Abstract
In the cross-coupling reactions of unprotected oxindoles with aryl halides, Pd- and Cu-based catalyst systems displayed orthogonal chemoselectivity. A Pd/dialkylbiarylphosphine-based catalyst system chemoselectively arylated oxindole at the 3-position, while arylation occurred exclusively at the nitrogen using a Cu/diamine-based catalyst system. Computational examination of the relevant L1Pd(Ar)(oxindolate) and diamine-Cu-(oxindolate) species were performed to gain mechanistic insight into the controlling features of the observed chemoselectivity.
Keywords: Palladium, Copper, Amidation, Enolate Arylation, Oxindole, Aryl Halide, Diamine, Biarylmonophosphine, Goldberg Reaction
Introduction
In recent years, Pd-1 and Cu-2 catalyzed nucleophilic substitution reactions of aryl halides have been areas of intensive research. Our laboratory has been intimately involved in designing and developing highly-efficient and user-friendly Pd- and Cu-based catalyst systems to cross-couple aryl halides with a wide variety of nucleophiles, including amides3–4 and ketone enolate derivatives.5–6
Generally, the Cu- and Pd-catalyzed arylation reactions of both linear and cyclic aliphatic amides react at the more acidic N—H moiety as opposed to the less acidic C—Hα position. For instance, when reacting 2-pyrrolidinone with aryl halides, both Cu-diamine- and Pd-biarylmonophosphine-based catalyst systems provide the N-aryl amide in excellent yield (Scheme 1).3–4 Ongoing work in our and other laboratories7 has identified oxindole as a unique substrate for chemoselective metal-catalyzed cross-coupling reactions with aryl halides. Due to the identical acidities of the protons in positions C3 and N1 (pKa = 18.5),8 the cross-coupling reactions of oxindole with aryl halides might provide either the C-aryl or N-aryl products.
Scheme 1.
Pd- and Cu-Catalyzed C- and N-Arylation of 2-Pyrrolidinone and Oxindole.
Importantly, N1-aryl and C3-aryl oxindole products of the type generated from the reactions described in this manuscript display interesting biological activities with therapeutic applications (Figure 1).9 In addition, the amide of the N-aryl oxindole can be cleaved to provide access to a variety of derivatives of 2-(2-phenylamino)-phenyl)ethanoic acid non-steroidal anti-inflammatory agents, such as Lumiracoxib10 and diclofenac.11
Figure 1.
Therapeutically Relevant C-Aryl and N-Aryl Oxindoles and Related Compounds.
Herein, we describe improved reaction conditions for the Cu-catalyzed N1-arylation reaction with aryl iodides and bromides, and general reaction conditions for the Pd-catalyzed C3-arylation reaction of unprotected oxindoles with aryl chlorides and tosylates. Further, we report computational studies that suggest reasonable explanations for the observed selectivity.
Results
Pd-Catalyzed C3-Arylation of Oxindoles
The use of 1% Pd2(dba)3 and 5% XPhos (Figure 2) was found to facilitate the cross-coupling of aryl chlorides with oxindoles unsubstituted at C3 using K2CO3 as the base in THF or 1,4-dioxane at temperatures ranging between 80 and 100 °C (Table 1). The use of bidentate or other dialkylbiarylmonophosphine ligands provided low conversion of starting material and yield of products. The Pd-catalyzed C3-arylation reaction of oxindoles with aryl chlorides tolerated a variety of functional groups on the meta- and para-positions of the electrophile (entries 1–10); however, ortho-substituted aryl chlorides provided low conversion of reactants (> 5%) even at slightly elevated temperatures (up to 120 °C) with a variety of biarylmonophosphine ligands. Under the standard reaction conditions, the use of 3-chlorobenzonitrile provided low yields of coupled product due to partial hydrolysis of the nitrile functional group to an amide (entry 4). This side reaction could be partially impeded by the addition of activated 4 Å molecular sieves to the reaction vessel. Using t-BuOH as a solvent, an unactivated aryl benzenesulfonate could be successfully cross-coupled to provide the C-aryl product in modest yield (entry 5). Substrates possessing substituents on the benzannulated backbone (entries 6–8) as well as on the nitrogen atom (entries 9–10) provided more highly substituted products. Using XPhos as a ligand, the reaction of a 3-substituted oxindole was unsuccessful;7b however, using re-optimized reaction conditions (RuPhos/NaOt-Bu/toluene), the cross-coupling reactions of 3-methyl- and 3-benzyl-oxindole were successfully accomplished to generate quaternary stereocenters at the C3 positions to produce racemic products (entries 11–12). In contrast to the previously reported catalyst systems for the C3-vinylation of unprotected oxindoles and –arylation of protected oxindoles, which required strong bases such as KHMDS and LHMDS, respectively, for the reactions to proceed,7b–c K2CO3 and NaOt-Bu were found to be suitable bases with our catalyst system.
