Abstract
Context
Stimulant medication can effectively treat 60–70% of youth with attention deficit hyperactivity disorder. Yet, many parents seek out alternative therapies, and Hypericum perforatum is one of the top three botanicals used.
Objective
To determine the efficacy and safety of Hypericum perforatum for the treatment of attention deficit hyperactivity disorder in children.
Design, Setting, and Participants
A randomized double-blind placebo-controlled trial of 54 children was conducted between March 2005 and August 2006 at Bastyr University. A volunteer sample of children aged 6–17 years met DSM-IV criteria for attention deficit hyperactivity disorder by structured interview. Other medications for ADHD were not allowed during the trial. One patient in the placebo group withdrew due to an adverse event.
Intervention
Participants were randomized to receive 300 mg of Hypericum perforatum or a matched placebo three times daily for eight weeks.
Main Outcome Measures
ADHD Rating Scale-IV (0 to 54), Clinical Global Impression scales for Improvement and Severity (0 to 7), and adverse events
Results
No significant difference in the change of ADHD Rating Scale-IV score from baseline to week 8 was found between treatment and placebo groups (inattention improved 2.6 points Hypericum (95% CI −4.6 to −0.6) vs. 3.2 points placebo (95% CI −5.7 to −0.8), p = 0.68; hyperactivity improved 1.8 points Hypericum (95% CI −3.7 to 0.04) vs. 2.0 points placebo (95% CI −4.1 to 0.1), p = 0.89). There was also no significant difference between groups in the proportion of participants who met criteria for improvement (score of 2 or less) on the Clinical Global Impression Improvement Scale (Hypericum 44.4% (95% CI 25.5 to 64.7) vs. placebo 51.9% (95% CI 31.9 to 71.3), p = 0.59). No difference between groups was found in the number of participants who experienced adverse effects during the study period (Hypericum 40.7% (95% CI 22.4 to 61.2) vs. placebo 44.4% (95% CI 25.5 to 64.7), p = 0.78).
Conclusions
In this study, use of Hypericum perforatum for the treatment of attention deficit hyperactivity disorder over the course of eight weeks did not improve symptoms.
Background
Attention deficit hyperactivity disorder (ADHD) affects 3–12% of children in the United States.1, 2 Up to 30% of these children will not respond to pharmaceutical medications or will have side effects, such as nausea, insomnia, or weight loss, from the medications.3 For these reasons, many parents seek out complementary or alternative medicine (CAM) for their children with ADHD.4 CAM treatments used for pediatric ADHD include massage, dietary changes, dietary supplements and herbal treatments.5–9 In the United States, the most common herbal treatments used by children with ADHD are St. John’s Wort, Echinacea species, and Ginkgo biloba.5
Extracts from St. John’s Wort, also known by its Latin botanical name Hypericum perforatum, have been studied extensively for the treatment of depression in adults with mixed results and in two open-label studies in children and adolescents with depression.10–16 Hypericum perforatum has been found to inhibit reuptake of serotonin, norepinephrine and dopamine.17 The only medication with similar actions is bupropion hydrochloride, which is sometimes used by pediatricians or child psychiatrists to treat children and adolescents with ADHD.17, 18 However, bupropion is not believed to strongly inhibit serotonin reuptake and it is not FDA approved for this indication. In the last decade, a new non-stimulant, selective norepinephrine reuptake inhibitor, atomoxetine, was approved by the FDA for the treatment of ADHD in children and adolescents.19–21 Because Hypericum is believed to act as a norepinephrine reuptake inhibitor, we hypothesized that Hypericum may be beneficial in the treatment of ADHD.
We conducted a small placebo-controlled trial of Hypericum perforatum in children and adolescents with ADHD. The primary goal of the study was to determine if Hypericum perforatum was effective in lessening the severity of ADHD symptoms, as measured by the ADHD Rating Scale-IV and the Clinical Global Impression (CGI) Improvement Scale.22, 23
Methods
An eight-week randomized placebo-controlled, double-blind trial of Hypericum perforatum for the treatment of ADHD in children and adolescents was conducted at Bastyr University. All screening appointments and study visits occurred in the clinical research facility there. The study was approved by the Office of Scientific and Ethical Review and Institutional Review Board (IRB) of Bastyr University and the Human Subjects Division of the University of Washington. The clinical trial was registered with the Protocol Registration System ClinicalTrials.gov prior to beginning recruitment (NCT00100295).
Participants
Healthy children and adolescents aged 6–17 years who met DSM-IV criteria for ADHD based on a structured diagnostic interview using the Kiddie-Schedule for Affective Disorders and Schizophrenia-Epidemiologic Version (K-SADS), were enrolled in the trial between March 2005 and August 2006.24, 25 To be eligible, participants scored more than 1.5 SD above age and gender norms on the ADHD Rating Scale-IV (ADHD RS-IV)22; parents and participants read the consent and assent forms in written English; parents and participants were able to attend all study visits; and participants were capable of swallowing pills. Children with severe depression or an active suicidal plan, a history or current diagnosis of bipolar disorder or severe conduct disorder, or psychotic symptoms were excluded from the trial. All structured interviews were conducted by the principal investigator, who had been trained in Dr. Joseph Biederman’s pediatric psychopharmacology research laboratory at Massachusetts General Hospital to a high degree of inter-rater reliability with experienced child and adult board-certified psychiatrists. Diagnostic uncertainties were resolved by consensus between Drs. Biederman, McClellan, and Weber. Participants at risk of becoming pregnant during the study period or who used medications or over the counter products that were metabolized by the CYP3A4 isoenzyme of the P450 system were also excluded. Hypericum is known to interact with the metabolism of up to 50% of medications, decreasing the circulating levels of these medications by inducing P450 isoenzymes in the liver including CYP3A4.26 No other ADHD treatments were allowed during the study period, including prescription pharmaceutical medications. A washout period was required for all participants who discontinued pharmaceutical medications prior to starting the trial (one week for stimulant medications and two weeks for all other medications). Children who had previously used Hypericum perforatum for more than two weeks were not allowed to participate. Multi-vitamins, essential fatty acid supplements, and counseling were allowed as long as the child had been consistently using the treatment for at least three months and was expected to continue at the same dose or frequency.
