Abstract
It is generally appreciated that platelets derived from diabetic patients display increased responsiveness to low levels of agonists. O-GlcNAcylation has been linked to hyperglycemia-related effects in other tissues; therefore we examined this modification in platelets to determine if O-GlcNAcylation affects platelet function. This post-translational modification consists of an N-acetylglucosamine attached to serine and/or threonine residues. We examined O-GlcNAc levels in platelets from a hyperglycemic murine model of Type I diabetes with known hypersensitivity to agonists and a Type II diabetes model (ob/ob) lacking detectable alterations in the aggregation profile. Neither model showed marked increases in protein O-GlcNAcylation. Treatment of platelets with multiple O-GlcNAcase inhibitors led to O-GlcNAc accumulation on multiple platelet proteins. However, the inhibitor-induced accumulation of this modification does not correlate with any gross alterations in platelet aggregation. These data suggest that while the modification occurs in platelets, their activity is not globally sensitive to O-GlcNAc levels.
Keywords: O-GlcNAc, protein modification, platelets, aggregation, diabetes
Introduction
Platelets are anucleate cell fragments responsible for maintaining vascular integrity by adhering to lesions and facilitating thrombus formation. Platelets respond to multiple extracellular signals leading to shape change, filopodial extensions, and granule exocytosis necessary for hemostasis [1]. Platelets that are either hyperactive or hypoactive pose a critical health risk. Hyperactive platelets form spurious clots that can cause loss of blood flow and lead to myocardial infarction or stroke. Hypoactive platelets fail to correctly adhere to lesions and do not form clots creating points for excessive blood loss.
Inappropriate thrombotic events are a leading cause of mortality in diabetics. It has been estimated that greater than 75% of diabetic patients eventually succumb to a thrombotic event [2]. Platelets obtained from diabetics have been shown to be hyperactive ex vivo and to aggregate in the presence of agonist considered substimulatory to platelets obtained from non-diabetics [3]. It is not clear at this time if the cause this hyperactivity is an intrinsic defect in platelets or an extrinsic factor contributing to their being maintained in a primed state.
While multiple signaling pathways have been identified, the precise protein interactions that result in platelet activation, granule release, and clot formation have yet to be fully characterized. Alterations in post-translational modifications are likely to be critical in regulating this complex set of events. In 1984, Torres et al. described a protein modification consisting of a single β-N-acetylglucosamine attached to serine and/or threonine residues on cytosolic and nuclear proteins (O-GlcNAc) [4]. O-GlcNAc is added by UDP-GlcNAc:polypeptide β-N-acetylglucosaminyl transferase (OGT) [5] and is removed by an O-GlcNAc-selective β-N-acetylglucosaminidase (O-GlcNAcase), also known as hexosaminidase C [5]. The importance of this modification is most obviously illustrated by the embryonic lethality seen in attempts to knock-out the OGT gene in mice [6]. Numerous proteins that regulate a broad range of cellular functions have been shown to be O-GlcNAcylated, including altering DNA binding affinity, promoting survivability during environmental stress, and increasing basal cellular motility and chemotaxis (for review [7]). Since its discovery, the importance of O-GlcNAcylation has increased as its roles in protein regulation and cellular outcomes have been better characterized.
Hyperglycemia associated with diabetes is currently thought to induce an increase in protein O-GlcNAcylation through a branch of hexose metabolism known as the hexosamine biosynthesis pathway (HBP) [8]. The HBP converts two to five percent of cellular glucose to UDP-GlcNAc, the sugar-nucleotide substrate for the OGT. An increase in glucose uptake leads to increased flux through the HBP resulting in increased production of UDP-GlcNAc. By mass-action, increased UDP-GlcNAc is thought to increase protein O-GlcNAcylation. Based on these relationships, we hypothesized that the hyperglycemia associated with diabetes might lead to an increase in O-GlcNAcylation on platelet proteins. This proposed increase in O-GlcNAc may account for an intrinsic defect in platelets leading to the observed hyperactivity of platelets from diabetic patients. The experiments presented here seek to address this hypothesis.
