Abstract
Objective
Increased concentrations of amino-terminal prohormone brain-type natriuretic peptide (NT-proBNP) are associated with cardiovascular morbidity and mortality, but little is known about their relationship to chronic inflammation. Patients with rheumatoid arthritis (RA) have chronic inflammation, increased arterial stiffness and accelerated coronary atherosclerosis. We tested the hypothesis that NT-proBNP concentrations are elevated in patients with RA, and are associated with coronary artery calcification and markers of inflammation.
Methods
In 159 subjects with RA (90 patients with early RA and 69 patients with longstanding RA) without heart failure and 88 control subjects, we measured serum concentrations of NT-proBNP, interleukin (IL)-6, and tumor necrosis factor-α (TNF-α), and coronary calcification.
Results
NT-proBNP concentrations were elevated in patients with long-standing RA [median (IQR): 142.8 (54.8–270.5) pg/mL] and those with early RA [58.1 (19.4–157.6) pg/mL] compared to controls [18.1 (3.2–46.0) pg/mL, P<0.001]. In patients with RA, NT-proBNP concentrations were associated with age (ρ=0.35, P<0.001), IL-6 (ρ=0.33, P<0.001), TNF-α (ρ=0.23, P=0.003), CRP (ρ=0.21, P=0.01), coronary calcium score (ρ=0.30, P<0.001), systolic blood pressure (ρ=0.30, p<0.001), and disease activity (ρ=0.29, P<0.001). After adjustment for age, race and sex the associations between NT-proBNP concentrations and disease activity (P<0.001), TNF-α (P<0.001), IL-6 (P=0.04) and CRP concentrations (P=0.02) remained significant, but those with systolic blood pressure (P=0.10) and coronary calcium score (P=0.27) were attenuated.
Conclusions
NT-proBNP concentrations are increased in patients with RA without clinical heart failure and may indicate subclinical cardiovascular disease and a chronic inflammatory state.
Keywords: rheumatoid arthritis, inflammation, atherosclerosis, B-type natriuretic peptide, NT-proBNP
Patients with rheumatoid arthritis (RA) suffer premature mortality that is largely attributable to coronary heart disease.1, 2 We and others have shown that patients with RA have increased coronary3 and extra-coronary 4, 5 atherosclerosis. Increased atherosclerosis in rheumatoid arthritis is not explained by traditional cardiovascular risk factors alone.6, 7 Thus, the relationship between chronic inflammation and coronary atherosclerosis is of interest, particularly considering the poorly understood interplay of their pathogenic mechanisms in vascular and coronary remodelling, as well as the cross-talk among the endocrine, autocrine, immune and circulatory systems.8, 9
B-type natriuretic peptide (BNP), a hormone synthesized and secreted mainly in the cardiac ventricles in response to myocyte stretch, has natriuretic, diuretic and vasodilating properties.10, 11 Originally it was considered a specific marker for ventricular dysfunction with prognostic significance in heart failure, although more recently, its prognostic significance in acute coronary syndrome has also been recognized.12, 13 Serum concentrations of BNP, or its amino terminal fragment (NT-proBNP), also predict mortality in patients with stable coronary disease14 and in the general population without symptoms of heart failure,15 suggesting that, in addition to being a marker of ventricular dysfunction, it is also a marker of cardiovascular risk. In a recent analysis of the Framingham Heart study, after adjustment for conventional cardiovascular risk factors, BNP was a stronger predictor of major cardiovascular events than C-reactive protein (CRP).16
In addition, inflammatory cytokines induce BNP production in vitro 17 and in the HOPE study of patients with coronary artery disease, NT-proBNP concentrations were correlated with soluble TNF receptors. 18 There is no information about the relationship between NT-proBNP and subclinical coronary atherosclerosis and inflammation in patients with RA.We examined the hypotheses that NT-proBNP concentrations are increased in RA and associated with cardiovascular risk factors, markers of inflammation, and coronary artery calcification.
Methods
Patients and control subjects
The subjects are participants in an ongoing study of cardiovascular risk in patients with RA and detailed methods have been described.3, 6, 19 One-hundred and fifty-nine eligible patients, older than 18 years of age who met the classification criteria for rheumatoid arthritis,20 and 89 control subjects were studied. Control subjects did not have rheumatoid arthritis or any other inflammatory disease. Subjects with heart failure, determined by either a current history of heart failure requiring treatment, or at least two of the following three criteria: a past history of heart failure, use of digoxin, or use of diuretics were excluded from the study.
