Abstract
Background
Zinc accumulation diminishes early in the course of prostate malignancy and continues to decline during progression toward hormone-independent growth. In contrast, constitutive levels of NF-κB activity increase during progression of prostate cells toward greater tumorigenic potential. We have reported previously that physiological levels of zinc suppress NF-κB activity in prostate cancer cells and reduce expression of pro-angiogenic and pro-metastatic cytokines VEGF, IL-6, IL-8, and MMP-9 associated with negative prognostic features in prostate cancer.
Methods
Intracellular zinc levels were examined by Atomic Absorption Spectroscopy. NF-κB activity was examined by TransAm and Luciferase reporter assays, and Western Blot analysis of p50 nuclear translocation. VEGF, IL-6 and IL-8 levels were assessed by ELISA.
Results
Selective zinc deficiency induced by the membrane-permeable zinc chelator N,N,N’,N’-tetrakis(2-pyridylmethyl)-ethylenediamine (TPEN) increases activation of NF-κB and up-regulates expression of the NF-κB controlled pro-angiogenic and pro-metastatic cytokines VEGF, IL-6 and IL-8 in androgen-independent PC-3 and DU-145 prostate cancer cells. Pre-incubation with IκBα dominant mutant adenovirus efficiently blocks expression of these cytokines in zinc deficient cells indicating that the observed effects are NF-κB dependent.
Conclusions
Our findings suggest that zinc deficiency may contribute to the tumor progression via augmented expression of the NF-κB dependent pro-tumorigenic cytokines.
Keywords: zinc, NF-κB, prostate, cancer, cytokines
Introduction
Multiple studies indicate that the loss of the unique ability to retain high intracellular levels of zinc may be an important factor in the development and progression of prostate cancer. The loss of zinc accumulation is the most consistent and persistent characteristic of prostate malignancy. The zinc content of normal prostate epithelium, benign prostatic hyperplasia, and prostate adenocarcinoma has been measured at 1018, 1142 and 146 μg/g dry tissue, respectively [1]. Importantly, zinc accumulation decreases early in the course of prostate malignancy and continues to decline during progression toward hormone-independent growth. In addition, recent studies have revealed a strong association between prostate cancer in African American men and down-regulation of the zinc uptake transporters hZIP1 and hZIP2 [2]. Moreover, expression of the hZIP1 gene and transporter protein is markedly down-regulated in adenocarcinomatous glands and in prostate intra-epithelial neoplastic foci when compared with normal peripheral zone glandular epithelium and benign hyperplastic glands [3]. Conversely, studies have associated zinc accumulation with suppression of prostate cancer cell growth [4,5], inhibition of prostate tumor cell invasion [6] and mitochondrial apoptogenesis [4], suggesting that high intraprostatic zinc levels may protect against prostate carcinogenesis.
The Rel/NF-κB family of eukaryotic transcription factors is comprised of several structurally related proteins that form homo- and heterodimers. The most common Rel/NF-κB dimer in mammals contains the p50-RelA subunits and is specifically called NF-κB. The activity of NF-κB is regulated by interaction with inhibitory IκB proteins, which block the ability of NF-κB to enter the nucleus and bind to DNA. Upon activation, IκB is phosphorylated and marks the inhibitor for ubiquitination and degradation by the proteasome-dependent pathway. This process allows translocation of active NF-κB complexes into the nucleus [7,8]. Multiple studies in a wide variety of tumors have established the role of NF-κB regulated genes in malignant transformation [9], progression of cancer to hormone-independent growth [10–14] and resistance to therapeutic regimens [15–17]. Suppression of NF-κB activity in human prostate cancer cells inhibits their tumorigenic and metastatic properties in nude mice by suppressing angiogenesis and invasion via down-regulation of VEGF, IL-8 and MMP-9. Decreased expression of these molecules in vivo directly correlates with decreased neovascularization and a reduction in lymph node metastases [18]. Thiol-reactive metals such as zinc, copper and gold have been shown to block degradation of IκB proteins and activation of a multi-subunit IκB kinase (IKK), providing a molecular mechanism for suppression of NF-κB activity [19].
In the present study we provide experimental evidence that selective intracellular zinc deficiency is sufficient to augment expression of pro-angiogenic and pro-metastatic cytokines in established prostate cancer cells.
Materials and Methods
Cell lines and culture conditions
PC-3 and DU-145 cell lines were obtained from ATCC (Rockville, MD). Cells were cultured in RPMI 1640 (Bio-Whittaker, Walkersville, MD) medium supplemented with 10% FBS (Hyclone, Logan, UT), gentamicin (50 mg/l), sodium pyruvate (1 mM) and non-essential amino acids (0.1mM) under conditions indicated in the figure legends. RWPE-1 and RWPE-2 cells were maintained in Keratinocyte-Serum Free medium supplemented with 5 ng/ml of human recombinant EGF and 0.05 mg/ml of bovine pituitary extract.
Antibodies and Reagents
Antibodies to p50, IκBα TOPO I and actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Secondary horseradish peroxidase-conjugated donkey anti-rabbit antibodies were purchased from Amersham (Arlington Hts., IL). TPEN was obtained from Sigma (St. Louis, MO). IκBα dominant mutant (IκBαM) adenovirus was purchased from Imgenex (San Diego, CA).