Figure 2.
Ligands Employed for the Metal-Catalyzed C- and N-Arylation of Oxindole.
Table 1.
Pd-Catalyzed C-Arylation of Oxindoles.a
 | ||||
|---|---|---|---|---|
| entry | product | solvent | temp. (°C) | % yield |
| 1 |  |
THF | 80 | 92 |
| 2 |  |
1,4-dioxane | 100 | 81 |
| 3 |  |
1,4-dioxane | 100 | 89b |
| 4 |  |
THF | 80 | 55c |
| 5 |  |
t-BuOH | 110 | 67d |
| 6 |  |
1,4-dioxane | 100 | 80 |
| 7 |  |
THF | 80 | 77 |
| 8 |  |
THF | 80 | 63 |
| 9 |  |
THF | 80 | 82 |
| 10 |  |
1,4-dioxane | 80 | 94e |
| 11 |  |
toluene | 100 | 90f |
| 12 |  |
toluene | 100 | 90g |
Reactions Conditions: 1.0–1.2 mmol oxindole, 1.2-1.0 mmol ArCl, 2.0 mmol K2CO3, 0.010 mmol Pd2dba3, 0.050 mmol XPhos, 1.0 mL solvent, in a sealed tube under an Ar atmosphere. Yields reported are an average of at least two runs determined to be > 95% pure by elemental analysis or 1H NMR.
3.0 mmol K2CO3.
4 Å mol Sieves.
From ArOSO2Ph.
K3PO4 used as base.
From ArBr. RuPhos and NaOt-Bu employed as ligand and base. 9 h reaction time.
From ArBr. RuPhos and NaOt-Bu employed as ligand and base. 20 h reaction time.
Cu-Catalyzed N-Arylation of Oxindoles
The Cu-catalyzed N-arylation reactions of meta- and para-substituted aryl iodides generally proceeded smoothly at temperatures ranging from 80 to 100 °C using 1–5% catalyst loading, 4–10% CyDMEDA (Figure 2) as the ligand, K2CO3 as the base, and 1,4-dioxane as the solvent (Table 2, entries 1–8). In these reactions, the C3-aryl product was not detected by GCMS analysis of the crude reaction mixtures. Using this catalyst system, ortho-substituted aryl iodides were unreactive, even at temperatures up to 150 °C in high boiling-point solvents. This serves to reinforce the notion that ortho-substituted aryl halides can be quite difficult to activate in Cu-catalyzed C-heteroatom bond-forming reactions. At 60–100 °C, the cross-coupling reaction of 1-bromo-4-iodobenzene with oxindole provided a complex mixture of products; however, by lowering the reaction temperature to 40 °C, the bromide-containing product could be isolated in acceptable yield (entry 3). The addition of activated 4 Å molecular sieves to the reaction mixtures was necessary for substrates containing hydroxide- or water-sensitive functional groups (entries 4 and 7). As anticipated, substituents on the nucleophile were also tolerated (entries 6–7).
Table 2.
Cu-Catalyzed N-Arylation of Oxindoles.a
 | ||||||
|---|---|---|---|---|---|---|
| entry | Product | Y | X | Z | temp. (°C) | % yield |
| 1 |  |
5 | I | 10 | 100 | 86 |
| 2 |  |
1 | I | 4 | 100 | 94 |
| 3 |  |
5 | I | 10 | 40 | 61 |
| 4 |  |
5 | I | 10 | 80 | 72b |
| 5 |  |
1 | I | 4 | 80 | 85 |
| 6 |  |
5 | I | 10 | 80 | 69 |
| 7 |  |
5 | I | 10 | 80 | 87b |
| 8 |  |
10 | Br | 20 | 100 | 62 |
| 9 |  |
10 | Br | 20 | 100 | 71 |
| 10 |  |
10 | Br | 20 | 100 | 77 |
| 11 |  |
10 | Br | 20 | 100 | 72 |
Reactions Conditions: 1.0–1.2 mmol oxindole, 1.2-1.0 mmol ArI, 2.0 mmol K2CO3, 1.0 mL 1,4-dioxane, in a sealed tube under an Ar atmosphere. Yields reported are an average of at least two runs determined to be > 95% pure by elemental analysis or 1H NMR.
4 Å mol Sieves.