Study participants were referred from multiple sites including the Bastyr Center for Natural Health (BCNH) and naturopathic physician offices in the Seattle area. Recruitment advertisements were published in a Seattle area parenting magazine, a Seattle area co-op grocery store newsletter, the BCNH newsletter, the Bastyr University student and staff bulletin, and on the Bastyr University website. Interested parents telephoned the study line for details and were asked screening questions over the phone. Eligible participants were scheduled for two screening visits with the principal investigator. At the first screening visit, the study was explained, and informed consent and assent were obtained. At the screening visits, parents provided information on the child’s medical and family history; participants were given a physical exam by the principal investigator; the parent completed a structured interview (K-SADS); and the ADHD RS-IV was completed by interview of the parent. Participants who were 12 years of age or older were also interviewed directly by the principal investigator using a structured interview (K-SADS). Participants and parents were remunerated and could receive up to $75 each for attending all study visits and completing the trial.
Intervention
A placebo run-in phase of one week was built in from the time of the screening visit to the baseline visit. Participants who were less than 80% compliant as assessed by pill count, or who had a dramatic placebo response (>25% decrease in ADHD RS-IV or score of 1 on the CGI Improvement Scale) during the run-in phase, were excluded from the trial prior to randomization.22, 23 Participants who remained eligible after the placebo run-in were randomized to Hypericum perforatum or placebo for eight weeks of treatment. The Hypericum product was standardized to 0.3% hypericin and was free of heavy metals, pesticides, and adulterants. The placebo pills contained a mixture of rice protein powder and a small amount of activated charcoal (for coloring purposes). Hypericum and placebo were encapsulated in identical opaque capsules and provided by Vital Nutrients Inc. An Investigational New Drug Application for this clinical trial was filed with the Food and Drug Administration (IND #65162 Protocol #2). Participants were instructed to take one capsule (300 mg) three times every day for the duration of the study, ideally before school, after school, and before bed.
Outcomes
Once randomized, participants were evaluated by the principal investigator during study visits at baseline and weeks 1, 2, 4, 6, and 8. The primary outcomes for the study were changes in ADHD symptoms from baseline to week eight as measured by the ADHD RS-IV, changes on the CGI Improvement scale from baseline to week eight, and safety assessed by monitoring children for side effects.22, 23 The ADHD RS-IV is an 18 item standardized, valid, reliable instrument for the diagnosis and weekly assessment of treatment response for children and adolescents with ADHD.22 Each item on the scale describes one of the symptoms of ADHD rated on a 0 to 3 likert scale (never or rarely, sometimes, often, or very often).22 The principal investigator, blinded to treatment assignment, administered the ADHD RS-IV to the parent at each study visit. Nationally representative norms are available for the scale and were used to determine eligibility.22 Participants in the study were at least 1.5 standard deviations above the norm for the child’s age and gender on at least one of the subscales (total, inattentive, or hyperactive/impulsive). The Clinical Global Impression Improvement Scale was used at weeks 4 and 8 to rate the worsening, maintenance, or improvement in global impairment of subjects enrolled in the study compared to baseline.23 The CGI Improvement Scale includes eight options for scoring: 0 not assessed; 1 very much improved; 2 much improved; 3 minimally improved; 4 no change; 5 minimally worse; 6 much worse; and 7 very much worse.23 Clinical response at week eight was defined as a rating of “much” or “very much improved” (1 or 2), which is considered to be a clinically meaningful response.
Safety was the third primary outcome for the study and was assessed by administering the Monitoring of Side Effects Scale (MOSES) to ascertain whether participants had experienced any adverse events since the last visit.27 The MOSES lists 76 possible adverse effects, which are rated on a six point likert scale: 0 not present; 1 minimal no care required; 2 mild over the counter treatment needed; 3 moderate needed to see a health care provider; 4 severe prevented function for more than 2 hours; and 5 FDA “serious” adverse effect. Expected potential adverse events included rash, nausea/vomiting, headache, and sunburn.
Sample Size
Sample size calculations were performed to determine the number of participants needed to detect effect sizes similar to those that have been reported in recent ADHD medication trials.19, 28 A 5 point reduction in the ADHD RS-IV total score was expected in the placebo group, and a 13 point change was expected in the Hypericum group, which had been used to define a clinically meaningful effect in a previous study.28 Therefore, this study was powered to detect an 8 point difference between the groups.28 A sample size of 26 per group was required to achieve 80% power with a two-tailed significance level of 0.05, assuming an equivalent standard deviation of 10.1 in both groups. Estimating a 10% drop out during the study, a minimum of 58 total subjects was needed to reach the target of 26 participants per group.
Randomization and blinding
The study medication allocation sequence was applied in random blocks of 4 and 6 to ensure that approximately equal numbers of subjects received Hypericum and placebo. An independent pharmacy technician placed the study medication in consecutively numbered bottles that were identical in appearance. An independent data manager created the randomization sequence allowing the principal investigator and recruitment staff to remain blinded to the randomization code until the database was locked. Two independent data safety officers were provided with a summary of adverse events (with groups coded as A or B) twice during the study to evaluate if the study needed to be terminated due to the occurrence of disproportionately more adverse events in one of the groups. To assess the success of the blinding procedure at the end of the study, the participant, parent and investigator were asked whether they believed the child was taking Hypericum or placebo. To determine compliance, pills were counted both prior to being dispensed to participants and upon return of study medication at the next study visit.
Statistical methods
Descriptive statistics were performed to characterize the study participants. Baseline characteristics included age, gender, race/ethnicity, median household income, comorbid mental health conditions, and ADHD RS-IV scores. Parents were asked to identify the NIH clinical trial race/ethnicity category that most closely described their child’s race/ethnicity or were allowed to select more than one category of race/ethnicity. Median household income was obtained by searching the US Census Bureau 2000 census data for median household income based on participant address.29 Baseline characteristics were examined as potential confounders in adjusted analyses if binary variables yielded ≥15% difference in absolute risk between the groups. Age and household income were also examined as potential confounders because they are commonly controlled for in studies of childhood psychopathology.
