Abstract
Background
Experts recommend that clinicians target mammography and colon cancer screening to individuals with at least 5 years life expectancy. Immunizations and exercise counseling are generally recommended for all women aged ≥ 65 while PAP smears are generally not encouraged for these women.
Methods
We used the 2005 National Health Interview Survey to examine receipt of several preventive health measures simultaneously among community dwelling US women aged ≥ 65 by age and health status. We used functional status, significant diseases, and perceived health to categorize women into those most likely to be in above average, average, or below average health status. We used age and health status to estimate life expectancy.
Results
Of 4,683 participants, 25.8% were ≥ 80 years; 81.8% were non-Hispanic white; 21% were in above average and 20% were in below average health status. Receipt of mammography and colon cancer screening declined with age and was not associated with health status for women aged ≥80. Nearly half (49%) of women aged ≥80 in below average health received mammography screening while 19% of women aged 65–79 in above average health did not report receiving mammography. Nearly half of women aged 65–79 (49%) in above average health did not report receiving colon cancer screening. PAP smear screening was common among older women. Few (34%) reported receiving exercise counseling. Many did not report receiving pneumococcal (43%) or flu vaccinations (40%).
Conclusions
In our comprehensive review of preventive health measures for older women, we found evidence to suggest a need to improve delivery and targeting of preventive health services.
Introduction
Many preventive health measures are available to elderly women.1 Since life expectancy greatly varies with health status, geriatricians recommend that services be targeted to elderly individuals most likely to benefit.2 In the case of breast and colon cancer screening, experts recommend that screening be targeted to those with at least 5 years of life expectancy.3–4 Other preventive health measures such as pneumococcal and influenza vaccinations and exercise counseling are generally recommended for all women aged 65 and older while PAP smears are generally not encouraged for these women.5–7
Several studies have examined targeting of mammography and PAP smear screening to older women according to health.8–10 These studies found that decisions to screen older women for cancer are generally related to health; however, they also showed that many older women in good health are not screened while many in poor health are screened. No studies have examined how a wide range of preventive health services are targeted to older women by health status. Yet, in practice, primary care physicians are faced with the responsibility of discussing a large scope of preventive services with their older female patients and prioritizing which services to pursue. Since our previous work found that clinicians often find discussing stopping cancer screening uncomfortable and there are many competing demands during clinic visits, older women in poor health may receive some preventive health measures from which they are unlikely to benefit (e.g., pap smears) while not receiving other preventive health measures from which they would benefit (e.g., flu shots, exercise counseling).11 Our study considers multiple preventive health measures simultaneously to closely represent the complexities of decision-making and service delivery faced in primary care. We hypothesized that cancer screening tests would be poorly targeted to elderly women with regards to health status while immunizations and exercise counseling would be underutilized across all age and health strata.
Methods
Data Source
We used the 2005 National Health Interview Survey (NHIS), a continuing, in-person household survey of the civilian, non-institutionalized US population, conducted by the Census Bureau for the National Center for Health Statistics.12 The NHIS collects information from a nationally representative sample including sociodemographic factors, functional status, insurance coverage, and access to health care for all household members. One randomly selected adult from each responding family is asked to complete “the sample adult module,” which includes questions about medical conditions, receipt of immunizations, and exercise recommendations (N=31,428). In 2005, these respondents were also asked to complete a cancer module which included questions on receipt of cancer screening. The overall response rate was 69.0%.
Study Sample
The 2005 NHIS interviewed 5,601 women 65 years or older. We excluded 594 women who had not seen a health care provider in the past year since we planned to examine reported receipt of several preventive services delivered in the past year. We also excluded 281 women with proxy respondents and 43 women with dementia. Our final sample consisted of 4,683 women, representing an estimated 30 million, US community dwelling women aged 65 or older. We categorized women into five age groups (65–69, 70–74, 75–79, 80–84, and ≥85).
Outcomes
Preventive Health Services
We examined receipt of cancer screening (mammography, colon cancer screening, and PAP smears), immunizations (pneumococcal and influenza), and exercise counseling. We defined women as having undergone mammography screening if they did not have a history of breast cancer and they reported having a mammogram as part of a routine exam within 2 years. We defined women as having undergone colon cancer screening if they did not have a history of colon cancer and they reported 1) a colonoscopy within 10 years, 2) a sigmoidoscopy within 5 years, or 3) a fecal occult blood test (FOBT) within one year, all as part of a routine exam. We defined women as having received cervical cancer screening if they still had a uterus and reported having a PAP smear as part of a routine exam within 3 years. Women who reported having a flu shot in the past year and women who reported ever receiving a pneumonia shot were defined as having received these immunizations. We considered women to have received exercise counseling if they reported that a medical professional recommended that they begin or continue to do any type of exercise or physical activity in the past year. We excluded women who refused, did not answer, or were missing whether or not they had received the specific measure.
