombinant proteins were characterized by molecular mass determination and on immunoblots using specific antibodies which revealed the presence of αV, β3 and Fc peptides at the calculated molecular mass (Figure 3S-A, see Supplementary Material). αVβ3-Fc functionality was tested using an enzyme-linked immunosorbent assay (ELISA) and the protein showed to bind to fibronectin and to different recombinant RGD containing snake venom peptides (Kis RGD-GFP, Kis peptide) whereas it did not bind to Kis RGE-GFP (Figure 3S-B, see Supplementary Material).
2.3. Western blots and immunoprecipitations
Whole cell lysates were obtained from rat DI TNC1 astrocytes in ice-cold-lysis buffer as described [7]. Protein extracts (50µg/lane) or immunoprecipitated proteins were separated by SDS-PAGE (10% gels) and transferred to nitrocellulose. Membranes were blocked with 5% fat-free milk or 5% gelatin (for goat antibodies) and then incubated with goat anti-β3 pAb or mouse anti-αV mAb followed by a second antibody coupled to HRP. The peroxidase activity was revealed by enhanced chemiluminescence.
For immunoprecipitation, the lysates were clarified by centrifugation and pre-cleared with Protein A-Sepharose beads for 30min. Afterwards, pre-cleared protein extracts were incubated with 1–2µg of anti-β3 integrin mAb or control IgG coupled to Protein A-Sepharose beads during 1h at 4°C. In a different experiment, cells were first surface biotinylated using non-permeable NHS-biotin (according to manufacturer’s instructions) prior to preparing the lysates, and proteins were immunoprecipitated as indicated above. Proteins bound to the Ab-coupled beads were solubilized in Laemmli buffer and processed for immunoblotting. In the case of biotinylated proteins, nitrocellulose membranes were blocked with 2% BSA in PBS and then incubated for 1h with streptavidin-HRP.
2.4. Cell-cell adhesion assay
The relevance of Thy-1-integrin binding in interactions between neurons and astrocytes was assessed as described [8]. In brief, PC12 cells were labelled with the CellTracker (Molecular probes) CMTMR red following the manufacturer’s instructions. Once labelled, cells were added to a monolayer of astrocytes in a 24-well plate for 20min at 37°C and then gently washed with PBS to remove unbound cells. Bound neuronal cells were counted with an inverted microscope equipped with epifluorescence. To interfere with the cellular interaction, astrocytes were pretreated as reported [8] with soluble chimeric Thy-1-Fc proteins, anti-αV or anti-β3 integrin antibodies. TRAIL-R2-Fc or anti-β1 integrin antibodies were used as the respective controls.
2.5. Cell-cell stimulation assay
Astrocytes were seeded on coverslips in 24-well plates and grown for 24h to 70–80% confluency. Then, cells were washed in serum-free medium and CAD cells (106 cells/well) were added in the same medium to stimulate focal adhesion formation for 10min. Upon simulation, cells were washed twice and then fixed for indirect immunofluorescence. To test specificity of the interaction, CAD cells were pre-treated with hybridoma supernatants of anti-Thy-1 mAbs (V8 clone) for 30min to block Thy-1 on the cell surface. Cells were rinsed twice with serum-free medium to remove excess of antibody and serum, prior to their addition to the astrocytes. Alternatively, CAD cells were treated with a hybridoma supernatant containing a control antibody (MTS-35). To identify signaling mechanisms involved in the astrocyte response to Thy-1, astrocytes were pre-treated with the ROCK inhibitor Y-27632 (10µM) for 30min at 37°C prior to stimulation with CAD cells.
2.6. Indirect immunofluorescence
Astrocytes were stimulated for 10min with CAD cells and the formation of focal adhesions and stress fibers were evaluated as reported [20]. To test whether integrin engagement was sufficient to stimulate focal adhesion formation, astrocytes were also incubated with 10µm microspheres coated with RLD peptide. Polybead microspheres were prepared by incubating 1×106 polybeads with 4µg of either RLD, Thy-1-Fc or TRAIL-R2-Fc. After eliminating excess peptide and washing with PBS, remaining polybead binding sites were blocked by incubating with 2% fatty acid-free BSA for 60min. Then, peptide-coated polybeads were washed again twice with PBS and once with serum-free medium. Finally, polybeads were added to astrocytes for 10min. Focal adhesions were stained with anti-paxillin or anti-vinculin mAbs followed by anti-mouse IgG-Alexa Fluor 488. Stress fibers were visualized with Rhodamine-conjugated phalloidin that binds to F-actin. Samples were examined with a Carl Zeiss Axiovert-135M confocal microscope (LSM Microsystems) following excitation at 488 or 543 nm. Optical sections obtained and acquired image z-stacks were processed with Imaris software (Bitplane AG, Zuerich, Switzerland). The number of focal adhesions per cell was quantified as previously described [20].
2.7. Surface plasmon resonance analysis
Protein-protein interaction studies were carried out using a Biacore X (Biacore, Uppsala, Sweden) as previously described [18]. Wild type (RLD) and mutated (RLE) Thy-1-Fc proteins of human or mouse origin, or BSA as a control were covalently immobilized on the carboxy methyl dextran surface of CM5 chips via primary amino groups, using the amine coupling kit. For all biosensor assays, HBS was employed as running buffer [10mM HEPES, 150mM sodium chloride, (pH 7.4)] containing 1mM MgCl2 or other divalent cation.
Human αVβ3-Fc from a concentrated culture supernatant diluted in HBS buffer as indicated in Figure 6, were run over the sensor chip at 30µl/min, 25°C. The effect of divalent cations, all at 1mM was tested. To repeat the experiments, CM5 chips were regenerated using 5mM EDTA as described [18].
Figure 6. Mouse and human Thy-1-Fc wild type proteins directly interacted with human αvβ3-Fc recombinant protein in a cation-dependent manner.
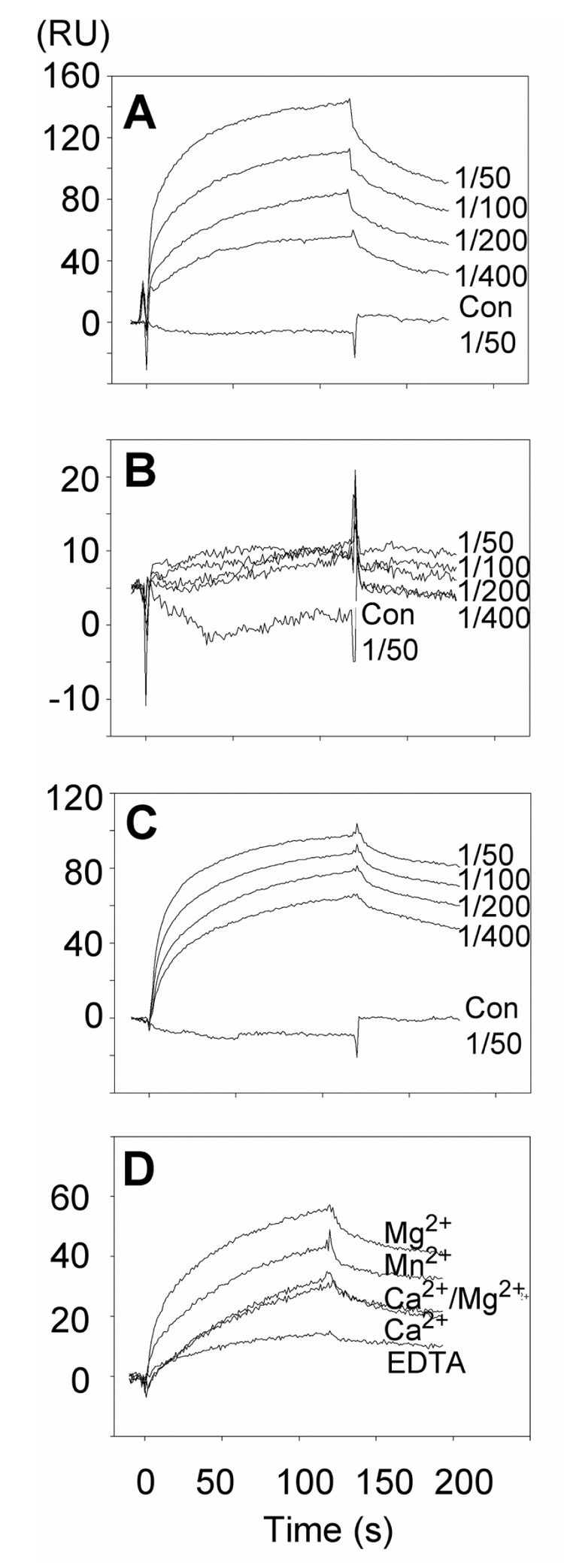
Concentrated supernatant from the cells expressing αvβ3-Fc was diluted sequentially (1/50 – 1/400) in Hepes buffer saline (HBS) containing 1mM MgCl2. Concentrated supernatant from non-transfected HEK293T cells diluted 1/50 in the same HBS buffer was used as a control (Con 1/50). A) Human Thy-1(RLD)-Fc, B) human Thy-1(RLE)-Fc, C) and D) mouse Thy-1(RLD)-Fc were immobilized on CM5 chips. Concentrated supernatants were diluted as mentioned (or 1/400 in D) in HBS buffer containing 1mM MgCl2 or other cations as indicated (all at 1mM). EDTA (5mM) was used as a control. Binding of αvβ3-Fc was monitored at different time points and the binding was expressed in Response Units (RU). Data shown are representative of at least 3 independent experiments.
