Abstract
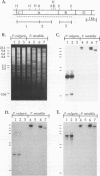
Two different hemolysins, HpmA and HlyA, have been reported in Proteus spp. To study the distribution of these hemolysins among Proteus strains, isolates from various infections and normal feces were screened for hemolysin production. All 63 Proteus mirabilis strains and 23 of the 24 Proteus vulgaris strains produced a calcium-independent hemolytic activity detectable in cell-free supernatants. The calcium-independent activity was due to HpmA; this activity correlated with the presence of hpmA sequences and the production of an extracellular 166-kilodalton (kDa) protein that reacted with anti-HpmA antiserum. HpmA- mutants, constructed by deletion of the central portion of the hpmA gene, did not produce the 166-kDa protein and were no longer hemolytic when compared with their respective parent strains. Among the 87 P. mirabilis and P. vulgaris isolates examined, calcium-dependent hemolytic activity was produced by only two P. vulgaris strains. These strains produced a 110-kDa protein which comigrated with the Escherichia coli hemolysin (HlyA) antibodies in immunoblots. These studies show that Proteus spp. produce two distinct extracellular hemolysins, with nearly all strains producing the calcium-independent hemolysin, HpmA, but only an occasional P. vulgaris isolate producing HlyA.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adegbola R. A., Old D. C., Senior B. W. The adhesins and fimbriae of Proteus mirabilis strains associated with high and low affinity for the urinary tract. J Med Microbiol. 1983 Nov;16(4):427–431. doi: 10.1099/00222615-16-4-427. [DOI] [PubMed] [Google Scholar]
- BRAUDE A. I., SIEMIENSKI J. Role of bacterial urease in experimental pyelonephritis. J Bacteriol. 1960 Aug;80:171–179. doi: 10.1128/jb.80.2.171-179.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind A., Naber K., Sauerwein D. Spectrum of bacterial pathogens in uncomplicated and complicated urinary tract infections. Eur Urol. 1987;13 (Suppl 1):9–12. doi: 10.1159/000472851. [DOI] [PubMed] [Google Scholar]
- Brooks H. J., O'Grady F., McSherry M. A., Cattell W. R. Uropathogenic properties of Escherichia coli in recurrent urinary-tract infection. J Med Microbiol. 1980 Feb;13(1):57–68. doi: 10.1099/00222615-13-1-57. [DOI] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Struck D. K. Cloning and characterization of a hemolysin gene from Actinobacillus (Haemophilus) pleuropneumoniae. DNA. 1989 Nov;8(9):635–647. doi: 10.1089/dna.1.1989.8.635. [DOI] [PubMed] [Google Scholar]
- Cooke E. M., Ewins S. P. Properties of strains of Escherichia coli isolated from a variety of sources. J Med Microbiol. 1975 Feb;8(1):107–111. doi: 10.1099/00222615-8-1-107. [DOI] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Welch R. A. Alterations of amino acid repeats in the Escherichia coli hemolysin affect cytolytic activity and secretion. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5269–5273. doi: 10.1073/pnas.85.14.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J., Nicolet J. Regulation of hemolysin expression in Actinobacillus pleuropneumoniae serotype 1 by Ca2+. Infect Immun. 1988 Oct;56(10):2570–2575. doi: 10.1128/iai.56.10.2570-2575.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Ladant D., Sezer O., Pichot F., Ullmann A., Danchin A. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):19–30. [PubMed] [Google Scholar]
- Kolodrubetz D., Dailey T., Ebersole J., Kraig E. Cloning and expression of the leukotoxin gene from Actinobacillus actinomycetemcomitans. Infect Immun. 1989 May;57(5):1465–1469. doi: 10.1128/iai.57.5.1465-1469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronakis V., Cross M., Senior B., Koronakis E., Hughes C. The secreted hemolysins of Proteus mirabilis, Proteus vulgaris, and Morganella morganii are genetically related to each other and to the alpha-hemolysin of Escherichia coli. J Bacteriol. 1987 Apr;169(4):1509–1515. doi: 10.1128/jb.169.4.1509-1515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronakis V., Hughes C. Identification of the promoters directing in vivo expression of hemolysin genes in Proteus vulgaris and Escherichia coli. Mol Gen Genet. 1988 Jul;213(1):99–104. doi: 10.1007/BF00333404. [DOI] [PubMed] [Google Scholar]
- Koronakis V., Koronakis E., Hughes C. Comparison of the haemolysin secretion protein HlyB from Proteus vulgaris and Escherichia coli; site-directed mutagenesis causing impairment of export function. Mol Gen Genet. 1988 Aug;213(2-3):551–555. doi: 10.1007/BF00339631. [DOI] [PubMed] [Google Scholar]
- Kotelko K., Kaca W., Rózalski A., Deka M. Some biological features of Proteus bacilli. 2. Haemolytic activities of Proteus mirabilis and Proteus vulgaris strains. Acta Microbiol Pol. 1983;32(4):345–351. [PubMed] [Google Scholar]
- MacLaren D. M. The significance of urease in proteus pyelonephritis: a histological and biochemical study. J Pathol. 1969 Jan;97(1):43–49. doi: 10.1002/path.1710970107. [DOI] [PubMed] [Google Scholar]