Health Status
Walter and Covinsky proposed that although it is impossible for physicians to predict the exact life expectancy of an individual patient, it is possible for physicians to make reasonable estimates of whether a patient is likely to live more, less, or as long as the average person their age.3 After deciding whether a patient is in above, average, or below average health, clinicians may then use life expectancy charts divided by age and quartiles of health to estimate individual life expectancy.3 For example, a woman aged 80–84 in above average health has an estimated life expectancy of 13 years while one in average health has an estimated life expectancy of 8.6 years and one in below average health has an estimated life expectancy of 4.6 years.3 Since the NHIS represents the non-institutionalized population of the US, our aim was to categorize the women in our sample into those most likely to be in above average, average, or below average health status to approximate life expectancy. We used functional status, significant diseases, and perceived health to categorize women into these groups of health status. We chose these measures since experts recommend that clinicians use functional status and comorbidity in addition to age when estimating patient life expectancy4 and perceived health is an independent predictor of survival.13 We only included significant diseases that are included in the Charlson Comorbidity Index and were available in the NHIS: heart disease, diabetes, stroke, cancer [excluding non-melanomatous skin cancer], COPD, kidney failure, and liver failure.14
We considered women to be in below average health status if they had three or more significant diseases or a functional limitation (dependent in a basic or instrumental activity of daily living) or perceived themselves to be in poor health. We considered women to be in above average health status if they did not have any significant diseases or functional limitations and perceived themselves to be in very good/excellent health. We considered women to be in average health status if they had 1 to 2 significant diseases and/or perceived themselves to be in good/fair health but had no functional limitations.
Finally, we examined appropriate receipt of all 6 preventive health measures in different populations of older women. Since most guidelines would recommend mammography and colon cancer screening as well as immunizations but not PAP smear screening to women aged 70–79 in above average health status, we examined the proportion of these women who received the 6 measures appropriately. Similarly, since experts would generally discourage cancer screening among women aged 80 and older in below average health status but would encourage they receive immunizations and exercise counseling we also examined receipt of appropriate care among these women.
Covariates
We considered factors previously found to be associated with receipt of preventive services as potential confounders.15–16 These included sociodemographic factors such as race/ethnicity (non-Hispanic white, non-Hispanic black, other), education (less than high school, high school graduate, some college or beyond), annual family income (<$20,000, $20,000–$34,999, $35,000+), insurance (Medicare Part A only/No coverage; Medicare Part A and B; Medicare plus Medicaid; and Medicare Plus Choice, Medicare plus private, and private insurance only), and geographical region (Northeast, Midwest, South, West). We considered two indicators of access to care including usual source of medical care (primary care physician or gynecologist, specialist, or having no usual source) and number of doctor visits in the past year.
Statistical Analysis
We used the Mantel-Haenszel test of trend to determine whether health status was a significant predictor of receipt of each preventive health measure within each age group. We also performed multivariable logistic regression models to compare receipt of each preventive health service by age group and health status adjusted for all covariates. We tested for interactions between age and health status on receipt of preventive health measures. All analyses used SAS-callable SUDAAN software, version 9.1 (Research Triangle Institute, Research Triangle Park, North Carolina). Results presented herein are weighted to reflect US population estimates and to adjust for non-response.
Results
Of the 4,683 women in our study, 30.3% were aged 65–69, 23.9% were aged 70–74, 20.0% were aged 75–79, 15.6% were aged 80–84 and 10.2% were aged 85 or older; the majority (81.8%) were non-Hispanic white. Ten percent had only seen their clinician once within the past year and this varied slightly by age (12.1% of women aged 65–79 vs. 7.6% of women aged 85 and older). With respect to health status, 21.3% were in above average health, 58.3% were in average health, and 20.4% were in below average health (Table 1).
Table 1.
