Abstract
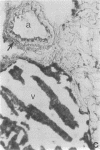
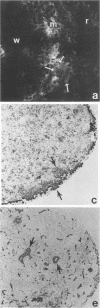
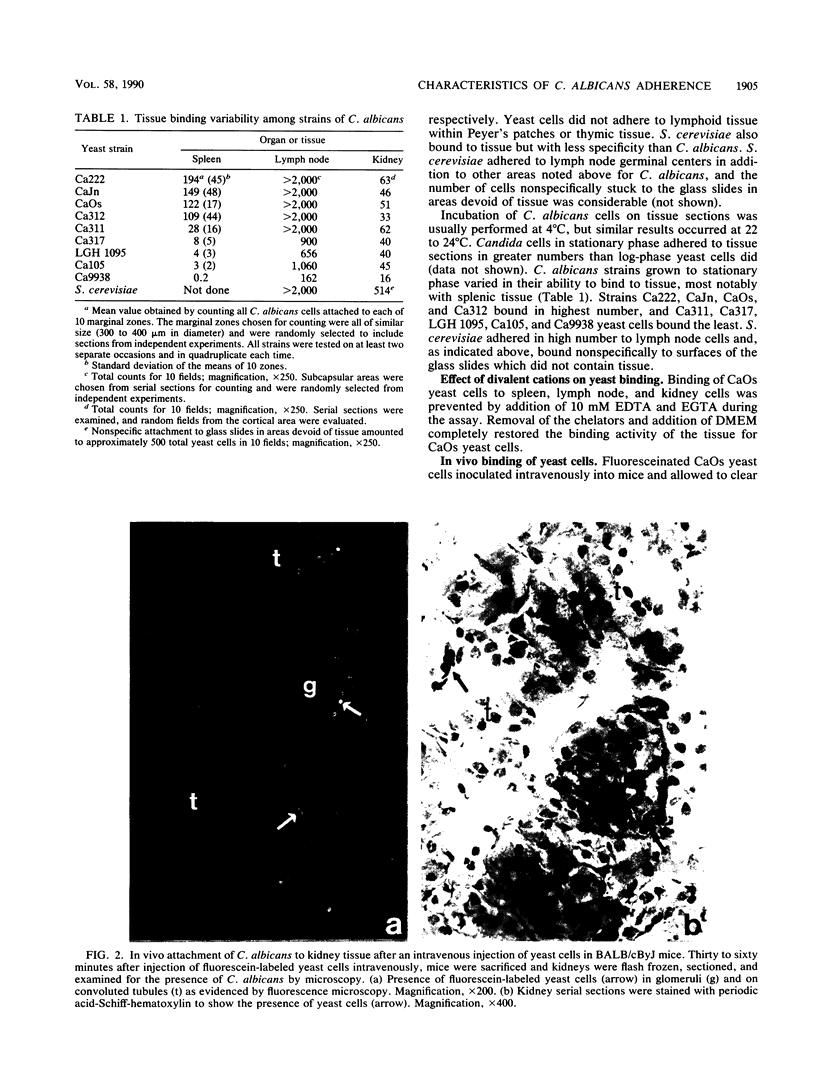
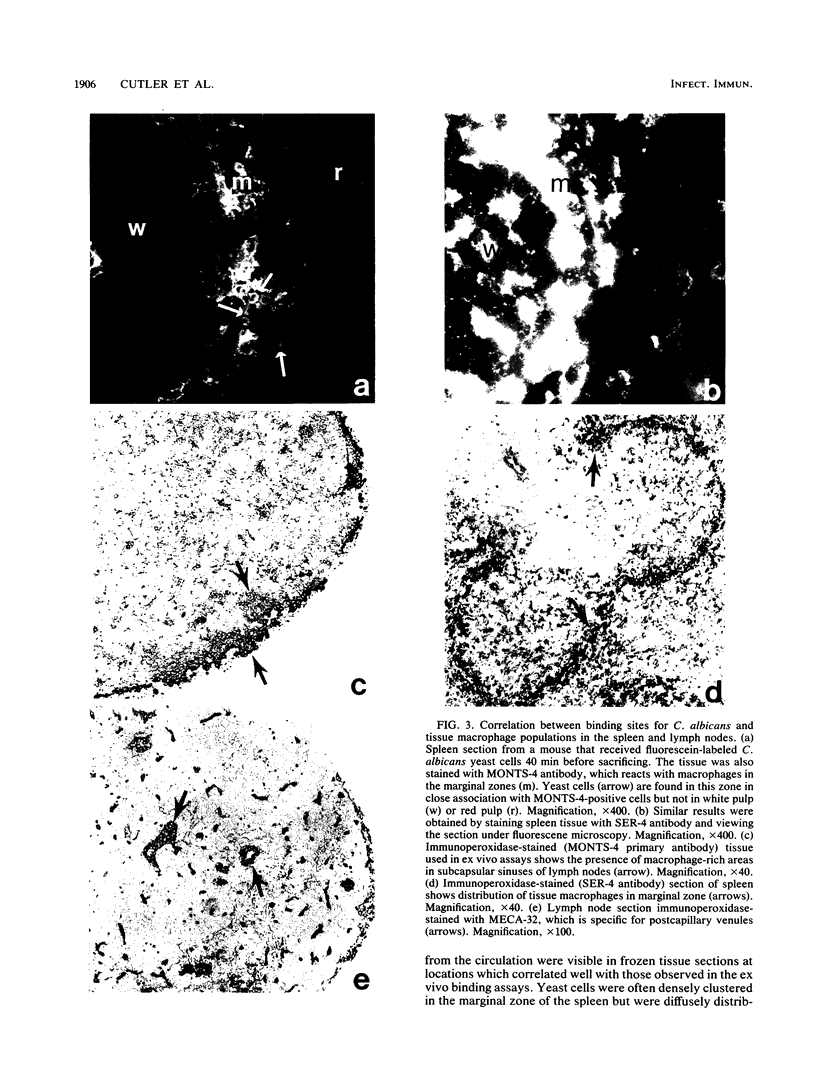
An ex vivo binding assay originally described for determining lymphocyte homing receptors was adapted for studying Candida albicans-host cell interactions in unfixed tissue sections. BALB/cByJ mice were sacrificed, and various organs were removed, rapidly frozen on dry ice, and sectioned. C. albicans yeast cells were suspended to 1.5 x 10(8) cells per ml in Dulbecco modified Eagle medium supplemented with 5% newborn calf serum, and 100 microliters of the suspension was added to tissue sections for 15 min with rotation at 4 degrees C or at 22 to 24 degrees C. The sections were then fixed in glutaraldehyde, washed, and examined. Stationary-phase yeast cells adhered better than log-phase cells, and adherence characteristics were similar at 4 degrees C and 22 to 24 degrees C. Yeast cells from nine strains of C. albicans showed similar tissue specificity. Adherence to lymph node tissue was confined to subcapsular spaces and trabecular sinuses. In the spleen, yeast cells bound to the marginal zones. In both tissues, an association of yeast cells with tissue macrophages was suggested by results with macrophage-specific monoclonal antibodies and fluorescent or immunoperoxidase staining techniques. C. albicans adhered to convoluted tubules, glomeruli, and the tunica media of arterioles in the kidney. During experimentally induced fungemia in mice, C. albicans yeast cells associated with the same tissue sites as in the ex vivo assay, except that binding to renal arterioles was not seen in the in vivo test. A strain of Saccharomyces cerevisiae showed some adherence patterns in common with C. albicans, which indicates that tissue adherence is not sufficient for virulence. Mechanisms of attachment were not determined, but strains of C. albicans varied quantitatively in their ability to attach, and binding was inhibited by chelators of divalent cations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes J. L., Osgood R. W., Lee J. C., King R. D., Stein J. H. Host-parasite interactions in the pathogenesis of experimental renal candidiasis. Lab Invest. 1983 Oct;49(4):460–467. [PubMed] [Google Scholar]