To determine whether any observed differences among patients with RA occurred early in the illness, and therefore could play a role in pathogenesis, or act as an early prognostic indicator, patients with early (less than 5 years duration) and established (more than 10 years duration) were studied. Patients were recruited from an early rheumatoid arthritis registry,21 by referral from area rheumatologists, and by local advertisement. Control subjects were recruited from patients’ acquaintances, by local advertisement, and from a database of volunteers maintained by the General Clinical Research Center. Patients with RA (combined early and long-standing) and controls were frequency-matched for age, sex and race. The study was approved by the Institutional Review Board of Vanderbilt University Hospital, and all subjects gave written informed consent.
Data collection
Clinical and demographic information was obtained through a structured interview, self-report questionnaires, physical examination, laboratory tests, and electron beam computer tomography and, for patients, review of medical records. Current and cumulative medication use was determined from both the information provided by patients and medical records. Blood pressure was determined as the average of two measurements obtained 5 minutes apart after subjects had rested in supine position for at least 10 minutes. Subjects were considered hypertensive if they were taking anti-hypertensive agents or if they had a systolic blood pressure of at least 140 mmHg and/or a diastolic pressure of at least 90 mmHg. In patients, disease activity was measured using the Disease Activity Score based on evaluation of 28 joints (DAS28). The DAS28 score is a validated composite index containing a 28-joint count for tenderness, a 28-joint count for swelling, erythrocyte sedimentation rate (ESR), and the patient’s overall assessment of well-being.22 Ability to perform activities of daily living was measured using the modified Health Assessment Questionnaire (0–3) with a score of 0 representing no impairment of function.23
Laboratory tests
Blood was collected after an overnight fast for the measurement of a complete blood count, creatinine, total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, lipoprotein-a (Lpa), and homocysteine and low-density lipoprotein (LDL) cholesterol was determined using the Friedewald formula by the Vanderbilt University Hospital clinical laboratory. Tumor necrosis factor alpha (TNFα), interleukin-6 (IL-6) and NT-proBNP concentrations were measured by multiplex ELISA (Linco Research/Millipore Corp.). In patients with rheumatoid arthritis, C-reactive protein (CRP) and Westergren erythrocyte sedimentation rate (ESR) were measured and medical records were reviewed to determine the presence or absence of rheumatoid factor.
Coronary artery calcification
All subjects underwent imaging with a C-150 scanner (GE/Imatron, South San Francisco, CA) as described previously.3 Scans were evaluated by a single expert investigator (PR), unaware of the subjects’ clinical status. The degree of coronary artery calcification was calculated as described previously.24 The area of each calcified plaque is multiplied by the peak attenuation inside such area expressed as a coefficient (1= for an attenuation of 130–199 Hounsfield units (HU); 2 =200–299 HU; 3= 300–400 HU and 4 >400 HU); the sum of the scores for all coronary arterial lesions provides an overall score for each individual.24
Statistical analysis
Analysis was performed in two phases. First, data from both patients and controls were used to assess the association between rheumatoid arthritis, cardiovascular risk factors and chronic inflammation and NT-proBNP. Second, data from only RA patients were used to assess the association between NT-proBNP concentrations and coronary calcification. In the first phase, NT-proBNP concentrations were compared among patients with early RA, long-standing RA, and control subjects using a Kruskal-Wallis test. The association between disease status and NT-proBNP concentrations was assessed in a multivariable linear regression model that included cardiovascular risk factors and previous cardiovascular disease (defined as the presence of myocardial infarction, angina, stroke, or a history of having undergone a coronary procedure such as angioplasty or stent). A sensitivity analysis excluding individuals with history of cardiovascular events was performed.
Next, we studied factors associated with NT-proBNP separately among patients with RA and controls. Spearman rank correlation coefficients were calculated to assess the relationship between NT-proBNP concentrations and markers of inflammation, cardiovascular risk factors, and coronary artery calcium scores. Multivariable linear regressions were used to adjust for age, sex, and race. To evaluate the relative contribution of continuous variables on a common scale, standardized beta coefficients were calculated. Among RA patients, we further assessed the association between NT-proBNP concentrations, disease activity, functional capacity, and TNF-α, adjusted for cardiovascular risk factors and cholesterol profile. To ensure regression power, HDL and LDL cholesterol, triglycerides, Lp(a) lipoprotein were combined using principal component methods into one factor (cholesterol profile) and included in the model.25 For all multivariable linear regression models, Box-Cox transformation of NT-proBNP concentrations were performed to achieve normality of residuals to satisfy regression assumptions.
In the second phase of analysis, proportional odds logistic regression models were used to estimate unadjusted and adjusted odds ratios (OR) to evaluate the association between NT-proBNP and coronary calcification in patients with RA.6 Regression covariates for adjustment chosen a priori included age, sex, and race, homocysteine, rheumatoid factor, disease activity, functional capacity, IL-6 and TNF-α.