Atomic Absorption Spectroscopy
Harvested cells were rinsed three times in PBS, digested in 2% SDS and boiled for 10 minutes. Protein concentrations were measured with BCA protein assay (Pierce, Rockford, IL). Zn levels were measured by flame mode using a Shimadzu AA-6300 atomic absorption spectrophotometer.
Stable Transfection of PC-3 cells with hZIP1
To create the hZip1 C-end FLAG-tagged expression vector, hZip1 ORF was obtained by PCR with 5′-atcttg aagctt gcc acc atg gga ccg tgg gga gag cca gag ctc ctg gtg-3′ (forward) and 5′-atcttg tctaga tta att aat cta ctt atc gtc gtc atc ctt gta atc gat ttg gat gaa gag cag gcc-3′ (reverse) primers using pRC-CMV-hZip1vector [20] (a kind gift from Dr. R. Franklin (University of Maryland)) as a template and then cloned into the HindIII/Xba1 restriction sites of the pRC-CMV plasmid. PC-3 cells were transfected with either the hZIP1 expression vector or the CMV control vector using the TransIT-Prostate transfection kit (Mirus Bio, Madison, WI). Selection was performed using G418 (1.5 mg/mL; Invitrogen/Life Technologies), and screening of clones was based on Western Blot analysis with anti-hZIP1 antibody to determine hZIP1 expression. Stable transfectants were maintained in medium containing G418 (500 μg/mL).
Colony formation assay in soft agar
A single cell suspension of 1.5×103 PC-3 cells in 0.3% low melting point agar was plated onto a base layer of hardened 0.6% agarose. Cultures were allowed to grow for 18 days with or without various concentrations of zinc in the form of ZnSO4, colonies were stained with Thiazolyl Blue Tetrazolium Bromide (Sigma, St. Louis, MO) and analyzed using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). Each experimental and control group consisted of three wells.
Western Blot Analysis
Nuclear and cytoplasmic extracts and whole cell lysates were prepared as described previously [21]. Protein concentrations were measured with BCA protein assay reagents (Pierce, Rockford, IL). Equivalent amounts of proteins (20 μg) were mixed with an equal volume of 2X Laemmli sample buffer, boiled and resolved by electrophoresis in 10% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE). The proteins were transferred from the gel to a nitrocellulose membrane using an electroblotting apparatus (Bio-Rad) (15 V, 3 mA/cm2 for 24 minutes). Membranes were incubated in blocking solution containing 5% nonfat dry milk overnight to inhibit nonspecific binding. The membranes were then incubated with specific antibody (1–3 μg/ml) for 2 hours. After washing in TRIS/0.1% Tween 20 for 30 minutes the membranes were incubated for another 30 minutes with horseradish peroxidase-conjugated secondary antibody. The membranes were then washed and developed with enhanced chemiluminescence (ECL Western Blotting Kit, Amersham, Arlington Heights, IL).
TransAM assay
Cells were pre-incubated with various concentrations of TPEN for 2 hours. DNA binding activity of NF-κB (p50) was examined in nuclear extracts according to the protocols provided with the TransAM kit (Active Motif, Carlsbad, CA).
Luciferase reporter assay
Cells were transfected with pNF-κB-luc (Stratagene, La Jolla, CA) and pRL-TK (Promega, Madison, WI) plasmids. Twenty-four hours after transfection, cells were treated with various concentrations of TPEN for 4 hours in fresh medium. Samples were assayed for firefly and renilla luciferase activities using Dual-Glo Luciferase assay System (Promega) and normalized as instructed by the manufacturer.
Measurement of VEGF, IL-6 and IL-8 proteins
Cells were treated in the presence or absence of IκBαM adenovirus (1:500 dilution) for 24 hours and then incubated with TPEN (6μM) for 18 hours. IL-6, IL-8 and VEGF levels in cell culture supernatants were determined by ELISA kits (R&D Systems, Minneapolis, MN).
Results
Intracellular zinc concentrations and basal levels of NF-κB activity in normal and transformed prostate cells
We first evaluated intracellular zinc levels in normal (RWPE-1), Ki-ras transformed (RWPE-2) and malignant (DU-145 and PC-3) prostate cell lines. Atomic absorption spectroscopy confirmed the reduction in cellular zinc levels between normal and malignant cells (Fig. 1A). Moreover, Ki-ras transformed RWPE-2 cells demonstrated diminished zinc accumulation compared to parental RWPE-1 normal prostate epithelial cells (Fig. 1A).
Figure 1.
