Abstract
Context
The Treatment for Adolescents with Depression Study evaluated fluoxetine (FLX), cognitive-behavioral therapy (CBT), and FLX/CBT combination (COMB), versus pill placebo (PBO) in 439 adolescents with major depressive disorder. Treatment consisted of three stages: (I) acute (12 weeks), (II) continuation (6 weeks), and (III) maintenance (18 weeks).
Objective
Examine rates of achieving and maintaining sustained response during continuation and maintenance treatments.
Design and Setting
Randomized controlled trial conducted in 13 US sites. Response was determined by blinded independent evaluators (IEs).
Clinical Trial Registry
Patients
242 FLX, CBT, and COMB patients in their assigned treatment at the end of Stage I.
Interventions
Stage II treatment varied based on Stage I response. Stage III consisted of 3 CBT and/or pharmacotherapy sessions, and, if applicable, continued medication.
Main Outcome Measures
Sustained response was defined as two consecutive Clinical Global Impression-Improvement (CGI-I) ratings of 1 or 2 (“full response”). Patients achieving sustained response were classified on subsequent non-response status.
Results
Among 95 patients (39.3%) who had not achieved sustained response by Week 12 (29.1% COMB, 32.5% FLX, 57.9% CBT), sustained response rates during Stages II/III were 80.0% COMB, 61.5% FLX, and 77.3% CBT (difference ns). Among the remaining 147 patients (60.7%) who achieved sustained response by Week 12, CBT patients were more likely than FLX patients to maintain sustained response through Week 36 (96.9% vs. 74.1%; p = 0.007; 88.5% of COMB patients maintained sustained response through Week 36). Total rates of sustained response by Week 36 were 88.4% COMB, 82.5% FLX, and 75.0% CBT.
Conclusions
Most depressed adolescents who had not achieved sustained response during acute treatment did achieve that level of improvement during continuation and maintenance therapies. The possibility that CBT may help the subset of depressed adolescents who achieve early sustained response maintain their response warrants further investigation.
The Treatment for Adolescents with Depression Study evaluated fluoxetine (FLX), cognitive-behavior therapy (CBT), and FLX/CBT combination (COMB) compared to pill placebo in 439 adolescents with major depressive disorder. Treatment consisted of acute, continuation, and maintenance stages. The present study examined rates of achieving and maintaining sustained response during continuation and maintenance therapy for the 242 active treatment patients who were in their assigned condition at the end of acute therapy. Sustained response was defined as two consecutive Clinical Global Impression-Improvement ratings by blinded assessors of 1 or 2 (“full response”). Among the 95 patients who had not achieved sustained response during acute therapy, sustained response rates during continuation and maintenance treatment were 80.0% COMB, 61.5% FLX, and 77.3% CBT (difference ns). Among the remaining 147 patients who had achieved sustained response during acute treatment, CBT patients were more likely than FLX patients to maintain sustained response through maintenance therapy (96.9% vs. 74.1%; p = 0.007; 88.5% of COMB patients maintained sustained response through Week 36). Most depressed adolescents achieved sustained response by the completion of treatment. The possibility that CBT may help the subset of depressed adolescents who achieve early sustained response maintain their response warrants further investigation.
Given the high probability of relapse and recurrence following favorable response to treatment in depressed adults and adolescents1,2, guidelines have been developed to extend beyond acute treatment. The goal of continuation treatment is to prevent relapse following remission (i.e., the initial period during which the person has responded to treatment3), whereas the goal of maintenance treatment is to prevent recurrence following recovery (i.e., once the person has been symptom-free long enough to consider the initial depressive episode over). Little is known concerning effective continuation and maintenance therapies for depressed adolescents, and practice guidelines for adolescents4,5 have been adopted from the larger body of guidelines for long-term treatment of depressed adults6,7,8,9.
Previous post-acute treatment research with depressed adolescents is extremely limited. A six-month course of cognitive behavioral therapy (CBT) continuation treatment in 17 patients (ages 11-17) following acute CBT10 was superior to no treatment in a historical control group of 12 patients, and fluoxetine continuation treatment was superior to pill placebo in reducing depression relapse among 40 patients (ages 8-18) who had responded to fluoxetine acute treatment11. In the former study, cumulative relapse risk was 20% among continuation CBT patients versus 50% among historical controls. In the latter study, relapse, which was defined as either a Children's Depression Rating Scale-Revised (CDRS-R12) score > 40 or physician opinion over a 32-week post-acute phase, occurred in 34% of patients who remained on fluoxetine versus 60% of those switched to placebo.
In the only study to our knowledge that evaluated a maintenance treatment for adolescent depression, Clarke and colleagues13 examined the degree to which an individual CBT booster intervention reduced depression recurrence. After an 8-week, 16-session group CBT intervention, one-third of the 54 adolescents (ages 14-18) who completed treatment (regardless of response status) were randomly assigned to individual boosters every four months over a 2-year follow-up. The booster condition was not associated with less depression recurrence among recovered patients (27% recurrence among booster patients versus 8% among assessment-only controls, ns) but did significantly improve the rate of recovery for adolescents who were still depressed following acute treatment (100% recovery among booster patients versus 50% among assessment-only controls, p < .05). This study suggests that an important function of post-acute treatments may be to facilitate continued improvement among partial responders.