Data are expressed as median and interquartile range [IQR]. All analyses used a 5% two sided significant level and were performed with R 2.1.0 (The R Project for Statistical Computing) and SAS 9.1 (SAS Insitute Cary, NC) statistical software packages. P-values were not adjusted for multiple comparisons.
Results
Patient characteristics
The clinical characteristics of patients with early RA (n=90), with median disease duration of 2 years [IQR:1–3 years], long-standing RA (n=69), with median disease duration of 20 years [IQR:14–24 years], and control subjects (n=88) are shown in Table 1. Patients with long-standing RA were slightly older, had higher systolic blood pressure and a higher percentage were more likely to be actively smoking compared to those with early disease and controls. Serum concentrations of TNF-α, and IL-6 were markedly higher in patients with RA than controls (Table 1).
Table 1.
Characteristics of Rheumatoid Arthritis Patients and Control Subjects
| Characteristics: | Long-standing RA (N=69) | Early RA (N=90) | Control subjects (N=88) | P-Value |
|---|---|---|---|---|
| Demography: | ||||
| Age (yrs) | 59 (51–67) | 51 (43–59) | 53 (45–59) | <0.001 |
| Female (%) | 74% | 64% | 64% | 0.33 |
| Caucasian (%) | 87% | 91% | 84% | 0.36 |
| Current smoker (%) | 26% | 21% | 9% | 0.02 |
| Hypertensive (%) | 67% | 40% | 41% | 0.001 |
| Diabetes mellitus (%) | 10% | 11% | 5% | 0.25 |
| Body mass index | 27 (24–31) | 28 (24–34) | 27 (25–32) | 0.37 |
| Family history CHD (%) | 28% | 27% | 30% | 0.91 |
| Coronary artery procedure* (%) | 12% | 7% | 6% | 0.35 |
| Cardiovascular disease** | 20% | 11% | 8% | 0.06 |
| Creatinine | 0.8 (0.6–0.9) | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | 0.53 |
| Homocysteine (μmol/l) | 10.8 (8.2–12.4) | 9.5 (8.1–11.4) | 8.2 (7.2–9.6) | <0.001 |
| TNF-α (pg/ml) | 6.5 (3.3–11.1) | 4.7 (2.6–11.2) | 3.2 (2.3–4.7) | <0.001 |
| IL-6 (pg/ml) | 16.8 (6.4–58.6) | 12.6 (3.7–34.6) | 4.0 (1.1–18.1) | <0.001 |
| NT-proBNP (pg/ml)*** | 142.8 (54.8–270.5) | 58.1 (19.4–157.6) | 18.1 (3.2–46.0) | <0.001 |
Coronary artery procedure represents the proportion of patients who had undergone a previous stent, balloon angioplasty or coronary artery bypass graft.
Cardiovascular disease represents the proportion of patients who had had a myocardial infarction, angina, stroke or coronary procedure
A sensitivity analysis excluding thirty two patients receiving anti TNF-α therapy at the time of the study showed essentially the same results: NT-proBNP concentrations (pg/ml) were 152.9 [61.5–315.8] in patients with long-standing RA, 58.1 [19.1–164.7] in patients with early RA and 18.1 [3.2–46.0] in control subjects, p<0.001
NT-proBNP concentrations in patients with RA and controls
NT-proBNP concentrations were elevated in patients with long-standing RA [median (IQR): 142.8 (54.8–270.5) pg/mL] and early RA [58.1 (19.4–157.6) pg/mL] compared to controls [18.1 (3.2–46.0) pg/mL, P<0.001 for both comparisons] (Figure 1). In a multiple linear regression model the association between RA and higher NT-proBNP concentrations remained significant (P <0.001) after adjustment for age, race, sex, cardiovascular risk factors and previous cardiovascular disease (defined as the presence of myocardial infarction, angina, stroke, or a history of having undergone a coronary procedure such as angioplasty, coronary artery bypass graft or stent). These results were consistent with the ones obtained when individuals with a previous history of cardiovascular disease were excluded (P<0.001). There was no significant difference between patients with early and long-standing RA (P = 0.51).
Figure 1. Serum Concentrations of NT-proBNP in patients with early RA, long-term RA, and controls.