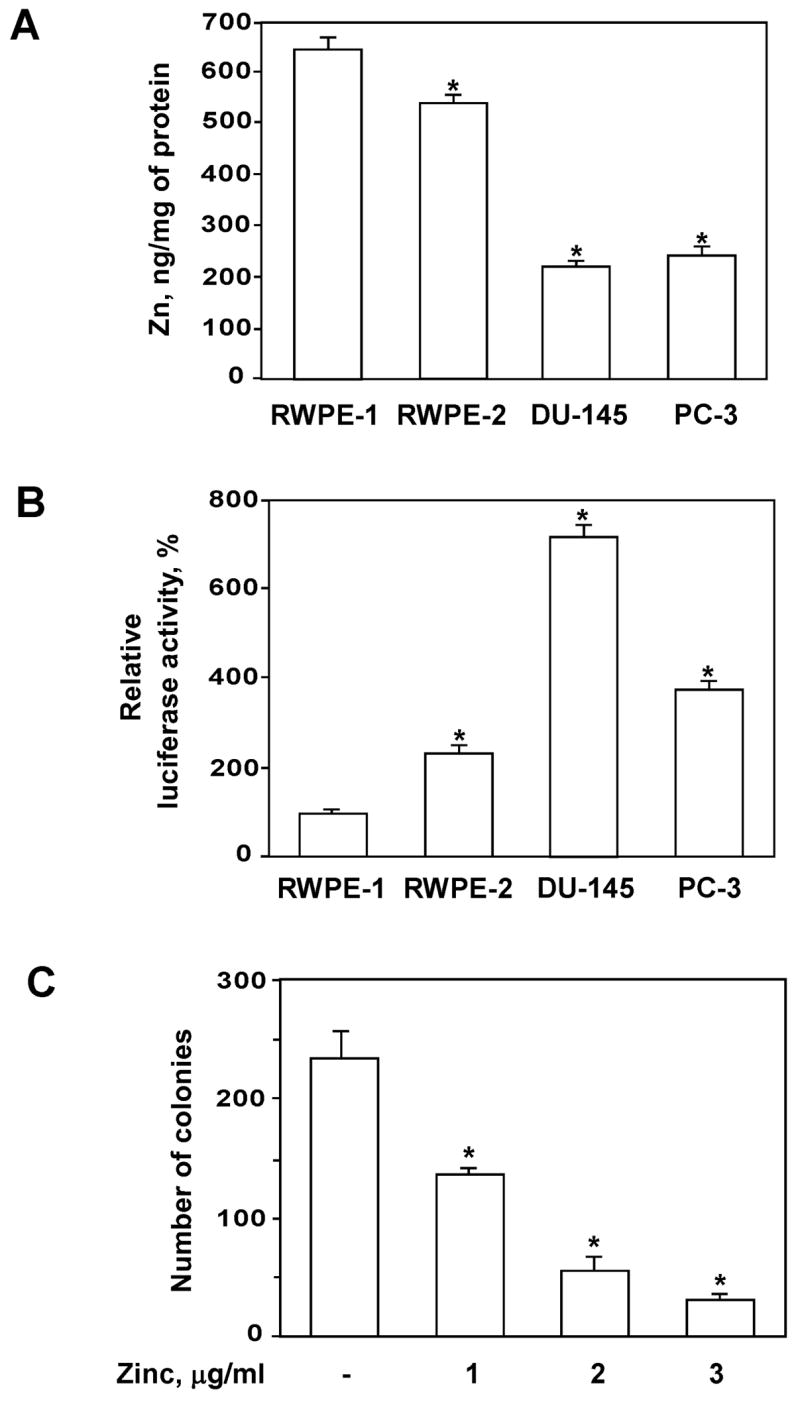
Intracellular zinc concentrations and basal levels of NF-κB activity in normal and transformed prostate cells. (A) Intracellular Zn levels were measured by flame mode using a Shimadzu AA-6300 atomic absorption spectrophotometer as described in Materials and Methods. (B) Basal levels of NF-κB activity were determined by luciferase reporter assay as described in Materials and Methods. Statistical analysis was performed by one-way ANOVA. * Statistically significant (p < 0.05) compared with RWPE-1 cells. RWPE-1 and RWPE-2 cells were maintained in Keratinocyte-Serum Free medium as described in Materials and Methods. Keratinocyte-Serum Free medium was replaced with RPMI 1640 medium containing 10% FCS 3 days prior to the actual experiments. (C) Physiologically relevant concentrations of zinc prevent colony formation in soft agar of PC-3 cells overexpressing hZIP1. * Statistically significant (p < 0.05) compared with cell cultured in medium only.
We have reported previously that physiological levels of zinc reduce NF-κB activity in prostate cancer cells and functionally suppress tumor cell invasiveness and adhesion [22,23]. Therefore the relationship between intracellular zinc concentrations and basal levels of NF-κB activity in prostate cancer cells was evaluated using a luciferase reporter assay. As shown in Figure 1B, basal NF-κB-dependent reporter activity was significantly higher in DU-145 and PC-3 prostate cancer cells compared with normal RWPE-1 cells. These results are in agreement with recent findings describing constitutive NF-κB activity in DU-145 and PC-3 cells [24]. Recent studies reveal that the NF-κB pathway is constitutively active in Ki-ras-transformed prostate epithelial cells [25]. Indeed, the basal level of NF-κB activity was notably higher in RWPE-2 cells than in parental RWPE-1 cells (Fig. 1B). RWPE-1 and RWPE-2 essentially share the same genotype differing only in their tumorigenic status. RWPE-1 cells neither grow in agar nor form tumors when injected into nude mice. However, RWPE-2 cells form colonies in agar and tumors in nude mice [26]. Therefore, our data support recent findings that progression of prostate cells toward greater tumorigenic potential is associated with decreasing levels of intracellular zinc and increasing constitutive levels of NF-κB activity [27,28].
To explore the effect of zinc on the malignant potential of prostate cancer cells, we evaluated colony-forming efficiency in soft agar. Prostate cancer cells have lost the ability to accumulate zinc. Recent studies demonstrate that cells overexpressing the zinc uptake transporter hZIP1 exhibit increased zinc accumulation compared to parental cells [20,27]. Therefore, we generated PC-3 cells overexpressing the hZIP1 transporter and exposed them to zinc. As demonstrated in Figure 1C, physiologically relevant concentrations of zinc (a reference interval for the serum zinc level is 0.5–1.5 μg/ml [29]) prevent colony formation of PC-3-hZIP1 cells in soft agar.