All primary analyses are intention to treat with the significance level set at p < 0.05 (two sided). Two sample t-tests were performed to evaluate the difference between the change in score in the Hypericum group versus the placebo group for each of the ADHD RS-IV subscores inattentive, hyperactive/impulsive, and total. For participants who dropped out of the study prior to week 8, the last available ADHD RS-IV score was carried forward. Two sample t-tests were also performed to compare the difference from baseline to week 8 in age and gender-normed percentile score change between the two groups for each of the subscales. The chi-square test was performed to compare the number of individuals with a CGI-Improvement Score of 2 or less versus those with higher scores in the Hypericum and placebo groups. Chi square tests were performed to compare the difference in numbers of total potential adverse events and each of the specific potential adverse events (rash, nausea/vomiting, headache, and sunburn). Kappa coefficients were calculated to measure the chance-corrected agreement between study medication allocation and predicted medication status as reported by the parent, participant, and principal investigator.
Secondary analyses examined the effect of Hypericum versus placebo in participants who completed the trial according to the protocol. Participants were excluded from this analysis if they dropped out of the study early (n=3), were inadvertently randomized to study medication despite an early placebo response during the run-in period (see results for explanation) (n=6), or if they took less than 75% of their study medication over the entire trial (n=4). All analyses were conducted with Stata/SE version 10.0.30
Additional analyses explored the effect of Hypericum on the child’s behavior as measured by the parent-report Child Behavior Checklist (CBCL) for all children and the Youth Self Report Form (YSR) for children older than 11 years of age.31 These measures have been used extensively in pediatric research due to their high reliability and validity.31 The effect of the treatment on behavior as measured by the Conners’ Parent Rating Scale was also evaluated.32 Finally, the effect of Hypericum on quality of life was examined, using both parent and child-report versions of the Pediatric Quality of Life Inventory Version 4.0 Generic Core Scales (PedsQL), a standardized measure used in the pediatric population.33 For all of these additional measures, differences in scores between baseline and end of study were computed for each participant, and mean differences were compared with two sample t-tests.
Results
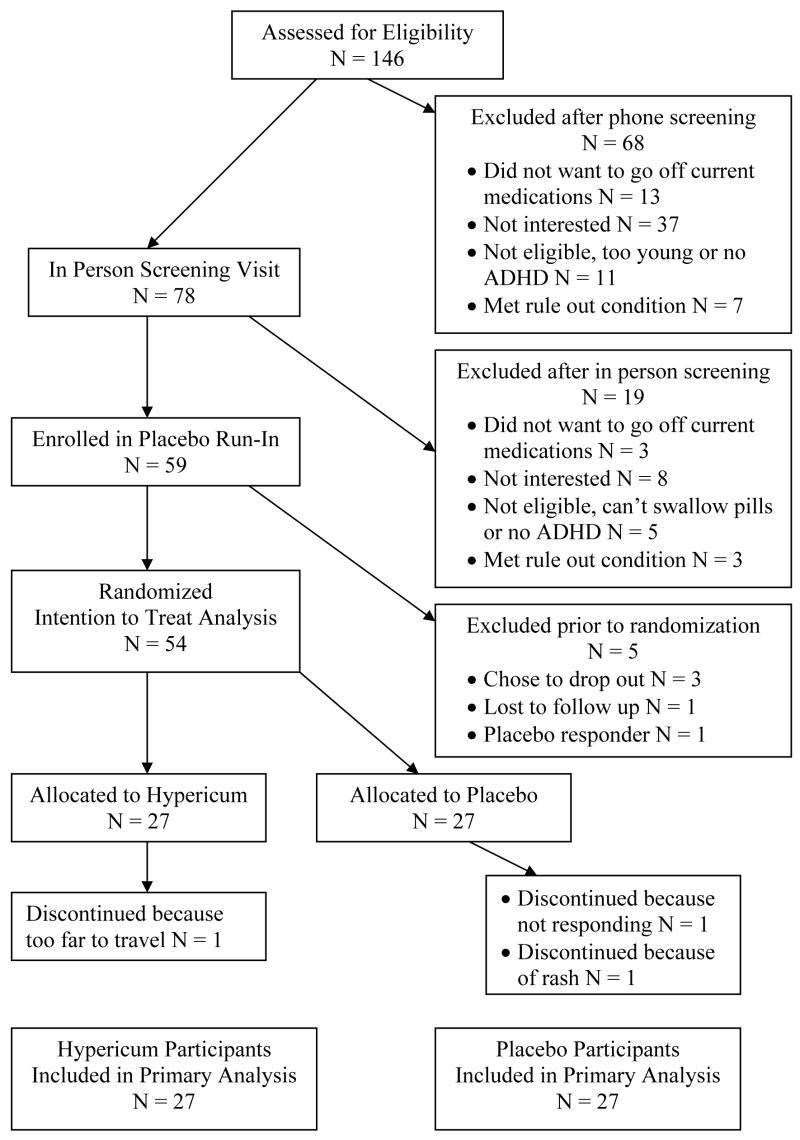
Of the 146 screened potential participants, 104 met eligibility criteria and of those 59 agreed to participate in the study. A total of 59 participants were enrolled in the trial, and 54 (27 per group) were randomized to study medication (see figure 1 for participant disposition). Six participants had a large response during the placebo-run-in period and should have been dropped from the study prior to randomization, however they were erroneously randomized to study medication. Because they had been randomized, these participants were allowed to remain in the trial and are included in the intent to treat analysis. Fifty percent of participants were referred from advertisements in parenting or co-op newsletters, 20% from Bastyr University publications or websites, and 30% from other sources.
Figure 1.
Participant Flow Diagram For Screening, Enrollment And Completion Of Study
The demographic characteristics of the participants are displayed in Table 1. Forty-one percent of participants randomized to Hypericum and 44% of participants randomized to placebo had previously used medications for the treatment of their ADHD symptoms. There were small differences between most demographic characteristics of the two groups. The Hypericum group did, however, have a higher percentage of boys (20 of 27 in the Hypericum group vs. 14 of 27 in the placebo group) and a lower percentage with co-occurring oppositional defiant disorder (9 of 27 in the Hypericum group vs. 15 of 27 in the placebo group). Randomized study participants took a mean of 82.0% of study medication (95% CI 77.5 to 86.4%) during the study, and there was no significant difference in medication adherence between the Hypericum and placebo groups.