- Bishop D. K., Jutila M. A., Sedmak D. D., Beattie M. S., Orosz C. G. Lymphocyte entry into inflammatory tissues in vivo. Qualitative differences of high endothelial venule-like vessels in sponge matrix allografts vs isografts. J Immunol. 1989 Jun 15;142(12):4219–4224. [PubMed] [Google Scholar]
- Bistoni F., Vecchiarelli A., Cenci E., Puccetti P., Marconi P., Cassone A. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect Immun. 1986 Feb;51(2):668–674. doi: 10.1128/iai.51.2.668-674.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Cell surface and intracellular expression of two Candida albicans antigens during in vitro and in vivo growth. Microb Pathog. 1987 Apr;2(4):249–257. doi: 10.1016/0882-4010(87)90123-9. [DOI] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Variability in expression of cell surface antigens of Candida albicans during morphogenesis. Infect Immun. 1986 Jan;51(1):337–343. doi: 10.1128/iai.51.1.337-343.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C., Scollay R. G., Weissman I. L. Lymphocyte adherence to high endothelial venules: characterization of a modified in vitro assay, and examination of the binding of syngeneic and allogeneic lymphocyte populations. J Immunol. 1979 Nov;123(5):1996–2003. [PubMed] [Google Scholar]
- Calderone R. A., Scheld W. M. Role of fibronectin in the pathogenesis of candidal infections. Rev Infect Dis. 1987 Jul-Aug;9 (Suppl 4):S400–S403. doi: 10.1093/clinids/9.supplement_4.s400. [DOI] [PubMed] [Google Scholar]
- Chaffin W. L., Sogin S. J. Germ tube formation from zonal rotor fractions of Candida albicans. J Bacteriol. 1976 May;126(2):771–776. doi: 10.1128/jb.126.2.771-776.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Gordon S. Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J Exp Med. 1989 Apr 1;169(4):1333–1346. doi: 10.1084/jem.169.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- Herman M. A., Soll D. R. A comparison of volume growth during bud and mycelium formation in Candida albicans: a single cell analysis. J Gen Microbiol. 1984 Sep;130(9):2219–2228. doi: 10.1099/00221287-130-9-2219. [DOI] [PubMed] [Google Scholar]
- Hurley D. L., Fauci A. S. Disseminated candidiasis. i. an experimental model in the guinea pig. J Infect Dis. 1975 May;131(5):516–527. doi: 10.1093/infdis/131.5.516. [DOI] [PubMed] [Google Scholar]