Materials and Methods
Antibodies and Reagents
The anti-O-GlcNAc antibody, RL2, was purchased from Affinity BioReagents (Golden, CO). The anti-O-GlcNAc antibody, CTD110.6, has been previously characterized [9]. The actin antibody and all secondary antibodies were purchased from Sigma (St. Louis, MO). Thrombin was purchased from Chrono-Log (Havertown, PA). O-(2-acetamido-2-deoxy-D-glucopyranosylidene) amino-N-phenylcarbamate (PUGNAc) was synthesized in the laboratory of Dr. Gerald Hart. The O-GlcNAcase inhibitors (referred to as 9c and 9d) were a kind gift from Dr. David Vocadlo (Simon Fraser University, Vancouver, BC). Wild type C57BL/6 mice and leptin knockout (ob/ob) were purchased from Jackson Labs (Bar Harbor, ME) and Streptozotocin (STZ) was purchased from Sigma. All animal studies were approved by the University of Kentucky IACUC.
Antibody generation
Polyclonal antibodies to OGT and O-GlcNAcase were generated using full length recombinant protein as the antigens. OGT was expressed using a previously generated construct [10]. O-GlcNAcase was subcloned from a mammalian expression vector [11] to a pProEX-HT bacterial expression vector (Invitrogen, Carlsbad, CA). These proteins were expressed in Rosetta DE3 E. coli (Stratagene, La Jolle, CA). Purified proteins were dialyzed against PBS and concentrated to 1 mg/mL. Protein purity was confirmed by SDS-PAGE. Antibodies were generated in New Zealand white rabbits using standard protocols. The OGT antibody was used as sera. The O-GlcNAcase antibody was affinity-purified using O-GlcNAcase coupled to CNBr-activated Sepharose beads (Sigma).
Western blotting
Following resolution by SDS-PAGE, proteins were transferred to PVDF membranes (Millipore, Billerica, MA). Membranes were incubated with the indicated primary antibody followed by incubation with the appropriate secondary antibody conjugated to alkaline phosphatase. Blots were imaged on a Typhoon 9400 using Enhanced Chemi-Fluorescent (ECF) substrate (GE Biosciences, Piscataway, NJ). Quantification of western blots was done using Image-Quant 5.2 software (GE Biosciences, Piscataway, NJ). Equal loading was confirmed by immunodetection of β-actin.
Generation of hyperglycemia in mice using streptozotocin
Hyperglycemia was induced in eight-week-old C57BL/6 mice by injecting STZ intraperitoneally for six consecutive days. The STZ was prepared immediately prior to use in citrate buffer. Animals were injected with 60 mg of STZ per kg of body weight. Control mice received an equal volume of buffer alone. The STZ-treated mice were held in a hyperglycemic state for 14 days. Since platelets have a half-life of 7 days [12], it is estimated that >75% of the platelets would have been exposed to hyperglycemic conditions for their entire lifespan.
Preparation of murine platelets
Mice were euthanatized by CO2 inhalation. Blood was collected from the right ventricle and was mixed with sodium citrate to a final concentration of 0.38%. The citrated blood was mixed with an equal volume of PBS, pH 7.4. Platelet-rich plasma (PRP) was prepared by centrifugation at 250 × g for 10 min. After adding 10 ng/ml prostaglandin I2 (Sigma) for 5 min, the PRP was centrifuged at 500 × g for 15 min, and the platelet pellet was suspended in HEPES-Tyrode's buffer [13] to a concentration of 3.5 × 108 platelets / mL.
Aggregation of murine platelets
Washed platelets were allowed to equilibrate at 37°C for 2 min with stirring prior to the addition of agonist. Aggregation was measured under constant stirring conditions in a Chrono-Log Model 460VS Lumi-aggregometer (Havertown, PA).
Glucose measurements
Plasma glucose was measured using a coupled reaction containing peroxidase-glucose oxidase enzyme and O-dianisidine dihydrochloride solution based on a protocol developed by Raabo and Terkildsen [14].
