Abstract
Arrays of electrospray ionization (ESI) emitters have been reported previously as a means of enhancing ionization efficiency or signal intensity. A key challenge when working with multiple, closely spaced ESI emitters is overcoming the deleterious effects caused by electrical interference among neighboring emitters. Individual emitters can experience different electric fields depending on their relative position in the array, such that it becomes difficult to operate all of the emitters optimally for a given applied potential. In this work, we have developed multi-nanoESI emitters arranged with a circular pattern, which enable the constituent emitters to experience a uniform electric field. The performance of the circular emitter array was compared to a single emitter and to a previously developed linear emitter array, which verified that improved electric field uniformity was achieved with the circular arrangement. The circular arrays were also interfaced with a mass spectrometer via a matching multi-capillary inlet, and the results were compared with those obtained using a single emitter. By minimizing inter-emitter electric field inhomogeneities, much larger arrays having closer emitter spacing should be feasible.
INTRODUCTION
Liquid chromatography (LC) coupled with electrospray ionization mass spectrometry (ESI-MS) is a powerful and widely used analytical platform for separating and identifying large numbers of species from complex mixtures. The operational flow rate for LC-ESI-MS is typically a compromise: while the ESI process becomes increasingly efficient as the flow rate is reduced, particularly at low nL/min flows,1 the LC sample loading capacity and the robustness of the system decrease as flow becomes smaller. We recently developed capillary-based multi nanoelectrospray emitters (multi- emitters),2,3 which allow the eluent from a capillary LC separation to be divided post-column among the emitters comprising the array, thus extending the benefits of nanoESI to higher flow rate LC separations. Combined with a heated multi-capillary inlet, the multi-emitters provided a >10-fold increase in sensitivity relative to a single emitter/single inlet configuration,3 and have been shown to reduce ion suppression effects, thus improving quantitation.2 Importantly, separation efficiency was preserved when the multi-emitters were used, and the chromatographic signal to noise ratio increased dramatically for trace peptide species.3
Besides ESI-MS related applications,2-5 multiplexed electrospray sources stand to benefit a number of other applications as well, including nanoparticle synthesis,6 thin film deposition,7 and the development of thrusters for microsatellite positioning.8-10 Regardless of the application, a key challenge impeding the practical implementation of multi-electrospray sources is inhomogeneous electrical shielding that arises due to interference from neighboring electrosprays. In a two dimensional or linear array, the outer emitters experience higher electric fields than the interior emitters for the same applied voltage, which can lead to emitters functioning in different regimes. A number of researchers have sought to better understand and overcome such shielding.9-16 The most common solution is to use an extractor electrode,9,10,13,14,16 in which a modified ring counter electrode forms an integral part of the emitter itself, taking advantage of the fact that shielding effects are minimized at small emitter–counter electrode spacings.14 While effective, extractor electrodes have not been demonstrated for arrays in which individual emitters operate in the nanoelectrospray regime. Alternatively, it should be possible to contour the counterelectrode or the emitters in the array such that the interior emitters are closer to the counterelectrode,14 thus increasing the electric field at the center of the array and compensating for shielding, but emitter fabrication constraints and the difficulty of accurately modifying the shape of the mass spectrometer inlet make this approach problematic. In our previous work with linear emitter arrays,2,3 we mitigated the effects of shielding by minimizing the emitter–counter electrode (−MS inlet) spacing, with the disadvantage that the narrow spacing may be insufficient for efficient droplet desolvation at higher flow rates.17 Also, further scale-up of the multi-emitters using greater emitter densities would be challenging, as the electric field inhomogeneities caused by shielding become more pronounced as the emitter density increases.
Here, we report the development of capillary-based nanoelectrospray emitter arrays having a circular arrangement, enabling all of the emitters to experience the same electric field and operate optimally with a given applied potential. The new geometry affords greater flexibility in terms of emitter–counter electrode spacing and should enable the fabrication of denser arrays. The circular layout also facilitates comparison of the multi-emitters with individual emitters using a single instrument configuration, which provides additional insight into the benefits of the multiplexed ESI sources for ionization and challenges associated with ion transmission.
EXPERIMENTAL SECTION
Sample Preparation
Acetic acid (HOAc), syntide 2, kemptide acetate salt, dynorphin A fragment 1–13, and fibrinopeptide A were purchased from Sigma-Aldrich (St. Louis, MO). Methanol (MeOH; HPLC grade) and 49% hydrofluoric acid were from Fisher Scientific (Fair Lawn, NJ), and water was purified in-house using a Barnstead Nanopure Infinity system (Dubuque, IA).