Aryl bromides also proved to be reactive in the Cu-catalyzed cross-coupling reactions with oxindoles (entries 8–11), though higher catalyst loadings were necessary to ensure full conversion of the substrates within a 24 h time period. Although the C3-aryl oxindole product was not observed, up to 5% of the N1,C3-bis-arylated product (10% of aryl halide consumption) was isolated in the reaction of 5-bromo-m-xylene (entry 9). A second common side-product, when using aryl bromides, was the reduced arene. Aryl chlorides were not suitable coupling partners for this reaction. These substrates are generally unreactive in Cu-catalyzed C-heteroatom bond-forming reactions due to the high energy required for activating the CAryl–Cl bond relative to the –Br or –I bonds.2
Computational Studies of Ligand-Metal-Oxindole Complexes
The catalytic cycle of Pd-catalyzed nucleophilic substitution reactions of aryl halides involve three steps: 1) oxidative-addition; 2) transmetallation; 3) reductive-elimination (Scheme 2, Cycle A).1
Scheme 2.
Mechanisms of Pd- and Cu-Catalyzed Nucleophilic Substitution Reactions of Aryl Halides.
Since oxindole does not participate in the oxidative addition step of the cycle, the selectivity-controlling feature for the Pd-based catalyst system must involve either the transmetallation or reductive-elimination steps. To gain further insight into the observed selectivity, the relative energies and structures of various L1Pd(Ph)(oxindolate) complexes were calculated by DFT methods (Figure 3 and Figure 4, see Computational Methods for full details).
Figure 3.
Calculated Geometries and Energies of C- and N-bound XPhos·Pd(Ph)(oxindolate) Complexes with B3LYP.a
a Calculated at 298 K in THF.
Figure 4.
Calculated Geometries and Energies of the κ2- O- and η3-oxyallyl-bound XPhos·Pd(Ph)(oxindolate) Complexes with B3LYP.a,b
a Calculated at 298 K in THF.
b ΔErel values are relative to complex 1 in Figure 3.
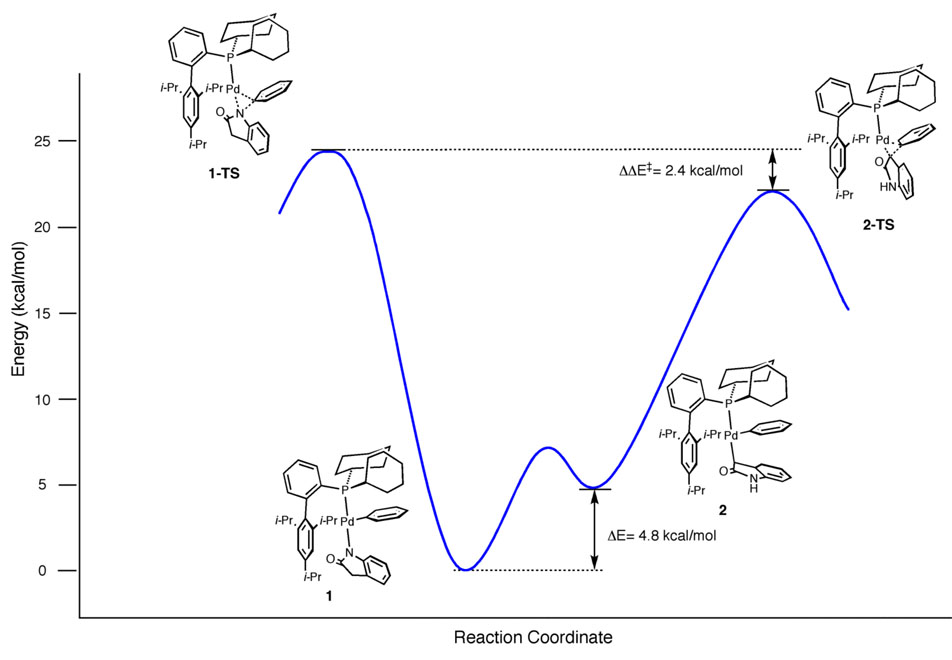
In light of the experimental findings that catalysts derived from XPhos were the ones that produced high yields of the C3-arylated of oxindole product, it was critical to model structures that contained the entire ligand without any approximations. The geometry of the XPhos·Pd(Ph)(oxindolate) complex formed following transmetallation was minimized with the Pd bound to either the nitrogen or α-carbon of the oxindole (Figure 3). This minimization was performed with structures in which the Pd points towards or away from the lower biaryl ring. Consistent with previous computational studies from our group,12 three-coordinate Pd(II)/dialkylbiarylmonophosphine intermediates prefer the orientation shown in structures 1 and 2 with the Pd sitting above the lower biaryl ring. In both cases, the C-bound oxindolate was significantly higher in energy than the N-bound complex. This energy difference was 4.8 kcal/mol between 1 and 2 and 7.0 kcal/mol between 3 and 4. We then determined the energies of the κ2-amidate (5,9), O-bound amidate and enolate (6–7, 10–11), and η3-oxyallyl (8,12) structures as, they may be intermediates in the N to C isomerization process (Figure 4). As expected, the three-coordinate O-bound enolate and amidate structures are lower in energy when the Pd is pointing towards the lower ring. However, the four-coordinate κ2-amidate and η3-oxyallyl bound structures are lower in energy when the Pd is distal to the lower ring.