Selective zinc deficiency induced by TPEN stimulates activation of NF-κB in prostate cancer cells
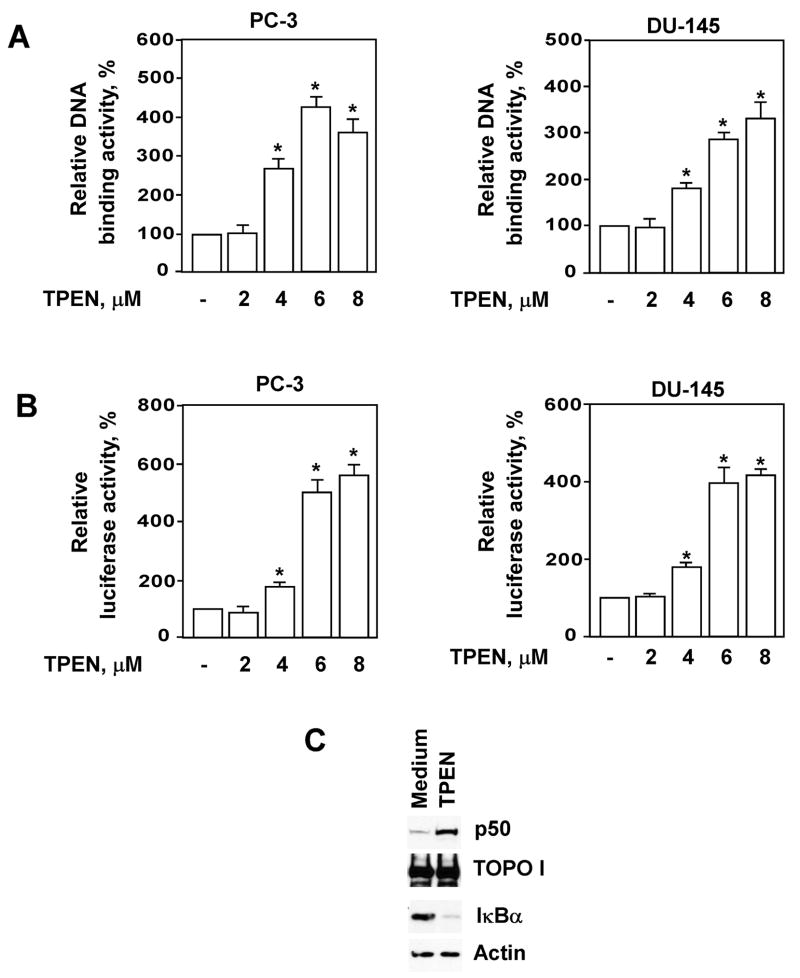
NF-κB regulated genes contribute to neoplastic transformation, metastatic tumor progression and evolution of cancer cells to hormone-independent growth [9,10,18]. Our previous studies demonstrate that physiologic concentrations of zinc inhibit NF-κB activity in prostate cancer cells, reduce expression of pro-angiogenic and pro-metastatic VEGF, IL-6, IL-8, and MMP-9 cytokines, and functionally suppress tumor cell invasiveness and adhesion [22,23]. Treatment with TPEN-induced concentration-dependent NF-κB activation in PC-3 and DU-145 cells as assessed by TransAM (Fig. 2A) and luciferase reporter (Fig. 2B) assays when concentrations were equal to or greater than 4μM. These observed effects were not cell line specific, occurring in both DU-145 and PC-3 cells. To further evaluate the effect of zinc deficiency on NF-κB activation in prostate cancer cells, we performed Western Blot analysis of nuclear and cytoplasmic extracts isolated from TPEN-treated PC-3 cells using anti-p50 and anti-IκBα antibodies. Figure 2C demonstrates that TPEN at 6μM induces nuclear translocation of p50, which coincides with degradation of the inhibitory subunit IκBα.
Figure 2.
Treatment with TPEN activates NF-κB in PC-3 and DU-145 prostate cancer cells. (A) DNA binding activity of NF-κB (p50) was determined by TransAm assay after incubation of prostate cancer cells with indicated concentrations of TPEN for 2 hours. (B) NF-κB activity was determined by luciferase reporter assay after incubation of cells with indicated concentrations of TPEN for 4 hours. Statistical analysis was performed by one-way ANOVA. * Statistically significant (p < 0.05) compared with cells cultured in medium alone. (C) TPEN triggers degradation of IκBα and nuclear translocation of p50 in PC-3 cells. Cells were pre-incubated with 6μM TPEN for 2 hours and the p50 protein level was determined in nuclear extracts by Western blotting analysis with specific antibodies. Expression of TOPO I was used to control equal protein loading. Cytoplasmic extracts from the same samples were subjected to SDS-PAGE followed by Western Blotting with anti-IκBα antibody. Expression of actin was used to control equal protein loading.