Table 1.
Baseline Characteristics Of Study Participants
| Study Medication | ||
|---|---|---|
| Characteristics | Placebo N = 27 | Hypericum N = 27 |
| Gender No. (% Male) | 14 (51.9) | 20 (74.1) |
| Hispanic | 4 (14.8) | 4 (14.8) |
| Race | ||
| White | 21 (77.8) | 25 (92.6) |
| Native American | 0 (0) | 1 (3.7) |
| > 1 Race Reported | 6 (22.2) | 1 (3.7) |
| Home Environment | ||
| Married Biological Parents | 18 (69.2) | 17 (63.0) |
| Biological Parents Divorced | 4 (15.4) | 1 (3.7) |
| Biological Parents Never Married | 3 (11.5) | 4 (15.8) |
| Adoptive Parents | 1 (3.9) | 2 (7.4) |
| Other Home Environment | 0 (0) | 3 (11.1) |
| Household Income, Mean | $61,452 | $58,264 |
| Age, Mean | 9.7 | 9.9 |
| Duration of ADHD, Mean | 6.2 | 7.0 |
| Parent Rating of ADHD Severity at Baseline | ||
| Mild No. (%) | 2 (7.4) | 1 (3.7) |
| Moderate | 17 (63.0) | 14 (51.9) |
| Severe | 8 (29.6) | 12 (44.4) |
| Previous Treatment for ADHD | ||
| Counseling Only | 2 (7.4) | 3 (11.1) |
| Medication Only | 2 (7.4) | 2 (7.4) |
| Medications and other treatment | 10 (37.0) | 9 (33.3) |
| Natural Treatment Only | 3 (11.1) | 3 (11.1) |
| No previous Treatment | 10 (37.0) | 10 (37.0) |
| Co-occuring Conditions | ||
| Current Depression | 2 (7.4) | 1 (3.7) |
| Past Depression | 4 (14.8) | 3 (11.1) |
| Any Current Anxiety | 12 (44.4) | 11 (40.7) |
| Current Oppositional Defiant | 15 (55.6) | 9 (33.3) |
| Disorder | ||
| Current Sleep Disturbance | 6 (22.2) | 3 (11.1) |
In the primary intention-to-treat analysis, no significant difference was seen between the two groups in the change in ADHD RS-IV scores from baseline to week 8. The improvement in ADHD RS-IV total score was 5.2 points (95% CI −9.4 to −1.1) in the placebo group, whereas the improvement in ADHD RS-IV total score in the Hypericum group was 4.4 points (95% CI −7.9 to −0.9) (see figure 2). When the inattentive and hyperactive scales were examined for differences, again there was no significant difference in the change in scores between the groups (Table 2). Analysis of age and gender-normalized percentile scores revealed no differences between the groups. There was no difference in the proportion of participants who were rated as much or very much improved on the CGI Improvement scale (12 of 27 in the Hypericum group (95% CI 25.5 to 64.7%) and 14 of 27 in the placebo group (95% CI 31.9 to 71.3%), p=0.59).
Figure 2.
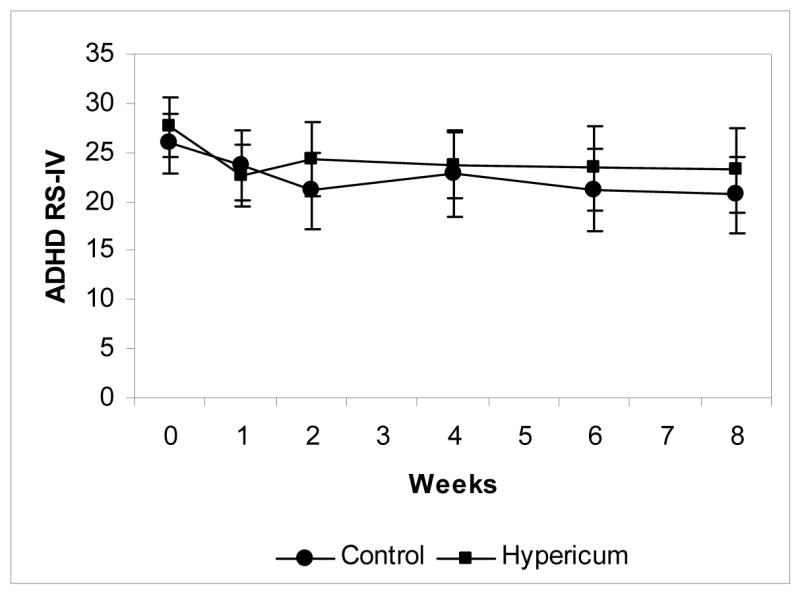
Mean ADHD-IV Rating Scale Total Score for Each Study Visit, error bars depict the 95% confidence interval around the mean score for each study visit. N= 27 for each group at each time point, with last observation carried forward for missing data.
Table 2.