If the N-bound and C-bound structures exist in rapid equilibrium, then the barriers for reductive elimination should be product determining. Thus, transition states for both C–C and C–N reductive elimination processes were calculated (Figure 5). The mechanism for reductive elimination from Pd-bound enolates has not been well studied, and could occur by different mechanisms involving O-bound, C-bound, or η3-oxyallyl Pd intermediates. Hartwig and Culkin13 have put forth circumstantial evidence in support of a simple reduction elimination between an η1-bound enolate and the arene. However, these studies were primarily performed using bidentate ligands, which may prevent η3-bound intermediates and relevant transition states. Several reports have appeared, which indicate η3-oxyallyl Pd intermediates may be involved in some Pd-enolate based processes.14
Figure 5.
Calculated Reductive-elimination Transition States for XPhos·Pd(Ph)(oxindolate) with B3LYP.a,b
a Calculated at 298 K in THF.
b ΔErel values are relative to complex 1 in Figure 3.
Reasonable starting geometries for the transition states of enolate C–C reductive elimination were arrived at by examining calculations reported by others for methyl-methyl reductive elimination from Pd.15 A prior publication by our group on the mechanism of aryl amination provided us with reasonable starting geometries for amidate C–N reductive-elimination.12 We were then able to quickly find transition states for both C–C and C–N reductive elimination towards and away from the lower biaryl ring. The ΔE‡ for 1→1-TS was calculated to be 21.7 kcal/mol while the ΔE‡ for 2→2-TS was significantly less at 14.5 kcal/mol. For the structures with the Pd swung away, the ΔE‡ of 3→3-TS was calculated to be 20.9 kcal/mol and the ΔE‡ of 4→4-TS was 12.6 kcal/mol. Although these barriers are lower than when the Pd is pointed towards the lower biaryl ring, their absolute energies are much higher. Therefore, it is unlikely that 3-TS and 4-TS contribute to the reaction course. We also attempted to find a transition state for C–C reductive elimination, which proceeds through an η3 pathway, but one could not be located.
Cu-catalyzed amidation reactions of aryl halides initiate by addition of the nucleophile to a L2Cu(I)X complex to provide an L2Cu(I)amidate, followed by aryl halide activation and subsequent product formation.16,17 Although the Guo group has recently published a computational study that calculates the transition states and intermediates of the catalytic cycle for the Goldberg reaction,18 this study only considered a mechanism based on an insertion reaction between an L2Cu(I)-(amidate) and an aryl halide to generate an L2Cu(III)-(Ar)(X)(amidate) species, and neglects to evaluate evidence that an electron-transfer mechanism might be occurring, as suggested by Hida.19 Therefore, we will assume that the mechanism of the rate-limiting aryl halide activation step is yet to be fully elucidated (Scheme 2, Cycle B). According to this paradigm, reaction of a molecule of oxindole with the CyDMEDA-CuI complex and base may provide multiple regioisomeric products, which could react with aryl halides to provide the N-aryl and C3-aryl products, respectively. In order to gain insight into the features that control the selectivity of the reaction, the energies of relevant CyDMEDA-Cu(I)(oxindolate) complexes were examined (Figure 6).
Figure 6.
Calculated Geometries and Energies of CyDMEDA·Cu(Oxindolate) Complexes with B3LYP.a
a Calculated at 298 K.