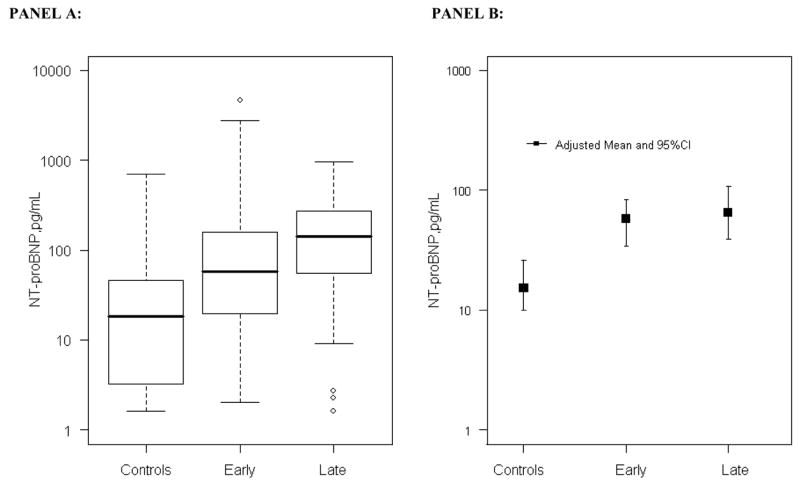
PANEL A: Concentrations of NT-proBNP in patients with early and late RA and control subjects. The box represents the 25th –75th percentile. The median is shown as a line across the box. The whiskers represent the lower quartile minus 1.5 times the interquartile range, or the upper quartile plus 1.5 times the interquartile range. Concentrations were higher in patients with early and late RA than control subjects, (P<0.001) for both comparisons after adjustment for age, race and sex.
PANEL B: Model predicted concentrations of NT-proBNP after adjustment for age, sex, homocysteine, HDL and LDL cholesterol, triglycerides, lipoprotein (a), hypertension, BMI, diabetes, smoking, creatinine, family history of coronary heart disease, and past history of cardiovascular disease. Adjusted means and 95% confidence intervals are represented.
Association between NT-proBNP concentrations and clinical variables in patients with RA and controls
In patients with RA but not control subjects, in univariate Spearman correlations NT-proBNP concentrations were associated with inflammatory markers (IL-6, ρ=0.33, P<0.001; TNF-α, ρ=0.23, P=0.003; CRP, ρ=0.21, P=0.01), systolic blood pressure (ρ=0.30, P<0.001), disease activity (ρ=0.29, P<0.001), and coronary calcium (ρ=0.30, P<0.001). NT-proBNP concentrations were significantly associated with age in both control subjects and patients with RA. After adjustment for age, race and sex (Table 2) the associations of Box-Cox transformed NT-proBNP concentrations with disease activity (DAS28) (P<0.001), TNF-α (P<0.001), IL-6 (P=0.04) and CRP concentrations (P=0.02) remained significant, but those with systolic blood pressure (P=0.10) and Agatston score (P=0.27) were attenuated in patients with RA. Because anti TNF-α therapy could affect the biological function of endogenous TNF-α, in a sensitivity analysis the results from thirty two patients receiving anti TNF-α therapy at the time of the study were excluded from analyses involving TNF-α concentrations and the results were essentially unchanged.
Table 2.
Relationship between NT-proBNP concentration and clinical variables
| Patients with RA n = 159 | Control subjects n = 88 | ||||||
|---|---|---|---|---|---|---|---|
| Categorical variables | n | Median (IQR) | P* | n | Median (IQR) | P* | |
| Sex | Female | 109 | 92.0 (34.0–200.0) | 0.48 | 56 | 20.7 (3.9–63.4) | 0.38 |
| male | 50 | 85.0 (23.0–234.0) | 32 | 14.6 (3.1–36.0) | |||
| Caucasian | yes | 142 | 101.0 (36.9–260.0) | <0.001 | 74 | 18.8 (3.3–70.5) | 0.34 |
| no | 17 | 16.0 (3.2–65.4) | 14 | 14.7 (4.8–29.2) | |||
| Hypertension | present | 82 | 145.0 (45.3–328.7) | 0.06 | 36 | 22.7 (9.2–152.5) | 0.12 |
| absent | 77 | 53.0 (18.8–140.3) | 52 | 12.6 (2.2–36.0) | |||
| Family history of coronary disease | present | 43 | 83.6 (30.4–333.8) | 0.69 | 26 | 21.7 (5.4–37.9) | 0.83 |
| absent | 116 | 91.0 (29.1–178.0) | 62 | 17.8 (2.8–46.0) | |||
| Coronary Procedure | present | 14 | 186.4 (117.5–368.7) | 0.05 | 5 | 201.6 (2.2–433.8) | 0.33 |
| absent | 145 | 87.0 (26.0–190.5) | 83 | 18.0 (3.4–37.7) | |||
| Diabetes | present | 17 | 111.8 (39.0–169.2) | 0.42 | 4 | 22.7 (17.9–44.4) | 0.95 |
| absent | 142 | 88.0 (29.4–216.9) | 84 | 17.8 (3.1–46.0) | |||
| Patients with RA n = 159 | Control subjects n = 88 | ||||||
| Continuous variables | Standardized Beta | P* | Standardized Beta | P* | |||
| Age | <0.0001 | <0.001 | |||||
| Systolic blood pressure | 0.14 | 0.10 | 0.09 | 0.44 | |||
| Diastolic blood pressure | 0.05 | 0.48 | 0.01 | 0.95 | |||
| Agatston score | NA | 0.27† | NA | 0.08† | |||
| Homocysteine | 0.02 | 0.81 | −0.03 | 0.79 | |||
| IL-6 | 0.15 | 0.04 | −0.01 | 0.96 | |||
| TNF-α** | 0.25 | 0.001 | −0.07 | 0.52 | |||
| Disease duration | 0.05 | 0.56 | NA | NA | |||
| DAS28 | 0.28 | <0.001 | NA | NA | |||
| CRP | 0.16 | 0.02 | NA | NA | |||
| MHAQ | 0.26 | <0.001 | −0.17 | 0.11 | |||
n = number of subjects
P-values from linear regressions adjusted for race, age and sex.
