Abstract
The [Fe]-hydrogenase enzymes are highly efficient H2 catalysts found in ecologically, and phylogenetically diverse microorganisms, including the photosynthetic green alga, Chlamydomonas reinhardtii. Although these enzymes can occur in several forms, H2 catalysis takes place at a unique [FeS] prosthetic group, or H-cluster, located at the active site. Significant to the function of hydrogenases is how the surrounding protein structure facilitates substrate-product transfer, and protects the active site H-cluster from inactivation. To elucidate the role of protein structure in O2 inactivation of [Fe]-hydrogenases, experimental and theoretical investigations have been performed. Molecular dynamics was used to comparatively investigate O2 and H2 diffusion in [Fe]-hydrogenase CpI. The results are compared to initial investigations of H2 diffusion in [NiFe]-hydrogenase [1]. Our preliminary results suggest that H2 diffuses more easily and freely than O2, which is restricted to a small number of allowed pathways to and from the active site. These O2 pathways are located in the conserved active site domain, shown experimentally to have an essential role in active site protection.
Keywords: hydrogenase, gas diffusion, oxygen sensitivity
Introduction
Hydrogen production in the green alga, C. reinhardtii, is a strictly anaerobic process. Coupling to photosynthesis can be achieved under conditions of sulfur deprivation that results in a series of physiological events leading to reduced PSII activity, the respiratory consumption of residual O2, and establishment of an anaerobic state [2,3]. Induction of hydrogenase gene expression and enzyme synthesis ultimately results in H2-production. Although metabolic sequestration of O2 overcomes the severe sensitivity of algal [Fe]-hydrogenase to O2, the H2-production efficiency is lower than theoretical maximum [2,3]. In order to develop an efficient, large-scale, photobiological H2-production system, the O2 sensitivity of algal [Fe]-hydrogenases needs to be addressed [2].
Inactivation of [Fe]-hydrogenase by O2 involves diffusion of the diatomic gas from solvent to the active site, and subsequent chemical oxidation of the catalytic H- cluster [4]. The initial stages of diffusion involve molecular interactions between O2 and protein, suggesting that the protein structure influences how O2 approaches the active site. Previous studies of H2 diffusion in [NiFe]-hydrogenase suggest that H2 diffusion in the enzyme is not a random process, but rather tends to occur through a specific pathway, the H2-channel [1]. To further investigate gas diffusion in hydrogenase, and to elucidate the initial events in the O2-inactivation process, molecular mechanics methods were used to study O2 and H2 diffusion in [Fe]-hydrogenase CpI.
Methods and Results
Expression and O2-sensitivity determination of [Fe]-hydrogenase
The expression of active the [Fe]-hydrogenases HydA1, HydA and HydAΔN were carried out in E. coli as previously reported [5]. Whole cell samples were solubilized, exposed to air, and aliquots assayed for residual H2 production activity using the reduced methyl viologen assay [5].
Early biochemical studies of O2-inactivation of [Fe]-hydrogenase suggested that differences exist in the O2 sensitivities of enzymes isolated from different sources. The partially purified [Fe]-hydrogenase from C. reinhardtii exhibited an I50 value of ~1 second when exposed to atmospheric levels of O2 [6], whereas under similar conditions purified CpI exhibited an I50 of 120–300 seconds [7] (Table 1). Our recombinantly expressed C. reinhardtii HydA1 [Fe]-hydrogenase was ~415-fold more sensitive to atmospheric O2 (21%) than the C. acetobutylicum HydA (Table 1). To date the algal enzymes represent the simplest [Fe]-hydrogenases known, consisting solely of an active site domain. In contrast, the homologous bacterial enzymes, CpI and HydA, are more complex, possessing an additional electron-transfer domain [8]. This domain may also contribute to protection of the active site, and may explain the higher observed O2- tolerance levels of these enzymes. Removal of the electron-transfer domain from HydA (HydAΔN), however, resulted in only a 3-fold decrease in O2 tolerance (Table 1), and remained ~140-fold more tolerant to O2 than HydA1. The differences in enzyme sensitivities conferred by the conserved active site domain strongly suggests that the amino acid composition of this domain is critical to protection of the H-cluster from O2.
Table 1.
Comparison of algal and bacterial [Fe]-hydrogenase O2 sensitivities.
Simulation setup and methods
Our CpI model is based on the X-ray crystal structure of [Fe]-hydrogenase CpI from Clostridium pasteurianum [8]. Missing H-cluster atoms from the CpI structure are modeled as a di(thiomethyl)amine as in [9]. The partial charges for the rest of H-cluster atoms were based on Ref. [10], with modifications of up to ±0.02e to preserve charge neutrality. The model was then embedded in a water box, resulting in a 57,000-atom system consisting of 9,000 hydrogenase atoms, 16,000 water molecules and 15 sodium ions. The full system was then equilibrated at constant temperature (310K) and pressure (1 atm) for 1ns.