Sample Characteristics (n=4,683)*
| Overall % | 65–69 years (n=1,330) | 70–74 years (n=1,115) | 75–79 years (n=971) | 80–84 years (n=749) | 85+ (n=518) | P value | ||
|---|---|---|---|---|---|---|---|---|
| Race: | Non-Hispanic White | 81.8 | 78.7 | 78.7 | 83.3 | 85.1 | 84.3 | 0.29 |
| Non-Hispanic Black | 7.6 | 8.0 | 8.3 | 7.5 | 6.2 | 7.3 | ||
| Other | 10.6 | 13.4 | 10.5 | 9.3 | 8.7 | 8.4 | ||
| Education: (4,602) | <High-School | 24.9 | 19.9 | 21.7 | 28.5 | 28.2 | 35.3 | 0.0001 |
| High-School graduate | 37.1 | 38.1 | 37.8 | 38.2 | 34.8 | 33.4 | ||
| Some college | 21.6 | 22.1 | 22.4 | 19.2 | 23.9 | 19.2 | ||
| College or beyond | 16.5 | 19.9 | 18.1 | 14.2 | 13.1 | 12.2 | ||
| Income: (4,287) | <$20K | 27.5 | 22.1 | 23.4 | 32.3 | 32.2 | 37.0 | <0.0001 |
| $20–<$35K | 40.4 | 40.1 | 40.7 | 39.2 | 42.6 | 39.2 | ||
| >$35K | 32.1 | 37.9 | 35.9 | 28.5 | 25.2 | 23.8 | ||
| Insurance (4,664) | Medicare Part A only/No Coverage | 2.1 | 2.4 | 2.9 | 2.1 | 1.6 | 0.13 | <0.0001 |
| Medicare Parts A and B only | 21.5 | 21.8 | 20.1 | 22.6 | 19.9 | 23.7 | ||
| Medicaid | 7.5 | 8.0 | 8.1 | 6.6 | 7.8 | 5.9 | ||
| Private/Medicare+Choice | 69.0 | 67.8 | 68.8 | 68.8 | 70.8 | 70.3 | ||
| Region | Northeast | 21.2 | 20.6 | 18.4 | 21.8 | 24.0 | 24.0 | 0.05 |
| Midwest | 24.6 | 26.3 | 23.0 | 22.2 | 24.3 | 28.1 | ||
| South | 36.8 | 36.0 | 40.3 | 38.4 | 36.3 | 28.5 | ||
| West | 17.5 | 17.1 | 18.3 | 17.6 | 15.5 | 19.4 | ||
| Usual Care | Primary Care MD | 90.9 | 90.5 | 89.5 | 90.0 | 92.6 | 94.2 | 0.01 |
| Specialist | 5.0 | 4.7 | 6.4 | 6.7 | 3.0 | 2.1 | ||
| No Usual Source | 4.2 | 4.8 | 4.2 | 3.3 | 4.4 | 3.8 | ||
| MD visits in past year | 1 visit | 10.3 | 12.1 | 11.5 | 8.7 | 8.8 | 7.6 | 0.01 |
| 2–5 | 47.3 | 49.0 | 42.8 | 50.5 | 44.5 | 51.1 | ||
| 6+ visits | 42.4 | 38.9 | 45.8 | 40.8 | 46.7 | 41.3 | ||
| Health Characteristics: | ||||||||
| Significant Diseases† | None | 45.3 | 51.3 | 46.1 | 42.3 | 39.5 | 40.6 | 0.001 |
| 1–2 | 49.1 | 43.8 | 47.0 | 51.7 | 54.9 | 55.5 | ||
| 3+ | 5.6 | 4.9 | 6.9 | 6.0 | 5.6 | 3.9 | ||
| Functional Dependency‡ (4,682) | None | 86.5 | 93.7 | 90.3 | 86.2 | 80.8 | 66.0 | <0.0001 |
| IADL only | 7.0 | 3.6 | 4.4 | 7.6 | 10.3 | 16.4 | ||
| ADL | 6.5 | 2.7 | 5.3 | 6.3 | 8.9 | 17.6 | ||
| Perceived Health (4,682) | Very Good/Excellent38.0 | 42.9 | 41.5 | 35.7 | 31.4 | 29.4 | <0.0001 | |
| Good | 35.2 | 34.8 | 33.6 | 33.6 | 37.9 | 38.9 | ||
| Fair | 19.3 | 16.8 | 17.8 | 22.0 | 21.0 | 22.5 | ||
| Poor | 7.6 | 5.5 | 7.2 | 8.7 | 9.7 | 9.3 | ||
| Health Status§ | Above Average 21.3 | 26.8 | 24.1 | 18.8 | 15.2 | 12.9 | <0.0001 | |
| Average | 58.3 | 60.5 | 58.0 | 59.3 | 59.0 | 49.7 | ||
| Below Average 20.4 | 12.7 | 18.0 | 21.9 | 25.8 | 37.5 | |||
All proportions were weighted to reflect national estimates.