The goal of the present study was to examine the rates of sustained improvement during continuation and maintenance therapies in the Treatment for Adolescents with Depression Study (TADS; The TADS Team14,15,16). TADS is a randomized controlled trial that evaluated the effectiveness of three treatments for adolescents with major depressive disorder (MDD): fluoxetine pharmacotherapy with clinical management (FLX), cognitive-behavior therapy (CBT), and the combination of FLX and CBT (COMB), relative to clinical management with pill placebo (PBO). TADS treatment was provided in three stages: (I) acute treatment lasting 12 weeks, (II) continuation treatment lasting 6 weeks and varying in intensity based on the patient's response to acute treatment, and (III) maintenance treatment lasting 18 weeks, in which patients met with their clinician(s) three times and, if applicable, continued medication. Previous publications reported that the COMB and FLX conditions had higher response rates than CBT or PBO during Stage I14, and COMB performed better during Stages II and III than either of the monotherapies, which had very similar response rates17.
Our outcome measure in the present study is Sustained Response, which is defined as two consecutive ratings of a full response in assessments conducted 6 weeks apart. TADS follow-up did not include the collection of weekly symptom ratings necessary to determine recovery and recurrence3,18. Our creation of the sustained response variable provides an alternative outcome that answers important questions in clinical decision making that must be answered before a patient is considered recovered (e.g., Is my patient responding to this treatment? Is this apparent response more than transitory? Should I augment treatment or change modalities?). Although it was not an original aim of TADS, data during TADS continuation and maintenance therapies provided a unique and valuable opportunity to address two issues: (1) to what degree do patients who have not achieved sustained response during acute therapy subsequently achieve sustained response during continuation and maintenance therapies, and (2) among patients who achieved sustained response during acute therapy, how many maintain their sustained response through continuation and maintenance therapies. The present study extends the previous Stage III TADS publication17 by requiring a maintained response rather than the one time achievement of a positive response and by examining the degree to which sustained response is maintained once achieved. In addition, although data on treatment adherence and compliance following acute treatment were not available, we examine, as a secondary aim, whether treatment modalities differed in rates of attendance, which serves as one basic indicator of treatment utilization. Retaining the patient is a basic gauge of the perceived value of post-acute care. In addition, differences in the rates of sustained response may be influenced by condition differences in attendance. One possible explanation for the nonsignificant recurrence prevention finding of the booster protocol in Clarke et al.13 was that many adolescents assigned to booster therapy (perhaps more than half) failed to attend post-acute treatment. Although we are unable to evaluate the degree to which patients complied with continuation and maintenance therapies in TADS, we compared whether treatment attendance varied across conditions.
Method
The TADS design have been extensively documented in previous publications14,15,16, so will be only briefly reviewed here.
Stage I Participants and Procedures
Between 2000 and 2003, 439 adolescents at 13 sites were enrolled. Both adolescents and parents provided written informed assent/consent. Institutional review boards at each site approved and monitored the protocol. Diagnoses of MDD and associated comorbidities at baseline were established using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL19). TADS employed two primary measures of depression status assessed at baseline, week 6, 12, 18, 24 and 36 by an Independent Evaluator (IE) blind to condition: (1) the 17-item Children's Depression Rating Scale-Revised (CDRS-R11) as a continuous measure of depression severity in the past week, and (2) responder status on the 7-point Clinical Global Impression-Improvement (CGI-I20).
Inclusion criteria were (a) 12-17 years old, (b) current DSM-IV MDD, (c) CDRS-R total score ≥ 45, (d) stable mood symptoms for at least 6 weeks, and (e) impairment in at least two settings. Exclusion criteria included (a) psychiatric disorders requiring out-of-protocol treatments, (b) one failed CBT trial or two failed SSRI trials for depression, (c) current psychiatric treatment (other than stable dose of stimulant medication for Attention-Deficit/Hyperactivity Disorder), (d) non-English speaking, (e) confounding medical condition, (f) previous intolerance to FLX, (g) pregnant or sexually active while refusing acceptable birth control, or (h) danger to self or others.
Eligible participants were randomly assigned to FLX, CBT, COMB, or PBO using stratified randomization at the Coordinating Center. Patients and staff remained masked in the “pills only” conditions (FLX and PBO) until week 12, at which time patients and clinicians were unblinded. At the end of Stage I, PBO nonresponders were provided with the TADS treatment of their choice and nonresponders to active treatment (6 in COMB, 11 in FLX, 11 in CBT) were withdrawn from treatment and provided with referrals for alternative care.