Oxygen and hydrogen gas diffusion in CpI were investigated by all-atom molecular dynamics simulations of the outward diffusion of either O2 or H2 from the active site, in a similar manner to previous studies of gas diffusion across proteins [1, 11]. A charge-free model for O2 and H2 is used. In addition, for reasons of numerical stability, and to facilitate comparison between the two gases, H2 with equivalent mass to O2 (hH2) was used in place of H2. This way, O2 and H2 differ solely by their van der Waals (Lennard-Jones) parameters, molecular bond lengths, and spring constants.
In order to increase the sampling, the method of locally enhanced sampling (LES), otherwise known as the time-dependent Hartree approximation [12], was used to simulate 1,000 mutually invisible, simultaneous copies of the gas molecules (hH2 or O2) within a single protein-water system. Gas temperatures were regulated independently at 310K. Each of the 1,000 copies of either O2 or hH2 was initially placed at the same location as the H-cluster bound CO molecule in the CpI structure [4]. For hH2, our simulations correspond to the realistic transport of H2 out of the protein, but for O2, we are observing a reverse of the normal O2 diffusion from the bulk solvent to the active site.
LES simulations were performed with NAMD2 [13] using the CHARMM22 force-field [14]. A constant volume and temperature were used. The system was simulated with periodic boundary conditions, and Particle-Mesh Ewald method.
MD simulation of H2 and O2 diffusion in CpI
During the 1 ns equilibration, we observed a permanent and almost continuous tunnel- shaped cavity (using a 1 Å-radius probe) connecting the active site binding location to the solvent outside the protein at the location of the “H2-channel” detailed for the Desulfovibrio desulfuricans [Fe]-hydrogenase reported by Nicolet et. al. [9,15].
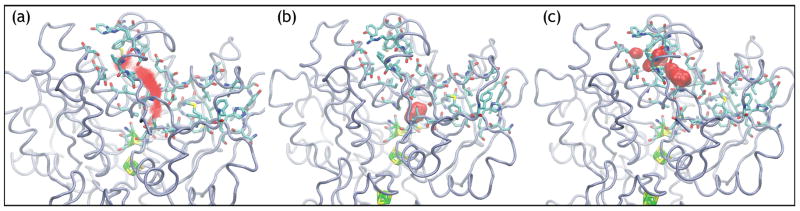
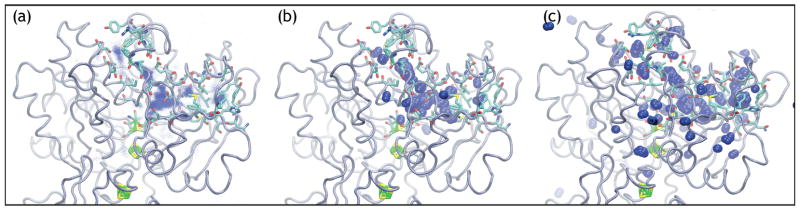
Our preliminary simulations (6 or 4 independent 2 ns simulations, for either O2 or H2 diffusion respectively) suggest that the diffusion pathways of O2 and hH2 between the active site of CpI and the protein-solvent interface are different. More specifically, on one hand, O2 is observed to transit across the protein barrier through a limited set of precisely defined channels, of which one is displayed in Fig 1a. We have observed O2 motion across only two such channels, and in only one simulation did O2 reach the solvent within the simulated time of 2 ns. On the other hand, in all four H2 simulations, H2 was observed exiting the protein. A sample of the allowed hH2 pathways is shown in Fig 2a. In addition, hH2 is occasionally observed (in 2–15% of all observed exits per simulation) to cut through the bulk protein by unique paths where no channel has been detected. A second important difference between H2 and O2 diffusion is that, during LES simulations with 1000 copies of the gas molecule sharing a unique protein trajectory, the different simultaneous hH2 molecules are observed to spread out in the protein and take a multitude of exits (see Fig 2b–c). Oxygen molecules, in stark contrast, stay clumped together for most or all of the duration of the trajectories (see Fig 1b–c).
Figure 1.
Caption Sample trajectory of molecular diffusion of 1000 simultaneous and mutually invisible copies of di-oxygen (O2) from the active site of CpI hydrogenase. Shown is (a) a superposition of all the O2 positions over the 2.3 ns trajectory and snapshots of the O2 trajectories after (b) 20 ps and (c) 2.3 ns. Figures were made using VMD [16].
Figure 2.
Caption Same as in Figure 1 for hH2. Shown is (a) a superposition of all the hH2 positions of the 2.3 ns trajectory and snapshots of the hH2 trajectories after (b) 300 ps and (c) 2.2 ns.
