Abstract
Addiction is characterized by compulsive or inflexible behavior, observed both in the context of drug-seeking and in contexts unrelated to drugs. One possible contributor to these inflexible behaviors may be drug-induced dysfunction within circuits that support behavioral flexibility, including the basolateral amygdala (ABL) and the orbitofrontal cortex (OFC). Here we describe data demonstrating that chronic cocaine exposure causes long-lasting changes in encoding properties in the ABL and the OFC during learning and reversal in an odor-guided task. In particular, these data suggest that inflexible encoding in ABL neurons may be the proximal cause of cocaine-induced behavioral inflexibility, and that a loss of outcome-expectant encoding in OFC neurons could be a more distal contributor to this impairment. A similar mechanism of drug-induced orbitofrontal–amygdalar dysfunction may cause inflexible behavior when animals and addicts are exposed to drug-associated cues and contexts.
Keywords: addiction, cocaine, orbitofrontal cortex, basolateral amygdala, reversal, associative learning
Addiction is characterized by poor decision making and a loss of control over drug-seeking. Memories for cues or contexts that occur in close proximity to drug-taking, such as syringes, crack pipes, people, or places, are often prominent features of these behaviors.1–3 Activating these drug-associated memories elicits drug-seeking behavior and relapse in animal models and, it is theorized, in human addicts.4–9 The influence of drug-associated memories persists long into abstinence,10,11 through extinction,12 and, perhaps most strikingly, even in the face of adverse outcomes or cues that represent adverse outcomes.13,14 These memories' persistence and apparent invulnerability to change have been argued to contribute to the lack of control that characterizes decision making in addiction.8,15
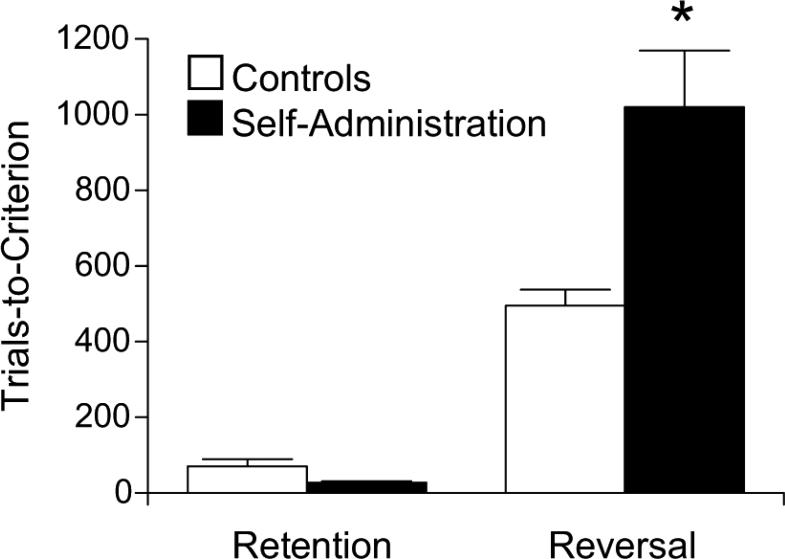
Addicts and drug-experienced animals also exhibit poor, inflexible decision making in experimental settings, far removed from drug-taking. For example, addicts exhibit an inability to shift or to reverse their response strategy in several gambling tasks.16–18 Animal models, primarily of psychostimulant addiction, indicate that these deficits may be the result of drug exposure rather than a pre-existing condition. Thus, monkeys and rats exposed to chronic noncontingent cocaine show inflexible behavior in reversal-learning tasks up to a month after cocaine exposure.19,20 As illustrated in Figure 1, we have recently found a similar reversal deficit in rats previously trained to self-administer cocaine.21 The go, no-go odor discrimination task used in studies in our lab, illustrated in Figure 2, requires the rat to learn to associate one odor with a sucrose reward and a second odor with a bitter quinine punishment. Rats previously exposed to cocaine, either passively by 14 days of daily ip injections (30 mg/kg) or via 14 daily self-administration sessions (see Fig.1 legend for details), learn these problems normally but require many more trials than controls to learn serial reversals of the final problem. Interestingly, drug-associated behavioral deficits, both in gambling tasks in humans and in reversal tasks, are similar to those caused by damage to the orbitofrontal cortex (OFC).22–24
FIGURE 1.