As observed with the Pd-based catalyst system, the N-bound (CyDMEDA)·Cu(oxindolate) 13 was found to be significantly lower in energy than both C3-bound and O-bound structures. In this structure, the geometry around Cu is a distorted T-shape, consistent with known neutral tricoordinate Cu(I) structures.20 Interestingly, the calculation predicts a hydrogen bonding interaction between the carbonyl and one hydrogen of the amine (O–H distance of 1.9 Å). The C3-bound oxindolate 14 is significantly higher in energy by 14.1 kcal/mol. It is noteworthy that the geometry about Cu is no longer planar but trigonal pyramidal and equidistant to both nitrogen atoms. There also appears to be a hydrogen bond between the carbonyl oxygen and amine hydrogen (O–H distance of 2.0 Å). The O-bound amidate 15 and enolate 16 are 9.3 kcal/mol and 19.2 kcal/mol higher in energy respectively than the N-bound structure. We also optimized κ2-amidate and η3-oxyallyl bound structures but no reasonable stationary points could be found.
Discussion
In metal-catalyzed amidation reactions of aliphatic amides, such as 2-pyrrolidinone, coordination and deprotonation of the nucleophile during the transmetallation step of the catalytic cycle occur at the more acidic N–H position as opposed to the less acidic C–Hα position (Scheme 1). In the case of oxindole, both the N–H and the C–Hα protons are significantly acidified due to the conjugation of the deprotonated anion with the aromatic ring. Further, the predisposition for the anion to reside on the more electronegative nitrogen atom is overcome by the aromatic stabilization gained from isomerization of the anion to generate an enolate.8 Thus, a 1:1 ratio of amidate : enolate exists in solution. As such, the difference in reactivity between typical aliphatic amides and oxindole demonstrated by Cu- and Pd-based catalyst systems might not be entirely unexpected.
Since a weak base was employed (K2CO3), the large pKa difference (~5) between oxindole and the base indicates that an appreciable quantity of an anionic amidate species does not exist in solution; thus, an intermolecular ligand exchange between an oxindolate anion and XPhos·Pd(Ar)(Cl) 17 is unlikely. As such, it is likely that the oxindole is further acidified by reversible coordination of Pd(II) intermediate 17 to the oxindole carbonyl to form 18 (Scheme 3). Deprotonation of the acidified oxindole should occur at N as opposed to C3, since deprotonation is kinetically faster from N–H than from C–H bonds, in which rehybridization must occur at the carbon atom.21 Thus, deprotonation of 18 would initially lead to 6, followed by an intramolecular migration of Pd from O to either N or C. If intramolecular isomerization is the preferred pathway, then a plausible reaction sequence to form a C-bound Pd enolate that does not involve formation a Pd–N bond may be 17→18→6→7→8→2. If a Pd–N bond does transiently form, then the reaction pathway might proceed as such, 17→18→6→5→1→5→6→7→8→2.
Scheme 3.
Transmetallation of oxindole to XPhos·Pd(Ph)(Cl).
For the Pd-catalyzed C-arylation reaction of oxindole with aryl halides, transmetallation of a molecule of oxindole to the L1Pd(Ar)(X) complex can provide multiple isomeric species. Two of these isomers, namely 1 and 2, would reductively eliminate to provide the N-aryl and C3-aryl oxindole products, respectively. The energy profile illustrating the reaction course with the relative energies of the key intermediates and transition states is shown in Figure 7. The C3-aryl product, which is exclusively observed in the Pd-catalyzed reaction, must result from a rapid reductive elimination from the higher-energy Pd-C-bound enolate, 2, as opposed to the more-stable Pd-N-bound amidate, 1. Therefore, the selectivity demonstrated by the Pd-catalyzed reaction is kinetically governed according to the Curtin-Hammett principle.22 The 2.4 kcal/mol difference in energy between 1-TS and 2-TS is consistent with the observed selectivity of the catalytic reaction. This difference in energy is likely a reflection of the relative electronegativities of nitrogen and carbon and the overlap of the relevant molecular orbitals with those of Pd.23 Since the halide anion is not present during the proposed selectivity-determining event of the cycle, the change in identity of the electrophile from an aryl chloride to a bromide, or sulfonate ester does not have an effect on the chemoselectivity observed in the catalytic reaction (e.g., Table 1, entries 11–12). However, the change in the nature of the electrophile should have an effect on the relative rate of a reaction.
Figure 7.
Energy Diagram for XPhos·Pd(Ph)(oxindolate) Reductive-elimination.