ADHD Rating Scale-IV Scores Of Study Participants
| Control N=27 | Hypericum N=27 | P Value | |
|---|---|---|---|
| Hyperactivity Subscale | |||
| ADHD Rating Scale-IV | |||
| Mean (95% CI) | |||
| Baseline | 10.3 (8.0–12.6) | 11.8 (9.4–14.2) | 0.34 |
| Week 4 | 8.8 (6.3–11.2) | 10.4 (7.7–13.0) | 0.37 |
| Week 8 | 8.3 (5.9–10.6) | 10.0 (7.1–12.9) | 0.34 |
| Difference Baseline to | −2.0 (−4.1–0.1) | −1.8 (−3.7–0.1) | 0.89 |
| Week 8 | |||
| Inattentive Subscale | |||
| ADHD Rating Scale-IV | |||
| Mean (SD) | |||
| Baseline | 15.6 (14.0–17.3) | 15.8 (14.1–17.5) | 0.87 |
| Week 4 | 14.0 (11.6–16.4) | 13.4 (11.6–15.1) | 0.66 |
| Week 8 | 12.4 (10.2–14.7) | 13.2 (11.1–15.4) | 0.59 |
| Difference Baseline to | −3.2 (−5.7– −0.8) | −2.6 (−4.6– −0.6) | 0.68 |
| Week 8 | |||
| Total Subscale ADHD | |||
| Rating Scale-IV | |||
| Mean (SD) | |||
| Baseline | 25.9 (22.9–28.8) | 27.6 (24.6–30.7) | 0.32 |
| Week 4 | 22.8 (18.5–27.0) | 23.7 (20.3–27.2) | 0.72 |
| Week 8 | 20.7 (16.7–24.6) | 23.22 (18.9–27.6) | 0.37 |
| Difference Baseline to | −5.2 (−9.4– −1.1) | −4.4 (−7.9– −1.0) | 0.76 |
| Week 8 | |||
No statistically significant difference was found in the proportion of participants who experienced one or more of the following: rash, nausea/vomiting, headache, and sunburn during the trial between the two groups (Table 3). Participants’ weight was measured at each study visit. Participants in the Hypericum group gained 3.3 pounds (95% CI 2.2 to 4.5 pounds), and those in the placebo group gained 2.3 pounds (95% CI 1.3 to 3.4 pounds) over the eight week trial (p=0.22). No significant difference was seen in the change in height between the groups during the eight-week trial.
Table 3.
Percentage (95% CI) of Participants Experiencing Adverse Events
| Control N=27 | Hypericum N=27 | P Value | |
|---|---|---|---|
| Rash | 15% (4–34) | 0% (0–13) | 0.04 |
| Nausea/Vomiting | 11% (2–29) | 26% (11–46) | 0.16 |
| Headache | 22% (9–42) | 15% (4–34) | 0.48 |
| Sunburn | 4% (0.1–19) | 4% (0.1–19) | >0.99 |
| Any of the Above | 44% (25–65) | 41% (22–61) | 0.78 |
Additional analyses were conducted to determine if use of Hypericum was associated with improved ADHD RS-IV scores in the participants who completed the study according to protocol. As shown in Figure 1, three participants, one from the Hypericum group and two from the placebo group, dropped out of the study and were excluded from the per protocol analysis. In addition, four participants were excluded from the Hypericum group (2 for poor compliance and 2 due to a large placebo response during the run-in phase) and 6 placebo participants (2 for poor compliance and 4 with a large placebo run-in response). In the per protocol analyses, no statistically significant differences were seen between the groups in the ADHD RS-IV scores (difference from week 8 to baseline on the total ADHD RS-IV the Hypericum group improved 4.8 points (95% CI −8.7 to −0.9) and placebo group improved 6.1 points (95% CI −11.7 to −0.4), p=0.69) or the proportion of participants who were responders on the CGI Improvement scale (Hypericum group 40.9% (95% CI 20.7 to 63.6%) and placebo 42.1% (95% CI 20.3 to 66.5%), p=0.94).
Medication status was not associated with a statistically significant improvement in total ADHD RS-IV score in the regression analysis that controlled for age, gender, household income, parental rating of ADHD severity, and co-occurring oppositional defiant disorder at baseline (beta coefficient for effect of medication status −0.68, 95% CI −5.54 to 4.18, p=0.78).
In the blinding analysis, parents correctly identified the medication status 52.9% (95% CI 38.5 to 67.1%) of the time (kappa 0.07, p=0.31). Children correctly identified their medication status 43.1% (95% CI 29.3 to 57.8%) of the time (kappa 0.17, p=0.84) and the principal investigator correctly identified the medication status 56.9% (95% CI 42.2 to 70.7%) of the time (kappa 0.14, p=0.16).
In analyses conducted to determine if use of Hypericum had an effect on other behavioral problems, as measured by the CBCL and YSR, there were no significant differences between the Hypericum and placebo groups for the a priori selected scales: internalizing problems, externalizing problems, total problems, DSM-IV affective, DSM-IV anxiety, DSM-IV oppositional, and DSM-IV conduct (Table 4). No differences between the Hypericum and placebo groups were found in the subscales of the CPRS: Conners’ ADHD Index difference from week 8 to baseline Hypericum group improved 4.6 points (95% CI −8.5 to −0.8) and placebo group improved 7.8 points (95% CI −12.3 to −3.2), p=0.29; and Conners’ DSM-IV Total ADHD Scale Hypericum group improved 3.7 points (95% CI −7.9 to 0.5) and placebo group improved 6.9 points (95% CI −11.5 to −2.2), p=0.30. Finally, there were no differences in the quality of life of participants in the Hypericum and placebo groups, as measured by the PedsQL. Difference from week 8 to baseline on the parent rated total PedsQL score the Hypericum group improved 1.1 points (95% CI −2.5 to 4.8) and placebo group improved 5.1 points (95% CI 1.1 to 9.2), p=0.13; and in the child rated total PedsQL score the Hypericum group improved 5.0 points (95% CI 1.4 to 8.6 points) and the placebo group improved 6.1 points (95% CI 1.5 to 10.8), p=0.69. Analysis of the subgroup of children who had never previously taken pharmaceutical medication for their ADHD symptoms revealed no significant improvement of ADHD symptoms with the use of Hypericum compared to placebo (difference from week 8 to baseline on the total ADHD RS-IV in Hypericum group improved 6.4 points (95% CI −10.7 to −2.1) and placebo group improved 7.6 points (95% CI −13.0 to −2.1), p=0.71).
Table 4.