2.8. Statistical Analysis
Where appropriate, experimental data were compared to control conditions using non-parametric statistical analysis for unpaired samples (Mann-Whitney U Test).
3. Results
Two different approaches were used to demonstrate that the αv subunit heterodimerizes with β3 integrin in astrocytes. Cell surface proteins were biotinylated and subsequently immunoprecipitated with anti-β3 mAb, yielding two major bands of 120 and 65 kDa, likely corresponding to αv and β3, respectively (Figure 1A). To demonstrate that the 120 kDa protein associated with the β3 subunit was αv integrin, and also that the band of lower molecular weight was indeed β3 integrin, non-biotinylated lysates were immunoprecipitated with either anti-β3 mAb or a control IgG. Complexes obtained were analyzed by immunoblotting with the anti-αv antibody (Figure 1B) and a polyclonal anti-β3 antibody (Figure 1C). Bands corresponding to αv (Figure 1B, 120 kDa band) and β3 (Figure 1C, 65 kDa band), were readily observed when the anti-β3 mAb was employed to immunoprecipitate the integrin heterodimer. Neither αv or β3 integrins were detected in control immunoprecipitations with an unrelated IgG (Figure 1B, 1C). Thus, the αvβ3 integrin heterodimer was present at the cell surface of DI TNC1 astrocytes although the β3 integrin identified was smaller than anticipated.
Figure 1. αv integrin dimerized with β3 integrin in rat astrocytes.
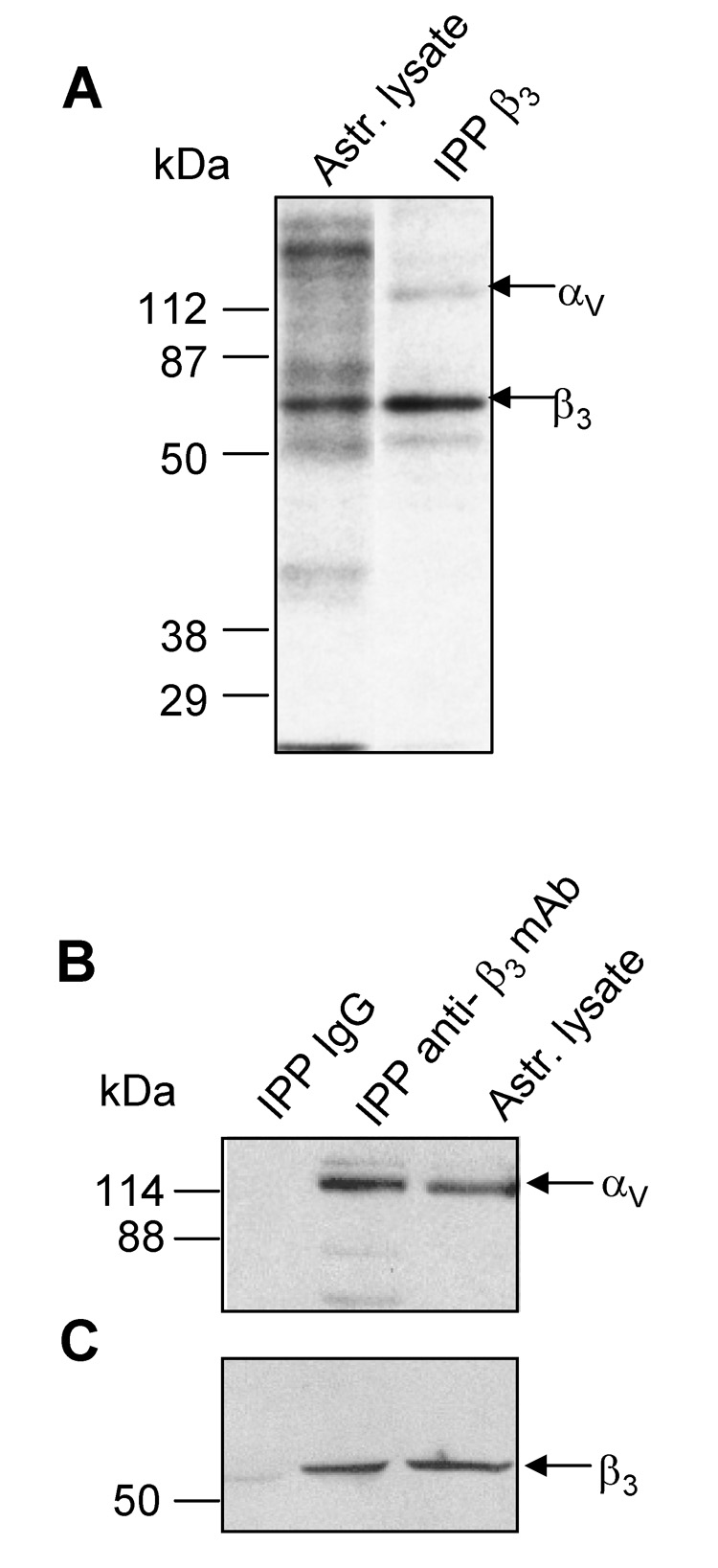
A) Surface biotinylated proteins were immunoprecipitated (IPP) with anti-β3 integrin mAb. Labelled proteins from astrocyte lysates and IPP proteins were revealed with streptavidin-HRP. B) and C) Proteins in a whole cell lysate were precipitated with anti-β3 mAb or a control IgG. IPP complexes were separated by SDS-PAGE in parallel with astrocyte lysate. Proteins transferred to nitrocellulose were revealed with anti-αv mAb (B) and anti-β3 pAb (C). Values for molecular mass in kDa are shown to the left of each panel.
To test whether the αvβ3 integrin heterodimer was required for neuron-astrocyte association, a previously described cell-cell adhesion assay was employed [8]. Furthermore, Thy-1 interaction with β3-containing integrin has been demonstrated between either EL-4 thymoma cells or Thy-1-Fc recombinant protein and astrocytes. Thus, this assay was employed to demonstrate that Thy-1-integrin binding participated in neuron-astrocyte interaction as well. Indeed, binding of rat PC12 cells to a monolayer of rat DI TNC1 astrocytes was detected. This interaction was inhibited in a dose-dependent manner, by pretreatment with either human or mouse Thy-1-Fc proteins (Figure 2A). As expected, pre-treatment with either anti-αv or anti-β3 integrin antibodies reduced significantly cell-cell interaction detected by this assay (Figure 2B), whereas no effect was observed upon pre-incubation with equivalent amounts of TRAIL-R2-Fc (Figure 2A) or anti-β1 integrin (Figure 2B) antibodies used as negative controls. In this context, it is important to note that expression of β1 integrin and presence at the cell surface were verified by immunoblotting and flow cytometric analysis, respectively, using the same antibody (data not shown). Thus, the rat PC12 bound to rat DI TNC1 cells and this association was diminished by Thy-1-Fc recombinant proteins. Additionally, given that not only anti-β3 integrin antibody but also anti-αv antibody reduced the level of interaction, these results indicate that neuron-astrocyte association in this in vitro assay is dependent to a considerable extent on Thy-1-αvβ3 integrin interaction.