Results
O-GlcNAc modified proteins are present in murine platelets
O-GlcNAc has been detected on many proteins in nucleated cells [7] and in human erythrocytes [15]; however, it is unknown whether it is present on proteins in anucleate platelets. To determine if these cell fragments contain O-GlcNAc modified proteins, murine platelet extracts were analyzed by western blotting with two different, modification-specific, monoclonal antibodies, RL2 and CTD110.6. Both antibodies recognize approximately 15–20 major bands in the extracts (Fig. 1). The majority of the detected proteins migrate between 50 kDa and 150 kDa. The two reagents detected many of the same proteins but with slightly differing intensities. The differences between these two reagents are consistent with previous reports from other cell types [9]. As a specificity control, membranes were immuno-probed in the presence of 20 mM free GlcNAc. This free sugar effectively competes the CTD110.6 antibody as previously reported [9] (Fig. 1, far right lane). Based on this analysis, it appears that murine platelets contained a number of O-GlcNAcylated proteins. Similar experiments showed that human platelets also contain O-GlcNAcylated proteins (data not shown).
Figure 1.
Murine platelets contain proteins modified with O-Linked β-N-acetylglucosamine. Platelet extracts were analyzed by western blotting with two anti-O-GlcNAc antibodies, RL2 and CTD110.6. Specificity of the CTD110.6 antibody for O-GlcNAcylation was confirmed by co-incubation of the primary antibody with 20 mM GlcNAc. Western blots are representative of five separate platelet preparations.
Characterization of murine diabetic models
To probe the relationship between hyperglycemia and O-GlcNAcylation in platelets, we examined two murine models of diabetes. The first, mimicking Type I diabetes, was generated using the β-cell toxin STZ. Platelets from STZ-treated animals are hyperactive and respond to lower doses of agonist than their normo-glycemic, control littermates [16]. The second model uses leptin deficient (ob/ob) mice. Leptin plays a key role in regulating metabolism and appetite. These animals display a Type II diabetic phenotype, which includes excessive weight gain, hyperglycemia, elevated plasma insulin levels, and hyperlipidemia [17]. Control, STZ-treated, and ob/ob mice were on the C57BL/6 background and were analyzed at 10–12 weeks of age. As expected for the Type I phenotype, the STZ-treated mice consistently weigh less than control. The ob/ob mice weigh significantly more than wild-type mice, consistent with the Type II phenotype (Fig. 2A). Analysis of blood glucose confirmed that both models are hyperglycemic but the Type I animals are more extremely so, showing an almost three fold increase relative to control (Fig. 2B). Using both ob/ob and STZ-injected models, we have two distinct levels of hyperglycemia allowing for a better assessment of the relationship between blood glucose and alterations in protein O-GlcNAcylation.
Figure 2.
Characterization of diabetic murine models. The weights (A) and blood glucose levels (B) of the animals at the time of euthanasia were determined (n=5). P-values were determined using t-test. (*; p < 0.005; **, p < 0.001). Platelets from multiple mock-treated or STZ-treated animals (C) or from wild type or ob/ob animals (D) were pooled and examined by aggregometry using the indicated doses of thrombin. Platelet extracts from mock-treated or STZ treated animals (E) or from wild type or ob/ob animals (F) were analyzed by western blotting with the CTD110.6 antibody.
Platelets from the three groups were collected and their response to low levels of thrombin was determined by aggregometry. Platelets from the STZ-treated mice are hypersensitive and display marked aggregation to lower doses of agonists compared to control (Fig 2C). Platelets from the ob/ob mice show no significant differences in reactivity when compared to control, even at the lowest doses of thrombin used (Fig. 2D). These results are consistent with previously published observations using these and similar animal models [18]. Though both animal models have high glucose levels, only the severely hyperglycemic, STZ-treated animals had hyperactive platelets.
O-GlcNAc levels in platelets from the murine diabetic models
In an attempt to understand the differences in the platelet responses from these mice, we examined the extent of protein O-GlcNAcylation. Increased O-GlcNAcylation is thought to be a sequela of hyperglycemia due to increased production of UDP-GlcNAc that results from increased flux of glucose through the hexosamine biosynthesis pathway [19]. Indeed, several studies in animal models and in humans have shown that hyperglycemia and hyperinsulinemia are correlated with increased O-GlcNAcylation, particularly in muscle and adipose tissue [20]. If high glucose alone contributes to the development of increased protein O-GlcNAcylation, platelets obtained from both diabetic models are also expected to show global increases in O-GlcNAc that correlates to their degree of hyperglycemia. Such a correlation could provide a basis for the observed hyperactivity.