Achenbach Measures Of Behavior Completed By Parent (CBCL) And Children Older Than 11 Years Of Age (YSR)
| Baseline | Difference Baseline to Follow Up | ||||
|---|---|---|---|---|---|
| Child Behavior Checklist Problems Mean (95% CI) | Control N=27 | Hypericum N=26 | Control N=25 | Hypericum N=25 | P Value |
| Internalizing | 59.0 (49.7–68.4) | 63.8 (52.8–74.8) | −13.6 (−24.1– −3.1) | −3.8 (−12.0–4.4) | 0.14 |
| Externalizing | 73.1 (63.5–82.7) | 67.7 (56.9–78.4) | −12.5 (−21.2– −3.9) | −3.9 (−10.3–2.4) | 0.10 |
| Total | 80.1 (74.3–85.9) | 81.0 (74.4–87.6) | −15.2 (−23.8– −6.6) | −4.8 (−10.3–0.7) | 0.04 |
| DSM-IV Scales | |||||
| Affective | 69.4 (63.1–75.7) | 75.9 (68.9–83.0) | −4.8 (−11.3–1.7) | −1.9 (−7.3–3.4) | 0.48 |
| Anxiety | 67.0 (60.0–74.1) | 69.3 (62.1–76.4) | −5.3 (−10.4– −0.3) | −2.9 (−7.5–1.7) | 0.47 |
| Oppositional | 77.7 (71.5–84.0) | 75.8 (68.3–83.4) | −4.0 (−9.9–1.8) | −4.0 (−9.7–1.6) | 0.99 |
| Conduct | 74.7 (66.9–82.4) | 72.8 (65.0–80.6) | −3.6 (−9.4–2.2) | −0.7 (−6.8–5.4) | 0.48 |
|
| |||||
| Youth Self Report Problems Mean (SD) | Control N=12 | Hypericum N=10 | Control N=12 | Hypericum N=10 | P Value |
|
| |||||
| Internalizing | 37.6 (17.7–57.5) | 48.2 (21.3–75.1) | −2.5 (−12.9–8.0) | −13.2 (−30.2–3.8) | 0.23 |
| Externalizing | 52.4 (37.7–67.2) | 47.5 (26.1–68.9) | −5.4 (−13.9–3.2) | −4.1 (−21.1–12.9) | 0.88 |
| Total | 51.7 (34.3–69.1) | 54.5 (31.2–77.8) | −5.0 (−13.4–3.4) | −12.7 (−26.7–1.3) | 0.29 |
| DSM-IV Scales | |||||
| Affective | 66.6 (54.1–79.0) | 67.9 (52.5–83.3) | −8.9 (−16.6– −1.2) | −7.6 (−19.1–3.9) | 0.83 |
| Anxiety | 56.3 (52.1–60.4) | 62.0 (50.4–73.6) | 1.7 (−9.9–13.4) | −5.1 (−16.7–6.5) | 0.36 |
| Oppositional | 65.1 (57.8–72.3) | 68.6 (57.3–79.9) | −3.9 (−10.7–3.0) | −1.4 (−14.4–11.6) | 0.70 |
| Conduct | 62.3 (52.8–71.9) | 62.5 (52.0–73.0) | 3.2 (−2.4–8.8) | −1.1 (−12.5–10.3) | 0.44 |
Discussion
To our knowledge, this is the first placebo controlled trial of Hypericum perforatum in children and adolescents. The results of this study suggest that administration of Hypericum perforatum has no additional benefit beyond that of placebo for treating symptoms of child and adolescent ADHD. In our study, those in the Hypericum group experienced neither more nor fewer adverse events than the placebo group.
Participants were recruited from the general population with advertisements in Seattle parenting magazines, as well as from Bastyr University’s publications and website. To facilitate comparisons to pharmaceutical ADHD medication trials, this trial used similar enrollment criteria and the same rating scales as previous trials to monitor improvement in symptoms over the course of the study. The placebo response seen on the ADHD RS-IV is nearly identical to the placebo response seen in one of the atomoxetine trials.19 The participants in this clinical trial were similar in age, gender, and comorbidity status to those treated in other ADHD clinical trials.19, 21, 34, 35 This study enrolled a lower percentage of participants who had previously been treated with stimulant medications than children with ADHD in national surveys (41–44% versus 55–74%).36–38 As with other trials, this trial excluded participants with a history of bipolar disorder, severe depression, active suicidal plan, severe conduct disorder, and psychosis.19, 20, 28, 34, 35, 39 Thus, the results of this study cannot necessarily be generalized to children with these co-occurring conditions.
This trial was designed as a single agent clinical trial, so the results only pertain to the use of Hypericum in isolation for the treatment of ADHD. It is possible that Hypericum may work synergistically with other botanicals, vitamins, minerals or supplements. In addition, independent testing at the beginning of the trial confirmed that the product was standardized to 0.3% hypericin. Initially in the study of Hypericum, focus was on the constituent hypericin, a napthodianthrone, which was believed to work as a monoamine oxidase inhibitor, but it was not found to reach levels in the blood which would be physiologically active. More recently, the attention has turned to hyperforin, a phloroglucinol derivative, which is believed to be responsible for the reuptake inhibition of serotonin, dopamine, and norepinephrine.17 The product used for this study was not one of the newly marketed “high hyperforin” products, ranging from 3–5% hyperforin. In fact, the product used in this trial was tested for hypericin and hyperforin content at the end of the trial, and it contained only 0.13% hypericin and 0.14% hyperforin. Hyperforin is a very unstable constituent, which quickly oxidizes and then becomes inactive, which is likely what happened to the product used in this clinical trial.40 The majority of Hypericum products on the market are at risk of oxidation due to their delivery as two part capsules. It is possible that a product standardized to ≥3% hyperforin could benefit children with ADHD symptoms, if it were delivered in a method that limits oxidation.
Finally, the relatively short duration (8 weeks) and small sample size of this trial are limitations. The placebo group did somewhat, though not significantly better than the Hypericum group in this study, with a mean 5.2 point reduction in total symptoms on the ADHD RS-IV (1.1–9.4 point reduction, 95% CI) versus a 4.4 point reduction (0.9–7.9 point reduction, 95% CI). The number of participants who took part in this study was too small to completely reject the possibility of a modest benefit in terms of symptom reduction, compared to placebo. The study is also too small to state that there are no side effects with the use of Hypericum in children. With a sample size of 27 participants per group, our study was powered to detect a 40% difference in the occurrence of adverse events between the two groups. Larger trials of Hypericum perforatum in children would be needed to assess less common events. The results of this study do not support further research on the use of Hypericum as formulated in this study for the treatment of ADHD in children. Nonetheless, if a Hypericum product with stable and high hyperforin content became available for investigation, it would be worthwhile to conduct a study to determine whether a clinically meaningful benefit could be achieved.