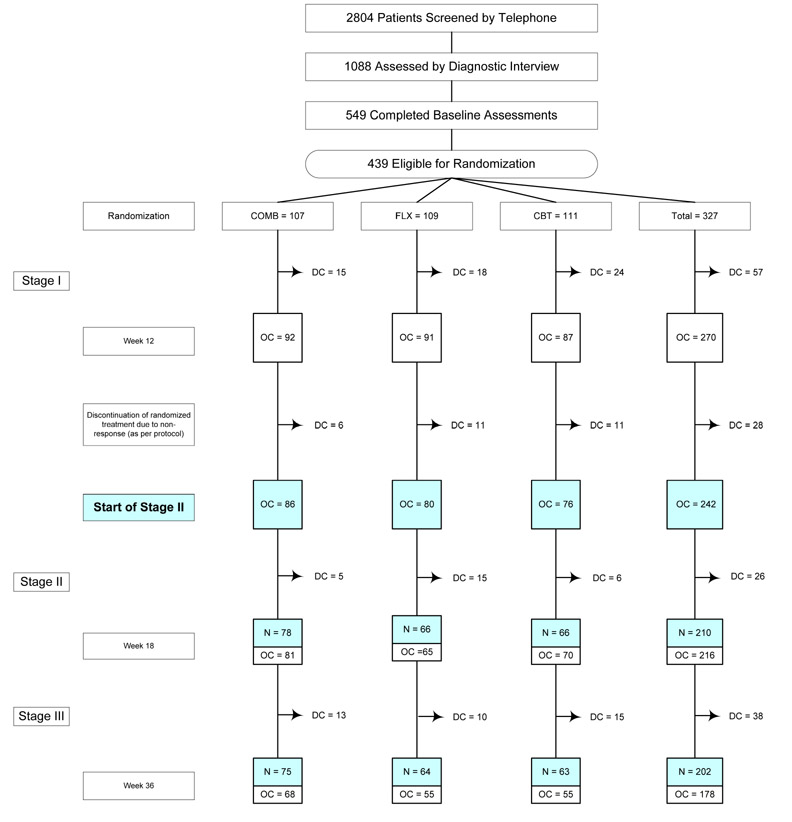
Given our focus on Stages II and III in the present study, patients initially assigned to placebo were not included. Rates of participation at each project stage are shown in the CONSORT diagram (Figure 1). A total of 327 adolescents were randomized to an active treatment; 270 completed the study through Week 12. The present sample (N = 242) consisted of observed cases (i.e., adolescents in their randomized treatment arm with no treatment augmentation) at the start of Stage II. The sample had a mean age at intake of 14.6 years (SD = 1.5) and included 140 (57.9%) female adolescents. Participants classified their race/ethnicity status as 190 (78.5%) Caucasian, 21 (8.7%) African-American, 17 (7.0%) Hispanic-White, 4 (1.7%) Hispanic-Black, 3 (1.2%) Asian, and 7 (2.9%) Other. Participants in the present study were compared on demographic factors (age, gender, race/ethnicity, income) and depression severity (CDRS-R; CGI-S) to the 85 patients assigned to active treatment who had been excluded. Differences were nonsignificant, with one exception: retained patients consisted of a higher proportion of White adolescents (78.5% vs. 62.1%; p = .002). Data were available for 210 participants (86.8%) at the end of Stage II and 202 (83.5%) at the end of Stage III.
Figure 1.
Treatment for Adolescents with Depression Study (TADS): Flow diagram for the N = 242 who continued randomized treatment after 12 weeks of acute treatment.
Reasons for discontinuation prior to randomization have been previously reported (TADS Team, 2004, JAMA, p. 811). COMB = combination of fluoxetine and cognitive behavior therapy; FLX = fluoxetine; CBT = cognitive behavior therapy; PBO = pll placebo; DC = discontinuation of randomized treatment due to premature termination, non-response at the end of Stage I, or study exit; OC (observed case) = youth still in randomized treatment arm;
The 242 youths who started Stage II as an observed case were evaluated in the current analysis; N = Number of CGI-1 assessments completed for 242 cases at Week 18 and Week 36. All 112 patients assigned to the PBO condition discontinued randomized treatment at the end of Stage I as per protocol and are excluded from the current analysis.
Interventions
Medication
Stage I medication management included monitoring status and medication effects during 20-30 minute visits. Doses began at 10 mg/day, titrated as necessary, up to 40 mg/day by week 8. At the week 12 visit, the dose was raised to 50 or 60 mg/day in patients rated by the clinician as partial responders (i.e., CGI-I = 3). Full responders at the end of Stage I (CGI-I = 1 or 2 by clinician rating) were maintained at the same FLX dose. In Stage II, full responders had 2 office visits; partial responders had 4 office visits. In Stage III, patients were followed every 6 weeks with a 20-30 minute medication visit. Other than reductions due to major side effects, medication dose did not change without special approval from the conference call peer supervision group.
CBT
TADS CBT in Stage I included fifteen sessions (a combination of adolescent only, parent psychoeducation, and conjoint). Patients rated by their clinician as partial responders at the end of Stage I received six additional weeks of weekly CBT in Stage II, whereas Stage I full responders shifted to biweekly CBT sessions. During Stage III, patients met with their therapist every 6 weeks for three CBT “booster” sessions.
Combination Treatment
COMB consisted of all components from the medication management and CBT protocols.
Definitions of Depression Status
Treatment Response
Treatment response status, relative to baseline, was determined at each assessment based on IE CGI-I scores. When a Week 12 IE score was missing, last observation carried forward procedures were implemented. Adolescents with CGI-I scores of 1 (“very much improved”) or 2 (“much improved”) were considered Full Responders, those with a CGI-I score of 3 (“minimally improved”) were considered Partial Responders, and those with a CGI-I score of 4 (“no change”) and higher (indicating worsening) were considered Non-Responders. Thirty-one adolescents rated by the IE as Week 12 Non-Responders were included in the present study because the patient had been rated by the clinician(s) as either a partial or full responder and had remained in his or her treatment arm without treatment augmentation. CGI-I ratings were made by the IE after the CDRS-R and scores between the two measures were highly correlated (i.e., Spearman correlations between CGI-I and CDRS-R at Weeks 12, 18, and 36 were r = .81, .80, and .73, respectively, all p < .001).
Sustained Response Status
Sustained response status was based on the maintenance of IE CGI-I scores. The following two assumptions were applied when response status data were missing: (1) adolescents missing Week 6 data were assumed to be non-responders at Week 6, and (2) when one or more consecutive assessments were missing, and response scores before and after the missing assessment(s) were identical, we assumed the same response status for the missed assessments.