For the Cu-catalyzed N-arylation reaction, the computational studies of the relevant CyDMEDA-Cu(oxindolate) species suggest that N-bound species 13 is favored by 14.1 kcal/mol over the C-bound isomer 14 (Scheme 4). Therefore, the selectivity observed for the Cu-catalyzed N-arylation of oxindole might be governed by two different factors: (1) aryl halide activation from 13 proceeds faster than from 14, and/or (2) aryl halide activation proceeds faster than the isomerization process. If the first factor is selectivity-determining, there could be a dynamic equilibrium in solution between 13 and 14. The nature of the aryl halide activation step in Cu-catalyzed C-heteroatom bond-forming substitution reactions of aryl halides is not well understood.17–19 Kinetic studies for the reaction of 2-pyrrolidinone with 4-iodo-m-xylene estimate ΔG‡ to be 19.4 kcal/mol.17 Therefore, it is plausible that the C-bound enolate does exist in small portions in solution, and that the selectivity is governed by the aryl halide activation processes (k3 > k4). If the second factor is selectivity-determining, then the absence of a low energy pathway for the interconversion of 13 and 14 controls the reaction’s outcome. A better understanding of the mechanism of aryl halide activation is required to properly estimate the transitions state energies for this step and to gain a full understanding for the observed chemoselectivity of the Cu-catalyzed reaction.
Scheme 4.
Mechanistic Considerations for the Cu-Catalyzed N-Arylation of Oxindole.
Conclusions
In summary, we have reported efficient and complementary Pd- and Cu-based catalyst systems for the C3- and N-arylation reactions of unprotected oxindoles using aryl halides. The use of a weak base allows for the presence of a wide variety of functional groups and substitution patterns that are not tolerated with stronger bases.7b–c Theoretical calculations suggest that for both the Pd- and Cu-based catalyst systems, the respective metallated oxindoles have a strong preference for the oxindole moiety to coordinate as an N-bound amidate as opposed to a C3-bound enolate. For the Pd-based catalyst system, the energy difference between the Pd-amide and Pd-enolates is ~ 5 kcal/mol, however, the selectivity is governed by a rapid C–C reductive elimination compared to C–N reductive elimination based on calculated transition state energies. For the Cu-based catalyst system, the preference for the metal to bind at N1 is stronger (~ 14 kcal/mol). In this case, the selectivity might be governed by rapid aryl halide activation from the diamine-Cu(I)-amidate complex compared to the diamine-Cu(I)-enolate. Alternatively, a low energy pathway for Cu to isomerize from N to C may not exist, and the C-bound enolate might never form. The implications of this study should be useful for those chemists interested in understanding the inherent differences between Pd- and Cu-based catalyst systems for nucleophilic substitution reactions of aryl halides.
Computational Methods
All calculations were performed with the Gaussian ’0324 suite of programs. DFT calculations employed the B3LYP functional25 using the 6-31G(d) basis set for all atoms in the Cu complexes. Due to the size of the XPhos-Pd complexes, geometry optimization was first performed using a two-layered ONIOM26 calculation (B3LYP/6-31g(d):UFF) with the oxindole, phenyl, Pd and P at a high level and the rest of the ligand at the low level. The resulting structures were then reoptimized using all-atom DFT B3LYP/6-31g(d) with the LANL2DZ basis set and the Hay-Wadt effective core potential27 (ECP) for Pd. To obtain the final ΔE values, single point energy calculations were performed with the 6-311g(d,p) basis set with implicit solvation included. Frequency calculations were performed on all optimized structures to confirm that the minima had no negative frequencies and transition states had a single imaginary frequency. The Gibbs free energies were calculated at 298.15 K and 1 atm.
Supplementary Material
Supporting Information Available Experimental procedures and characterization data for all new and known compounds, computational data for complexes, complete reference 24. This material is available free of charge via the Internet at http://pubs.acs.org
Acknowledgements
We thank the National Institutes of Health (GM58160 and GM46059) for financial support for this project. RAA is grateful to Pfizer and the National Institutes of Health (F31GM081905) for predoctoral fellowships. AMH thanks Aldrich for a Graduate Student Innovation in Organic Chemistry Award. We thank Amgen, Boehringer-Ingelheim and Merck for additional unrestricted financial contributions. The XPhos used for this publication was generously provided by Saltigo. The NMR instruments used for this publication were furnished by funds from the National Science Foundation (CHE 9808061 and DBI 9729592).