Significant diseases included heart disease, diabetes, cancer (excluding non-melanomatous skin cancer), Chronic Obstructive Pulmonary Disease (COPD), liver disease, and kidney failure.
IADL– unable to perform an Instrumental Activity of Daily Living but able to perform all Activities of Daily Living.
ADL - unable to perform an Activity of Daily Living.
Perceived health, functional limitations, and a comorbidity count were used to classify women as either above average, average, or below average in health (page 7).
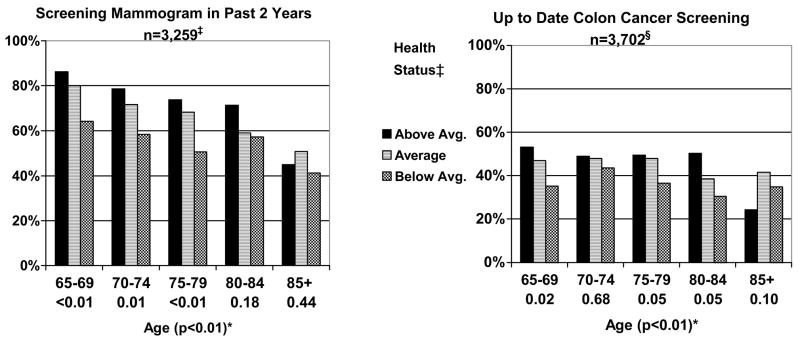
Cancer Screening (Figure 1)
Figure 1.
Reported Receipt of Cancer Screening Tests by Age and Health Status for Women aged 65 and Older (n=4,683).* †‡§
* Below each age category (65–69, 70–74, 75–79, 80–84, and 85+) is the P value for the test of trend for the influence of health on receipt of preventive health measures within each age group. In parenthesis next to age is the P value for the test of trend for the influence of age across all age groups.
† Perceived health, functional limitations, and a comorbidity count were used to classify women as either above average, average, or below average in health (page 7).
‡ Excluded 310 women with a history of breast cancer, 81 who had a recent mammogram for reasons besides screening, 281 who were missing data on their last mammogram and 752 who were missing completely from the cancer control module.
§ Excluded 92 women with a history of colon cancer, 328 missing whether or not they ever had a sigmoidoscopy or colonoscopy or home blood stool test, 600 who had a recent sigmoidoscopy/colonscopy or home blood stool test for reasons besides screening, and 43 who were missing completely from the cancer control module.
Reported receipt of mammography screening declined with age. Above average health status was a significant predictor of receipt of mammography screening among women aged 65–79. Although there were no significant associations between health status and receipt of mammography screening among women aged 80 and older, women aged 80–84 in above average health tended to be more likely to be screened than those in below average health. There were no trends in receipt of mammography screening by health status among women aged 85 and older. Overall, 18.8% of women aged 65–79 in above average health did not report being screened while 48.9% of women aged 80 and older in below average health reported being screened.
In the case of colon cancer screening, we found that 44.7% of women aged 65 and older reported receiving colon cancer screening in the past 10 years; the majority with colonoscopy (78.5%) and in the past 5 years (91.6%). Reported receipt of colon cancer screening declined with increasing age. Above average health status was a significant predictor of receipt of colon cancer screening for women aged 65–69 and similar trends were evident for women aged 75–84. However, health status was not significantly associated with reported receipt of colon cancer screening among women aged 70–74 and 85 and older. Nearly half (49.0%) of women aged 65–79 in above average health did not report receiving any form of colon cancer screening.
PAP smears were common but declined with advancing age; 68.3% of women aged 65–79 and 45.6% of women aged 80 and older reported being screened. Reported receipt of PAP smear screening was significantly associated with above average health among women aged 75–79 (p<0.01). There were no other significant associations between health status and receipt of PAP smear screening (data not shown).