For P value calculations Agatson score was included as a non-linear parameter and adjusted for race, age and sex.
A sensitivity analysis excluding thirty two patients receiving anti TNF-α therapy at the time of the study showed similar results: standardized beta=0.43, p<0.001
The highest age, race and sex adjusted correlations between components of the DAS28 score and NT-proBNP were for ESR (standardized beta=0.26, P<0.001) and global assessment (standardized beta=0.24, P=0.001), and the association was weaker for the number of tender (standardized beta=0.18, P=0.01) and swollen joints (standardized beta=0.12, P=0.11). When cardiovascular risk factors and previous coronary artery disease were included in the model, the relationship between BNP and DAS28 (P=0.02) and mHAQ remained significant (P=0.04).
Association between NTproBNP and coronary calcification in patients with RA
In the proportional odds logistic regression models the association between NT-proBNP and coronary calcification in patients with RA was no longer significant after adjusting for age and sex (OR=1.11, 95%CI=0.99–1.25, P value=0.08), and after further adjustment for race, homocysteine, rheumatoid factor, DAS28, MHAQ, IL-6 and TNF-α (P=0.84).
Discussion
The major findings of this study are that serum concentrations of NT-proBNP, a biomarker of ventricular dysfunction, myocardial ischemia and atherosclerosis, are increased in patients with RA and associated with inflammation and disease activity.
Marked elevation of B-type natriuretic peptide concentrations, measured either as BNP or the NT-proBNP fragment, occurs in patients with heart failure or acute coronary syndrome.13, 26, 27 BNP is produced primarily by ventricular myocytes in response to myocardial stretch and is released into the bloodstream to compensate for left ventricular dysfunction.28–32 Elevated concentrations in patients with acute ischemia may result from ventricular dysfuntion, but in patients with stable ischemic heart disease BNP was associated with inducible ischemia even after adjustment for ventricular and diastolic dysfunction.33 Thus, either ischemia or ventricular dysfunction could lead to an increase in BNP concentration.
Recently, concentrations of BNP originally considered to be within the normal range, and not associated with heart failure or acute myocardial ischemia, were found to be predictive of major cardiovascular events and mortality, both in patients with a high risk of ischemic heart disease13, 14 and in the general population.14, 15, 34 Ischemia may stimulate BNP release directly, and BNP is expressed in the intima of human coronary arteries, particularly of diseased vessels.31, 32 More recently, BNP immunoreactivity has been found in cardiomyocytes, endothelial cells, infiltrating T-cells and macrophages in cardiac biopsies from patients with severe heart failure supported by a left ventricular assist device.35 Also, inflammatory cytokines increase the production and secretion of BNP in cultured cardiac myocytes,17 and TNF-α is an inducer of BNP mRNA expression in human peripheral blood mononuclear cells.35 Thus, circulating concentrations of BNP may not only be integrally related to ventricular function and ischemia, but also to atherosclerosis and inflammation.
In the HOPE study of patients with previous coronary artery disease but no heart failure, NT-proBNP concentrations were related to cardiovascular risk and were correlated with soluble TNF-α receptors (r= 0.2 – 0.3) but not with CRP, IL6, and sICAM-1 (r all <0.2).18 There is little information about BNP concentrations in the setting of chronic inflammation, particularly in a population of patients with chronic inflammation and an increased risk of ischemic heart disease, such as occurs in RA.
In a letter to the editor, Harney reported that BNP concentrations were approximately four times higher in 26 patients with RA than in 32 controls who were on average 13 years younger; also, BNP concentrations appeared related to a high proportion of patients having occult cardiac dysfunction.36 In a study of 226 patients with RA, definite left ventricular systolic dysfunction (ejection fraction <40%) and any systolic dysfunction (ejection fraction <50%) were present in 5.3% and 10.2% of patients, respectively.37 Although BNP concentrations were higher in patients with ventricular dysfunction, the authors noted substantial overlap among those with and without ventricular dysfunction, and that the marker performed poorly in identifying ventricular dysfunction in RA.37 Our findings, showing a relationship between NT-proBNP concentrations and inflammatory markers, provide a potential explanation for their observation.