Patients were categorized into one of three Sustained Response groups at each assessment. Sustained Response was defined as two consecutive ratings of “full responder.” Possible Sustained Response was defined as one rating of “full responder” followed by no additional Stage II/III data. All other response patterns were defined as Non-Sustained Response. At Week 12, only four patients (1 COMB, 1 FLX, 2 CBT) were classified as Possible Sustained Response. For ease of presentation, they were combined with the Non-Sustained Response group at week 12.
Maintenance of Sustained Response
Once patients experienced Sustained Response, Sustained Response status at subsequent assessments was classified as (a) “Failed to Maintain,” given a subsequent assessment of “non-responder” or “partial responder” (CGI-I = 3-7); (b) “Maintained Sustained Response,” given continued assessments of “full responder;” or (c) “Unknown,” if additional IE data following Sustained Response were not available.
Statistical Methods
Analyses were conducted by the second and third authors. Fisher's Exact tests were conducted to test for between-treatment differences in rates. The level of significance was set at 0.05 for each two-tailed statistical test.
Differences in the amount of missing data between the three conditions could impact our ability to detect treatment effects. Differences in amount of missing IE data across conditions and assessments were nonsignificant, with one exception: Week 24, p < .01. Paired contrasts indicated more missing data for FLX compared to COMB patients (22.5% vs. 5.8%, p < .01); CBT patients were intermediate (13.2% missing) and did not differ from the other two groups. Given the lack of systematic differences across conditions in missing data, no adjustments were made.
Results
Sustained Response in Stage I
Among the 242 participants, 147 (60.7%) were classified as Sustained Response by Week 12: 70.9% COMB, 67.5% FLX, and 42.1% CBT. The overall difference was significant, χ2(2, n = 147) = 16.35, p = 0. 0003. Paired contrasts indicated that, compared to CBT, rates of Sustained Response by Week 12 were significantly higher for COMB (p = .0002) and FLX (p = .001) patients, which did not differ.
Sustained Response in Stages II/III for Patients who had not Achieved Sustained Response in Acute Treatment
Ninety-five of the 242 participants (39.3%) were classified as Non-Sustained Response by Week 12: 29.1% COMB, 32.5% FLX, and 57.9% CBT. Table 1 shows whether Sustained Response during Weeks 18-36 was achieved for these patients as a function of condition. The majority of adolescents who had not achieved Sustained Response by Week 12 achieved either definite or possible Sustained Response by Week 36: 80.0% COMB, 61.5% FLX, and 77.3% CBT. Sustained response rates by week 18 are not presented in the Table but indicated that treatment response tended to occur slowly (48.0% COMB, 34.6% FLX, 31.8% CBT). Differences in Sustained Response status across the three treatment conditions at both Weeks 18 and 36 were nonsignificant.
Table 1. Sustained Response During Weeks 18-36 for Patients who had not Achieved Sustained Response by Week 12.
| Treatment Arm | Number (%) of Patients who had not Achieved Sustained Response by Week 12 | Week 18-36 Sustained Response Status | n (%) |
|---|---|---|---|
| COMB | 25 of 86 (29.1%) | Sustained Response
Possible Sustained Response Non-Sustained Response |
15 (60.0%)
5 (20.0%) 5 (20.0%) |
| FLX | 26 of 80 (32.5%) | Sustained Response
Possible Sustained Response Non-Sustained Response |
12 (46.2%)
4 (15.4%) 10 (38.5%) |
| CBT | 44 of 76 (57.9%) | Sustained Response
Possible Sustained Response Non-Sustained Response |
25 (56.8%)
9 (20.5%) 10 (22.7%) |
| All Active Tx | 95 of 242 (39.3%) | Sustained Response
Possible Sustained Response Non-Sustained Response |
52 (54.7%)
18 (18.9%) 25 (26.3%) |
Note: Week 18-36 Sustained Response Status indicates whether the patient achieved Sustained Response at any point during the period. If the patient had multiple response classifications across the period, the best response status was counted. The clinical ordering of response status from best to worse is: Sustained Response (i.e., 2 consecutive ratings of “full responder,” defined as CGI-I = 1 or 2), Possible Sustained Response (i.e., one rating of “full responder” either at the end of Stage III or followed by missing assessments only), and Non-Sustained Response (all other responder patterns).
Maintenance of Sustained Response in Stages II/III for Patients who Achieved Sustained Response in Acute Treatment
The next analyses examined whether the 147 patients who achieved Sustained Response by Week 12 maintained that status during TADS continuation and maintenance therapy. The maintenance of Sustained Response from Weeks 18-36 for Week 12 Sustained Responders is shown in Table 2. Among this subset of patients who responded well to acute therapy, the majority (82.3%) maintained their sustained response throughout Stages II/III (i.e., assessments at Weeks 18, 24, 30, 36). Fifteen percent, however, failed to maintain their Sustained Response, with rates differing as a function of treatment modality: 11.5% COMB, 25.9% FLX, and 3.1% CBT. The overall difference was significant, χ2(3, n = 242), p = 0.01. Paired contrasts indicated that the maintenance of Sustained Response was higher for CBT compared to FLX (p = 0.007) and approached significance in COMB compared to FLX (p = 0.06). Rates of maintaining Sustained Response in COMB versus CBT did not differ.