Figure 2. Rat PC12 cell binding to DI TNC1 astrocytes involved Thy-1/αvβ3 integrin interaction.
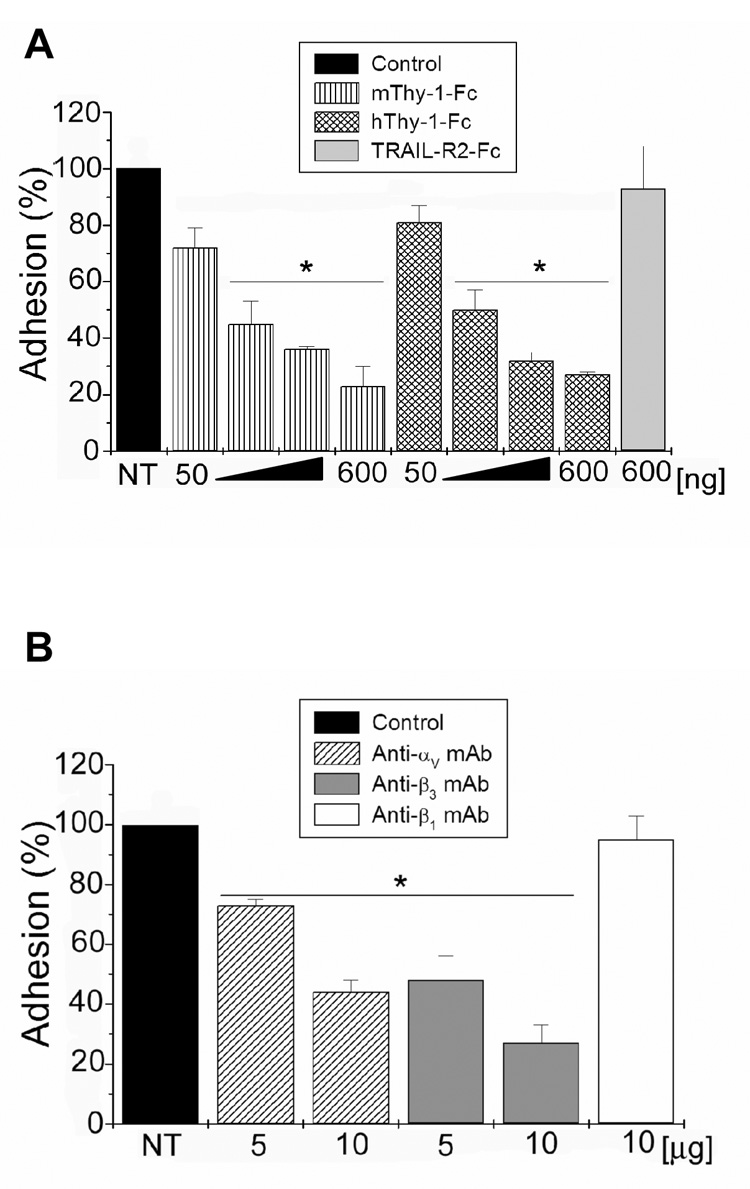
Fluorescently labelled, live PC12 cells were incubated over a monolayer of DI TNC1 astrocytes. In A) astrocytes in a 24-well plate were treated with 50,100, 300 and 600ng of recombinant Thy-1-Fc proteins of human or mouse origin prior to incubation with labelled PC12 cells. TRAIL-R2-Fc recombinant protein was used as a negative control at 600ng. In B) astrocytes were treated with 5 or 10µg of different anti-integrin antibodies as indicated, for 30min, before the addition of labelled PC12 cells. Treatment with anti-β1 integrin antibody was used as a negative control in this case. PC12 cells were then incubated for 20min with astrocytes, and subsequently washed gently. Bound labelled cells were counted using an inverted microscope equipped with epifluorescence. Values shown correspond to the percentage of PC12 cells bound to astrocytes whereby non-treated cells (NT) were assigned the value 100%. Averages ± SE of 3 independent experiments are shown. *, p < 0.05 compared to TRAIL-R2-Fc (A) or to anti-β1 integrin (B) treated cells.
A second cell line (CAD cells) of mouse origin was used to test whether neuronal cells not only bind to, but additionally stimulate a response in rat DI TNC1 astrocytes. As shown in Figure 3B, the formation of substantially larger focal adhesions (green/yellow dots) was observed in astrocytes exposed to CAD cells (arrowheads) than in non-stimulated astrocytes (Figure 3A). Quantification of the number of focal adhesions per cell and of the average area per focal adhesion showed that CAD cells stimulated both the formation of additional and larger focal adhesions in astrocytes (Figure 3C). Such increased adhesion was inhibited by an anti-Thy-1 (clone V8) monoclonal antibody but not by a control antibody (Figure 3C; see also Figure 4). Other controls included treatment of astrocytes for the same period of time (10min)with conditioned medium from CAD cells or conditioned medium obtained from co-cultures of CAD-DI TNC1 cells. None of these media had an effect on the formation of focal adhesions and stress fibers in astrocytes (data not shown), suggesting that the astrocytic response to CAD cells is not caused by soluble factors but rather by the direct interaction of these two cell types.
Figure 3. Mouse CAD cells stimulated focal adhesion and stress fiber formation in rat astrocytes in a Thy-1-dependent manner.
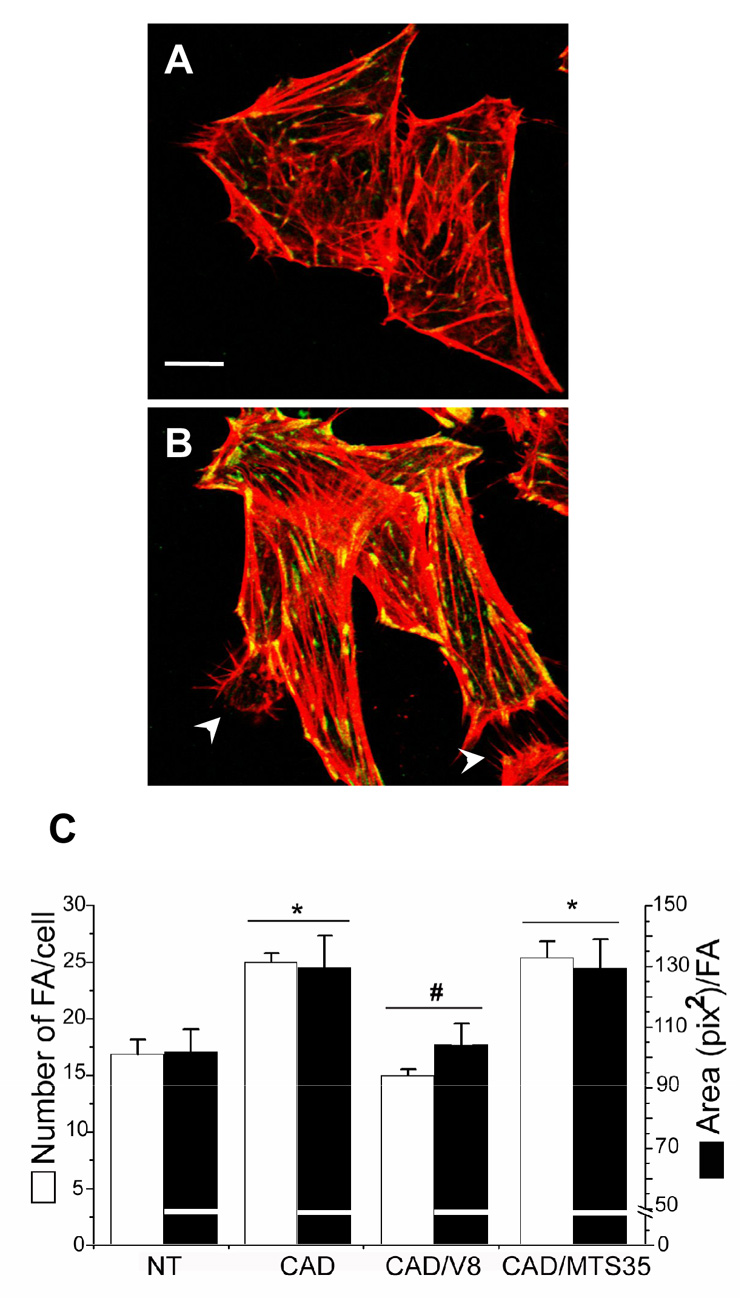
Astrocytes were washed with serum-free medium and left as is (A) or stimulated with CAD cells (B). Utilized CAD cells were either not treated or previously treated with hybridoma supernatant containing anti-Thy-1 mAb (clone V8) or a control antibody (MTS-35) for 30min. After 10min incubation with CAD cells, astrocytes were gently washed, fixed and permeabilized for immunofluorescence analysis. Focal adhesions were stained with anti-vinculin antibodies followed by anti-mouse IgG-Alexa 488 (green). Stress fibers were marked with Rhodamine-labelled phalloidin (red). White arrowheads indicate CAD cells bound to astrocytes (B). The graph (C) shows the quantification of focal adhesions per cell (white bars), as well as the average area/focal adhesion (black bars). CAD cells stimulated the formation of more and larger focal adhesions. This effect was inhibited by V8 but not by MTS-35 antibodies (C). CAD cells also stimulated the formation of stress fibers (B compared to A). Results shown are representative of 3 individual experiments performed in duplicates. *, p < 0.05 with respect to the control non-treated (NT) cells. #, p < 0.05 compared to CAD-stimulated (CAD) cells. Bar in A is 10µm.