We found that patients with RA had higher serum NT-proBNP concentrations than control subjects, and this association was independent of age, race and sex. Concentrations of BNP were increased both in patients with long-standing RA and those with relatively early disease, and furthermore, among the patients with RA, were associated with coronary calcium score. However, this relationship was attenuated after adjustment for age, race and sex. NT-proBNP concentrations were significantly associated with markers of inflammation and disease activity such as DAS28, mHAQ,IL-6, CRP and TNF-α after adjustment for age and sex in patients with RA. When we additionally included cardiovascular risk factors and previous coronary artery disease in the model, the relationship between NT-proBNP and DAS28 and mHAQ remained significant. This suggests that some of the relationships between NT-proBNP and IL-6, TNF-α and CRP in patients with RA are explained by coronary risk factors and coronary disease, but significant associations between RA disease activity and disability and NT-proBNP remain.
The biological mechanisms by which NT-proBNP predicts cardiovascular risk are unclear. Interestingly, in the prospective Heart and Soul study concentrations of NT-proBNP predict cardiovascular outcomes after adjustment for systolic and diastolic function, left ventricular mass, exercise capacity, inducible ischemia and CRP concentrations. 38 This suggests that NT-proBNP may capture additional components and mechanisms of cardiovascular risk.
In the general population the sensitivity/specificity of NT-proBNP concentrations of 300 pg/ml and 1000 pg/ml and above for the diagnosis of heart failure are 99%/68% and 87%/86%, respectively. 39 Relatively few patients with RA had concentrations in this range - nevertheless, it is likely that the specificity of elevated NT-proBNP concentrations in RA will be lower. Thus, an elevated NT-proBNP concentration in a patient with RA may be less likely to equate with a diagnosis of heart failure. In addition, increasing evidence indicates that NTproBNP is also a marker of atherosclerosis; indeed NT-proBNP, inflammation and atherosclerosis are related in patients with RA. However, the relationship between NT-proBNP and coronary calcium was not independent and it is therefore unlikely to be a useful biomarker for the early detection of atherosclerosis in RA.
The findings of increased concentrations of NT-proBNP in patients with RA, and their association with disease activity and inflammatory markers, raise several possibilities that may not be mutually exclusive. Inflammation in patients with RA may result in subclinical ventricular dysfunction, and this manifests as an increase in BNP concentration. This cannot be excluded since, although patients did not have clinical heart failure, we did not perform specific test of cardiac function. Myocardial dysfunction is well-recognized in the setting of severe sepsis, but milder, chronic inflammation is not generally believed to cause heart failure. However, the prevalence of heart failure is increased almost two-fold in patients with RA,40 and in echocardiographic studies, diastolic dysfunction has been a relatively common finding in unselected populations of patients with RA.41
Another possibility is that RA is associated with structural heart disease such as ischemic heart disease, as reflected by the higher coronary calcium scores in patients with RA3 and their association with NT-proBNP concentrations. If true, then the association between NT-proBNP and inflammatory markers could be indirect, through an association between inflammation and atherosclerosis. However, this seems unlikely since the relationship between NT-proBNP concentrations and coronary calcium was weak, particlularly after statistical adjustment for age and sex. A third possibility is that NT-proBNP is increased directly by inflammation, as suggested by its independent association with RA disease activity. Additional longitudinal studies with measures of cardiac function will be helpful to define the relationship between inflammation and increased BNP concentrations in patients with RA.
These results need to be interpreted in the light of the limitations of the study. Since echocardiographic studies were not performed, we cannot exclude the possibility that subclinical myocardial dysfunction (either systolic or diastolic) was related to the increased concentrations of NTproBNP in patients with RA. Current guidelines support the use of NTproBNP as a marker of heart failure in the general population but additional studies will be required to define its relationship to cardiac function in patients with RA.
In conclusion, NT-proBNP concentrations are increased in patients with RA without clinical heart failure and may reflect subclinical cardiovascular risk and inflammation.
Acknowledgments
This study was supported by grants (HL04012, HL67964 and GM5M01-RR00095) from the National Institutes of Health and by the Arthritis Foundation. Dr. Avalos is partially supported by a grant from the American College of Rheumatology.
We thank Ms. Carol Brannon and Ms. Elizabeth Simpson who assisted with subject recruitment.