Table 2. Maintenance of Sustained Response During Weeks 18-36 for Patients who Achieved Sustained Response by Week 12.
| Treatment Arm | Number (%) of Patients Experiencing Sustained Response by Week 12 | Week 18-36 Maintenance Status | n (%) |
|---|---|---|---|
| COMB | 61 of 86 (70.9%) | Failed to Maintain
Maintained Sustained Response Unknown |
7 (11.5%)
53 (86.9%) 1 (1.6%) |
| FLX | 54 of 80 (67.5%) | Failed to Maintain
Maintained Sustained Response Unknown |
14 (24.9%)
38 (70.4%) 2 (3.7%) |
| CBT | 32 of 76 (42.1%) | Failed to Maintain
Maintained Sustained Response Unknown |
1 (3.1%)
30 (93.8%) 1 (3.1%) |
| All Active Tx | 147 of 242 (60.7%) | Failed to Maintain
Maintained Sustained Response Unknown |
22 (15.0%)
121 (82.3%) 4 (2.7%) |
Note: Patients who experienced Sustained Response were classified on the degree to which they maintained their Sustained Response status for subsequent assessments. Maintenance status consisted of: Failed to Maintain (i.e., a subsequent assessment of “non-responder” or “partial responder;” CGI-I = 1-3), Maintained Sustained Response (i.e., continued assessments of “full responder”), or Unknown (i.e., no assessment data were available following Sustained Response).
Potential Differences in Depressive Severity Associated with Sustained Response for Different Treatment Conditions
Given that a smaller proportion of CBT patients reached Sustained Response during Stage I compared to COMB and FLX patients (42% vs. 71% and 68%, respectively), it was possible that this subset of CBT patients had been less depressed at baseline than COMB and FLX patients who achieved Sustained Response during Stage I. CDRS-R scores at baseline for the COMB, FLX, and CBT patients who achieved Sustained Response by Week 12 were compared and did not differ (baseline mean = 60.4, 59.2, and 57.5; SD = 12.2, 10.4, 7.9; respectively); Overall GLM results: F(2, 144) = 0.77, p = 0.46. As a secondary check, we compared the three groups of Sustained Responders on depression level at Week 12. Differences between COMB, FLX, and CBT groups were nonsignificant (Week 12 CDRS-R mean = 28.3, 29.7, and 29.6; SD = 7.7, 6.2, 6.5; respectively); Overall GLM results: F(2, 141) = 0.67, p = 0.51. Previously, Curry and colleagues21 identified three moderators of treatment condition: annual family income, severity of depression (CGI-S), and cognitive distortions (Children's Negative Cognitive Errors Questionnaire22) and we compared Sustained Responders in the three conditions on baseline levels of these three moderators. Differences on family income and depression severity as per CGI-S were nonsignificant, although group differences on cognitive distortions were present; F(2, 144) = 3.92, p = 0.22, with higher cognitive distortions for COMB compared to FLX patients (mean = 68.3 vs. 58.8, SD = 19.3 vs. 19.0; p = 0.008; CBT patients did not differ from either group, mean [SD] = 60.8 [17.3]).
Treatment Attendance in Stages II/III
Condition differences in the rates of maintaining Sustained Response may have been influenced by differential attendance. Because Stage II treatment was designed to provide more treatment for partial responders compared to full responders, attendance was examined as a function of Stage I response. Using the Wilcoxon Rank Sums Test, all differences in attendance during Stages II/III between conditions were nonsignificant. Full Responders at Week 12 (n = 161) attended an average of 4.3 (SD = 2.0) of the five pharmacotherapy sessions and 4.9 (SD = 2.3) of the six CBT sessions. Week 12 Partial Responders 12 (n = 50) attended 4.3 (SD = 2.6) of the six pharmacotherapy sessions and 6.0 (SD = 2.2) of the nine CBT sessions.
Treatment provided outside of TADS was infrequent (6.2% began non-TADS antidepressants; 12.0% began non-TADS psychotherapy during Stages II/III). Analyses examining the achievement and maintenance of Sustained Response were re-computed after deselecting the 34 patients who received adjunctive treatment and the pattern of results remained identical.
Discussion
The purpose of this study was to describe the rates at which depressed adolescents in three treatments were able to achieve and maintain a sustained response during continuation and maintenance therapy as developed in TADS. Information on sustained response can be used by the clinician and the family to guide important decisions regarding the optimal course of treatment after the acute phase.
Rates of sustained response during acute therapy were significantly higher for the two conditions that included fluoxetine compared to CBT monotherapy. Regarding the achievement of sustained response during continuation and maintenance therapies, 55% of depressed adolescents who had not reached sustained response by the end of acute treatment subsequently achieved this outcome during additional therapy, with an additional 19% possibly reaching this threshold. By the end of maintenance therapy, rates of sustained response across the three conditions were comparable, suggesting that CBT has a slower effect in treating depression. The findings suggest that approximately three-quarters of depressed adolescents who have not fully responded after 12 weeks of acute treatment will experience sustained response with further treatment. As noted earlier, Clarke and colleagues13 found that additional treatment produced further symptom reduction in those who were partially recovered during acute treatment. Also consistent with the present findings, from a study of 107 adolescents treated with various psychosocial interventions23, among the subset of 18 patients who had subsyndromal depression (i.e., 2-3 MDD symptoms) at the end of acute treatment, 94% experienced recovery in the two–year follow-up period, which included 2-4 booster sessions for all patients and open treatment for approximately half. In conclusion, these findings emphasize the value of ongoing treatment in facilitating the depression recovery process, even if psychosocial treatment occurs on a significantly less frequent basis. The findings also suggest that continuation and maintenance treatment guidelines explicitly recognize the value of “continued response among partial responders” as a stated goal.