- Minshew B. H., Jorgensen J., Swanstrum M., Grootes-Reuvecamp G. A., Falkow S. Some characteristics of Escherichia coli strains isolated from extraintestinal infections of humans. J Infect Dis. 1978 May;137(5):648–654. doi: 10.1093/infdis/137.5.648. [DOI] [PubMed] [Google Scholar]
- Peerbooms P. G., Verweij A. M., MacLaren D. M. Investigation of the haemolytic activity of Proteus mirabilis strains. Antonie Van Leeuwenhoek. 1983 Apr;49(1):1–11. doi: 10.1007/BF00457874. [DOI] [PubMed] [Google Scholar]
- Peerbooms P. G., Verweij A. M., MacLaren D. M. Uropathogenic properties of Proteus mirabilis and Proteus vulgaris. J Med Microbiol. 1985 Feb;19(1):55–60. doi: 10.1099/00222615-19-1-55. [DOI] [PubMed] [Google Scholar]
- Peerbooms P. G., Verweij A. M., MacLaren D. M. Vero cell invasiveness of Proteus mirabilis. Infect Immun. 1984 Mar;43(3):1068–1071. doi: 10.1128/iai.43.3.1068-1071.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerbooms P. G., Verweij A. M., Oe P. L., MacLaren D. M. Urinary pathogenicity of Proteus mirabilis strains isolated from faeces or urine. Antonie Van Leeuwenhoek. 1986;52(1):53–62. doi: 10.1007/BF00402687. [DOI] [PubMed] [Google Scholar]
- Pellett S., Boehm D. F., Snyder I. S., Rowe G., Welch R. A. Characterization of monoclonal antibodies against the Escherichia coli hemolysin. Infect Immun. 1990 Mar;58(3):822–827. doi: 10.1128/iai.58.3.822-827.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K., Braun V. Iron regulation of Serratia marcescens hemolysin gene expression. Infect Immun. 1988 Nov;56(11):2967–2971. doi: 10.1128/iai.56.11.2967-2971.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K., Schiebel E., Braun V. Molecular characterization of the hemolysin determinant of Serratia marcescens. J Bacteriol. 1988 Jul;170(7):3177–3188. doi: 10.1128/jb.170.7.3177-3188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiebel E., Schwarz H., Braun V. Subcellular location and unique secretion of the hemolysin of Serratia marcescens. J Biol Chem. 1989 Sep 25;264(27):16311–16320. [PubMed] [Google Scholar]
- Senior B. W., Albrechtsen M., Kerr M. A. Proteus mirabilis strains of diverse type have IgA protease activity. J Med Microbiol. 1987 Sep;24(2):175–180. doi: 10.1099/00222615-24-2-175. [DOI] [PubMed] [Google Scholar]
- Senior B. W., Bradford N. C., Simpson D. S. The ureases of Proteus strains in relation to virulence for the urinary tract. J Med Microbiol. 1980 Nov;13(4):507–512. doi: 10.1099/00222615-13-4-507. [DOI] [PubMed] [Google Scholar]
- Senior B. W., Leslie D. L. Rare occurrence of Proteus vulgaris in faeces: a reason for its rare association with urinary tract infections. J Med Microbiol. 1986 Mar;21(2):139–144. doi: 10.1099/00222615-21-2-139. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J. Host-parasite interaction in the rat renal pelvis: a possible role for pili in the pathogenesis of pyelonephritis. J Exp Med. 1974 Dec 1;140(6):1696–1711. doi: 10.1084/jem.140.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm W. E., Martin S. M., Bennett J. V. Epidemiology of nosocomial infection due to Gram-negative bacilli: aspects relevant to development and use of vaccines. J Infect Dis. 1977 Aug;136 (Suppl):S151–S160. doi: 10.1093/infdis/136.supplement.s151. [DOI] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989 Feb;171(2):916–928. doi: 10.1128/jb.171.2.916-928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Extensive homology between the leukotoxin of Pasteurella haemolytica A1 and the alpha-hemolysin of Escherichia coli. Infect Immun. 1987 Dec;55(12):3233–3236. doi: 10.1128/iai.55.12.3233-3236.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swihart K. G., Welch R. A. Cytotoxic activity of the Proteus hemolysin HpmA. Infect Immun. 1990 Jun;58(6):1861–1869. doi: 10.1128/iai.58.6.1861-1869.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uphoff T. S., Welch R. A. Nucleotide sequencing of the Proteus mirabilis calcium-independent hemolysin genes (hpmA and hpmB) reveals sequence similarity with the Serratia marcescens hemolysin genes (shlA and shlB). J Bacteriol. 1990 Mar;172(3):1206–1216. doi: 10.1128/jb.172.3.1206-1216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A. Identification of two different hemolysin determinants in uropathogenic Proteus isolates. Infect Immun. 1987 Sep;55(9):2183–2190. doi: 10.1128/iai.55.9.2183-2190.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Pellett S. Transcriptional organization of the Escherichia coli hemolysin genes. J Bacteriol. 1988 Apr;170(4):1622–1630. doi: 10.1128/jb.170.4.1622-1630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S. K., Hull S. I., Cook R. G., Barrish J., Hull R. A. Identification and characterization of a uroepithelial cell adhesin from a uropathogenic isolate of Proteus mirabilis. Infect Immun. 1986 Oct;54(1):43–49. doi: 10.1128/iai.54.1.43-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]