Reference List
- 1.Wolfe F, Mitchell DM, Sibley JT, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37(4):481–494. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 2.Pincus T, Callahan LF, Sale WG, Brooks AL, Payne LE, Vaughn WK. Severe functional decline, work disability and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum. 1984;27(8):864–872. doi: 10.1002/art.1780270805. [DOI] [PubMed] [Google Scholar]
- 3.Chung CP, Oeser A, Raggi P, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: Relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52(10):3045–3053. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 4.Park YB, Choi HK, Lee SH, et al. Atherosclerosis in rheumatoid arthritis: morphologic evidence obtained by carotid ultrasound. Arthritis Rheum. 2003;46(7):1714–1719. doi: 10.1002/art.10359. [DOI] [PubMed] [Google Scholar]
- 5.Roman MJ, Moeller E, Davis A, et al. Preclinical Carotid Atherosclerosis in Patients with Rheumatoid Arthritis. Ann Intern Med. 2006;144(4):249–256. doi: 10.7326/0003-4819-144-4-200602210-00006. [DOI] [PubMed] [Google Scholar]
- 6.Chung CP, Oeser A, Avalos I, et al. Utility of the Framingham risk score to predict the presence of coronary atherosclerosis in patients with rheumatoid arthritis. Arthritis Res Ther. 2006 doi: 10.1186/ar2098. Ref Type: Electronic Citation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dessein PH, Joffe BI, Veller MG, et al. Traditional and nontraditional cardiovascular risk factors are associated with atherosclerosis in rheumatoid arthritis. J Rheumatol. 2005;32(3):435–442. [PubMed] [Google Scholar]
- 8.Kogler H, Schott P, Toischer K, et al. Relevance of brain natriuretic peptide in preload-dependent regulation of cardiac sarcoplasmic reticulum Ca2+ ATPase expression. Circulation. 2006;113(23):2724–2732. doi: 10.1161/CIRCULATIONAHA.105.608828. [DOI] [PubMed] [Google Scholar]
- 9.Clerico A, Recchia FA, Passino C, Emdin M. Cardiac endocrine function is an essential component of the homeostatic regulation network: physiological and clinical implications. Am J Physiol Heart Circ Physiol. 2006;290(1):H17–H29. doi: 10.1152/ajpheart.00684.2005. [DOI] [PubMed] [Google Scholar]
- 10.Levin ER, Gardner DG, Samson WK. Natriuretic Peptides. N Engl J Med. 1998;339(5):321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 11.Pandey KN. Biology of natriuretic peptides and their receptors. Peptides. 2005;26(6):901–932. doi: 10.1016/j.peptides.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Costello-Boerrigter LC, Boerrigter G, Redfield MM, et al. Amino-Terminal Pro-B-Type Natriuretic Peptide and B-Type Natriuretic Peptide in the General Community: Determinants and Detection of Left Ventricular Dysfunction. Journal of the American College of Cardiology. 2006;47(2):345–353. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omland T, Persson A, Ng L, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in acute coronary syndromes. Circulation. 2002;106(23):2913–2918. doi: 10.1161/01.cir.0000041661.63285.ae. [DOI] [PubMed] [Google Scholar]
- 14.Kragelund C, Gronning B, Kober L, Hildebrandt P, Steffensen R. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352(7):666–675. doi: 10.1056/NEJMoa042330. [DOI] [PubMed] [Google Scholar]
- 15.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350(7):655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 16.Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355(25):2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 17.Ma KK, Ogawa T, de Bold AJ. Selective upregulation of cardiac brain natriuretic peptide at the transcriptional and translational levels by pro-inflammatory cytokines and by conditioned medium derived from mixed lymphocyte reactions via p38 MAP kinase. J Mol Cell Cardiol. 2004;36(4):505–513. doi: 10.1016/j.yjmcc.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Blankenberg S, McQueen MJ, Smieja M, et al. Comparative impact of multiple biomarkers and N-Terminal pro-brain natriuretic peptide in the context of conventional risk factors for the prediction of recurrent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation. 2006;114(3):201–208. doi: 10.1161/CIRCULATIONAHA.105.590927. [DOI] [PubMed] [Google Scholar]
- 19.Chung CP, Oeser A, Solus JF, et al. Prevalence of the Metabolic Syndrome is Increased in Rheumatoid Arthritis and is Associated with Coronary Atherosclerosis. Atherosclerosis. 2007 Jan 29; doi: 10.1016/j.atherosclerosis.2007.01.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 21.Sokka T, Pincus T. Contemporary disease modifying antirheumatic drugs (DMARD) in patients with recent onset rheumatoid arthritis in a US private practice: methotrexate as the anchor drug in 90% and new DMARD in 30% of patients. J Rheumatol. 2002;29(12):2521–2524. [PubMed] [Google Scholar]