The second method of evaluating continuation and maintenance therapies consisted of the degree to which patients who achieved sustained response during acute treatment were able to maintain that response through continuation and maintenance therapies. TADS is the first study to contrast the impact of psychosocial and pharmacotherapy treatments on depression response in adolescents. Although the rate of sustained response improvement during acute treatment for patients receiving CBT were significantly lower than combination therapy or fluoxetine monotherapy, among the subset of CBT patients who did achieve this measure of improvement by Week 12, only 3.1% failed to maintain their sustained response during the following 24 weeks. This rate was significantly lower than that of patients receiving fluoxetine (25.9%), a rate similar to the relapse rate of 34% over 32-weeks of continued fluoxetine management reported by Emslie et al.10. This difference is the first TADS finding in which CBT monotherapy significantly outperformed the fluoxetine monotherapy condition, although it only applied to the subset of patients still in their assigned treatment arm. The sustaining of response, which is consistent with findings from some adult studies24, is promising and warrants further investigation.
Although patients were assigned randomly to treatment modalities, the rate and degree to which patients across conditions achieved Sustained Response in acute therapy may have unbalanced the groups with respect to pre-existing characteristics, a phenomenon that has been referred to as the “differential sieve effect”25. Thus, while one interpretation of the present findings is that CBT improves the ability of depressed adolescents who respond to maintain their positive response to treatment, it is also possible that these results were due to other factors, such as patients who are at risk for relapse and recurrence being more likely to respond to pharmacotherapy compared to CBT. We examined four factors that might bias the results (i.e., two measures of depression severity, family income, cognitive distortions). We found no evidence suggesting that the group of CBT patients who achieved Sustained Response during Stage I entered treatment less severely depressed than Sustained Responders in either the COMB or FLX conditions or differed on other variables previously found to moderate TADS treatment21. Nonetheless, sustained responders across conditions could have differed on a number of other pre-treatment factors and the phenomenon of a differential sieve effect needs to be carefully examined in future research.
Although quite limited, previous research with depressed adolescents has failed to detect a protective effect for CBT. For example, among 54 child/adolescent patients treated with either CBT or an expanded assessment procedure who were followed for 2 years, 20% experienced recurrence with no differential treatment effects26, although patients received only 6 sessions of CBT on average. As noted earlier, Clarke et al.13 found no effect for CBT booster sessions in the prevention of depression recurrence. In a follow-up study of the three psychosocial interventions evaluated by Brent and colleagues, treatment conditions did not predict recurrence27. Conversely, in the study with depressed adults that most closely parallels the TADS design, Hollon et al.28 compared relapse/recurrence rates for adult patients who remitted during cognitive therapy (CT) to patients who remitted on antidepressant medication and found that CT continuation therapy at a greatly reduced dose (3 booster sessions over a 12-month period) was as effective as continued full dosage pharmacotherapy in preventing relapse.
We also examined attendance rates in continuation and maintenance therapy as a basic aspect of treatment utilization. Both full and partial responders by Week 12 appeared to find value in both continuation and maintenance treatments as designed by TADS and there was no indication that rates of sustained response were differentially impacted across treatments by attendance. In addition, attendance rates were comparable for patients assigned to combination therapy relative to either monotherapy, suggesting that patients engaged in two forms of treatment did not reduce their commitment to either monotherapy.
Several important limitations to the present study need to be acknowledged. First, our outcome measure of Sustained Response is related to, but not synonymous with, the criteria of recovery or recurrence. While the achievement of Sustained Response represents a significant reduction in depression severity, a subset of these adolescents were still experiencing persisting depressive symptoms or some degree of functional impairment. The TADS assessment battery did not track the week-by-week course of depression symptomatology necessary to diagnose depression recovery, relapse, or recurrence with absolute confidence. Thus, comparison of the present findings to previous research is somewhat limited. Second, we had no placebo or untreated group following acute treatment. This design decision was made purposefully, for both scientific and ethical reasons, but it meant that we do not know how many patients would have achieved and maintained a sustained response with no post-acute intervention. That said, given the lower rates of sustained response among CBT patients at the end of acute treatment and the higher rates of failure to maintain sustained response for patients receiving fluoxetine monotherapy, the present findings clearly support the value of continuation and maintenance therapies. A third, and very important, limitation of the present study is the lack of data on treatment compliance or adherence for either CBT or pharmacotherapy in the continuation and maintenance phases of TADS. It is possible that adolescents receiving fluoxetine discontinued or were more erratic with medication following the positive response, as the discontinuation of antidepressant therapy among adults is frequent.29 We recognized that treatment attendance serves as a necessary but not sufficient factor in CBT and is not even required for adequate pharmacotherapy, if the patients continue to take their medication. A fourth limitation is that a small subset of TADS participants (n = 28, or 10%) had been evaluated by the treatment provider to be clear non-responders at the end of Stage I acute treatment and were referred for treatment outside of TADS prior to Stage II. Thus, the present findings may be generalized to patients who have demonstrated at least a partial response to medications or CBT within 12 weeks. Lastly, some attrition had occurred during Stage I, which may have influenced the present findings. Although not significant across conditions, approximately 17% of TADS patients discontinued or augmented treatment during Stage I.
Given that a larger proportion of patients recover from fluoxetine than CBT but the sustained response may not be as enduring, one hypothesis for future research is that fluoxetine monotherapy needs to be augmented once depression recovery has occurred, and CBT would seem to be a reasonable place to start. Continuation and maintenance treatments in TADS were designed to extend the acute treatment modality rather than to introduce new treatment modalities. While the issue of a stepped program of care has not, to our knowledge, been evaluated in depressed adolescents, there has been a recent shift toward the use of sequencing treatments to achieve remission in adults, as in the landmark study, Sequenced Treatment Alternatives to Relieve Depression (STAR*D)30. Stepped care protocols are based on the rationale of limiting the use of more expensive and time consuming treatments, such as combination treatment, to the more severely depressed or treatment resistant patients. Although much of the literature describes the sequencing of medication strategies, there is some support for the sequencing of pharmacotherapy and psychosocial treatments31,32.