Figure 4. The formation of focal adhesions but not neuron-astrocyte interaction per se was completely dependent on Thy-1-integrin binding.
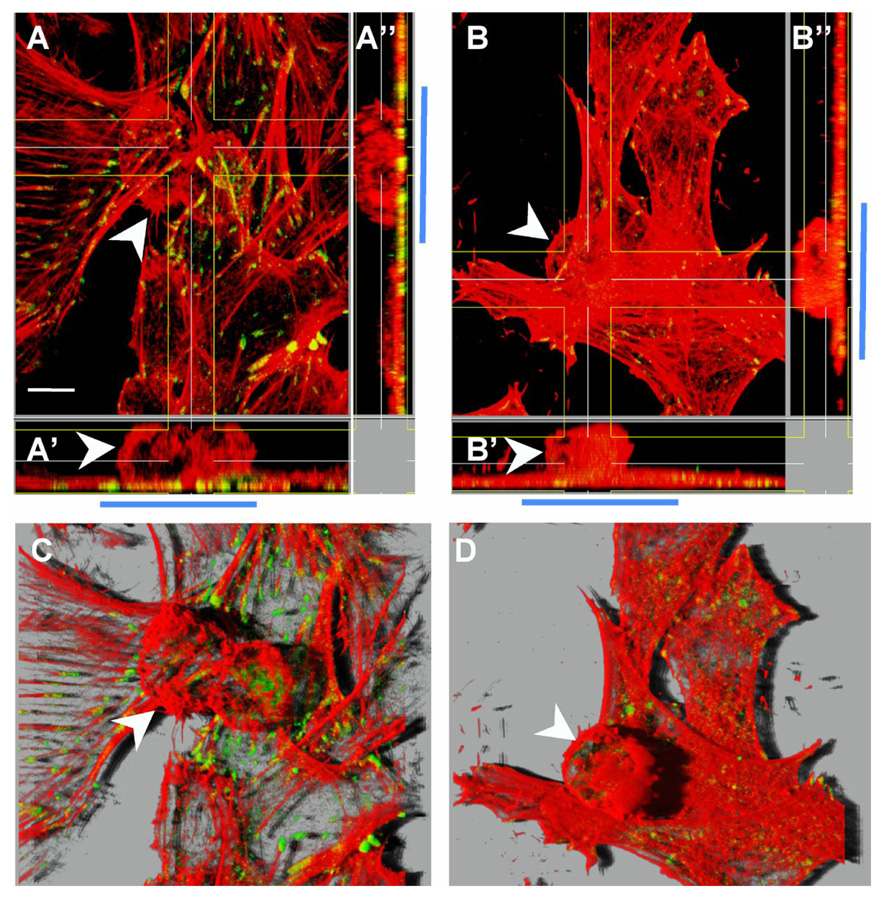
Astrocytes were washed with serum-free medium and stimulated with CAD cells either not treated (A and C) or treated with hybridoma supernatant containing anti-Thy-1 mAb (clone V8) (B and D) as indicated in Figure 3. After 10min of neuron-astrocyte incubation, cells were gently washed and then prepared for immunofluorescence analysis of focal adhesions (green) and F-actin (red). White arrowheads indicate CAD cells bound to a monolayer of astrocytes. These are easier to observe in orthogonal sections that show cells in the xz plane (A’ and B’) and xy plane (A’’ and B’’). Blue bars mark the area where CAD cells (round cells) were bound to the astrocyte monolayer. In A’ and A’’ green/yellow dots corresponding to focal adhesions are apparent in the area of cell-cell contact. In B’ and B’’, on the other hand, very few and small focal points are detectable in the area of contact. C and D correspond to the same fields shown in A and B, respectively. However here, z-stack-sections are visualized in 3D projections obtained using Imaris software (Bitplane AG, Zurich, Switzerland) to show that CAD cells bound to the astrocyte monolayer induced focal adhesion and stress fiber formation only when Thy-1 was available (C) and not in the presence of an anti-Thy-1 antibody (D). Results shown are representative of 3 individual experiments performed in duplicates. Magnification bar is 10µm.
Staining of polymerized actin with rhodamine-labelled phalloidin permitted visualization of CAD cells adhering to the astrocyte monolayer (white arrowheads in Figure 3B and Figure 4). This becomes more apparent when optical sections obtained in the z-axis are projected in three dimensions as shown in Figure 4C and 4D. Orthogonal sections of the same images showing the xz-plane (Figure 4A’) and xy-plane (Figure 4A’’) indicated that focal adhesions were exclusively detected in astrocytes and were enriched in the zone of CAD cell-astrocyte contact (green/yellow spots in Figure 4A’ and 4A’’). This effect was not detectable when CAD cells were pre-treated with the monoclonal anti-Thy-1 antibody (compare areas indicated by the blue bar in Figures 4B’, 4B’’ with 4A’, 4A’’; see also Figure 3C for data quantification), whereas a control antibody (clone MTS-35) had no effect (Figure 3C). Interestingly, despite the ability of the anti-Thy-1 antibody to block signaling events linked to focal adhesion formation, CAD cell-astrocyte binding was still detectable (see also Figures 2A and 2B). This most likely reflects the participation of additional cell adhesion molecules in neuron-astrocyte interaction (white arrowheads in Figures 4B and 4D). These results underscore the importance of Thy-1-integrin binding both in neuron-astrocyte association and communication.
Recombinant Thy-1-Fc fusion protein induces focal adhesion and stress fiber formation via integrin-mediated phosphorylation of FAK and p130Cas, the recruitment of vinculin, paxillin and FAK to focal adhesion sites and further activation of RhoA and ROCK [8, 19, 20]. To assess whether CAD cells activate signaling pathways similar to those detected with Thy-1 alone, a ROCK inhibitor was employed. Indeed, in astrocytes pre-treated with the Y-27632 compound, no increase in the formation of focal adhesions was observed following stimulation with CAD cells. Furthermore, in this case, focal adhesions were less abundant than those observed for non-stimulated astrocytes (Figure 5A).
Figure 5. Effects of ROCK inhibition and RLD-coated polybeads in astrocytes.