- King R. D., Lee J. C., Morris A. L. Adherence of Candida albicans and other Candida species to mucosal epithelial cells. Infect Immun. 1980 Feb;27(2):667–674. doi: 10.1128/iai.27.2.667-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Berg E. L., Butcher E. C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989 Sep 15;245(4923):1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- Klotz S. A., Drutz D. J., Zajic J. E. Factors governing adherence of Candida species to plastic surfaces. Infect Immun. 1985 Oct;50(1):97–101. doi: 10.1128/iai.50.1.97-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer N., Segal E., Lis H., Gov Y. Effect of Candida albicans cell wall components on the adhesion of the fungus to human and murine vaginal mucosa. Mycopathologia. 1988 May;102(2):115–121. doi: 10.1007/BF00437448. [DOI] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., van Furth R. Kinetics of phagocytosis and intracellular killing of Candida albicans by human granulocytes and monocytes. Infect Immun. 1977 Aug;17(2):313–318. doi: 10.1128/iai.17.2.313-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn D. M., Bargatze R. F., Butcher E. C. Leukocyte-endothelial cell recognition: evidence of a common molecular mechanism shared by neutrophils, lymphocytes, and other leukocytes. J Immunol. 1987 Jun 15;138(12):4313–4321. [PubMed] [Google Scholar]
- Maisch P. A., Calderone R. A. Adherence of Candida albicans to a fibrin-platelet matrix formed in vitro. Infect Immun. 1980 Feb;27(2):650–656. doi: 10.1128/iai.27.2.650-656.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch P. A., Calderone R. A. Role of surface mannan in the adherence of Candida albicans to fibrin-platelet clots formed in vitro. Infect Immun. 1981 Apr;32(1):92–97. doi: 10.1128/iai.32.1.92-97.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattia E., Cassone A. Inducibility of germ-tube formation in Candida albicans at different phases of yeast growth. J Gen Microbiol. 1979 Aug;113(2):439–442. doi: 10.1099/00221287-113-2-439. [DOI] [PubMed] [Google Scholar]
- McCourtie J., Douglas L. J. Relationship between cell surface composition, adherence, and virulence of Candida albicans. Infect Immun. 1984 Jul;45(1):6–12. doi: 10.1128/iai.45.1.6-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel G. J., Phelps C. L. Factors influencing the interaction of Candida albicans with fibroblast cell cultures. Infect Immun. 1988 Apr;56(4):792–801. doi: 10.1128/iai.56.4.792-801.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S., Odds F. C. Binding of plasma proteins to Candida species in vitro. J Gen Microbiol. 