Another direction for future research consists of the adolescent's depressive course once treatment has ended. Adult patients who received CT, with or without continuing clinical management, have been found to have significantly lower relapse and recurrence rates than medication patients following withdrawal from medication35,36,37. The rates of MDD recurrence in the one-year open follow-up (Stage IV) of TADS will be addressed in a subsequent report.
Acknowledgments
The Treatment for Adolescents with Depression Study (TADS) is supported by contract RFP-NIH-NIMH 98-DS-0008 from the National Institute of Mental Health to Duke University Medical Center (Principal Investigator: John S. March, MD, MPH.). Lilly, Inc. provided FLX and matching PBO under an independent educational grant to Duke University, but otherwise had no role in the design or implementation of the study, data analysis, or in authoring this manuscript. The opinions and assertions contained in this report are the private views of the authors and are not to be construed as official or as reflecting the views of the National Institute of Mental Health, the National Institutes of Health, or the Department of Health and Human Services. The first author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The TADS protocol and all of the TADS manuals are available on the Internet at: https://trialweb.dcri.duke.edu/tads/index.html.
Contributor Information
Paul Rohde, Oregon Research Institute, Eugene
Susan G. Silva, Duke Clinical Research Institute, Duke University Medical Center
Simon T. Tonev, Duke Clinical Research Institute, Duke University Medical Center
Betsy D. Kennard, University of Texas Southwestern Medical Center at Dallas
Benedetto Vitiello, National Institute of Mental Health
Christopher J. Kratochvil, University of Nebraska Medical Center, Omaha
Mark A. Reinecke, Northwestern University
John F. Curry, Department of Psychiatry & Behavioral Sciences, Duke University Medical Center
Anne D. Simons, University of Oregon
John S. March, Duke Clinical Research Institute, Duke University Medical Center
References
- 1.Mueller TI, Leon AD, Keller MG, Solomon DA, Endicott J, Coryell Y, Warshaw M, Maser JD. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. 1999;156:1000–1006. doi: 10.1176/ajp.156.7.1000. [DOI] [PubMed] [Google Scholar]
- 2.Emslie GJ, Rush A, Weinberg W, Gullion D, Rintelmann J, Hughes C. Recurrence of major depressive disorder in hospitalized children and adolescents. J Am Acad Child Adoles Psychiatry. 1997;36:785–792. doi: 10.1097/00004583-199706000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder: Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48:851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 4.Birmaher B, Brent D. Practice parameters for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 1998;37:63S–83S. doi: 10.1097/00004583-199810001-00005. [DOI] [PubMed] [Google Scholar]
- 5.Park RJ, Goodyer IM. Clinical guidelines for depressive disorders in childhood and adolescence. Eur Child Adolesc Psychiatry. 2000;9:147–161. doi: 10.1007/s007870070038. [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Practice guideline for major depressive disorder in adults. Am J Psychiatry. 1993;150(supplement):1–26. doi: 10.1176/ajp.150.4.1. [DOI] [PubMed] [Google Scholar]
- 7.Depression Guideline Panel. Depression in Primary Care, Vol I: Treatment of Major Depression Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; 1993. [Google Scholar]
- 8.Kutcher S. Practitioner review: The pharmacotherapy of adolescent depression. J Child Psychology Psychiatry. 1997;38:755–767. doi: 10.1111/j.1469-7610.1997.tb01594.x. [DOI] [PubMed] [Google Scholar]
- 9.Prien RF, Kocsis JH. Long-term treatment of mood disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press; 1995. pp. 1067–1079. [Google Scholar]
- 10.Kroll L, Harrington R, Jayson D, Fraser J, Gowers S. Pilot study of continuation cognitive-behavioral therapy for major depression in adolescent psychiatric patients. J Am Acad Child Adoles Psychiatry. 1996;35:1156–1161. doi: 10.1097/00004583-199609000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Emslie GJ, Heiligenstein JH, Hoog SL, Wagner KD, Findling RL, McCracken JT, Nilsson ME, Jacobson JG. Fluoxetine treatment for prevention of relapse of depression in children and adolescents: A double-blind, placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 2004;43:1397–1405. doi: 10.1097/01.chi.0000140453.89323.57. [DOI] [PubMed] [Google Scholar]
- 12.Poznanski EO, Mokros HB. Manual for the Children's Depression Rating Scale-Revised. Los Angeles, CA: Western Psychological Services; 1996. [Google Scholar]
- 13.Clarke GN, Rohde P, Lewinsohn PM, Hops H, Seeley JR. Cognitive-behavioral treatment of adolescent depression: Efficacy of acute group treatment and booster sessions. J Am Acad Child Adolesc Psychiatry. 1999;38:272–279. doi: 10.1097/00004583-199903000-00014. [DOI] [PubMed] [Google Scholar]