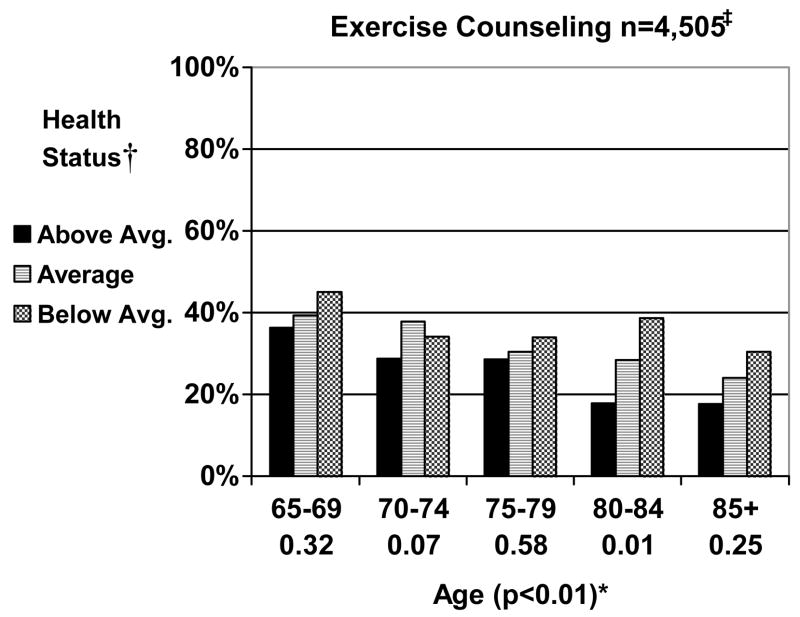
Exercise Counseling (Figure 2)
Figure 2.
Reported Receipt of Exercise Counseling by Age and Health Status for Women aged 65 and Older (n=4,683). * †‡
* Below each age category (65–69, 70–74, 75–79, 80–84, and 85+) is the P value for the test of trend for the influence of health on receipt of preventive health measures within each age group. In parenthesis next to age is the P value for the test of trend for the influence of age across all age groups.
† Perceived health, functional limitations, and a comorbidity count were used to classify women as either above average, average, or below average in health (page 7).
‡ Excluded 178 women who were missing whether or not they received exercise counseling.
Overall, 33.7% of women reported receiving exercise counseling. Exercise counseling declined with advancing age and tended to be more common among women in below average health status. Exercise counseling was significantly more common among women aged 80–84 in below average health than for those in above average health. Only 17.9% of women age 80 and older in above average health status reported receiving exercise counseling.
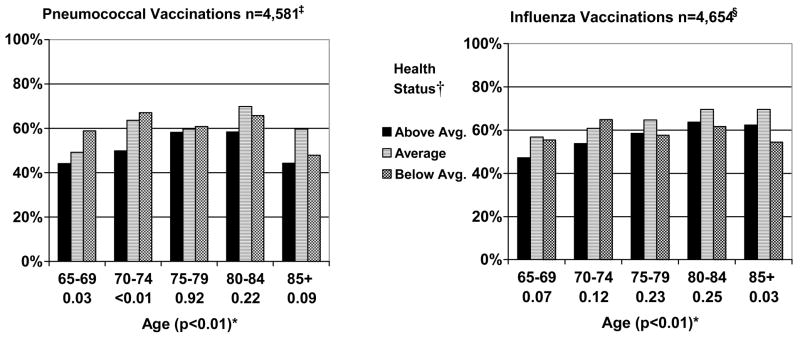
Immunizations (Figure 3)
Figure 3.
Reported Receipt of Immunizations by Age and Health Status for Women aged 65 and Older (n=4,683). * †‡§
* Below each age category (65–69, 70–74, 75–79, 80–84, and 85+) is the P value for the test of trend for the influence of health on receipt of preventive health measures within each age group. In parenthesis next to age is the P value for the test of trend for the influence of age across all age groups.
† Perceived health, functional limitations, and a comorbidity count were used to classify women as either above average, average, or below average in health (page 7).
‡ Excluded 103 women who were missing whether or not they ever received a pneumonia vaccination.
§ Excluded 29 women who were missing whether or not they had a flu shot in the past year.
Many women aged 65 and older did not report ever receiving pneumonia (42.7%) or flu immunizations in the past year (40.2%). Reported receipt of immunizations generally increased with age until 85. Women aged 65–74 in below average health status were significantly more likely to receive pneumococcal immunizations than those in above average health status. Otherwise there were no significant associations between health status and receipt of pneumococcal immunizations. There were no significant associations between health status and receipt of influenza vaccinations, except that women aged 85 and older in below average health were less likely to receive influenza vaccinations than women aged 85 and older in better health.
In multivariable analyses, reported receipt of mammography, PAP smears, and exercise counseling declined significantly with age while reported receipt of immunizations increased with age until 85 (Table 2). Those in worse health status were less likely to report receiving mammography and colon cancer screening and were more likely to report receiving immunizations. The health trends in the adjusted models approximate trends among women in younger age groups. No interactions between age and health status were significant in any of the models.
Table 2.