References
- 1.(a) Culkin DA, Hartwig JF. Acc. Chem. Res. 2003;36:234. doi: 10.1021/ar0201106. [DOI] [PubMed] [Google Scholar]; (b) Jiang L, Buchwald SL. Palladium-Catalyzed Aromatic Carbon-Nitrogen Bond Formation. In: de Meijere A, Diederich F, editors. Metal-Catalyzed Cross-Coupling Reactions. 2nd ed. Weinheim: Wiley-VCH; 2004. pp. 699–760. [Google Scholar]
- 2.(a) Monnier F, Taillefer M. Angew. Chem. Int. Ed. 2008;47:3096. doi: 10.1002/anie.200703209. [DOI] [PubMed] [Google Scholar]; (b) Beletskaya IP, Cheprakov AV. Coord. Chem. Rev. 2004:2337. doi: 10.1021/cr9903048. [DOI] [PubMed] [Google Scholar]; (c) Ley SV, Thomas AW. Angew. Chem. Int. Ed. 2003;42:5400. doi: 10.1002/anie.200300594. [DOI] [PubMed] [Google Scholar]; (d) Kunz K, Scholz U, Ganzer D. Synlett. 2003:2428. [Google Scholar]
- 3.For Cu-catalyzed N-arylation of amides using N,N’-dimethyl-1,2-ethylenediamine-derived ligands, SeeKlapars A, Antilla JC, Huang X, Buchwald SL. J. Am. Chem. Soc. 2001;123:7727. doi: 10.1021/ja016226z.; Klapars A, Huang X, Buchwald SL. J. Am. Chem. Soc. 2002;124:7421. doi: 10.1021/ja0260465. [DOI] [PubMed] [Google Scholar]
- 4.For Pd-catalyzed amidation of aryl halides using Xantphos and dialkylbiarylmonophosphine ligands, seeYin J, Buchwald SL. J. Am. Chem. Soc. 2002;124:6043. doi: 10.1021/ja012610k.Huang X, Anderson KW, Zim D, Jiang L, Klapars A, Buchwald SL. J. Am. Chem. Soc. 2003;125:6653. doi: 10.1021/ja035483w.Ikawa T, Barder TE, Biscoe MR, Buchwald SL. J. Am. Chem. Soc. 2007;129:13001. doi: 10.1021/ja0717414.
- 5.(a) Palucki M, Buchwald SL. J. Am. Chem. Soc. 1997;119:11108. [Google Scholar]; (b) Fox JM, Huang X, Chieffi A, Buchwald SL. J. Am. Chem. Soc. 2000;122:1360. [Google Scholar]; (c) Hamada T, Chieffi A, Ahman J, Buchwald SL. J. Am. Chem. Soc. 2002;124:1261. doi: 10.1021/ja011122+. [DOI] [PubMed] [Google Scholar]; (d) Nguyen HN, Huang X, Buchwald SL. J. Am. Chem. Soc. 2003;125:11818. doi: 10.1021/ja036947t. [DOI] [PubMed] [Google Scholar]
- 6.Hennessy EJ, Buchwald SL. Org. Lett. 2002;4:269. doi: 10.1021/ol017038g. [DOI] [PubMed] [Google Scholar]
- 7.(a) Phillips DP, Hudson AR, Nguyen B, Lau TL, McNeill MH, Dalgard JE, Chen JH, Penuliar RJ, Miller TA, Zhi L. Tetrahedron Lett. 2006;47:7137. [Google Scholar]; (b) Huang J, Bunel E, Faul MM. Org. Lett. 2007;9:4343. doi: 10.1021/ol7019839. [DOI] [PubMed] [Google Scholar]; (c) Durbin MJ, Willis MC. Org. Lett. 2008;10:1413. doi: 10.1021/ol800141t. [DOI] [PubMed] [Google Scholar]; (d) van den Hoogenband A, Lange JHM, Iwema-Bakker WI, den Hartog, Jack AJ, van Schaik J, Feenstra RW, Terpstra JW. Tetrahedron Lett. 2006;47:4361. [Google Scholar]
- 8.Bordwell FG, Fried HE. J. Org. Chem. 1991;56:4218. [Google Scholar]
- 9.(a) Luk KC, So SS, Zhang J, Zhang Z. (F. Hoffman-LaRoche AG) Oxindole Derivatives. 2006. Dec 28, WO 2006/136606 A3. [Google Scholar]; (b) Hewawasam P, Gribkoff VK, Pendri Y, Dworetzky SI, Meanwell NA, Martinez E, Boissard CG, Post-Munson DJ, Trojnacki JT, Yeleswaram K, Pajor LM, Knipe J, Gao Q, Perrone R, Starrett JE., Jr Bioorg. Med. Chem. Lett. 2002;12:1023. doi: 10.1016/s0960-894x(02)00101-4. [DOI] [PubMed] [Google Scholar]; (c) Sarges R, Howard HR, Koe K, Weissman A. J. Med. Chem. 1989;32:437. doi: 10.1021/jm00122a025. [DOI] [PubMed] [Google Scholar]
- 10.Karnachi AA, Bateman SD. (Novartis-AG) Pharmaceutical Composition Comprising Lumiracoxib. 2003 March 13; WO 03/020261 A1. [Google Scholar]