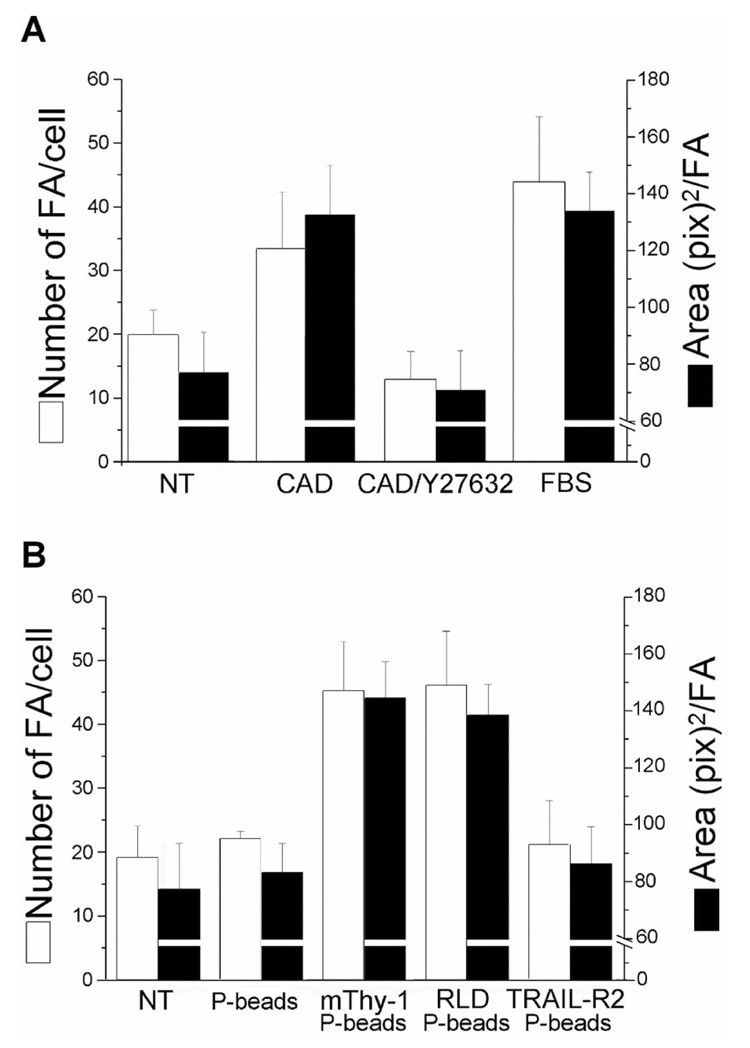
A. Astrocytes seeded on coverslips in 24-well plates were washed with serum-free medium and treated or not for 30min with Y-27632 (10µM) prior to stimulating with CAD cells, as indicated in Figure 3. After 10min in the presence of CAD cells, DI TNC1 cells were gently washed and then prepared for immunofluorescence analysis of focal adhesions. Data show the number of focal adhesions per cell (white bars), as well as the average area/focal adhesion (black bars). The presence of CAD cells increased size and number of focal adhesions in DI TNC1 cells, but had no effect when astrocytes were pre-treated with Y-27632. Values are averages ± SD from 2 different experiments. B. DI TNC1 cells left overnight to adhere to coverslips were washed with serum-free medium and treated with polybeads coated with fatty acid-free BSA, Thy-1-Fc, RLD peptide or TRAIL-R2-Fc. Focal adhesions were quantified after stimulating with coated-polybeads for 10min and preparing the cells for immunofluorescence as indicated in Figure 3. Results are representative of 3 independent experiments.
To determine whether the RLD peptide present in Thy-1 was sufficient to stimulate astrocytes, Thy-1-Fc- or RLD-coated microspheres were added to astrocytes. A two-fold increase in focal adhesion number and area was observed in both cases, whereas, no effect was detected in cells treated with control TRAIL-R2-Fc-coated microspheres or microspheres alone (Figure 5B).
To evaluate whether the interaction between αvβ3 and Thy-1 proteins was direct, surface plasmon resonance (SPR) analysis was utilized. Human αvβ3-Fc, as well as human and murine Thy-1-Fc recombinant proteins, were obtained and characterized as indicated in Materials and methods. Recombinant αvβ3-Fc protein containing only the extracellular domains of the integrin subunits was obtained by co-expressing both αv-Fc and β3-Fc in HEK293T cells. Soluble recombinant integrin was used in serum-free supernatants, since attempts to purify this protein resulted in a non-functional integrin. Transiently transfected HEK293T cells secreted the αvβ3-Fc heterodimer, however, αv-Fc was not detected when overexpressed in the absence of β3-Fc and, β3-Fc was not detected in the absence of αv-Fc (see Figure 3S-A in Supplementary Material).
αvβ3-Fc bound to both human and mouse Thy-1-Fc proteins in a concentration-dependent manner (Figures 6A and 6C, respectively), whereas, no interaction was observed with either the mutated human (Figure 6B) or mouse Thy-1(RLE)-Fc protein (not shown). Likewise, no binding was observed with control supernatants (Figure 6A–C). These results indicate that αvβ3 integrin and Thy-1 proteins interact directly and that the Thy-1 RLD motif molecule is important for interaction with the integrin.
Coordination of divalent metal ions induces conformational changes of integrins that initiate the low- to higher-affinity transition [21, 28]. To study cation-dependence, the interaction between mouse Thy-1-Fc and αvβ3-Fc was tested by SPR in the presence of three individual cations, EDTA or a Ca2+/Mg2+ mix. The observed order of cation preference in the binding assay was as follows: Mg2+ > Mn2+ > Ca2+= Ca2+/Mg2+ (Figure 6D). A similar cation-dependence was observed for the human Thy-1-Fc protein in that no binding was detected in the presence of either Ca2+ alone or the Ca2+/Mg2+ mix. Indeed, cation preference for binding between the proteins of human origin was similar to that observed for the mouse proteins (Mn2+ > Mg2+ > Ca2+= Ca2+/Mg2+, data not shown). In all cases, the presence of EDTA completely abrogated binding indicating the critical role of specific divalent cations in this interaction. Similar cation requirements have previously been reported for αvβ3 integrin activation in cells [29].
These experiments also suggested that the interaction was not species-specific, at least in vitro. However, absence of specificity may have been due to the fact that these fusion proteins were all produced by HEK293 or HEK293T cells. Thus, to characterize species-specificity further, Thy-1-Fc proteins were used to trigger a cellular response in rat DI TNC1 astrocytes. Mouse wild type (RLD) Thy-1-Fc protein was previously shown to stimulate focal adhesion and stress fiber formation, while the mutated (RLE) form did not [8, 19, 20]. These results were confirmed here: mouse Thy-1(RLD)-Fc protein induced focal adhesion and stress fiber formation (Figure 7D). Alternatively, astrocytes treated with either mouse Thy-1(RLE)-Fc protein (Figure 7C) or TRAIL-R2-Fc used as a control (Figure 7B) focal adhesions were similar to those in non-stimulated cells (Figure 7A). In addition, results obtained here employing human Thy-1-Fc proteins indicated that the wild type human Thy-1(RLD)-Fc protein also activated the formation of focal adhesions and stress fibers (Figure 7F), while the mutated Thy-1(RLE)-Fc had no effect on the rat astrocytic cell line (Figure 7E). Thus, Thy-1-elicited effects in astrocytes require the RLD motif and were species-independent.
Figure 7. Mouse and human Thy-1-Fc wild type proteins stimulated focal adhesion and stress fiber formation in rat astrocytes.