TPEN has been used as a zinc-specific chelator in multiple studies [30–32]. However, TPEN has the potential to chelate several other biologically important metals as well. TPEN has a very high affinity for Zn2+ (Kd = 2.6 × 10−16 M), Cu2+ (Kd = 3 × 10−20 M), Fe2+ (Kd = 2.4 × 10−15 M) and Mn2+ (Kd = 5.4 × 10−11 M) and low affinity for Ca2+ (Kd = 4 × 10−5 M) and Mg2+ (Kd = 2 × 10−2 M) [30,33]. To investigate whether zinc depletion specifically caused TPEN-induced NF-κB activation, PC-3 cells cultured in RPMI 1640 medium supplemented with 10% FBS were exposed to TPEN (6kM) with the addition of equimolar concentrations of zinc, copper, iron or manganese ions (Fig. 3). The addition of equimolar concentrations of zinc and copper but not iron or manganese completely blocked TPEN-induced NF-κB activation. In Fetal Bovine Serum (FBS), the total copper concentration is 2.5μM [34]. When FBS is omitted, the RPMI medium contains no detectable copper. In our experiments, because we used medium supplemented with 10% FBS, the concentration of copper in our culture medium was 0.25μM. We have determined zinc concentration in RPMI 1640 medium supplemented with 10% FCS by atomic absorption spectrometry and found this concentration to be 3.8μM. The total zinc (zinc in culture medium plus cellular zinc) in our experiments has been calculated to be approximately 4.2μM. A noticeable effect upon NF-κB activity was detected only when TPEN was used at concentrations equal to or greater than 4μM. Therefore the effect of TPEN on prostate cancer cells was mediated primarily by chelation of zinc and not other biologically relevant metals.
Figure 3.
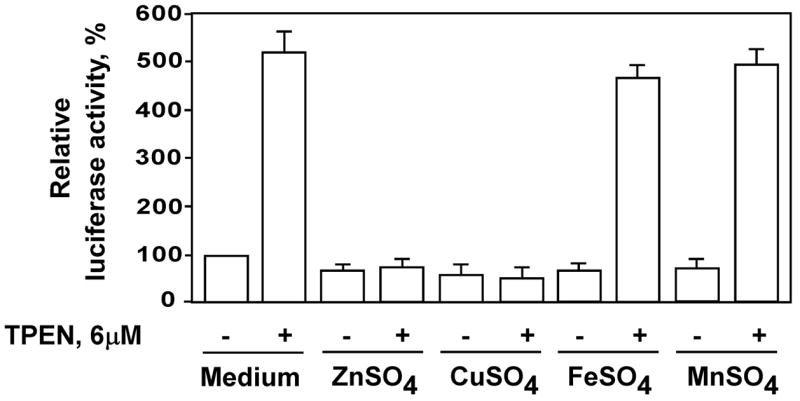
PC-3 cells were pre-incubated with 6μM of ZnSO4, CuSO4, FeSO4 or MnSO4 followed by incubation with equimolar concentration of TPEN for 2 hours. DNA binding activity of NF-κB (p50) was determined by TransAm assay.
NF-κB dependent expression of IL-6, IL-8 and VEGF is up-regulated in zinc-deficient prostate cancer cells
Prostate cancer progression is mediated by secretion of NF-κB-regulated angiogenic factors including VEGF, IL-6 and IL-8. Expression of these molecules by prostate cancer cells has been shown to correlate with malignant potential [35–37]. Since TPEN activates NF-κB, we tested whether TPEN also stimulates expression of these molecules by prostate cancer cells. Expression of VEGF, IL-6 and IL-8 was examined in cell culture supernatants of PC-3 cells incubated with TPEN using ELISA. As shown in Figure 4, supernatants collected from PC-3 cells that were treated with 6μM TPEN had significantly increased amounts of secreted VEGF, IL-6 and IL-8, when compared with supernatants obtained from cells cultured in medium alone.
Figure 4.
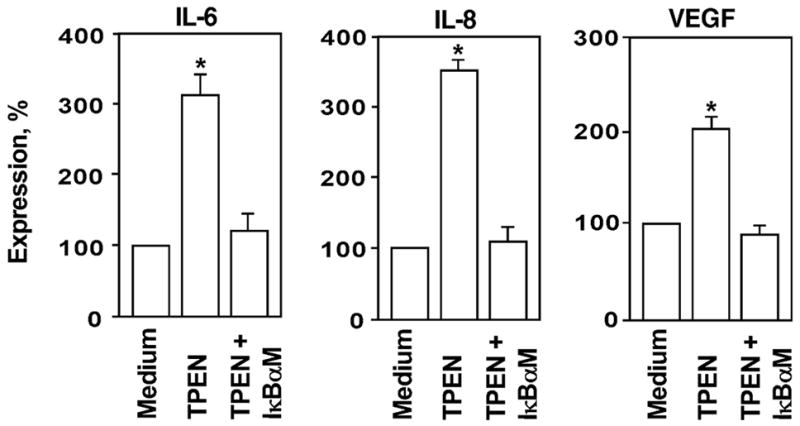
Expression of IL-6, IL-8 and VEGF is up-regulated via NF-κB-dependent mechanism in zinc-deficient PC-3 cells. Cells were cultured in the presence of TPEN (6 μM) for 18 hours. Expression of cytokines was examined in the cell culture supernatants using ELISA kits. To evaluate the contribution of NF-κB signaling for TPEN-induced IL-6, IL-8 and VEGF expression, PC-3 cells were pre-incubated with IκBαM adenovirus (1:500) for 24 hours prior to TPEN stimulation. Statistical analysis was performed by one-way ANOVA. * Statistically significant (p < 0.05) compared with cells cultured in medium alone.