Effect of previous cocaine self-administration training on reversal learning. Training included 14 daily 3-hour sessions, with 0.75 mg/kg cocaine-HCl per infusion and an average of 24 infusions per day, and ended at least 1 month prior to behavioral testing on the go, no-go odor discrimination task described in the text. Rats first showed retention of a previously learned odor discrimination, and then acquired a reversal of that odor discrimination. Shown are average trials to criterion for two serial retention/reversals. Error bars indicate SEMs.*P < 0.01, compared to controls (data from Calu et al.21).
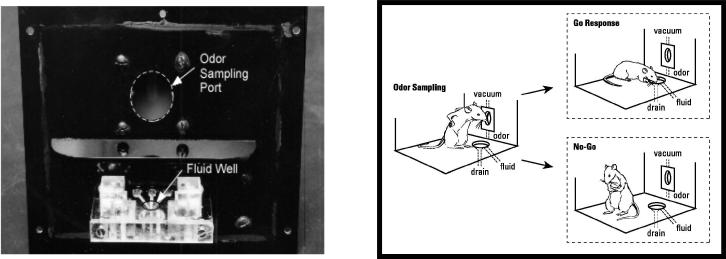
FIGURE 2.
Schematic diagram demonstrating the odor-guided, go, no-go task described in the text. On the left is the panel used to train rats on this task. In each trial within a particular session, one of two odors was delivered to the odor port at the top of the panel. Odor 1 indicated that if the rat went to the fluid well below the odor port, sucrose solution would be available. Odor 2 indicated that if the rat went to the fluid well, unpalatable quinine solution would be available. Rats learned to go to the well (go response, shown on the right) after sampling odor 1, and to avoid going to the well (no-go response, shown on the right) after sampling odor 2. After rats had reached and maintained a behavioral criterion of 18 correct out of 20 trials, the odor-outcome contingencies were reversed until rats again reached criterion performance.
Thus addiction involves inflexible behaviors both in drug-associated contexts and in normal learning contexts. What are the neural bases of these inflexible behaviors? One possibility is that addiction involves abnormalities in brain circuits that normally support flexible behavior. Below, we will describe evidence that chronic cocaine exposure causes long-lasting changes in information processing in a circuit including the OFC and the basolateral amygdala (ABL) that may contribute to the inflexibility of behavior in addiction.
INFLEXIBLE ASSOCIATIVE ENCODING IN BASOLATERAL AMYGDALA: A PROXIMAL CAUSE OF INFLEXIBLE DECISION MAKING IN ADDICTION
The ABL has been implicated in the persistent effects of drug-associated cues on drug-seeking, both in human cocaine addicts and in animal models of cocaine addiction.25–29 For instance, imaging studies frequently reveal activations of the amygdala during exposure to cues that elicit craving in addicts,25,27,28 and, in animal models, lesions or pharmacological manipulations of the ABL block cue-induced relapse.9,30,31 Recently it has been shown that pharmacological manipulations of the ABL that block memory reconsolidation also impair cue-induced relapse, suggesting that memories stored in the amygdala may mediate this phenomenon.32,33
We have reported recently that the reversal-learning deficits that result from damage to the OFC are also mediated through the ABL. As reviewed elsewhere,34 OFC lesions result in inflexible associative encoding in the ABL during reversal learning, and selective neurotoxic lesions of ABL, which eliminate these inflexible correlates, restore normal reversal learning in OFC-lesioned rats.35,36 Based on these data, we have suggested that signals from the OFC normally facilitate changes in associative representations in other brain areas, particularly in the face of novel or unexpected outcomes37; damage to the OFC eliminates these signals, resulting in slower changes in encoding downstream in the ABL and slower reversal learning.