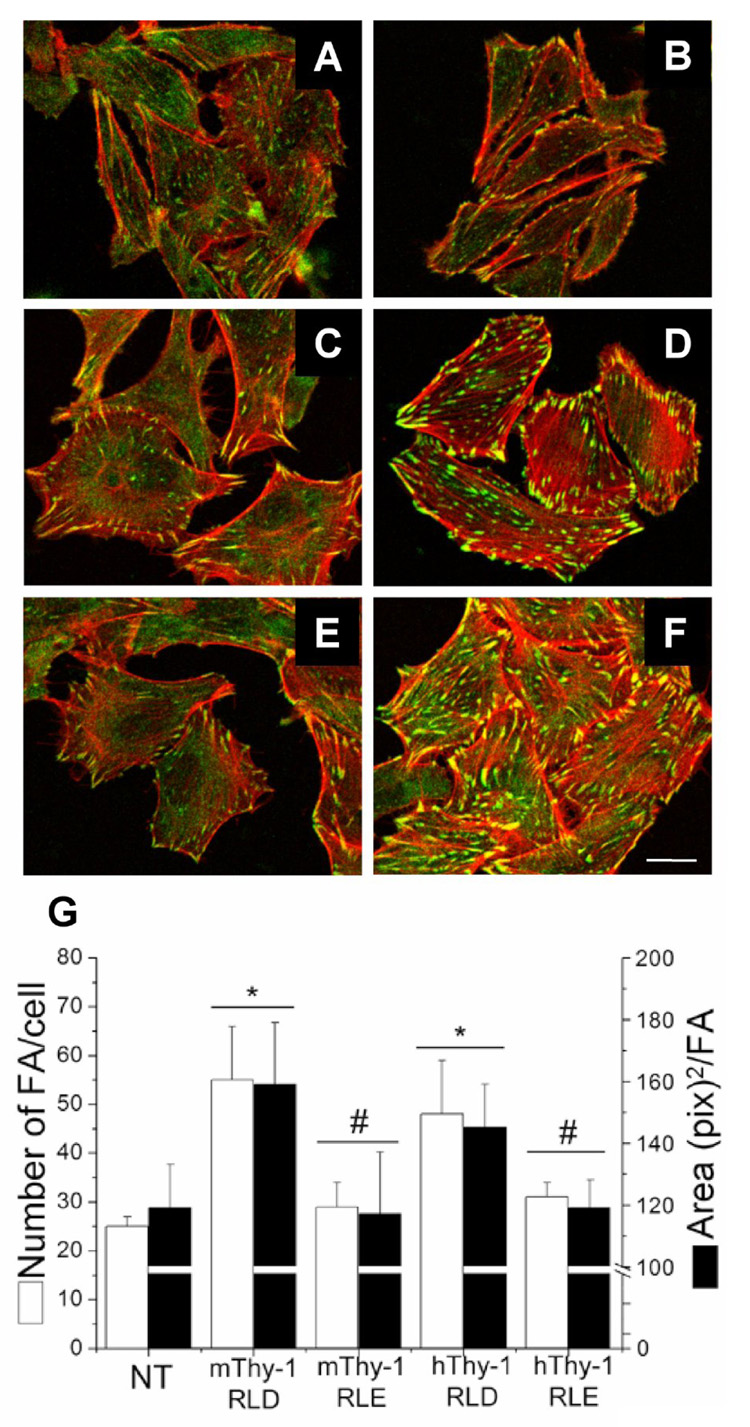
Astrocytes either not treated (A) or treated for 10min with Protein-A Sepharose beads containing TRAIL-R2-Fc (B), mouse Thy-1(RLE)-Fc (C), mouse Thy-1(RLD)-Fc (D), human Thy-1(RLE)-Fc (E) or human Thy-1(RLD)-Fc (F) were fixed, permeabilized and stained for focal adhesions with anti-paxillin mAb (green) and stress fibers with Rhodamine-coupled phalloidin (red). G) Number of focal adhesions/cell (white bars), as well as the average area/focal adhesion (black bars) were quantified using Image J software from NIH public domain. Only mouse and human Thy-1(RLD)-Fc significantly stimulated the formation of new and larger focal adhesions. Images are representative of results obtained in at least 3 different experiments. *, p < 0.05 with respect to the control non-treated (NT) cells. #, p < 0.05 compared to the values obtained when stimulating with the respective mouse or human RLD protein. Magnification bar is 20µm.
Taken together, these data indicate that a) αvβ3 integrin is a receptor for Thy-1, b) neurons induce focal adhesion formation in astrocytes only if neuronal Thy-1 engages αvβ3 integrin in astrocytes, c) αvβ3 integrin binds directly to Thy-1 in a manner that depends on the presence of the Thy-1 RLD motif and divalent cations (Mg2+ and Mn2+ but not Ca2+), d) neuron-astrocyte association mediated by Thy-1-integrin interaction triggers focal adhesion and stress fiber formation via a signaling pathway that involves ROCK activation, and e) these interactions are not species-specific, at least in vitro and in situ in cells.
4. Discussion
The abundantly expressed neuronal glycoprotein Thy-1 binds to a β3 integrin on astrocytes and triggers the formation of focal contact sites [8]. In the present study, upon verifying the presence of the αvβ3 integrin heterodimer on these astrocytes (Figure 1), the requirement of both subunits for neuron-astrocyte interaction was demonstrated in a cell-binding assay (Figure 2). Most importantly, the mouse CAD cell line stimulated focal adhesion formation in astrocytes in a Thy-1-dependent manner but interacted with astrocytes even when Thy-1-integrin binding was blocked with antibodies (Figure 3 and Figure 4). Signaling triggered by Thy-1 required the RLD motif to engage integrins and involved downstream activation of ROCK in astrocytes, since the inhibition of this kinase by a specific inhibitor precluded CAD cell stimulated focal adhesion formation (Figure 5). Furthermore, we expressed recombinant, soluble and functional αvβ3 integrin and showed by SPR analysis that Thy-1 binding was direct and required Mg2+ or Mn2+ but was not species-specific (Figure 6). Additionally, no species specificity was detected in cellular assays evaluating responses in rat astrocytes induced by human and mouse Thy-1-Fc proteins (Figure 7) or using a mouse neuronal cell line (Figure 3 and Figure 4).
Expression of β3 integrin subunits on the surface of DI TNC1 astrocytes had been previously determined by our laboratory using flow cytometry. However, due to the unavailability of anti-rat αv integrin antibodies at the time, the presence and participation of this subunit in Thy-1-induced astrocyte responses could not be confirmed [8]. The unequivocal identification of both integrin subunits in DI TNC1 astrocytes is shown here (Figure 1). Unexpectedly, the molecular mass of the β3 subunit was different to that reported for this integrin, while that observed for the αv integrin of approximately 120 kDa is in agreement with previous studies [30]. Monoclonal and polyclonal antibodies from two different sources recognized the same 65 kDa protein by immunoprecipitation and immunoblotting, respectively. Cell surface biotinylation indicated that the β3 subunit possessed an extracellular domain and the assays using cells [immunofluorescence for focal contact identification (Figure 7), as well as cell-cell adhesion and stimulation assays (Figure 2–Figure 5)] pointed towards the presence of a functionally active αvβ3 heterodimer in DI TNC1 astrocytes. Heterogeneity of αvβ3 integrin obtained from different tissues is generally attributed to alternative mRNA splicing [31–33]. Alternatively, proteolytic cleavage has also been reported [34]. Thus, a possible explanation for these results is that astrocytes express an as yet unidentified β3 variant or that β3 integrin in these cells is sensitive to protease degradation after cell lysis.
Thy-1-αvβ3 integrin binding is one of many interactions mediating adhesion between neurons and astrocytes. Accordingly, antibodies or fusion proteins that bound to either Thy-1 or the integrin never blocked cell-cell adhesion completely ([8] and Figure 2). Interestingly, an anti-Thy-1 antibody that bound to the surface of CAD cells blocked the Thy-1-induced signaling response in astrocytes. However, CAD cells were still able to bind to astrocytes (Figure 4B and 4D), albeit less efficiently (data not shown). Importantly, unlike wild type thymoma cells (EL-4), cells deficient in synthesis of the GPI anchor (EL4−f), which lack Thy-1 on their surface, also adhere less to astrocytes and do not trigger morphological changes in these cells [8]. These results highlight the selective ability of Thy-1 to trigger focal adhesion and stress fiber formation as a consequence of integrin binding during neuron-astrocyte association.
Signaling events involved in neuronal stimulation of astrocytes via Thy-1-αvβ3 integrin interaction are likely to include FAK and p130Cas tyrosine phosphorylation, as well as activation of Rho and ROCK to induce focal adhesion and stress fiber formation. This sequence was previously reported for astrocytes stimulated with recombinant Thy-1-Fc [8, 19, 20], and is infered here, since the specific ROCK inhibitor Y-27632 precludes astrocyte stimulation by CAD cells.
Since Thy-1 in neurons has been implicated in the inhibition of neurite extension [6], the possibility that signaling events in astrocytes triggered by neuronal Thy-1 may also play a role in axonal growth is intriguing. Therefore, Thy-1-αvβ3 integrin interaction could act bimodaly inducing morphological changes in astrocytes and, at the same time, triggering signals to neurons via Thy-1. The availability of a functional αvβ3-Fc recombinant protein is an invaluable tool that we are at present employing to test this possibility.
Truncated αvβ3 has been previously expressed and purified in a baculovirus system and proven to be functional by ligand-recognition experiments [35]. Here, the co-expression of human αv-Fc and β3-Fc also generated a functional heterodimer with binding capacity for its RGD-containing ligands (see Figure 3S-B in Supplemantary Material) and for Thy-1 of human and mouse origin by SPR analysis (Figure 6). Interestingly, αv-Fc was not secreted in the absence of β3-Fc and vice-versa, in agreement with existing evidence demonstrating that uncomplexed α or β subunits remain trapped in the endoplasmic reticulum [36, 37].