To evaluate the contribution of NF-κB signaling to TPEN-induced cytokine expression, PC-3 cells were pre-incubated with the IκBαM [38] adenovirus prior to TPEN stimulation. Pretreatment with IκBαM adenovirus efficiently blocked TPEN-stimulated expression of all tested cytokines (Fig. 4). Identical findings were also noted after treatment with the pharmacological NF-κB inhibitor BAY 11–7085 (data not shown). These data reinforce the critical role of the NF-κB pathway under zinc deficient conditions in prostate cancer and suggest that zinc deficiency augments the malignant phenotype in part through increased expression of pro-tumorigenic cytokines.
Discussion
The precise mechanisms involved in the development and progression of prostate cancer remain unclear. Multiple studies demonstrate that the inability to retain normal intracellular levels of zinc is a hallmark of prostatic malignancies. Indeed, the drop in intracellular zinc concentration occurs early in the development of prostate cancer, precedes histopathological changes, and continues during progression toward hormone-independent growth [39,40]. Clinically, cancer cells that maintain higher levels of intracellular zinc have been shown to have significantly slower growth rates [20,27].
NF-κB activation is a major pathway contributing to prostate cancer development and progression (3–11). We have reported previously that physiological levels of zinc suppress NF-κB activity in prostate cancer cells, sensitize malignant cells to apoptosis induced by cytotoxic agents and reduce expression of pro-angiogenic and pro-metastatic cytokines VEGF, IL-6, IL-8, and MMP-9 associated with negative prognostic features in prostate cancer [22,23]. Importantly, expression of VEGF, IL-6 and IL-8 has been shown to correlate directly with malignant potential of prostate cancer [35–37]. Our current experiments reveal that zinc deficiency induced by zinc-specific chelation via TPEN increases DNA binding and transcriptional activity of NF-κB in prostate cancer cells. Moreover, TPEN-mediated NF-κB activation coincides with an increased amount of secreted VEGF, IL-6 and IL-8 by PC-3 prostate cancer cells. Pre-treatment with the IκBαM adenovirus efficiently blocked expression of VEGF, IL-6 and IL-8 in zinc deficient cells demonstrating the critical role of the NF-κB pathway in augmenting expression of these cytokines.
Normal human epithelial cells can be maintained as replicative cultures only for a short time before they undergo senescence. Several immortalized cell lines of prostatic origin have been established by transfection with a plasmid containing the entire HPV-18 genome including the non-tumorigenic prostate epithelial cell line, RWPE-1, which was further transformed by v-Ki-ras to a tumorigenic cell line, RWPE-2 [41,42]. RWPE-1 cells retain many of the characteristics of normal cells, such as contact inhibition, anchorage dependence, inability to form tumors in nude mice, and expression of epithelial and prostate-specific proteins. Yet, a possibility exists that immortalized cells do not always accurately recapitulate the genetic composition or biological behavior of primary cells. Nonetheless, recent studies demonstrate that the expression of the 19 zinc transporters is similar between the RWPE-1 cell line and the in situ prostate gland [43]. Moreover, while RWPE-1 and RWPE-2 cells essentially share the same genetic background, tumorigenic RWPE-2 cells accumulate less intracellular zinc and have reduced levels of the hZIP1 protein compared with non-tumorigenic RWPE-1 cells [27]. These findings suggest that immortalized RWPE-1 and RWPE-2 cell lines can serve as a valid model system to study the role of zinc in prostate cancer.
The relationship between intracellular zinc levels and status of NF-κB activity provides a rational basis for the concept that restoration of normal zinc levels in malignant cells may be efficacious in the treatment and secondary prevention of prostate cancer.
Conclusions
In summary, our findings demonstrate for the first time that selective zinc deficiency in malignant prostate tissues may augment expression of pro-tumorigenic cytokines via activation of NF-κB-regulated pathways and therefore may contribute to prostate tumor progression.
Footnotes
Grant sponsor: National Institutes of Health. Grant RO1 CA108890.