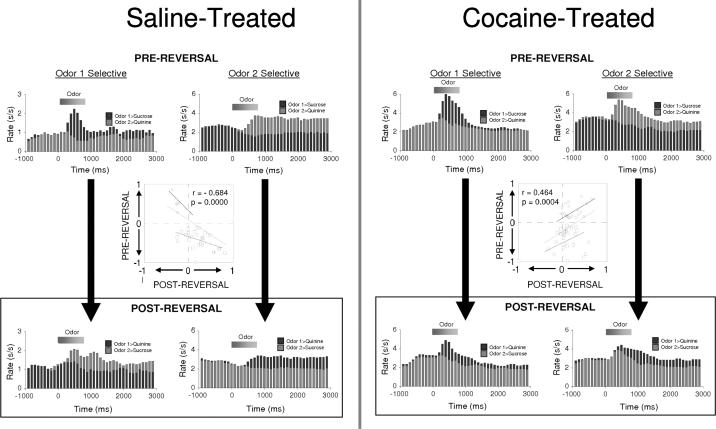
To ask whether a similar mechanism might mediate reversal-learning impairments caused by cocaine exposure, we recently compared neural correlates during reversal learning in the ABL in cocaine- and saline-treated rats.38 Rats were again exposed to 14 daily ip injections of cocaine (30 mg/kg), and then beginning approximately 1 month later, neural activity was recorded in these rats as they learned and reversed a series of two-odor discrimination problems using the same go, no-go task described above (illustrated in Fig.2). As we have reported previously for normal rats,35,39 ABL neurons in both saline- and cocaine-treated rats rapidly developed differential firing to the odor cues as the rats learned their meaning. In saline controls, these cue-selective populations reversed their cue-selectivity after reversal, such that they tracked the outcome predicted by the cue rather than the identity of the cue itself. In contrast, in cocaine-treated reversal-impaired rats, these populations failed to reverse their cue preference; instead they remained selective for the cue to which they fired before reversal. The contrast between the flexibility in cue-selectivity in controls and the inflexibility in cocaine-treated rats is illustrated by the population histograms and scatter plots shown in Figure 3.
FIGURE 3.
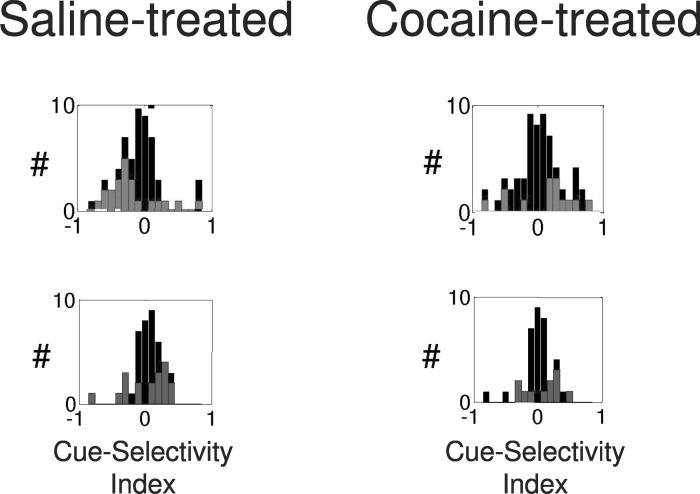
Population histograms before and after reversal for all neurons recorded in the ABL that were significantly selective for the sucrose-predictive cue (odor 1 selective) or the quinine-predictive cue (odor 2 selective) during the postcriterion prereversal-trial block. In saline-treated rats, neurons in both populations reversed their cue-selectivity across reversal. In contrast, in cocaine-treated rats, neurons that developed selectivity to the sucrose-predictive cue during learning remained selective for the same cue after reversal, even though it now predicted quinine. Neurons in cocaine-treated rats that developed selectivity to the quinine-predictive cue during learning failed to reverse their selectivity after reversal, instead showing a phasic response to both cues. Insets show a quantitative analysis of the flexibility of these populations across reversal. Neurons in saline-treated rats showed a negative correlation between their prereversal versus postreversal cue-selectivity indices; neurons in cocaine-treated rats showed a positive correlation between the two. Cue-selectivity index was defined as (frO1 − frO2)/(frO1 + frO2), where fr=firing rate during cue-sampling in the post-criterion trial block; O1 = odor cue that predicted sucrose before reversal; O2 = odor cue that predicted quinine before reversal (data adapted from Stalnaker et al.38). (In color in Annals online.)