1988 Oct;134(10):2693–2702. doi: 10.1099/00221287-134-10-2693. [DOI] [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Immunity to experimental renal candidiasis in rats. Infect Immun. 1978 Feb;19(2):737–740. doi: 10.1128/iai.19.2.737-740.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranayake L. P., McLaughlin L., MacFarlane T. Adherence of Candida species to fibrin clots in vitro. Mycopathologia. 1988 May;102(2):135–138. doi: 10.1007/BF00437451. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Altman L. C., Frankel A., Blaese R. M. Defective mononuclear leukocyte chemotaxis: a previously unrecognized immune dysfunction. Studies in a patient with chronic mucocutaneous candidiasis. Ann Intern Med. 1973 Apr;78(4):509–513. doi: 10.7326/0003-4819-78-4-509. [DOI] [PubMed] [Google Scholar]
- Sobel J. D., Myers P. G., Kaye D., Levison M. E. Adherence of Candida albicans to human vaginal and buccal epithelial cells. J Infect Dis. 1981 Jan;143(1):76–82. doi: 10.1093/infdis/143.1.76. [DOI] [PubMed] [Google Scholar]
- Sobel J. D., Myers P., Levison M. E., Kaye D. Comparison of bacterial and fungal adherence to vaginal exfoliated epithelial cells and human vaginal epithelial tissue culture cells. Infect Immun. 1982 Feb;35(2):697–701. doi: 10.1128/iai.35.2.697-701.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamper H. B., Jr, Woodruff J. J. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976 Sep 1;144(3):828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G., Bouchara J. P., Robert R., Senet J. M. Adherence of Candida albicans germ tubes to plastic: ultrastructural and molecular studies of fibrillar adhesins. Infect Immun. 1988 Aug;56(8):1987–1993. doi: 10.1128/iai.56.8.1987-1993.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey J. J., Waddell C. C., Krantz S., O'reilly R., L'esperance P., Good R. A. Chronic mucocutaneous candidiasis with macrophage dysfunction, a plasma inhibitor, and co-existent aplastic anemia. J Lab Clin Med. 1975 Jun;85(6):968–977. [PubMed] [Google Scholar]
- Winblad B. Experimental renal candidiasis in mice and guinea pigs. Acta Pathol Microbiol Scand A. 1975 Jul;83(4):406–414. doi: 10.1111/j.1699-0463.1975.tb01890.x. [DOI] [PubMed] [Google Scholar]
- Yednock T. A., Stoolman L. M., Rosen S. D. Phosphomannosyl-derivatized beads detect a receptor involved in lymphocyte homing. J Cell Biol. 1987 Mar;104(3):713–723. doi: 10.1083/jcb.104.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]