SPR, a sensitive tool to study specific binding events such as protein-protein interactions, was employed here to demonstrate direct interaction between Thy-1-Fc and αvβ3-Fc integrin. None of the mutated Thy-1(RLE)-Fc proteins bound integrin by this method. Additionally, these studies revealed that Mg2+ and Mn2+ but neither Ca2+ or Ca2+/Mg2+ promoted Thy-1/integrin interaction (Figure 6), These results are in agreement with our previous reports suggesting indirectly, the relevance of the RLD motif and of divalent cations for the interaction [8, 19]. Additionally, results presented here indicate that the RLD peptide alone attached to polybeads induces focal adhesion formation to the same extent as Thy-1-Fc-polybeads, likely indicating that the RLD motif is sufficient to trigger the response by integrin engagement. However, given that cells treated with the RLD-polybeads were adherent (bound to extracellular matrix), contributions from different ligand-receptor interactions cannot be ruled out.
The RLD tripeptide of Thy-1 is present in a highly conserved region of the glycoprotein. Indeed, Thy-1 of human, rat and mouse origin share roughly 70% homology in the amino acid sequence flanking the RLD region (10aa in each direction), as estimated using the program for multiple sequence alignment Clustal W [38]. Moreover, Thy-1 N-glycosylation is known to be highly conserved [39]. Thus, it is unlikely that the N-glycosylation site located close to the RLD segment confers diversity to this region. Since integrin-Thy-1 interaction requires this tripeptide, and integrins are reportedly promiscuous with respect to ligand recognition (Reviewed by [22]), the lack of species-specificity shown to occur here between isolated proteins (Figure 6) and cells (Figure 3 and Figure 4) is not surprising. Our previous report showing binding between a mouse thymoma cell line and rat astrocytes [8], already suggested this lack of species-specificity which is corroborated here using isolated recombinant proteins and cells of different origin. The preference of Thy-1 for particular integrins, such as αvβ3 and αXβ2 integrins [8, 18] is likely due to the presence of an RLD motif instead of the more typical RGD integrin-binding site (Reviewed by [40]).
Brain injury leads to astrogliosis, a process that involves the activation of astrocytes which increase in size and migrate to the damaged site to form the glial scar. This structure strongly inhibits regeneration in the central nervous system (Reviewed by [41]) in part as the consequence of a change in the profile of protein expression whereby the number of proteins that inhibit axonal regeneration increases [42]. Morphological changes observed in reactive astrocytes in vivo may be related to those triggered by Thy-1 in astrocytes in culture, which occur very rapidly via integrin clustering and activation of RhoA [20]. Interestingly, αvβ3 integrin is reportedly absent in astrocytes obtained from postnatal and adult rat brains [43, 44]; however, following an ischemic insult to the central nervous system, αvβ3 integrin expression increases in astrocytes of the periinfarct region [45]. Additionally, our own data suggest that cytokines, a stimulus that triggers astrogliosis [41], induce the expression of β3 integrin in neonatal rat primary astrocytes (Herrera-Molina and Leyton, unpublished results). Therefore, if integrin up-regulation occurs upon brain damage, subsequent interaction with the abundantly expressed neuronal Thy-1 protein could lead to both morphological changes in astrocytes and inhibition of axonal regeneration.
In summary, the Thy-1-αvβ3 integrin interaction characterized here contributes to a better understanding of fundamental aspects of astrocyte/neuron interaction. Furthermore, since this interaction involves two cell adhesion molecules that are not only expressed in the brain, the observations reported here are likely to be relevant to other physiopathological situations where these proteins are implicated, including melanoma metastasis [16], leukocyte recruitment and extravasation [15, 18].
Supplementary Material
Acknowledgements
Work from these laboratories was supported by the following awards: Fogarty-NIH grant #1-R03-TW06024-03 (to KB and LL), FONDECYT grants #1040390 and #1070699 (to LL), NIH grant GM-29860 (to KB); FONDAP #15010006 (to AFGQ), Ph.D. fellowships from CONICYT (to RH-M and AV), Grant No. R05-2002-000-01395-0 from the Basic Research Program of the Korea Science and Engineering Foundation (to S-HN), Grants of the Swiss National Science Foundation (to PS). Dr. D. Vásquez provided constructive criticism that improved the manuscript greatly.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams AF. Many cells in rat bone marrow have cell-surface Thy-1 antigen. Eur J Immunol. 1976;6:526–528. doi: 10.1002/eji.1830060716. [DOI] [PubMed] [Google Scholar]
- 2.Morris RJ, Beech JN. Differential expression of Thy-1 on the various components of connective tissue of rat nerve during postnatal development. Dev Biol. 1984;102:32–42. doi: 10.1016/0012-1606(84)90172-6. [DOI] [PubMed] [Google Scholar]
- 3.Saalbach A, Kraft R, Herrmann K, Haustein UF, Anderegg U. The monoclonal antibody AS02 recognizes a protein on human fibroblasts being highly homologous to Thy-1. Arch Dermatol Res. 1998;290:360–366. doi: 10.1007/s004030050318. [DOI] [PubMed] [Google Scholar]
- 4.Saalbach A, Wetzig T, Haustein UF, Anderegg U. Detection of human soluble Thy-1 in serum by ELISA. Fibroblasts and activated endothelial cells are a possible source of soluble Thy-1 in serum. Cell Tissue Res. 1999;298:307–315. doi: 10.1007/s004419900079. [DOI] [PubMed] [Google Scholar]
- 5.He HT, Naquet P, Caillol D, Pierres M. Thy-1 supports adhesion of mouse thymocytes to thymic epithelial cells through a Ca2(+)-independent mechanism. J Exp Med. 1991;173:515–518. doi: 10.1084/jem.173.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiveron MC, Barboni E, Pliego Rivero FB, Gormley AM, Seeley PJ, Grosveld F, Morris RJ. Selective inhibition of neurite outgrowth on mature astrocytes by Thy-1 glycoprotein. Nature. 1992;355:745–748. doi: 10.1038/355745a0. [DOI] [PubMed] [Google Scholar]
- 7.Leyton L, Quest AFG, Bron C. Thy-1/CD3 coengagement promotes TCR signaling and enhances particularly tyrosine phosphorylation of the raft molecule LAT. Molec. Immunol. 1999;36:755–768. doi: 10.1016/s0161-5890(99)00086-3. [DOI] [PubMed] [Google Scholar]
- 8.Leyton L, Schneider P, Labra CV, Rüegg C, Hetz CA, Quest AFG, Bron C. Thy-1 binds to the integrin β3 on astrocytes and triggers formation of focal contact sites. Curr Biol. 2001;11:1028–1038. doi: 10.1016/s0960-9822(01)00262-7. [DOI] [PubMed] [Google Scholar]
- 9.Saalbach A, Hildebrandt G, Haustein UF, Anderegg U. The Thy-1/Thy-1 ligand interaction is involved in binding of melanoma cells to activated Thy-1-positive microvascular endothelial cells. Microvasc Res. 2002;64:86–93. doi: 10.1006/mvre.2002.2401. [DOI] [PubMed] [Google Scholar]
- 10.Barker TH, Grenett HE, MacEwen MW, Tilden SG, Fuller GM, Settleman J, Woods A, Murphy-Ullrich J, Hagood JS. Thy-1 regulates fibroblast focal adhesions, cytoskeletal organization and migration through modulation of p190 RhoGAP and Rho GTPase activity. Exp Cell Res. 2004;295:488–496. doi: 10.1016/j.yexcr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 11.Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. Faseb J. 2006;20:1045–1054. doi: 10.1096/fj.05-5460rev. [DOI] [PubMed] [Google Scholar]
- 12.Morris RJ. Thy-1, the enigmatic extrovert on the neuronal surface. BioEssays. 1992;14:715–722. doi: 10.1002/bies.950141014. [DOI] [PubMed] [Google Scholar]