References
- 1.Zaichick V, Sviridova TV, Zaichick SV. Zinc in the human prostate gland: normal, hyperplastic and cancerous. Int Urol Nephrol. 1997;29(5):565–574. doi: 10.1007/BF02552202. [DOI] [PubMed] [Google Scholar]
- 2.Rishi I, Baidouri H, Abbasi JA, Bullard-Dillard R, Kajdacsy-Balla A, Pestaner JP, Skacel M, Tubbs R, Bagasra O. Prostate cancer in African American men is associated with downregulation of zinc transporters. Appl Immunohistochem Mol Morphol. 2003;11(3):253–260. doi: 10.1097/00129039-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Kajdacsy-Balla A, Bagasra O, Costello LC. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer. 2005;4:32. doi: 10.1186/1476-4598-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate. 2002;52(4):311–318. doi: 10.1002/pros.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang JY, Liu YY, Zou J, Franklin RB, Costello LC, Feng P. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate. 1999;40(3):200–207. doi: 10.1002/(sici)1097-0045(19990801)40:3<200::aid-pros8>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishii K, Usui S, Sugimura Y, Yoshida S, Hioki T, Tatematsu M, Yamamoto H, Hirano K. Aminopeptidase N regulated by zinc in human prostate participates in tumor cell invasion. Int J Cancer. 2001;92(1):49–54. [PubMed] [Google Scholar]
- 7.Gilmore TD. The Rel/NF-kappaB signal transduction pathway: introduction. Oncogene. 1999;18(49):6842–6844. doi: 10.1038/sj.onc.1203237. [DOI] [PubMed] [Google Scholar]
- 8.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18(49):6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 9.Hsu TC, Nair R, Tulsian P, Camalier CE, Hegamyer GA, Young MR, Colburn NH. Transformation nonresponsive cells owe their resistance to lack of p65/nuclear factor-kappaB activation. Cancer Res. 2001;61(10):4160–4168. [PubMed] [Google Scholar]
- 10.Sweeney C, Li L, Shanmugam R, Bhat-Nakshatri P, Jayaprakasan V, Baldridge LA, Gardner T, Smith M, Nakshatri H, Cheng L. Nuclear factor-kappaB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin Cancer Res. 2004;10(16):5501–5507. doi: 10.1158/1078-0432.CCR-0571-03. [DOI] [PubMed] [Google Scholar]
- 11.Palayoor ST, Youmell MY, Calderwood SK, Coleman CN, Price BD. Constitutive activation of IkappaB kinase alpha and NF-kappaB in prostate cancer cells is inhibited by ibuprofen. Oncogene. 1999;18(51):7389–7394. doi: 10.1038/sj.onc.1203160. [DOI] [PubMed] [Google Scholar]
- 12.Supakar PC, Jung MH, Song CS, Chatterjee B, Roy AK. Nuclear factor kappa B functions as a negative regulator for the rat androgen receptor gene and NF-kappa B activity increases during the age-dependent desensitization of the liver. J Biol Chem. 1995;270(2):837–842. doi: 10.1074/jbc.270.2.837. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama T, Watanabe M, Suzuki H, Toyota M, Sekita N, Hirokawa Y, Mizokami A, Ito H, Yatani R, Shiraishi T. Epigenetic regulation of androgen receptor gene expression in human prostate cancers. Lab Invest. 2000;80(12):1789–1796. doi: 10.1038/labinvest.3780190. [DOI] [PubMed] [Google Scholar]
- 14.Li LC, Zhao H, Nakajima K, Oh BR, Filho LA, Carroll P, Dahiya R. Methylation of the E-cadherin gene promoter correlates with progression of prostate cancer. J Urol. 2001;166(2):705–709. [PubMed] [Google Scholar]
- 15.Bloem A, Lockhorst H. Bcl-2 antisense therapy in multiple myeloma. Pathol Biol (Paris) 1999;47(2):216–220. [PubMed] [Google Scholar]
- 16.Lashinger LM, Zhu K, Williams SA, Shrader M, Dinney CP, McConkey DJ. Bortezomib abolishes tumor necrosis factor-related apoptosis-inducing ligand resistance via a p21-dependent mechanism in human bladder and prostate cancer cells. Cancer Res. 2005;65(11):4902–4908. doi: 10.1158/0008-5472.CAN-04-3701. [DOI] [PubMed] [Google Scholar]
- 17.Tu Y, Renner S, Xu F, Fleishman A, Taylor J, Weisz J, Vescio R, Rettig M, Berenson J, Krajewski S, Reed JC, Lichtenstein A. BCL-X expression in multiple myeloma: possible indicator of chemoresistance. Cancer Res. 1998;58(2):256–262. [PubMed] [Google Scholar]
- 18.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20(31):4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 19.Jeon KI, Jeong JY, Jue DM. Thiol-reactive metal compounds inhibit NF-kappa B activation by blocking I kappa B kinase. J Immunol. 2000;164(11):5981–5989. doi: 10.4049/jimmunol.164.11.5981. [DOI] [PubMed] [Google Scholar]
- 20.Franklin RB, Ma J, Zou J, Guan Z, Kukoyi BI, Feng P, Costello LC. Human ZIP1 is a major zinc uptake transporter for the accumulation of zinc in prostate cells. J Inorg Biochem. 2003;96(2–3):435–442. doi: 10.1016/s0162-0134(03)00249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolenko V, Bloom T, Rayman P, Bukowski R, Hsi E, Finke J. Inhibition of NF-kappa B activity in human T lymphocytes induces caspase-dependent apoptosis without detectable activation of caspase-1 and -3. J Immunol. 1999;163(2):590–598. [PubMed] [Google Scholar]
- 22.Uzzo RG, Crispen P, Golovine K, Makhov P, Horwitz E, Kolenko VM. Diverse effects of zinc on NF-kB and AP-1 transcription factors: Implications for prostate cancer progression. Carcinogenesis. 2006 doi: 10.1093/carcin/bgl034. [DOI] [PubMed] [Google Scholar]