- 22.Prevoo ML, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 23.Pincus T, Sokka T, Kautiainen H. Further development of a physical function scale on a MDHAQ [corrected] for standard care of patients with rheumatic diseases. J Rheumatol. 2005;32(8):1432–1439. [PubMed] [Google Scholar]
- 24.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Vaimonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 25.Hoteling H. Analysis of a Complex of Statistical Variables into Principal Components. Journal of Educational Psychology. 1953;(24):417–441. [Google Scholar]
- 26.de Lemos JA, Morrow DA, Bentley JH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345(14):1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 27.Magga J, Puhakka M, Hietakorpi S, et al. Atrial natriuretic peptide, B-type natriuretic peptide, and serum collagen markers after acute myocardial infarction. J Appl Physiol. 2004;96(4):1306–1311. doi: 10.1152/japplphysiol.00557.2003. [DOI] [PubMed] [Google Scholar]
- 28.Magga J, Marttila M, Mantymaa P, Vuolteenaho O, Ruskoaho H. Brain natriuretic peptide in plasma, atria, and ventricles of vasopressin- and phenylephrine-infused conscious rats. Endocrinology. 1994;134(6):2505–2515. doi: 10.1210/endo.134.6.8194476. [DOI] [PubMed] [Google Scholar]
- 29.Hall C. NT-ProBNP: the mechanism behind the marker. J Card Fail. 2005;11(5 Suppl):S81–S83. doi: 10.1016/j.cardfail.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 30.Bruneau BG, Piazza LA, de Bold AJ. BNP gene expression is specifically modulated by stretch and ET-1 in a new model of isolated rat atria. Am J Physiol. 1997;273(6 Pt 2):H2678–H2686. doi: 10.1152/ajpheart.1997.273.6.H2678. [DOI] [PubMed] [Google Scholar]
- 31.Goetze JP, Christoffersen C, Perko M, et al. Increased cardiac BNP expression associated with myocardial ischemia. FASEB J. 2003;17(9):1105–1107. doi: 10.1096/fj.02-0796fje. [DOI] [PubMed] [Google Scholar]
- 32.Casco VH, Veinot JP, Kuroski de Bold ML, Masters RG, Stevenson MM, de Bold AJ. Natriuretic peptide system gene expression in human coronary arteries. J Histochem Cytochem. 2002;50(6):799–809. doi: 10.1177/002215540205000606. [DOI] [PubMed] [Google Scholar]
- 33.Bibbins-Domingo K, Ansari M, Schiller NB, Massie B, Whooley MA. B-type natriuretic peptide and ischemia in patients with stable coronary disease: data from the Heart and Soul study. Circulation. 2003;108(24):2987–2992. doi: 10.1161/01.CIR.0000103681.04726.9C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKie PM, Rodeheffer RJ, Cataliotti A, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide: biomarkers for mortality in a large community-based cohort free of heart failure. Hypertension. 2006;47(5):874–880. doi: 10.1161/01.HYP.0000216794.24161.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruggink AH, de JN, van Oosterhout MF, et al. Brain natriuretic peptide is produced both by cardiomyocytes and cells infiltrating the heart in patients with severe heart failure supported by a left ventricular assist device. J Heart Lung Transplant. 2006;25(2):174–180. doi: 10.1016/j.healun.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Harney SM, Timperley J, Daly C, et al. Brain natriuretic peptide is a potentially useful screening tool for the detection of cardiovascular disease in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65(1):136. doi: 10.1136/ard.2005.040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatia GS, Sosin MD, Patel JV, et al. Left ventricular systolic dysfunction in rheumatoid disease: an unrecognized burden? J Am Coll Cardiol. 2006;47(6):1169–1174. doi: 10.1016/j.jacc.2005.10.059. [DOI] [PubMed] [Google Scholar]
- 38.Bibbins-Domingo K, Gupta R, Na B, Wu AHB, Schiller NB, Whooley MA. N-Terminal Fragment of the Prohormone Brain-Type Natriuretic Peptide (NT-proBNP), Cardiovascular Events, and Mortality in Patients With Stable Coronary Heart Disease. JAMA. 2007;297(2):169–176. doi: 10.1001/jama.297.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felker GM, Petersen JW, Mark DB. Natriuretic peptides in the diagnosis and management of heart failure. CMAJ. 2006;175(6):611–617. doi: 10.1503/cmaj.060236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicola PJ, Maradit-Kremers H, Roger VL, et al. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum. 2005;52(2):412–420. doi: 10.1002/art.20855. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez-Juanatey C, Testa A, Garcia-Castelo A, et al. Echocardiographic and Doppler findings in long-term treated rheumatoid arthritis patients without clinically evident cardiovascular disease. Semin Arthritis Rheum. 2004;33(4):231–238. doi: 10.1053/j.semarthrit.2003.09.011. [DOI] [PubMed] [Google Scholar]