- 14.The TADS Team. Treatment for Adolescents with Depression Study (TADS): Rationale, design, and methods. J Am Acad Child Adolesc Psychiatry. 2003;42:531–542. doi: 10.1097/01.CHI.0000046839.90931.0D. [DOI] [PubMed] [Google Scholar]
- 15.The TADS Team. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents with Depression Study (TADS) randomized controlled trial. JAMA. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 16.The TADS Team. The Treatment for Adolescents with Depression Study (TADS): Demographic and clinical characteristics. J Am Acad Child Adolesc Psychiatry. 2005;44:28–40. doi: 10.1097/01.chi.0000145807.09027.82. [DOI] [PubMed] [Google Scholar]
- 17.The TADS Team. The Treatment for Adolescents with Depression Study (TADS): Long-Term effectiveness and safety outcomes. Arch Gen Psychiatry. doi: 10.1001/archpsyc.64.10.1132. in press. [DOI] [PubMed] [Google Scholar]
- 18.Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, Ninan PT, Thase ME, Gelenberg AJ, Kupfer DJ, Regier DA, Rosenbaum JF, Ray O, Schatzberg AF. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31:1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman J, Birmaher B, Brent DA, Rao U, Flynn C, Moreci P, Williamson D, Ryan ND. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Guy W. DHEW Publication ABM 76-388. 2nd. Washington, DC: US Government Printing Office; 1976. ECDEU Assessment Manual for Psychopharmacology. [Google Scholar]
- 21.Curry J, Rohde P, Simons A, et al. Predictors and Moderators of Acute Treatment Outcome in the Treatment for Adolescents with Depression Study (TADS) J Am Acad Ch Adolesc Psychiatry. 2006;45:1427–1439. doi: 10.1097/01.chi.0000240838.78984.e2. [DOI] [PubMed] [Google Scholar]
- 22.Leitenberg H, Yost LW, Carroll-Wilson M. Negative cognitive errors in children. J Consult Clin Psych. 1986;54:528–536. doi: 10.1037//0022-006x.54.4.528. [DOI] [PubMed] [Google Scholar]
- 23.Brent DA, Birmaher B, Kolko D, Baugher M, Bridge J. Subsyndromal depression in adolescents after a brief psychotherapy trial: Course and outcome. J Affect Disord. 2001;63:51–58. doi: 10.1016/s0165-0327(00)00189-0. [DOI] [PubMed] [Google Scholar]
- 24.Vittengl JR, Clark LA, Dunn TW, Jarrett RB. Reducing relapse and recurrence in unipolar depression: A comparative meta-analysis of cognitive-behavioral therapy's effects. J Consult Clin Psych. 2007;75:475–488. doi: 10.1037/0022-006X.75.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobson NS, Hollon SD. Cognitive-Behavior Therapy Versus Pharmacotherapy: Now That the Jury's Returned Its Verdict, It's Time to Present the Rest of the Evidence. J Consult Clin Psych. 1996;64:74–80. doi: 10.1037//0022-006x.64.1.74. [DOI] [PubMed] [Google Scholar]
- 26.Vostanis P, Feehan C, Grattan E. Two-year outcome of children treated for depression. Eur Child Adolesc Psychiatry. 1998;7:12–18. doi: 10.1007/s007870050039. [DOI] [PubMed] [Google Scholar]
- 27.Birmaher B, Brent DA, Kolko D, Baugher M, Bridge J, Holder D, Iyengar S, Ulloa RE. Clinical outcome after short-term psychotherapy for adolescents with major depressive disorder. Arch Gen Psychiatry. 2000;57:29–36. doi: 10.1001/archpsyc.57.1.29. [DOI] [PubMed] [Google Scholar]
- 28.Hollon SD, DeRubeis RJ, Shelton RC, Amsterdam JD, Salomon RM, O'Reardon JP, et al. Prevention of relapse following cognitive therapy vs. medications in moderate to severe depression. Arch Gen Psychiatry. 2005;62:417–422. doi: 10.1001/archpsyc.62.4.417. [DOI] [PubMed] [Google Scholar]
- 29.Olfson M, Marcus SC, Tedeschi M, Wan GJ. Continuity of antidepressant treatment for adults with depression in United States. J Psychiatry. 2006;163:101–108. doi: 10.1176/appi.ajp.163.1.101. [DOI] [PubMed] [Google Scholar]
- 30.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 31.Schatzberg AF, Rush AF, Arnow BA, Banks PLC, Blalock AJ, Borian FE, et al. Chronic depression: Medication (Nefazodone) or psychotherapy (CBASP) is effective when the other is not. Arch Gen Psychiatry. 2005;62:513–520. doi: 10.1001/archpsyc.62.5.513. [DOI] [PubMed] [Google Scholar]
- 32.Thase ME, Friedman ES, Biggs MM, Wisniewski SR, Trivedi MH, Luther JF, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: A STAR*D report. Am J Psychiatry. 2007;164:739–752. doi: 10.1176/ajp.2007.164.5.739. [DOI] [PubMed] [Google Scholar]
- 35.Simons AD, Murphy GE, Levine JL, Wetzel RD. Cognitive therapy and pharmacotherapy of depression: Sustained improvement over one year. Arch Gen Psychiatry. 1986;43:43–48. doi: 10.1001/archpsyc.1986.01800010045006. [DOI] [PubMed] [Google Scholar]
- 36.Evans MD, Hollon SD, DeRubeis J, Piasecki JM, et al. Differential relapse following cognitive therapy and pharmacotherapy for depression. Arch Gen Psychiatry. 1992;49:802–808. doi: 10.1001/archpsyc.1992.01820100046009. [DOI] [PubMed] [Google Scholar]
- 37.Blackburn IM, Eunson KM, Bishop S. A two-year naturalistic follow-up of depressed patients treated with cognitive therapy, pharmacotherapy and a combination of both. J Affect Disord. 1986;10:67–75. doi: 10.1016/0165-0327(86)90050-9. [DOI] [PubMed] [Google Scholar]