- 23.Uzzo RG, Leavis P, Hatch W, Gabai VL, Dulin N, Zvartau N, Kolenko VM. Zinc Inhibits Nuclear Factor-kappaB Activation and Sensitizes Prostate Cancer Cells to Cytotoxic Agents. Clin Cancer Res. 2002;8(11):3579–3583. [PubMed] [Google Scholar]
- 24.Suh J, Payvandi F, Edelstein LC, Amenta PS, Zong WX, Gelinas C, Rabson AB. Mechanisms of constitutive NF-kappaB activation in human prostate cancer cells. Prostate. 2002;52(3):183–200. doi: 10.1002/pros.10082. [DOI] [PubMed] [Google Scholar]
- 25.Kim BY, Gaynor RB, Song K, Dritschilo A, Jung M. Constitutive activation of NF-kappaB in Ki-ras-transformed prostate epithelial cells. Oncogene. 2002;21(29):4490–4497. doi: 10.1038/sj.onc.1205547. [DOI] [PubMed] [Google Scholar]
- 26.Bello D, Webber MM, Kleinman HK, Wartinger DD, Rhim JS. Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis. 1997;18(6):1215–1223. doi: 10.1093/carcin/18.6.1215. [DOI] [PubMed] [Google Scholar]
- 27.Huang L, Kirschke CP, Zhang Y. Decreased intracellular zinc in human tumorigenic prostate epithelial cells: a possible role in prostate cancer progression. Cancer Cell Int. 2006;6(1):10. doi: 10.1186/1475-2867-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suh J, Rabson AB. NF-kappaB activation in human prostate cancer: important mediator or epiphenomenon? J Cell Biochem. 2004;91(1):100–117. doi: 10.1002/jcb.10729. [DOI] [PubMed] [Google Scholar]
- 29.Henry J. Clinical Diagnosis and Management by Laboratory Methods. 19. Philadelphia, PA: WB Saunders Co; 1996. [Google Scholar]
- 30.Hyun HJ, Sohn JH, Ha DW, Ahn YH, Koh JY, Yoon YH. Depletion of intracellular zinc and copper with TPEN results in apoptosis of cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2001;42(2):460–465. [PubMed] [Google Scholar]
- 31.Kolenko V, Uzzo RG, Bukowski R, Bander NH, Novick AC, Hsi ED, Finke JH. Dead or dying: necrosis versus apoptosis in caspase-deficient human renal cell carcinoma. Cancer Res. 1999;59(12):2838–2842. [PubMed] [Google Scholar]
- 32.Kolenko VM, Uzzo RG, Dulin N, Hauzman E, Bukowski R, Finke JH. Mechanism of apoptosis induced by zinc deficiency in peripheral blood T lymphocytes. Apoptosis. 2001;6(6):419–429. doi: 10.1023/a:1012497926537. [DOI] [PubMed] [Google Scholar]
- 33.Arslan P, Di Virgilio F, Beltrame M, Tsien RY, Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas/ A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+ J Biol Chem. 1985;260(5):2719–2727. [PubMed] [Google Scholar]
- 34.Glassman AB, Rydzewski RS, Bennett CE. Trace metal levels in commercially prepared tissue culture media. Tissue Cell. 1980;12(4):613–617. doi: 10.1016/0040-8166(80)90016-6. [DOI] [PubMed] [Google Scholar]
- 35.Ferrer FA, Miller LJ, Andrawis RI, Kurtzman SH, Albertsen PC, Laudone VP, Kreutzer DL. Angiogenesis and prostate cancer: in vivo and in vitro expression of angiogenesis factors by prostate cancer cells. Urology. 1998;51(1):161–167. doi: 10.1016/s0090-4295(97)00491-3. [DOI] [PubMed] [Google Scholar]
- 36.Aalinkeel R, Nair MP, Sufrin G, Mahajan SD, Chadha KC, Chawda RP, Schwartz SA. Gene expression of angiogenic factors correlates with metastatic potential of prostate cancer cells. Cancer Res. 2004;64(15):5311–5321. doi: 10.1158/0008-5472.CAN-2506-2. [DOI] [PubMed] [Google Scholar]
- 37.Strohmeyer D, Rossing C, Bauerfeind A, Kaufmann O, Schlechte H, Bartsch G, Loening S. Vascular endothelial growth factor and its correlation with angiogenesis and p53 expression in prostate cancer. Prostate. 2000;45(3):216–224. doi: 10.1002/1097-0045(20001101)45:3<216::aid-pros3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 38.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278(5339):860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 39.Costello LC, Feng P, Milon B, Tan M, Franklin RB. Role of zinc in the pathogenesis and treatment of prostate cancer: critical issues to resolve. Prostate Cancer Prostatic Dis. 2004;7(2):111–117. doi: 10.1038/sj.pcan.4500712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Costello LC, Franklin RB. Novel role of zinc in the regulation of prostate citrate metabolism and its implications in prostate cancer. Prostate. 1998;35(4):285–296. doi: 10.1002/(sici)1097-0045(19980601)35:4<285::aid-pros8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 41.Rhim JS, Webber MM, Bello D, Lee MS, Arnstein P, Chen LS, Jay G. Stepwise immortalization and transformation of adult human prostate epithelial cells by a combination of HPV-18 and v-Ki-ras. Proc Natl Acad Sci U S A. 1994;91(25):11874–11878. doi: 10.1073/pnas.91.25.11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webber MM, Bello D, Quader S. Immortalized and tumorigenic adult human prostatic epithelial cell lines: characteristics and applications. Part I Cell markers and immortalized nontumorigenic cell lines. Prostate. 1996;29(6):386–394. doi: 10.1002/(SICI)1097-0045(199612)29:6<386::AID-PROS7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 43.Albrecht AL, Somji S, Sens MA, Sens DA, Garrett SH. Zinc transporter mRNA expression in the RWPE-1 human prostate epithelial cell line. Biometals. 2007 doi: 10.1007/s10534-007-9129-0. [DOI] [PubMed] [Google Scholar]