Adjusted Receipt of Preventive Health Measures by Age and Health Status.*
| Mammography | Colon Cancer | PAP Smears | Exercise | Pneumonia Vaccine | Flu Shot | ||
|---|---|---|---|---|---|---|---|
| aOR(95% CI) | aOR(95% CI) | aOR(95% CI) | aOR(95% CI) | aOR(95% CI) | aOR(95% CI) | ||
| Age: | 65–69 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 70–74 | 0.62 (0.45–0.87) | 0.98 (0.75–1.28) | 0.42 (0.27–0.65) | 0.84 (0.65–1.08) | 1.58 (1.26–1.98) | 1.24 (0.97–1.59) | |
| 75–79 | 0.54 (0.39–0.74) | 1.0 (0.76–1.31) | 0.32 (0.21–0.49) | 0.71 (0.56–0.91) | 1.51 (1.18–1.92) | 1.38 (1.07–1.78) | |
| 80–84 | 0.42 (0.29–0.60) | 0.75 (0.56–1.01) | 0.27 (0.18–0.43) | 0.65 (0.48–0.88) | 1.92 (1.46–2.53) | 1.65 (1.23–2.21) | |
| 85+ | 0.27 (0.19–0.39) | 0.87 (0.62–1.22) | 0.19 (0.12–0.30) | 0.52 (0.38–0.72) | 1.03 (0.76–1.39) | 1.41 (1.04–1.91) | |
| Health | Status: Above Average | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Average | 0.74 (0.57–0.96) | 0.87 (0.70–1.09) | 1.08 (0.77–1.52) | 1.16 (0.94–1.44) | 1.31 (1.06–1.62) | 1.33 (1.08–1.64) | |
| Below Average | 0.51 (0.37–0.70) | 0.80 (0.52–0.95) | 0.88 (0.54–1.25) | 1.27 (0.96–1.66) | 1.51 (1.13–2.02) | 1.17 (0.90–1.51) |
We compared receipt of the preventive health measures above by age and health status after adjusting for sociodemographic factors (race, education, income level, insurance status, and geographical region) and access to care (having a usual provider and number of clinic visits in the past year).
Perceived health, functional limitations, and a comorbidity count were used to classify women as either above average, average, or below average in health (page 7).
When we examined the effect of the other covariates on receipt of the preventive health measures in the models, we found that higher education, frequent office visits, and having a primary care doctor were generally associated with receipt of preventive health measures. Higher income levels were associated with receipt of cancer screening tests and non-Hispanic whites were more likely than other racial groups to receive immunizations. There was no consistent relationship between receipt of preventive health measures and region and insurance (data not shown).
Less than 1 percent (0.4%) of women aged 70–79 in above average health received all 6 preventive health measures appropriately (yes to mammography, colon cancer screening, immunizations, and exercise counseling, but no to PAP smear screening) and only 2.4% of women aged 80 and older in below average health received all 6 measures appropriately (no to cancer screening but yes to immunizations and exercise counseling).
Discussion
Our findings indicate that older women are not receiving the preventive health measures most likely to be effective based on their age and health status. Cancer screening was not targeted to women aged 80 and older in above average health even though many of these women have more than 5 years of life expectancy.3 Meanwhile, many women (49%) aged 80 and older in below average health were screened with mammography. We also found that many older women did not receive recommended immunizations. Notably, women aged 85 and older in below average health were less likely to get influenza vaccinations than women aged 85 and older in better health even though the oldest women in poor health are at the greatest risk of mortality from influenza.17 In addition, despite a growing and robust literature demonstrating that exercise can delay disability and help maintain independence, we found that older women, especially those in good health, infrequently reported receiving exercise counseling.7 Higher exercise counseling among older women in poor health may reflect disease specific guidelines, however, exercise recommendations are also important for those in good health. Finally, very few older women simultaneously receive all preventive health measures appropriately. Interventions are necessary to improve the delivery and targeting of preventive health measures to older women.
Targeting of preventive health services to elderly women by health status may be poor for several reasons. Clinicians may use age rather than health status when deciding whether or not to screen women in their 80s and 90s for cancer. Also, elderly women, regardless of their health status, may perceive limited life expectancy for themselves and be reluctant to accept preventive health measures. Although increasing age significantly impacts life expectancy, older women’s health can also strongly affect life expectancy.3 Cancer screening in older women in poor health is concerning because it places women at risk for complications and anxiety related to diagnosis and treatment even though the cancer would be unlikely to become clinically significant in their lifetime.3 Denying or forgoing a screening test to elderly women in good health is equally inappropriate since these women may live long enough to benefit.