Acknowledgments
The project described was supported by Grant Number K23AT000929 and T32AT00815 from the National Center for Complementary and Alternative Medicine. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Complementary and Alternative Medicine, National Institutes of Health.
Study medication and placebo were provided by Vital Nutrients Inc, Middletown CT. The formulation of Hypericum perforatum used in the study is marketed as Hypericum 0.3%.
The sponsor (NCCAM) was responsible for the funding of the study and Vital Nutrients Inc. was responsible for providing study medication, neither had a role in the design or conduct of the study; collection, management, analysis or interpretation of data, nor preparation or approval of the manuscript.
We are grateful to the Bastyr University staff who were compensated to enter data and manage the database of the study: Sandra L. Schildt, BA database manager; Eden K. Pudberry, MS naturopathic medical student; Cecily C. Schuler, BS naturopathic medical student; and Deborah S. Box, BA naturopathic medical student. Bastyr University Professor, Leanna J. Standish ND, PhD, LAc, provided assistance with the conception of the study design as an uncompensated mentor. We are also grateful to the families who participated in the study.
Footnotes
Author Contributions: Dr. Weber had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Weber, Vander Stoep, Weiss, Biederman, McClellan
Acquisition of data: Weber, McCarty
Analysis and interpretation of data: Weber, Vander Stoep, Weiss, Biederman, McClellan
Drafting of the manuscript: Weber
Critical revision of the manuscript for important intellectual content: Weber, McCarty, Vander Stoep, Weiss, Biederman, McClellan Statistical Analysis: Weber, Vander Stoep Obtaining funding: Weber, Biederman, McClellan
Administrative, technical, or material support: Weber, McCarty, Biederman, McClellan
Study Supervision: Weber, Biederman, McClellan
Financial Disclosures: Dr. Joseph Biederman is currently receiving research support from the following sources: Alza, Bristol Myers Squibb, Eli Lilly and Co., Janssen Pharmaceuticals Inc., McNeil, Merk, Otsuka, Shire, NIMH, and NICHD. Dr. Joseph Biederman is currently a consultant/advisory board member for the following pharmaceutical companies: Janssen, McNeil, Novartis, and Shire. Dr. Joseph Biederman is currently a speaker for the following speaker’s bureaus: Janssen, McNeil, Novartis, Shire, and UCB Pharma, Inc. In previous years, Dr. Joseph Biederman received research support, consultation fees, or speaker’s fees for/from the following additional sources: Abbott, AstraZeneca, Celltech, Cephalon, Eli Lilly and Co., Esai, Forest, Glaxo, Gliatech, NARSAD, NIDA, New River, Novartis, Noven, Neurosearch, Pfizer, Pharmacia, The Prechter Foundation, The Stanley Foundation, and Wyeth. The other authors have no competing interests to declare.
References
- 1.Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. American Academy of Pediatrics. Pediatrics. 2000 May;105(5):1158–1170. doi: 10.1542/peds.105.5.1158. [DOI] [PubMed] [Google Scholar]
- 2.Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003;2(2):104–113. [PMC free article] [PubMed] [Google Scholar]
- 3.Schachter HM, Pham B, King J, Langford S, Moher D. How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. Cmaj. 2001 Nov 27;165(11):1475–1488. [PMC free article] [PubMed] [Google Scholar]
- 4.Baumgaertel A. Alternative and controversial treatments for attention-deficit/hyperactivity disorder. Pediatr Clin North Am. 1999 Oct;46(5):977–992. doi: 10.1016/s0031-3955(05)70167-x. [DOI] [PubMed] [Google Scholar]
- 5.Cala S, Crismon ML, Baumgartner J. A survey of herbal use in children with attention-deficit-hyperactivity disorder or depression. Pharmacotherapy. 2003 Feb;23(2):222–230. doi: 10.1592/phco.23.2.222.32092. [DOI] [PubMed] [Google Scholar]
- 6.Bussing R, Zima BT, Gary FA, Garvan CW. Use of complementary and alternative medicine for symptoms of attention-deficit hyperactivity disorder. Psychiatr Serv. 2002;53(9):1096–1102. doi: 10.1176/appi.ps.53.9.1096. [DOI] [PubMed] [Google Scholar]
- 7.Stubberfield T, Parry T. Utilization of alternative therapies in attention-deficit hyperactivity disorder. J Paediatr Child Health. 1999;35(5):450–453. doi: 10.1046/j.1440-1754.1999.355401.x. [DOI] [PubMed] [Google Scholar]
- 8.Chan E. The role of complementary and alternative medicine in attention-deficit hyperactivity disorder. J Dev Behav Pediatr. 2002;23(1 Suppl):S37–45. doi: 10.1097/00004703-200202001-00007. [DOI] [PubMed] [Google Scholar]
- 9.Chan E, Rappaport LA, Kemper KJ. Complementary and alternative therapies in childhood attention and hyperactivity problems. J Dev Behav Pediatr. 2003;24(1):4–8. doi: 10.1097/00004703-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Kasper S, Gastpar M, Muller WE, et al. Efficacy of St. John’s wort extract WS 5570 in acute treatment of mild depression: a reanalysis of data from controlled clinical trials. Eur Arch Psychiatry Clin Neurosci. 2008 Feb;258(1):59–63. doi: 10.1007/s00406-007-0779-2. [DOI] [PubMed] [Google Scholar]
- 11.Linde K, Mulrow CD, Berner M, Egger M. St John’s wort for depression. Cochrane Database Syst Rev. 2005;(2):CD000448. doi: 10.1002/14651858.CD000448.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Davidson J. Effect of Hypericum perforatum (St John’s wort) in major depressive disorder: a randomized controlled trial. Jama. 2002 Apr 10;287(14):1807–1814. doi: 10.1001/jama.287.14.1807. [DOI] [PubMed] [Google Scholar]
- 13.Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St John’s wort in major depression: a randomized controlled trial. Jama. 2001;285(15):1978–1986. doi: 10.1001/jama.285.15.1978. [DOI] [PubMed] [Google Scholar]