To test whether the inflexibility of associative encoding in the ABL after reversal actually mediates the reversal-learning deficits in these rats, we exposed a second set of rats to cocaine or saline and then made bilateral neurotoxic or sham lesions of the ABL. After these rats recovered from surgery, we tested them in the same go, no-go odor discrimination task used earlier to assess reversal learning. As illustrated in Figure 4, we again found that cocaine exposure caused impaired reversal learning; however, this impairment was not observed in cocaine-exposed rats with ABL lesions. ABL lesions by themselves had no significant effect on reversal learning. Thus, in cocaine-exposed rats, encoding in the ABL seemed to be interfering with the ability to learn reversals quickly. These results are consistent with the hypothesis that rigid associative encoding in the ABL after reversal is the proximal cause of the cocaine-induced reversal impairment, just as it may be the proximal cause of cue-induced relapse to drug-seeking.
FIGURE 4.
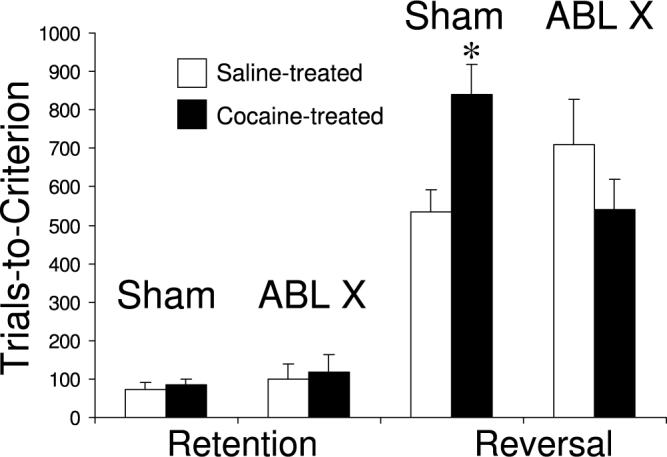
The effect of bilateral ABL lesions on retention and reversal learning in saline-treated rats (white bars) or cocaine treated rats (black bars). Previous cocaine treatment caused a severe reversal impairment that was abolished by ABL lesions. ABL lesions in the saline-treated rats had no significant effect. Shown is the average of three serial retention/reversals, in which rats first had to show retention of a previously learned odor discrimination, and then had to acquire a reversal of that odor discrimination. *P < 0.05 compared to the saline-treated sham-lesioned group and compared to the cocaine-treated ABL-lesioned group (data adapted from Stalnaker et al.38).
A FAILURE TO SIGNAL EXPECTED OUTCOMES IN THE ORBITOFRONTAL CORTEX: A DISTAL CAUSE OF INFLEXIBLE DECISION MAKING IN ADDICTION
But why is associative encoding in the ABL resistant to change after drug exposure? An answer to this question may lie in the effects of addictive drugs on the OFC. Imaging studies in cocaine,40 methamphetamine,41 and heroin42 users reveal altered metabolism in the OFC and abnormal neuronal activation in response to drug-associated cues.42 Furthermore, as reviewed above, drug addicts and animals exposed to addictive drugs, such as patients and animals with OFC damage,24,43,44 exhibit behavioral deficits on a variety of OFC-dependent tasks.16,17,20,45–48 These observations have led to the proposal that addictive drugs cause long-lasting disruptions to OFC function.49,50
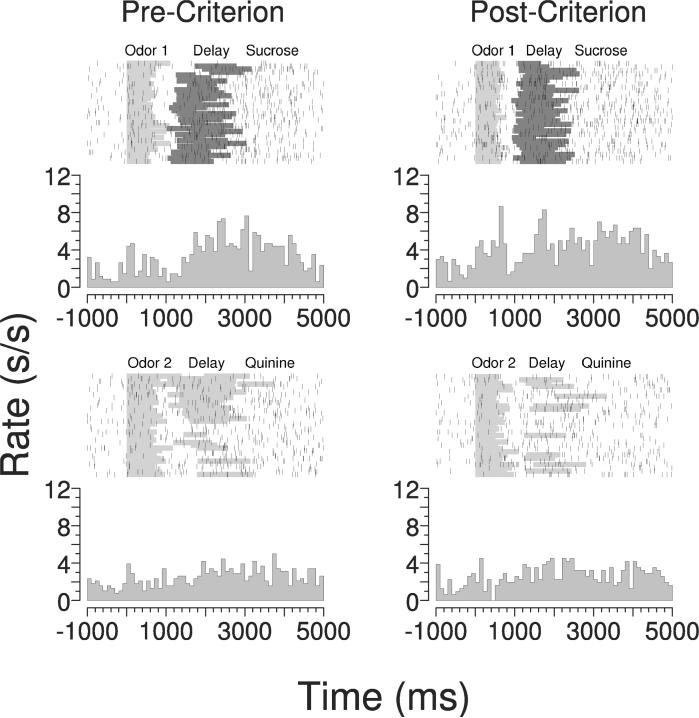
In the context of our reversal-learning task, we have proposed that the critical function of the OFC is to signal expected outcomes at the time a decision is made.34 This signal is particularly evident in subpopulations of OFC neurons, like the one shown in Figure 5, that fire in anticipation of one of the outcomes early in learning and then, as learning progresses, become active in the presence of the odor cue that predicts that outcome. By this pattern of activity, these neurons appear to activate a representation of the expected outcome at the time of odor sampling, when a decision about whether to respond or not must be made. Signaling expected outcomes might facilitate changes in associative encoding in the ABL after reversal by contributing to the generation of teaching signals that occur when actual outcomes fail to match expectations. In other words, such teaching signals require there to be some record of what outcome was expected; the OFC may provide this signal, either in whole or in part. With the loss of this signal, as after OFC lesions, changes in the outcome predicted by the cues would result in less-robust teaching signals, thereby causing associative encoding in downstream regions—such as the ABL—to change more slowly.