We wondered if greater number of clinic visits among the oldest women in poor health accounted for some of the poor targeting of preventive health services since additional clinic visits could be associated with more opportunities for physicians to recommend preventive services. However, in post hoc analyses we found that those in poor health status did not have significantly more clinic visits than those in good health status. Furthermore, physicians may need to use more clinic time to address active issues among older women in poor health.
We also found that many women aged 65–79 in good health did not report receiving breast (19%) or colon cancer screening (45%). Other studies that have examined receipt of colon cancer screening among US older adults have also found low rates.18 Interventions are necessary to increase breast and colon cancer screening among older women in good health. Ideally these interventions would focus on older women with life expectancies greater than 10 years. Interventions may also be necessary to reduce breast cancer screening among elderly women in poor health, especially since we found that 49% of women aged 80 and older in below average health were screened and these women likely have life expectancies of less than 5 years.
Interventions discussed in the medical literature designed to increase cancer screening among older women include physician education seminars,19 preventive health check lists,20 computer reminders,21 and support from non-physician staff. 22 We are unaware of interventions to reduce cancer screening among elderly women in poor health but such an intervention would likely require clinician counseling. Although discussing stopping screening may be uncomfortable, we recommend that clinicians discuss potential risks of screening and that there is little data showing any benefit of cancer screening among women aged 80 and older with multiple comorbidities.23;24 We also recommend that clinicians focus discussions on preventive measures whose benefits may be achieved in a short time (e.g., exercise or immunizations) so that elderly women do not feel like they are being “given-up on.”25 This may be particularly important since we found that many older women do not receive exercise counseling or immunizations. Finally, interventions designed to improve preventive health care delivery to older women may need to be comprehensive rather than focused on a specific service, since we found that so few older women receive all preventive health measures appropriately.
There are several limitations to this study. First, we relied on self-report, which can lead to recall bias and misclassification. Second, our measure of health status has not been validated and the survey only asks about a limited number of diseases and lacks information on severity and duration of disease. Third, the NHIS only releases an overall response rate to the survey, however sample weights are adjusted for non-response. Finally, the health of older women in our study may have changed since they underwent colonoscopy; however, 92% reported being screened for colon cancer in the past five years. Despite these limitations, this study represents a significant contribution to the literature by examining receipt of a wide range of preventive health measures simultaneously among a nationally representative sample of older US women stratified by health status.
In summary, we found evidence of poor targeting and inappropriate use of screening and preventive health measures among older women. Many older women in above average health were not screened for breast and/or colon cancer while many of the oldest women in below average health were screened. Regardless of health status, many older women did not receive immunizations from which they may benefit and many received PAP smears from which they are unlikely to benefit. Exercise counseling was uncommon and was least common among the oldest women in good health. Interventions are necessary to improve the quality and targeting of preventive health care delivered to older women.
Acknowledgments
Financial Disclosure Information: This research was conducted while Dr. Mara Schonberg was supported by a Hartford Geriatrics Health Outcomes Research Scholars Award from the AGS Foundation for Health in Aging.
Footnotes
Related Paper Presentations: This paper was presented in part at the National Meeting of the American Geriatrics Society, Seattle, Washington, May 4, 2007.
Contributor Information
Mara A. Schonberg, Division of General Medicine and Primary Care, Department of Medicine, Harvard Medical School, Beth Israel Deaconess Medical Center, Boston, MA.
Suzanne G. Leveille, Division of General Medicine and Primary Care, Department of Medicine, Harvard Medical School, Beth Israel Deaconess Medical Center, Boston, MA.
Edward R. Marcantonio, Division of General Medicine and Primary Care, Department of Medicine, Harvard Medical School, Beth Israel Deaconess Medical Center, Boston, MA.