Platelets, from mock-treated and STZ-treated mice or from wild type and ob/ob mice, were isolated from whole blood. These platelets were analyzed by quantitative western blotting using the anti-O-GlcNAc antibody CTD110.6. The immuno-decorated proteins display a similar array (Fig. 2E and 2F). Comparison of mock-treated and STZ-treated platelet extract by global quantification shows that there is no significant difference between these two samples (Fig. 2E). This is surprising considering the extent of hyperglycemia seen in the STZ-treated animals. Examination of the platelets derived from the ob/ob mice showed a similar lack of significantly increased O-GlcNAcylation compared to control platelets (Fig. 2F). These observations suggest that levels of O-GlcNAc in murine platelets are not solely driven by hyperglycemia. Additionally, these data in conjunction with the previous aggregation data, suggest that there is no direct correlation between increased protein O-GlcNAcylation and hyperactivity.
Murine platelets contain both enzymes responsible for O-GlcNAc cycling
One possible explanation for the previous observations is that platelets lack the ability to alter protein O-GlcNAc levels. Platelets do not undergo significant translation in their resting state, deriving the majority of their proteins during development from the megakaryocytes [21]. In order for dynamic cycling of O-GlcNAc to occur in platelets, both the enzymes, OGT and O-GlcNAcase, must be present. Platelet extracts from C57BL/6 mice were subjected to western blot analysis using polyclonal antibodies to either O-GlcNAcase or OGT generated in our laboratory to determine if these proteins were present. Probing with the anti-O-GlcNAcase antibody, a 130 kDa band was detected, consistent with the presence of the full-length enzyme [11]. Using the anti-OGT antibody, a single 135 kDa band was detected (Fig. 3), consistent with the presence of the full-length enzyme previously reported in rodents [22]. These data indicate that both enzymes, OGT and O-GlcNAcase, are present in murine platelets.
Figure 3.
Murine platelets contain the enzymes responsible for O-GlcNAc cycling. Murine platelet extracts were probed by western blotting with either anti-OGT sera (right) or affinity-purified anti-O-GlcNAcase (left) antibodies. Pre-immune sera for each rabbit were tested for reactivity to platelet proteins. Western blots are representative of three separate platelet preparations.
To assess the levels of OGT and O-GlcNAcase in platelets relative to nucleated cell, the two antibodies were used to probe extracts from TSA201 cells (an HEK293 cell derivative). Equivalent amounts of cellular proteins were compared and immunodecorated proteins were quantified by western blot. Based on this analysis, nucleated cells contain at least 5-fold more OGT and O-GlcNAcase per total protein than do platelets (data not shown). It is not clear at this time if this difference is due to the high concentration of platelet granule proteins [23] or a ramification of the extensive O-GlcNAcylation on nuclear proteins [24].
O-GlcNAcase inhibitors lead to an accumulation of O-GlcNAc modified proteins
Since both enzymes responsible for cycling this modification are present in platelets, we turned to available enzymatic inhibitors to modulate the level of O-GlcNAc and to further assess its role in platelet function. PUGNAc is a membrane permeant inhibitor of O-GlcNAcase and is readily available [25]. One of the drawbacks for utilizing PUGNAc is its partial specificity, as it also inhibits the lysosomal hexosaminidases [25]. To address this issue of specificity, we utilized two additional O-GlcNAcase inhibitors (referred to as 9c and 9d) developed in the laboratory of Dr. David Vocadlo [26]. These inhibitors show specificity for O-GlcNAcase that is over 1,500 times greater than for the lysosomal enzymes [26].