- 11.Acemoglu M, Allmendinger T, Calienni J, Cercus J, Loiseleur O, Sedelmier GH, Xu D. Tetrahedron. 2004;60:11571. [Google Scholar]
- 12.Barder TE, Buchwald SL. J. Am. Chem. Soc. 2007;129:12003. doi: 10.1021/ja073747z. [DOI] [PubMed] [Google Scholar]
- 13.(a) Culkin DA, Hartwig JF. J. Am. Chem. Soc. 2001;123:5816. doi: 10.1021/ja015732l. [DOI] [PubMed] [Google Scholar]; (b) Culkin DA, Hartwig JF. Organometallics. 2004;23:3398. [Google Scholar]
- 14.Ito Y, Nakatsuka M, Kise N, Saegusa T. Tetrahedron Lett. 1980;21:2873.Sodeoka M, Ohrai K, Shibasaki M. J. Org. Chem. 1995;60:2648.Albéniz AC, Catalina NM, Espinet P, Redón R. Organometallics. 1999;18:5571.For a DFT study which reports an η3-oxyallyl Pd transition state between O-bound and C-bound enolates, seeCámpora J, Maya CM, Palma P, Carmona E, Gutiérrez E, Ruiz C, Graiff C, Tiripicchio A. Chem.—Eur. J. 2005;11:6889. doi: 10.1002/chem.200500622.
- 15.(a) Low JJ, Goddard WA., III J. Am. Chem. Soc. 1984;106:8321. [Google Scholar]; (b) Ananikov VP, Musaev DG, Morokuma K. Organometallics. 2005;24:715. doi: 10.1021/om050255r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paine AJ. J. Am. Chem. Soc. 1987;109:1496. [Google Scholar]
- 17.Strieter ER. Ph.D. Thesis. Cambridge, MA: Massachusetts Institute of Technology; 2005. Jun, Mechanistic Studies on Metal-Catalyzed Carbon-Nitrogen Bond Forming Reactions. [Google Scholar]
- 18.Zhang SL, Liu L, Fu Y, Guo QX. Organometallics. 2007;26:4546. [Google Scholar]
- 19.Arai S, Hida M, Yamagishi T. Bull. Chem. Soc. Jpn. 1978;51:277. [Google Scholar]
- 20.(a) Jazdzewski BA, Young VG, Jr, Tolman WB. Chem. Comm. 1998:2521. [Google Scholar]; (b) Näther C, Beck A. Acta Crystallogr., Sect. E: Struct. Rep. Online. 2004;60:m1008. [Google Scholar]; (c) Blue ED, Davis A, Conner D, Gunnoe TB, Boyle PD, White PS. J. Am. Chem. Soc. 2003;125:9435. doi: 10.1021/ja0353659. [DOI] [PubMed] [Google Scholar]
- 21.(a) Eigen M. Angew. Chem. Int. Ed. 1964;3:1. [Google Scholar]; (b) Borwell FG, Boyle WJ, Hautala JA, Yee KC. J. Am. Chem. Soc. 1969;91:4002. [Google Scholar]; (c) Kresge AJ. Acc. Chem. Res. 1975;8:354. [Google Scholar]
- 22.Anslyn EV, Dougherty DA. Modern Physical Organic Chemistry. 3rd Ed. Sausalito, CA: University Science Books; 2006. pp. 378–379. [Google Scholar]
- 23.Hartwig JF. Inorganic Chemistry. 2007;46:1936. doi: 10.1021/ic061926w. [DOI] [PubMed] [Google Scholar]
- 24.Frisch MJ, et al. Gaussian 03. Wallingford, CT: Guassian Inc.; 2004. revision D.01. [Google Scholar]
- 25.(a) Becke AD. J. Chem. Phys. 1993;98:5648. [Google Scholar]; (b) Lee C, Yang W, Parr RG. Phys. Rev. B. 1988;37:785. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 26.(a) Svensson M, Humbel S, Froese RDJ, Matsubara T, Sieber S, Morokuma K. J. Phys. Chem. 1996;100:19357. [Google Scholar]; (b) Vreven T, Morokuma K, Farkas O, Schlegel HB, Frisch MJ. J. Comput. Chem. 2003;24:760. doi: 10.1002/jcc.10156. [DOI] [PubMed] [Google Scholar]
- 27.Hay PJ, Wadt WR. J. Chem. Phys. 1985;82:299. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available Experimental procedures and characterization data for all new and known compounds, computational data for complexes, complete reference 24. This material is available free of charge via the Internet at http://pubs.acs.org