- 13.Barker TH, Pallero MA, MacEwen MW, Tilden SG, Woods A, Murphy-Ullrich JE, Hagood JS. Thrombospondin-1-induced focal adhesion disassembly in fibroblasts requires Thy-1 surface expression, lipid raft integrity, and Src activation. J Biol Chem. 2004;279:23510–23516. doi: 10.1074/jbc.M402169200. [DOI] [PubMed] [Google Scholar]
- 14.Haeryfar SM, Al-Alwan MM, Mader JS, Rowden G, West KA, Hoskin DW. Thy-1 signaling in the context of costimulation provided by dendritic cells provides signal 1 for T cell proliferation and cytotoxic effector molecule expression, but fails to trigger delivery of the lethal hit. J Immunol. 2003;171:69–77. doi: 10.4049/jimmunol.171.1.69. [DOI] [PubMed] [Google Scholar]
- 15.Wetzel A, Chavakis T, Preissner KT, Sticherling M, Haustein UF, Anderegg U, Saalbach A. Human Thy-1 (CD90) on activated endothelial cells is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J Immunol. 2004;172:3850–3859. doi: 10.4049/jimmunol.172.6.3850. [DOI] [PubMed] [Google Scholar]
- 16.Saalbach A, Wetzel A, Haustein UF, Sticherling M, Simon JC, Anderegg U. Interaction of human Thy-1 (CD 90) with the integrin αvβ3 (CD51/CD61): an important mechanism mediating melanoma cell adhesion to activated endothelium. Oncogene. 2005;24:4710–4720. doi: 10.1038/sj.onc.1208559. [DOI] [PubMed] [Google Scholar]
- 17.Rege TA, Hagood JS. Thy-1, a versatile modulator of signaling affecting cellular adhesion, proliferation, survival, and cytokine/growth factor responses. Biochim Biophys Acta. 2006;1763:991–999. doi: 10.1016/j.bbamcr.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi J, Leyton L, Nham SU. Characterization of αX I-domain binding to Thy-1. Biochem Biophys Res Commun. 2005;331:557–561. doi: 10.1016/j.bbrc.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Avalos AM, Labra CV, Quest AF, Leyton L. Signaling triggered by Thy-1 interaction with β3 integrin on astrocytes is an essential step towards unraveling neuronal Thy-1 function. Biol Res. 2002;35:231–238. doi: 10.4067/s0716-97602002000200015. [DOI] [PubMed] [Google Scholar]
- 20.Avalos AM, Arthur WT, Schneider P, Quest AF, Burridge K, Leyton L. Aggregation of integrins and RhoA activation are required for Thy-1-induced morphological changes in astrocytes. J Biol Chem. 2004;279:39139–39145. doi: 10.1074/jbc.M403439200. [DOI] [PubMed] [Google Scholar]
- 21.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 22.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godwin JW, d'Apice AJ, Cowan PJ. Characterization of pig intercellular adhesion molecule-2 and its interaction with human LFA-1. Am J Transplant. 2004;4:515–525. doi: 10.1111/j.1600-6143.2004.00369.x. [DOI] [PubMed] [Google Scholar]
- 24.Qi Y, Wang JKT, McMillian M, Chikaraishi DM. Characterization of a CNS cell line, CAD, in which morphological differentiation is initiated by serum deprivation. J. Neurosci. 1997;17:1217–1225. doi: 10.1523/JNEUROSCI.17-04-01217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacDonald HR, Bron C, Rousseaux M, Horvath C, Cerottini J-C. Production and characterization of monoclonal anti-Thy-1 antibodies that stimulate lymphokine production by cytolytic T cell clones. Eur. J. Immunol. 1985;15:495–501. doi: 10.1002/eji.1830150514. [DOI] [PubMed] [Google Scholar]
- 26.Godfrey DI, Masciantonio M, Tucek CL, Malin MA, Boyd RL, Hugo P. Thymic shared antigen-1. A novel thymocyte marker discriminating immature from mature thymocyte subsets. J. Immunol. 1992;148:2006–2011. [PubMed] [Google Scholar]
- 27.Schneider P. Production of recombinant TRAIL and TRAIL receptors: Fc chimeric proteins. Meth. Enzymol. 2000;322:325–345. doi: 10.1016/s0076-6879(00)22031-4. [DOI] [PubMed] [Google Scholar]
- 28.Humphries MJ, Mould AP. Structure. An anthropomorphic integrin. Science. 2001;294:316–317. doi: 10.1126/science.1066240. [DOI] [PubMed] [Google Scholar]
- 29.Smith JW, Piotrowicz RS, Mathis D. A mechanism for divalent cation regulation of β3-integrins. J Biol Chem. 1994;269:960–967. [PubMed] [Google Scholar]
- 30.Berthet V, Rigot V, Champion S, Secchi J, Fouchier F, Marvaldi J, Luis J. Role of endoproteolytic processing in the adhesive and signaling functions of αvβ5 integrin. J Biol Chem. 2000;275:33308–33313. doi: 10.1074/jbc.M004834200. [DOI] [PubMed] [Google Scholar]
- 31.van Kuppevelt TH, Languino LR, Gailit JO, Suzuki S, Ruoslahti E. An alternative cytoplasmic domain of the integrin β3 subunit. Proc Natl Acad Sci U S A. 1989;86:5415–5418. doi: 10.1073/pnas.86.14.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Djaffar I, Chen YP, Creminon C, Maclouf J, Cieutat AM, Gayet O, Rosa JP. A new alternative transcript encodes a 60 kDa truncated form of integrin β3. Biochem J. 1994;300(Pt 1):69–74. doi: 10.1042/bj3000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar CS, James IE, Wong A, Mwangi V, Feild JA, Nuthulaganti P, Connor JR, Eichman C, Ali F, Hwang SM, Rieman DJ, Drake FH, Gowen M. Cloning and characterization of a novel integrin β3 subunit. J Biol Chem. 1997;272:16390–16397. doi: 10.1074/jbc.272.26.16390. [DOI] [PubMed] [Google Scholar]
- 34.Du X, Saido TC, Tsubukii S, Indig FE, Williams MJ, Ginsberg MH. Calpain Cleavage of the Cytoplasmic Domain of the Integrin β3 Subunit. J Biol Chem. 1995;270:26146–26151. doi: 10.1074/jbc.270.44.26146. [DOI] [PubMed] [Google Scholar]
- 35.Mehta RJ, Diefenbach B, Brown A, Cullen E, Jonczyk A, Gussow D, Luckenbach GA, Goodman SL. Transmembrane-truncated αvβ3 integrin retains high affinity for ligand binding: evidence for an 'inside-out' suppressor? Biochem J. 1998;330(Pt 2):861–869. doi: 10.1042/bj3300861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hotchin NA, Gandarillas A, Watt FM. Regulation of cell surface β1 integrin levels during keratinocyte terminal differentiation. J Cell Biol. 1995;128:1209–1219. doi: 10.1083/jcb.128.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conesa M, Prat A, Mort JS, Marvaldi J, Lissitzky JC, Seidah NG. Down-regulation of αvβ3 integrin via misrouting to lysosomes by overexpression of a β3Lamp1 fusion protein. Biochem J. 2003;370:703–711. doi: 10.1042/BJ20021301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams AF, Parekh RB, Wing DR, Willis AC, Barclay AN, Dalchau R, Fabre JW, Dwek RA, Rademacher TW. Comparative analysis of the N-glycans of rat, mouse and human Thy-1. Site-specific oligosaccharide patterns of neural Thy-1, a member of the immunoglobulin superfamily. Glycobiology. 1993;3:339–348. doi: 10.1093/glycob/3.4.339. [DOI] [PubMed] [Google Scholar]
- 40.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 41.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 42.Sandvig A, Berry M, Barrett LB, Butt A, Logan A. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46:225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- 43.Milner R, Huang X, Wu J, Nishimura S, Pytela R, Sheppard D, ffrench-Constant C. Distinct roles for astrocyte αvβ5 and αvβ8 integrins in adhesion and migration. J Cell Sci. 1999;112:4271–4279. doi: 10.1242/jcs.112.23.4271. [DOI] [PubMed] [Google Scholar]
- 44.Pinkstaff JK, Detterich J, Lynch G, Gall C. Integrin subunit gene expression is regionally differentiated in adult brain. J Neurosci. 1999;19:1541–1556. doi: 10.1523/JNEUROSCI.19-05-01541.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ellison JA, Velier JJ, Spera P, Jonak ZL, Wang X, Barone FC, Feuerstein GZ. Osteopontin and its integrin receptor αvβ3 are upregulated during formation of the glial scar after focal stroke. Stroke. 1998;29:1698–1707. doi: 10.1161/01.str.29.8.1698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