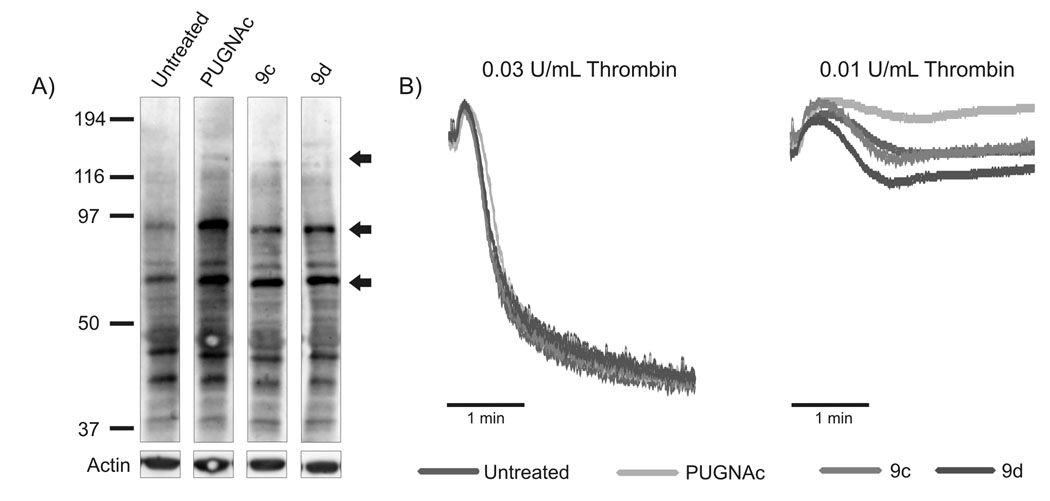
Platelets were isolated from C57BL/6 mice and either left untreated or incubated with 100 µM of the indicated O-GlcNAcase inhibitor (PUGNAc, 9c or 9d) for 6 hrs. Samples were analyzed by western blotting with the CTD110.6 antibody (Fig. 4A). Treatment with all three O-GlcNAcase inhibitors caused an increase in the levels of OGlcNAcylated proteins (most significant changes marked with arrows). Using Image-Quant 5.2 software, we determined that the indicated bands show at least a 50% increase in O-GlcNAcylation when compared to the untreated control. These data demonstrate that alterations in O-GlcNAcylation can be detected with the CTD110.6 antibody and suggest that the O-GlcNAc modification is cycling in resting platelets. Additionally, this analysis shows that it is possible to globally alter O-GlcNAc levels in platelets and thus be able to probe for the effects of increased O-GlcNAc on platelet function.
Figure 4.
Characterization of murine platelets treated with O-GlcNAcase inhibitors show increased O-GlcNAc levels. (A) Platelets were treated with the indicated O-GlcNAcase inhibitor and analyzed by western blotting with the CTD110.6 antibody. Arrows indicate the most significantly increased proteins. Western blots are representative of three separate platelet preparations. (B) Platelets were treated with the indicted O-GlcNAcase inhibitor and analyzed by aggregometry using the indicated doses of thrombin.
Aggregation of murine platelets following treatment with O-GlcNAcase inhibitors
Experiments so far indicate that platelets contain O-GlcNAcylated proteins and this modification cycles in resting platelets. To assess whether increased O-GlcNAcylation can affect platelet function, we examined the aggregation profiles of platelets treated with inhibitors to O-GlcNAcase. Murine platelets were either untreated or treated with each of three O-GlcNAcase inhibitors (100 µM PUGNAc, 9c, or 9d) for 6 hrs. Samples were stimulated with decreasing concentrations of thrombin in an attempt to identify any defects in aggregation (Fig.4B). At the concentrations of thrombin used, neither a hypoactive nor hyperactive phenotype can be detected from the platelets treated with O-GlcNAcase inhibitors compared to untreated platelets. Together with previous data, these results indicate that the hyperactivity seen in STZ-treated platelets is not a result of global alterations in protein O-GlcNAc levels.
Discussion
We have shown that murine platelets contain O-GlcNAcylated proteins (Fig. 1). This is not surprising given the ubiquitous nature of this modification. However, as platelets are anucleate, this does provide a novel model system to investigate O-GlcNAc function in the absence of the heavily modified nuclear pore proteins and various transcription factors. We have also shown here that platelets contain both of the enzymes responsible for cycling this modification (Fig. 3) and that inhibition of O-GlcNAcase resulted in the accumulation of O-GlcNAc (Fig. 4A), indicating that the modification is turning over in resting platelets.