Conclusion
In summary we have shown that the diffusion of H2 and O2 gases inside of an [Fe]-hydrogenase is governed by the physical properties of both the gas itself and the protein structure. The dynamic process of gas migration dictates that static representations of structures can unsatisfactorily predict pathway selection. In this study, althoug a majority of H2 was found to migrate through a series of conserved hydrophobic cavities, or a “H2-channel” [15], H2 is clearly able to diffuse during the same time period through a number of alternative routes. This is somewhat inconsistent with previous results shown for H2 diffusion in [NiFe]-hydrogenase [1].
In contrast to H2 diffusion which displays a degree of randomized behavior, O2 diffusion is clearly limited to specific regions located within the conserved active site domain. The importance of this domain in O2 diffusion agrees with the experimental results show here. The range of sensitivity levels found for algal and bacterial enzymes are also characterized by diversity among the amino acids that comprise the diffusion pathways. From the standpoint of engineering O2 tolerance in [Fe]-hydrogenases, the sequences and structures of naturally occurring enzymes offer important clues on how to overcome the O2-sensitivity of algal [Fe]-hydrogenases.
Acknowledgments
Research at NREL (MLG, MS and PK), and the Colorado School of Mines (MP) is supported by the Division of Energy Biosciences, Office of Science, U.S. Department of Energy, and by the Hydrogen, Fuel Cells and Infrastructure Technologies Program, U.S. Department of Energy.
Research at UIUC (JC and KS) is supported by National Institute of Health grant P41-RR05969, with computational time support by National Resource Allocations Committee grant MCA93S028.
Abbreviations used
- CpI
[Fe]-hydrogenase I from Clostridium pasteurianum
- HydA
Clostridium acetobutylicum [Fe]-hydrogenase I
- hH2
heavy di-hydrogen
- LES
locally enhanced sampling
References
- 1.Montet Y, Amara P, Volbeda A, Vernede X, Hatchikian EC, Field MJ, Frey M, Fontecilla-Camps JC. Nat Struc Biol. 1997;4:523–526. doi: 10.1038/nsb0797-523. 1997. [DOI] [PubMed] [Google Scholar]
- 2.Ghirardi ML, Zhang L, Lee JW, Flynn T, Seibert M, Greenbaum E, Melis A. Trends Biotech. 2000;18:506–511. doi: 10.1016/s0167-7799(00)01511-0. [DOI] [PubMed] [Google Scholar]
- 3.Melis A, Zhang L, Forestier M, Ghirardi ML, Seibert M. Plant Physiol. 2000;122:127–136. doi: 10.1104/pp.122.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemon BJ, Peters JW. Biochem. 1999;38:12969–12973. doi: 10.1021/bi9913193. [DOI] [PubMed] [Google Scholar]
- 5.Posewitz MC, King PW, Smolinski SL, Zhang L, Seibert M, Ghirardi ML. J Biol Chem. 2004;279:25711–25720. doi: 10.1074/jbc.M403206200. [DOI] [PubMed] [Google Scholar]
- 6.Erbes DL, King D, Gibbs M. Plant Physiol. 1979;63:1138–1142. doi: 10.1104/pp.63.6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams MWW, Mortenson LE. J Biol Chem. 1984;259:7045–7055. [PubMed] [Google Scholar]
- 8.Peters JW, Lanzilotta WN, Lemon BJ, Seefeldt LC. Science. 1998;282:1853–1858. doi: 10.1126/science.282.5395.1853. [DOI] [PubMed] [Google Scholar]
- 9.Nicolet Y, Piras C, Legrand P, Hatchikian CE, Fontecilla-Camps JC. Struc. 1999;7:12–23. doi: 10.1016/s0969-2126(99)80005-7. [DOI] [PubMed] [Google Scholar]
- 10.Torres RA, Lovell T, Noodleman L, Case DA. J Am Chem Soc. 2003;125:1923–1936. doi: 10.1021/ja0211104. [DOI] [PubMed] [Google Scholar]
- 11.Hofacker I, Schulten K. Prot Struct Func Gen. 1997;30:100–107. [PubMed] [Google Scholar]
- 12.Elber R, Karplus M. J Am Chem Soc. 1990;112:9161–9175. [Google Scholar]
- 13.Kalé L, Skeel R, Bhandarkar M, Brunner R, Gursoy A, Krawetz N, Phillips J, Shinozaki A, Varadarajan K, Schulten K. J Comp Phys. 1999;151:283–312. [Google Scholar]
- 14.MacKerell AD, Jr, et al. FASEB J. 1992;6:A143–A143. [Google Scholar]; MacKerell AD, Jr, et al. J Phys Chem B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 15.Nicolet Y, Cavazza C, Fontecilla-Camps JC. J Inorg Biochem. 2002;91:1–8. doi: 10.1016/s0162-0134(02)00392-6. [DOI] [PubMed] [Google Scholar]
- 16.Humphrey W, Dalke A, Schulten K. J Mol Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]