FIGURE 5.
Peri-event time histograms and raster plots show activity of a single-unit recorded from the OFC of a control rat during learning of an odor discrimination. The gray shading on the raster plots represents the period of odor delivery on each trial, the subsequent blue shading on the upper rasters represents the delay after the response and immediately before sucrose delivery, and the subsequent red shading on the lower rasters represents the delay after the response and immediately before quinine delivery. In trials before the rat had reached behavioral criterion (precriterion, left histograms and raster plots) this neuron fired selectively during and immediately preceding the delivery of sucrose. In trials after the rat had reached criterion (postcriterion, right histograms and raster plots) this neuron fired selectively for the odor that predicted sucrose, while continuing to fire during and immediately preceding sucrose. Thus in the postcriterion phase, this neuron activated a representation of the expected outcome at the time of odor delivery (data adapted from Roesch et al.54). (In color in Annals online.)
To test whether cocaine exposure causes a disruption of this outcome-expectant signaling in the OFC, we compared neural correlates in the OFC in cocaine- and saline-treated rats during acquisition of a series of two-odor go, no-go discrimination problems, using the same procedures as in our ABL recording experiment described above.51 Neural activity in saline-treated rats was similar to that reported previously,4,52,53 with some neurons firing selectively in anticipation of one of the two outcomes (sucrose or quinine) early in learning and then becoming activated by the appropriate odor cue after learning (as in Fig.5).54 Rats that had been exposed to cocaine exhibited similar proportions of neurons that fired in anticipation of one or the other of the outcomes. However, these populations were not consistently activated by the appropriate odor cue after learning, and instead were equally likely to become activated by either odor cue. Thus, for example, quinine-expectant neurons were equally likely to become selective for the quinine-predictive odor cue or for the sucrose-predictive odor cue. This pattern of results is quantified in the distributions of cue-selectivity indices for the outcome-expectant populations, shown in Figure 6. These distributions are significantly skewed to the left for quinine-expectant neurons and to the right for sucrose-expectant neurons in saline-treated rats, but they are symmetrically distributed around zero for cocaine-treated rats. Thus, chronic cocaine exposure caused OFC neurons to fail to signal the expected outcome during odor sampling in this task. This failure may explain why these rats are unable to bias behavior to reflect the value of expected outcomes, both during odor discrimination learning20 and possibly also after reinforcer devaluation.47 In addition, such a loss of outcome-expectant encoding may be a distal cause of the long-lasting reversal deficit seen after chronic exposure to cocaine.