- 14.Szegedi A, Kohnen R, Dienel A, Kieser M. Acute treatment of moderate to severe depression with hypericum extract WS 5570 (St John’s wort): randomised controlled double blind non-inferiority trial versus paroxetine. Bmj. 2005 Mar 5;330(7490):503. doi: 10.1136/bmj.38356.655266.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubner WD, Kirste T. Experience with St John’s Wort (Hypericum perforatum) in children under 12 years with symptoms of depression and psychovegetative disturbances. Phytother Res. 2001 Jun;15(4):367–370. doi: 10.1002/ptr.829. [DOI] [PubMed] [Google Scholar]
- 16.Findling RL, McNamara NK, O’Riordan MA, et al. An open-label pilot study of St. John’s wort in juvenile depression. J Am Acad Child Adolesc Psychiatry. 2003;42(8):908–914. doi: 10.1097/01.CHI.0000046900.27264.2A. [DOI] [PubMed] [Google Scholar]
- 17.Muller WE, Rolli M, Schafer C, Hafner U. Effects of hypericum extract (LI 160) in biochemical models of antidepressant activity. Pharmacopsychiatry. 1997;30(Suppl 2):102–107. doi: 10.1055/s-2007-979528. [DOI] [PubMed] [Google Scholar]
- 18.Biederman J, Spencer T, Wilens T. Evidence-based pharmacotherapy for attention-deficit hyperactivity disorder. Int J Neuropsychopharmacol. 2004;7(1):77–97. doi: 10.1017/S1461145703003973. [DOI] [PubMed] [Google Scholar]
- 19.Kratochvil CJ, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in children with ADHD: a prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry. 2002;41(7):776–784. doi: 10.1097/00004583-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Spencer T, Heiligenstein JH, Biederman J, et al. Results from 2 proof-of-concept, placebo-controlled studies of atomoxetine in children with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2002;63(12):1140–1147. doi: 10.4088/jcp.v63n1209. [DOI] [PubMed] [Google Scholar]
- 21.Michelson D, Allen AJ, Busner J, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry. 2002 Nov;159(11):1896–1901. doi: 10.1176/appi.ajp.159.11.1896. [DOI] [PubMed] [Google Scholar]
- 22.DuPaul G, Power T, Anastopoulos A, Reid R. ADHD Rating Scale - IV: Checklists, norms, and clinical interpretation. New York: The Guilford Press; 1998. [Google Scholar]
- 23.NIMH. Clinical Global Impressions. Psychopharmacology Bulletin. 1985;21(4):839–843. [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: APA; 1995. [Google Scholar]
- 25.Orvaschel H, Puig-Antich J. Schedule for Affective Disorders and Schizophrenia for School Age Children - Epidemiologic Version (K-SADS-E) 4. Pittsburgh: Western Psychiatric Institute and Clinic; 1987. [Google Scholar]
- 26.Markowitz JS, Donovan JL, DeVane CL, et al. Effect of St John’s wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. Jama. 2003 Sep 17;290(11):1500–1504. doi: 10.1001/jama.290.11.1500. [DOI] [PubMed] [Google Scholar]
- 27.Kalachnick J. Monitoring Of Side Effects System. 1986 [Google Scholar]
- 28.Michelson D, Faries D, Wernicke J, et al. Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics. 2001 Nov;108(5):E83. doi: 10.1542/peds.108.5.e83. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Census Bureau. American Fact Finder. [Accessed October 29, 2007];2007 website] http://factfinder.census.gov/home/saff/main.html?_lang=en.
- 30.Stata/SE Version 10.0 [computer program]. Version 10.0. College Station; TX: 2007. [Google Scholar]
- 31.Achenbach T, Rescorla L. Manual for the ASEBA school age forms & profiles. Burlington: ASEBA; 2001. [Google Scholar]
- 32.Conners K. Conners’ rating scales - revised user’s manual. New York: Multi-Health Systmes Inc; 1997. [Google Scholar]
- 33.Varni J, Seid M, Kurtin P. The PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory Version 4.0 Generic Core Scales in healthy and patient populations. Medical Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Kelsey DK, Sumner CR, Casat CD, et al. Once-daily atomoxetine treatment for children with attention-deficit/hyperactivity disorder, including an assessment of evening and morning behavior: a double-blind, placebo-controlled trial. Pediatrics. 2004;114(1):e1–8. doi: 10.1542/peds.114.1.e1. [DOI] [PubMed] [Google Scholar]
- 35.Prasad S, Harpin V, Poole L, Zeitlin H, Jamdar S, Puvanendran K. A multi-centre, randomised, open-label study of atomoxetine compared with standard current therapy in UK children and adolescents with attention-deficit/hyperactivity disorder (ADHD) Curr Med Res Opin. 2007;23(2):379–394. doi: 10.1185/030079906X167309. [DOI] [PubMed] [Google Scholar]
- 36.Robison LM, Sclar DA, Skaer TL, Galin RS. Treatment modalities among US children diagnosed with attention-deficit hyperactivity disorder: 1995–99. Int Clin Psychopharmacol. 2004 Jan;19(1):17–22. doi: 10.1097/00004850-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007 Sep;161(9):857–864. doi: 10.1001/archpedi.161.9.857. [DOI] [PubMed] [Google Scholar]
- 38.Visser SN, Lesesne CA, Perou R. National estimates and factors associated with medication treatment for childhood attention-deficit/hyperactivity disorder. Pediatrics. 2007 Feb;119(Suppl 1):S99–106. doi: 10.1542/peds.2006-2089O. [DOI] [PubMed] [Google Scholar]
- 39.Greenhill LL, Findling RL, Swanson JM. A double-blind, placebo-controlled study of modified-release methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2002 Mar;109(3):E39. doi: 10.1542/peds.109.3.e39. [DOI] [PubMed] [Google Scholar]
- 40.Orth HC, Rentel C, Schmidt PC. Isolation, purity analysis and stability of hyperforin as a standard material from Hypericum perforatum L. J Pharm Pharmacol. 1999 Feb;51(2):193–200. doi: 10.1211/0022357991772132. [DOI] [PubMed] [Google Scholar]