To probe O-GlcNAc’s role in platelet function, we examined platelets from two different diabetic models that presented with different degrees of hyperglycemia. It was our original hypothesis that at least one, if not both hyperglycemia models, would display an increase in protein O-GlcNAcylation. The STZ-treated mice had extremely high blood glucose levels (Fig. 2B) and their platelets were hypersensitive to low levels of agonist (Fig. 2C). The ob/ob mice had mildly elevated blood glucose (Fig. 2B) coupled with extreme weight gain (Fig. 2A); but their platelets lacked the hypersensitivity to agonist when measured ex vivo that is seen in the STZ-treated model (Fig. 2C and 2D). These observations confirm previous work examining aggregation in platelets from similar diabetic models [27] [18]. These previous studies show that the platelet hyperactivity in STZ-treated animals is most pronounced when using low doses of thrombin or ADP. Neither murine model showed significant alterations in platelet protein O-GlcNAcylation (Fig. 2E and 2F) as detected by western blotting with CTD110.6. However, treatment of platelets with O-GlcNAcase inhibitors did result in a detectable increase in O-GlcNAcylation (Fig. 4A), confirming that alterations in O-GlcNAc levels can be monitored by western blot analysis using the CTD110.6 antibody. The aggregation profiles from these drug treated platelets showed limited changes in functionality in response to the accumulation of O-GlcNAc (Fig. 4B). Although platelets utilized in these experiments were maintained ex-vivo for an extended period of time, there were no significant differences in their aggregation profiles throughout the course of the experiments (data not shown). Due to the limited number of platelets obtained from mice, similar experiments were also carried out using banked human platelets. Regardless of the agonist or conditions examined, results with human platelets were consistent with those presented here; platelets treated with O-GlcNAcase inhibitors showed accumulation of O-GlcNAc but there were no significant alterations in the aggregation profiles compared to untreated samples (data not shown).
In cell culture models, treatment with high glucose leads to increased protein O-GlcNAcylation and tissues derived from diabetic animal models have increased protein O-GlcNAcylation (for review [19]). These observations, coupled with the hyperactivity seen in platelets from STZ-treated animals, were the basis of our original hypothesis. Platelets possess transporters allowing glucose to enter the cells [28] where it is metabolized [29]. The accumulation of O-GlcNAc when using the O-GlcNAcase inhibitors implies that some level of UDP-GlcNAc must be generated as the modification is cycling. Therefore it seemed likely that platelets would respond to increased extracellular glucose as other cells do, by globally increasing O-GlcNAcylation. However, when examining the platelets from the two hyperglycemia models for alterations in O-GlcNAc and changes in functionality, we see no correlation between platelet hyperactivity, hyperglycemia, and increased O-GlcNAcylation. These results disprove our original hypothesis. Though platelet proteins are O-GlcNAcylated, when using platelet aggregation as a measure of functionality, they are elastic to changes in overall O-GlcNAc levels. It is possible that alterations on specific proteins may lead to subtle changes in functionality that are not appreciated when using aggregometry. To further address this point, it will be critical to identify specific O-GlcNAcylated platelet proteins and probe the role of the modification in regulating their functions.
Acknowledgments