FIGURE 6.
Cue-selectivity indices for neurons that developed outcome-expectant firing during the precriterion block, firing differentially after the rat's response in anticipation of either sucrose or quinine delivery. On the top row are shown the populations that developed quinine-expectant firing, and on the bottom row are shown the populations that developed sucrose-expectant firing. Red (top row) or blue (bottom row) bars represent neurons that were significantly selective for one or the other of the two odors. In both quinine-expectant and sucrose-expectant populations, neurons in control rats were more likely to develop cue-selectivity to the cue that predicted their preferred outcome. Thus, the distribution for quinine-expectant neurons is skewed to the left, and that in sucrose-expectant neurons is skewed to the right. In contrast, in both populations in cocaine-treated rats, neurons were equally likely to develop cue-selectivity to either cue. Thus, the distributions are symmetric around zero. Cue-selectivity indices were calculated from activity during odor sampling, using the same formula as in Figure 3 (data adapted from Stalnaker et al.51). (In color in Annals online.)
CONCLUSIONS
Here we have described data demonstrating that chronic cocaine exposure causes 1) inflexible encoding in the ABL across reversal of cue-outcome contingencies, 2) an ABL-dependent deficit in the ability to change behavior when cue-outcome contingencies change, and 3) long-lasting disruptions to outcome-expectant signaling in the OFC. These data support a model of cocaine-induced decision-making deficits in which cocaine exposure causes a critical loss of outcome-expectant encoding in the OFC, which leads to inflexible encoding of cue significance in the ABL. Such a model would be broadly consistent with data demonstrating that human addicts show abnormalities in the OFC and in OFC-dependent tasks, and with data from animal models of addiction suggesting that persistent ABL encoding underlies inflexible responding for drug-associated cues. Thus, while the changes in encoding properties described here were demonstrated during associative learning for nondrug outcomes, similar changes could also play a role in drug-seeking behavior itself. Future research should address whether the drug-induced changes to the encoding properties of the OFC–ABL circuit are similar in both drug and nondrug settings.
REFERENCES
- 1.Ehrman RN, Robbins SJ, Childress AR, O'Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl.) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- 2.Avants SK, Margolin A, Kosten TR, Cooney NL. Differences between responders and nonresponders to cocaine cues in the laboratory. Addict. Behav. 1995;20:215–224. doi: 10.1016/0306-4603(94)00066-2. [DOI] [PubMed] [Google Scholar]
- 3.Foltin RW, Haney M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology (Berl.) 2000;149:24–33. doi: 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J. Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 5.Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav. Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- 6.Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl.) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- 7.See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur. J. Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 8.Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr. Opin. Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs RA, Feltenstein MW, See RE. The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur. J. Neurosci. 2006;23:2809–2813. doi: 10.1111/j.1460-9568.2006.04806.x. [DOI] [PubMed] [Google Scholar]
- 10.Ciccocioppo R, Martin-Fardon R, Weiss F. Stimuli associated with a single cocaine experience elicit long-lasting cocaine-seeking. Nat. Neurosci. 2004;7:495–496. doi: 10.1038/nn1219. [DOI] [PubMed] [Google Scholar]
- 11.Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology. 2004;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- 12.Weiss F, Martin-Fardon R, Ciccocioppo R, et al. Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology. 2001;25:361–372. doi: 10.1016/S0893-133X(01)00238-X. [DOI] [PubMed] [Google Scholar]
- 13.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:951–953. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 14.Vanderschuren LJMJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 15.Vanderschuren LJ, Everitt BJ. Behavioral and neural mechanisms of compulsive drug seeking. Eur. J. Pharmacol. 2005;526:77–88. doi: 10.1016/j.ejphar.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 16.Rogers RD, Everitt BJ, Baldacchino A, et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 17.Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- 18.Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- 19.Jentsch JD, Olausson P, De La Garza R, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- 20.Schoenbaum G, Saddoris MP, Ramus SJ, et al. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. E. J. Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- 21.Calu DJ, Stalnaker TA, Franz TM, et al. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn. Mem. 2007;14:325–328. doi: 10.1101/lm.534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1294. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- 23.Schoenbaum G, Setlow B, Nugent SL, et al. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn. Mem. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izquierdo AD, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J. Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Childress AR, Mozley PD, Mcelgin W, et al. Limbic activation during cue-induced cocaine craving. Am. J. Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc. Natl. Acad. Sci. USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kilts CD, Schweitzer JB, Quinn CK, et al. Neural activity related to drug craving in cocaine addiction. Arch. Gen. Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 28.Bonson KR, Grant SJ, Contoreggi CS, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 29.Carelli RM, Williams JG, Hollander JA. Basolateral amygdala neurons encode cocaine self-administration and cocaine-associated cues. J. Neurosci. 2003;23:8204–8211. doi: 10.1523/JNEUROSCI.23-23-08204.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruzich PJ, See RE. Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J. Neurosci. 2001;21:RC155. doi: 10.1523/JNEUROSCI.21-14-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.See RE, McLaughlin J, Fuchs RA. Muscarinic receptor antagonism in the basolateral amygdala blocks acquisition of cocaine-stimulus association in a model of relapse to cocaine-seeking behavior in rats. Neuroscience. 2003;117:477–483. doi: 10.1016/s0306-4522(02)00665-6. [DOI] [PubMed] [Google Scholar]
- 32.Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J. Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoenbaum G, Saddoris MP, Stalnaker TA. Reconciling the roles of orbitofrontal cortex in reversal learning and the encoding of outcome expectancies. Ann. N. Y. Acad. Sci. 2007;1401:xx–xx. doi: 10.1196/annals.1401.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saddoris MP, Gallagher M, Schoenbaum G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron. 2005;46:321–331. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 36.Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54:51–58. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making, and drug addiction. Trends. Neurosci. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stalnaker TA, Roesch MR, Franz TM, et al. Cocaine-induced decision-making deficits are mediated by miscoding in basolateral amygdala. Nat. Neurosci. 2007;10:949–951. doi: 10.1038/nn1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J. Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volkow ND, Fowler JS, Wolf AP, et al. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am. J. Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- 41.Volkow ND, Chang L, Wang GJ, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am. J. Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 42.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 43.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J. Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mobini S, Body S, Ho M-Y, et al. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacol. 2002;160:290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- 45.Bechara A, Dolan S, Denburg N, et al. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- 46.Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp. Clin. Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- 47.Schoenbaum G, Setlow B. Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal-amygdalar function. Cerebral Cortex. 2005;15:1162–1169. doi: 10.1093/cercor/bhh216. [DOI] [PubMed] [Google Scholar]
- 48.Roesch MR, Takahashi Y, Gugsa N, et al. Previous cocaine exposure makes rats hypersensitive to both delay and reward magnitude. J. Neurosci. 2007;27:245–250. doi: 10.1523/JNEUROSCI.4080-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 50.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of orbitofrontal cortex. Cerebral Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 51.Stalnaker TA, Roesch MR, Franz TM, et al. Abnormal associative encoding in orbitofrontal neurons in cocaine-experienced rats during decision-making. Eur. J. Neurosci. 2006;24:2643–2653. doi: 10.1111/j.1460-9568.2006.05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat. Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- 53.Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- 54.Roesch MR, Stalnaker TA, Schoenbaum G. Associative encoding in anterior piriform cortex versus orbitofrontal cortex during odor discrimination and reversal learning. Cerebral Cortex. 2007;17:643–652. doi: 10.1093/cercor/bhk009. [DOI] [PMC free article] [PubMed] [Google Scholar]