References
- 1.Nicastri C, Fields S. Health promotion/disease prevention in older adults-an evidence-based update. Clinical Geriatrics. 2004;12 (11):17–25. [Google Scholar]
- 2.Wieland D. The art and science of targeting geriatrics programs. Scand J Soc Med. 1997;25(1):1–3. doi: 10.1177/140349489702500101. [DOI] [PubMed] [Google Scholar]
- 3.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher SW, Elmore JG. Clinical practice. Mammographic screening for breast cancer. N Engl J Med. 2003;348(17):1672–1680. doi: 10.1056/NEJMcp021804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Vaccine-preventable diseases: improving vaccination coverage in children, adolescents, and adults. A report on recommendations from the Task Force on Community Preventive Services. MMWR Morb Mortal Wkly Rep. 1999;44 (RR8):1–15. [PubMed] [Google Scholar]
- 6.Christmas C, Andersen RA. Exercise and older patients: guidelines for the clinician. J Am Geriatr Soc. 2000;48(3):318–324. doi: 10.1111/j.1532-5415.2000.tb02654.x. [DOI] [PubMed] [Google Scholar]
- 7.Screening for cervical cancer: recommendations and rationale. Am Fam Physician. 2003 Apr 15;67(8):1759–66. [PubMed] [Google Scholar]
- 8.Bynum JP, Braunstein JB, Sharkey P, et al. The influence of health status, age, and race on screening mammography in elderly women. Arch Intern Med. 2005;165(18):2083–2088. doi: 10.1001/archinte.165.18.2083. [DOI] [PubMed] [Google Scholar]
- 9.Schonberg MA, McCarthy EP, Davis RB, et al. Breast cancer screening in women aged 80 and older: results from a national survey. J Am Geriatr Soc. 2004;52(10):1688–1695. doi: 10.1111/j.1532-5415.2004.52462.x. [DOI] [PubMed] [Google Scholar]
- 10.Walter LC, Lindquist K, Covinsky KE. Relationship between health status and use of screening mammography and Papanicolaou smears among women older than 70 years of age. Ann Intern Med. 2004;140(9):681–688. doi: 10.7326/0003-4819-140-9-200405040-00007. [DOI] [PubMed] [Google Scholar]
- 11.Schonberg MA, Ramanan RA, McCarthy EP, et al. Decision-Making and Counseling around Mammography Screening for Women aged 80 or Older. J Gen Intern Med. 2006;21(9):979–985. doi: 10.1111/j.1525-1497.2006.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Health Interview Survey. [Accessed August 15, 2007];National Center for Health Statistics. [Public use data file and documentation] 2005 Available at: http://www.cdc.gov/nchs/nhis.
- 13.Kawada T. Self-rated health and life prognosis. Arch Med Res. 2003;34(4):343–7. doi: 10.1016/S0188-4409(03)00052-3. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Schonberg MA, Marcantonio ER, Wee CC. Receipt of exercise counseling by older women. J Am Geriatr Soc. 2006;54(4):619–26. doi: 10.1111/j.1532-5415.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 16.Burns RB, McCarthy EP, Freund KM, et al. Variability in mammography use among older women. J Am Geriatr Soc. 1996;44(8):922–6. doi: 10.1111/j.1532-5415.1996.tb01861.x. [DOI] [PubMed] [Google Scholar]
- 17.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 18.Wee CC, McCarthy EP, Phillips RS. Factors associated with colon cancer screening: the role of patient factors and physician counseling. Prev Med. 2005;41(1):23–29. doi: 10.1016/j.ypmed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Kerse NM, Flicker L, Jolley D, et al. Improving the health behaviours of elderly people: randomised controlled trial of a general practice education programme. BMJ. 1999;319(7211):683–687. doi: 10.1136/bmj.319.7211.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller KE, Zylstra RG, Standridge JB. The geriatric patient: a systematic approach to maintaining health. Am Fam Physician. 2000;61(4):1089–1104. [PubMed] [Google Scholar]
- 21.Dexter PR, Perkins S, Overhage JM, et al. A computerized reminder system to increase the use of preventive care for hospitalized patients. New Eng J Med. 2001;345(13):965–970. doi: 10.1056/NEJMsa010181. [DOI] [PubMed] [Google Scholar]
- 22.Moore AA, Siu A, Partridge JM, et al. A randomized trial of office-based screening for common problems in older persons. Am J Med. 1997;102(4):371–378. doi: 10.1016/s0002-9343(97)00089-2. [DOI] [PubMed] [Google Scholar]
- 23.Gross CP, McAvay GJ, Krumholz HM, et al. The effect of age and chronic illness on life expectancy after a diagnosis of colorectal cancer: implications for screening. Ann Intern Med. 2006;145(9):646–654. doi: 10.7326/0003-4819-145-9-200611070-00006. [DOI] [PubMed] [Google Scholar]
- 24.McPherson CP, Swenson KK, Lee MW. The effects of mammographic detection and comorbidity on the survival of older women with breast cancer. J Am Geriatr Soc. 2002;50(6):1061–1068. doi: 10.1046/j.1532-5415.2002.50261.x. [DOI] [PubMed] [Google Scholar]
- 25.Casarett DJ, Quill TE. “I’m not ready for hospice”: strategies for timely and effective hospice discussions. Ann Intern Med. 2007;146(6):443–449. doi: 10.7326/0003-4819-146-6-200703200-00011. [DOI] [PubMed] [Google Scholar]