Emitter Fabrication
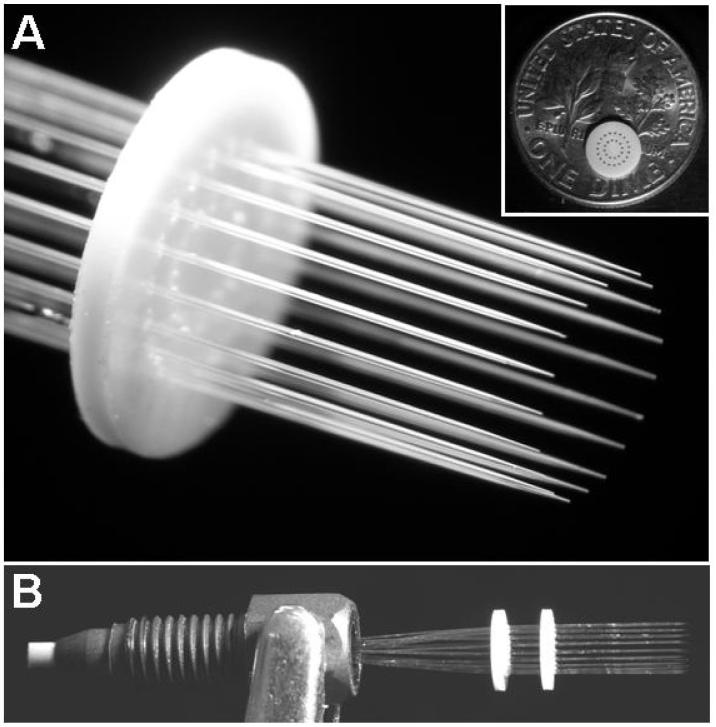
Individual electrospray emitters were chemically etched from fused silica capillaries (Polymicro Technologies, Phoenix, AZ) as described previously.18 Linear emitter arrays, which were made from 19 fused silica capillaries, have also been described elsewhere.2,3 To create the circular multi-emitters (Figure 1), two identical 0.5-mm-thick, 5-mm-diameter PEEK disks were machined with 200-μm-diameter drilled holes arranged in two concentric circles, as shown in Figure 1A (inset). The outer ring had 19 holes spaced 500 μm apart (center-to-center) to create circular multi-emitters with the same number and spacing of emitters as the previously characterized linear arrays. The inner ring had 12 drilled holes (410 μm center-to-center spacing) and was ∼1.6 mm in diameter, suitable for interfacing with a mass spectrometer via the custom-built multi-capillary heated inlet described in previous work.19 The holes in the disks were aligned and six-cm-long fused silica capillaries (20 μm i.d./150 μm o.d.) were threaded through the holes in either the inner or outer circle of the disks. The distal ends of the capillaries were inserted in a 750-μm-i.d., 1/16-in.-o.d. tubing sleeve (Upchurch Scientific, Oak Harbor, WA), sealed with epoxy and cut as described for the linear emitter arrays.3 A PEEK nut and ferrule (F-195, Upchurch Scientific) were fastened on the tubing sleeve to enable fluidic connection (Figure 1B). The two disks were separated from one another by 3–4 mm as shown in Figure 1B, which ensured that the capillaries comprising the array ran parallel to one another. Water was pumped through the capillaries at 100 nL/min/capillary, and the ends were inserted in a bath of Nanostrip 2X (Cyantek, Fremont, CA) at 90 °C for ∼20 min to remove the polyimide coating. The capillary ends were then etched in 49% HF2,3 to form externally tapered emitters of uniform length.
Figure 1.
Photographs of a circular array of nanoESI emitters. (A) Magnified view showing the uniform, externally tapered fused silica emitters. A PEEK disk used to arrange the emitters is shown in the inset with a dime for size comparison. (B) Side view of the multi-emitter showing the two PEEK disks separated by 3–4 mm to hold the emitters parallel to one another.
Electrospray Characterization and Mass Spectrometry
MS measurements were performed using a Finnigan TSQ Quantum Ultra triple quadrupole MS (ThermoFisher Scientific, Waltham, MA) that was modified to accept the greater ion currents from the multi-emitters. The standard skimmer interface was replaced with a tandem ion funnel interface consisting of a “high pressure” ion funnel19 that operated at 16.5 Torr, followed by a second ion funnel that operated at 1.0 Torr. The additional pumping stage afforded by the tandem ion funnel enabled a greater conductance, heated multi-capillary inlet to be used without requiring high speed pumps. The inlet employed was described previously19 and consisted of 18 capillaries, each 430 μm i.d., that were arranged in two concentric rings. The inner ring consisted of 6 capillaries and had a diameter of ∼1.5 mm on center, and the outer ring, having 12 inlets, was ∼2.5 mm in diameter. The inlet temperature was set at 120 °C.