We would like to thank the members of the Whiteheart laboratory: Dr. Elena A. Matveeva, Dr. Zubair A. Karim, Wangsun Choi, Qiansheng Ren, Chunxia Zhao, Shaojing Ye, and Rania Al Hawas for their helpful discussions and careful reading of this manuscript. This work was supported by grants from the National Institutes of Health (HL081614 and HL56652) (to S.W.W.) and from the Ohio Valley Affiliate of the American Heart Association (0415111B) (to G.L.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brass L. Chest Sep. 2003:18S–25S. doi: 10.1378/chest.124.3_suppl.18s. [DOI] [PubMed] [Google Scholar]
- 2.Lancet. 1999;354:617–621. [PubMed] [Google Scholar]
- 3.Mustard JF, Packham MA. N Engl J Med. 1977;297:1345–1347. doi: 10.1056/NEJM197712152972408. [DOI] [PubMed] [Google Scholar]
- 4.Torres CR, Hart GW. J Biol Chem. 1984;259:3308–3317. [PubMed] [Google Scholar]
- 5.Iyer SP, Hart GW. Biochemistry. 2003;42:2493–2499. doi: 10.1021/bi020685a. [DOI] [PubMed] [Google Scholar]
- 6.Shafi R, Iyer SP, Ellies LG, O'Donnell N, Marek KW, Chui D, Hart GW, Marth JD. Proc Natl Acad Sci U S A. 2000;97:5735–5739. doi: 10.1073/pnas.100471497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells L, Hart GW. FEBS Lett. 2003;546:154–158. doi: 10.1016/s0014-5793(03)00641-0. [DOI] [PubMed] [Google Scholar]
- 8.Marshall S, Bacote V, Traxinger RR. J Biol Chem. 1991;266:4706–4712. [PubMed] [Google Scholar]
- 9.Comer FI, Vosseller K, Wells L, Accavitti MA, Hart GW. Anal Biochem. 2001;293:169–177. doi: 10.1006/abio.2001.5132. [DOI] [PubMed] [Google Scholar]
- 10.Kreppel LK, Blomberg MA, Hart GW. J Biol Chem. 1997;272:9308–9315. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Wells L, Comer FI, Parker GJ, Hart GW. J Biol Chem. 2001;276:9838–9845. doi: 10.1074/jbc.M010420200. [DOI] [PubMed] [Google Scholar]
- 12.Harker LA. Prog Hemost Thromb. 1978;4:321–347. [PubMed] [Google Scholar]
- 13.Rutledge TW, Whiteheart SW. Methods Mol Biol. 2004;272:109–120. doi: 10.1385/1-59259-782-3:109. [DOI] [PubMed] [Google Scholar]
- 14.Raabo E, Terkildsen TC. Scand J Clin Lab Invest. 1960;12:402–407. doi: 10.3109/00365516009065404. [DOI] [PubMed] [Google Scholar]
- 15.Holt GD, Haltiwanger RS, Torres CR, Hart GW. J Biol Chem. 1987;262:14847–14850. [PubMed] [Google Scholar]
- 16.Winocour PD, Lopes-Virella M, Laimins M, Colwell JA. J Lab Clin Med. 1985;106:319–325. [PubMed] [Google Scholar]
- 17.Friedman JM. Nutr Rev. 2002;60:S1–S14. doi: 10.1301/002966402320634878. [DOI] [PubMed] [Google Scholar]
- 18.Paul W, Queen LR, Page CP, Ferro A. Br J Pharmacol. 2007;150:105–111. doi: 10.1038/sj.bjp.0706957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Love DC, Hanover JA. Sci STKE 2005. 2005:re13. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- 20.Buse MG. Am J Physiol Endocrinol Metab. 2006;290:E1–E8. doi: 10.1152/ajpendo.00329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weyrich AS, Lindemann S, Tolley ND, Kraiss LW, Dixon DA, Mahoney TM, Prescott SP, McIntyre TM, Zimmerman GA. Semin Thromb Hemost. 2004;30:491–498. doi: 10.1055/s-2004-833484. [DOI] [PubMed] [Google Scholar]
- 22.Konrad RJ, Tolar JF, Hale JE, Knierman MD, Becker GW, Kudlow JE. Biochem Biophys Res Commun. 2001;288:1136–1140. doi: 10.1006/bbrc.2001.5902. [DOI] [PubMed] [Google Scholar]
- 23.Maynard DM, Heijnen HF, Horne MK, White JG, Gahl WA. J Thromb Haemost. 2007;5:1945–1955. doi: 10.1111/j.1538-7836.2007.02690.x. [DOI] [PubMed] [Google Scholar]
- 24.Slawson C, Housley MP, Hart GW. J Cell Biochem. 2006;97:71–83. doi: 10.1002/jcb.20676. [DOI] [PubMed] [Google Scholar]
- 25.Haltiwanger RS, Grove K, Philipsberg GA. J Biol Chem. 1998;273:3611–3617. doi: 10.1074/jbc.273.6.3611. [DOI] [PubMed] [Google Scholar]
- 26.Macauley MS, Whitworth GE, Debowski AW, Chin D, Vocadlo DJ. J Biol Chem. 2005;280:25313–25322. doi: 10.1074/jbc.M413819200. [DOI] [PubMed] [Google Scholar]
- 27.Winocour PD, Kinlough-Rathbone RL, Mustard JF. J Lab Clin Med. 1986;107:148–153. [PubMed] [Google Scholar]
- 28.Craik JD, Stewart M, Cheeseman CI. Thromb Res. 1995;79:461–469. doi: 10.1016/0049-3848(95)00136-f. [DOI] [PubMed] [Google Scholar]
- 29.Farnararo M, Bruni P, Vasta V. Biochem Biophys Res Commun. 1986;138:666–672. doi: 10.1016/s0006-291x(86)80548-4. [DOI] [PubMed] [Google Scholar]