Electrospray current measurements were made by installing the multi-capillary inlet used for the MS experiments on a benchtop ESI interface that simulated the first vacuum stage of the mass spectrometer.2,17 The multi-capillary inlet was heated to 120 °C, the vacuum chamber was pumped using an E1M18 rough pump (BOC Edwards, Wilmington, MA), and electrospray currents were measured using a picoammeter (Keithley, Cleveland, OH). Transmitted electrospray currents were detected using a 4-cm-diameter charge collector inside the vacuum chamber that was placed 1 cm from the multi-capillary inlet. Another electrode was connected to the inlet itself to measure the current that was lost to the front of the interface or the inner walls of the heated capillaries. A separate charge collector was used to measure the total electrospray currents shown in Figure 2. A stereomicroscope (SMZ1500; Nikon, Tokyo, Japan) was used to view the electrosprays that formed at each emitter during operation.
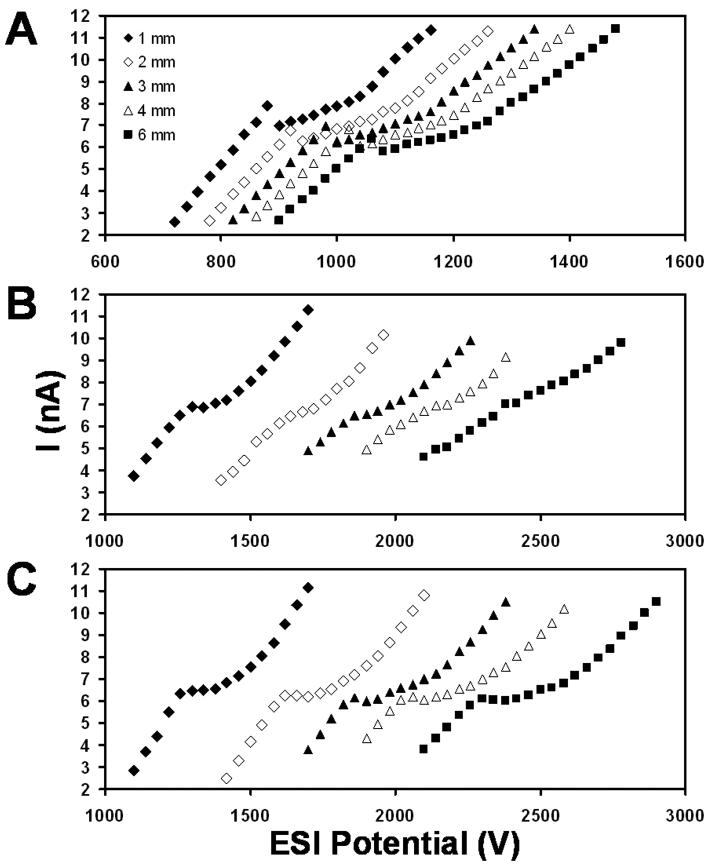
Figure 2.
Current vs. voltage curves at various emitter–counterelectrode distances for (A) a single emitter, (B) a linear array of 19 emitters, and (C) a circular array of 19 emitters. The flow rate was 50 nL/min in (A) and 950 nL/min in (B) and (C), and the plotted currents are the average current per emitter for (B) and (C). The solution was 1:1 H2O:MeOH + 0.1% HOAc.
Safety Information
Hydrofluoric acid is extremely hazardous and corrosive. Care must be taken to avoid exposure to HF liquid or vapor. HF solutions should be used in a ventilated hood and appropriate protective equipment should be worn. Nanostrip 2X is also corrosive and should be handled with similar care.
RESULTS AND DISCUSSION
The circular multi-emitters share many of the same characteristics as the previously reported linear arrays, including low dead volumes, straightforward coupling with capillary LC columns via standard fittings, and highly uniform emitter geometries afforded by the chemical etching procedure.18 In addition, the circular layout was implemented to overcome a major limitation of the linear nanoESI arrays: electric field inhomogeneities due to shielding. The shielding effects for both linear and two-dimensional arrays have been modeled and experimentally explored by other researchers for higher flow rate systems.11-16 The practical effect is that the outer emitters experience a greater electric field than those in the interior of the array, making it difficult or impossible for all of the emitters to operate optimally for a given applied ESI potential. It has also been shown that the effects of shielding are effectively minimized at closer distances to the counter electrode.14 In our previous work, the linear emitter arrays were typically positioned ∼1 mm from the inlet, effectively minimizing shielding effects, but likely also limiting droplet desolvation, which can be enhanced at greater distances. Also, when the density of emitters in the linear arrays was doubled, with inter-emitter spacing reduced to 250 μm (approaching the minimum spacing possible with this technology), shielding effects became far more pronounced (data not shown).
The reduction in inter-emitter electric field inhomogeneities afforded by the circular arrangement is shown in Figure 2. Characteristic electrospray current vs. voltage (I-V) curves3,20 were obtained at different emitter–counter electrode spacings for an individual emitter operated at 50 nL/min (Figure 2A), as well as for linear and circular emitter arrays (Figures 2B and 2C, respectively). The emitter arrays used a total flow of 950 nL/min, such that the average flow rate per emitter was the same in all cases. Spacing between emitters was 500 μm for both the linear and circular arrays. The individual and multi-emitters were all chemically etched from 20-μm-i.d., 150-μm-o.d. fused silica tubing. The shape of the I-V curve at 1 mm spacing with the linear array (Figure 2B) is similar to the curves for the single emitter (Figure 2A), indicating that shielding effects are minor at this close distance. However, the onset of the plateau region is still ∼300 V greater than for a single emitter. As the spacing is increased, the characteristic shape of the I-V curve is lost because each emitter in the array experiences a different electric field, such that the electrosprays change operating regimes at different potentials. In contrast, when the circular array is used (Figure 2C), the plateau region of the I-V curves is clearly visible and does not change substantially at greater emitter–counter electrode distances, indicating that this geometry successfully minimizes electric field inhomogeneities among the individual emitters. It should be noted that the circular arrays do not minimize shielding itself: larger potentials are required to achieve the same current/emitter relative to a single emitter. Rather, the emitters in the array are shielded uniformly, enabling all of the electrosprays to operate in the same regime at a given potential.
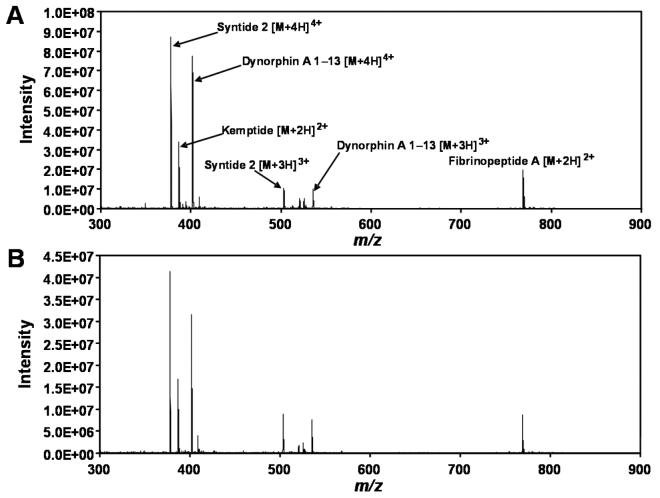
Emitter arrays require modification of the mass spectrometer inlet to effectively sample the greater ion currents. The linear arrangements of heated capillary inlets described previously2,3 were well suited for capturing ions from linear emitter arrays, but were clearly not ideal for use with single emitters. As a result, our previous work measured gains in ion signal relative to a single emitter/single inlet configuration. This made it difficult to determine what portion of the >10-fold signal enhancement was provided by the increased ionization efficiency afforded by the multi-emitters, and how much was simply due to the increased conductance of the inlet. Here, the circular multi-capillary inlet arrangement is well suited to accept the ion/droplet plume from either individual emitters or the circular emitter arrays, allowing the MS sensitivity to be directly compared under practical conditions using a single instrument configuration. A mixture of four peptides was thus introduced into an ion funnel-modified ESI interface using a single chemically etched emitter (50 μm i.d./360 μm o.d) and an array of 12 emitters made using the inner ring of the PEEK disks in Figure 1B (inset). The diameter of the 12-emitter array (1.6 mm) better matched the dimensions of the multi-capillary inlet, and was thus selected in favor of the circular array of 19 emitters. The peptide mixture (1 μM each in 1:1 H2O:MeOH + 1% HOAc) was infused at a total flow rate of 2 μL/min in both cases and optimized for maximum signal with respect to position and ESI potential. The optimum spacing between the emitter and the inlet was 5 mm for the single emitter and 3.5 mm for the array of 12 emitters. Mass spectra of the peptide mixture under optimized conditions for both the single emitter and the multi-emitter are shown in Figure 3 and indicate that, on average, the use of the multi-emitter provides a signal enhancement of approximately 2.2-fold. Similar sensitivity gains were obtained for other peptide mixtures and at different flow rates. For example, when the total flow rate was decreased from 2 μL/min to 0.5 μL/min, the average signal gain for the peptide mixture described above decreased only slightly, from 2.2× to 1.9×. These somewhat modest improvements in sensitivity are not completely unexpected, as studies using a single emitter have shown that multi-capillary inlets alone can provide up to a ∼5-fold improvement in ion transmission relative to a single inlet.19 However, considering the proportionately greater ion currents produced by the multi-emitters,2 it was desirable to determine whether additional ion losses were occurring, and how such losses could be minimized.
Figure 3.
Mass spectra of a peptide mixture obtained using (A) a circular array of 12 emitters and (B) a single emitter under optimized conditions. Additional description is in the text.
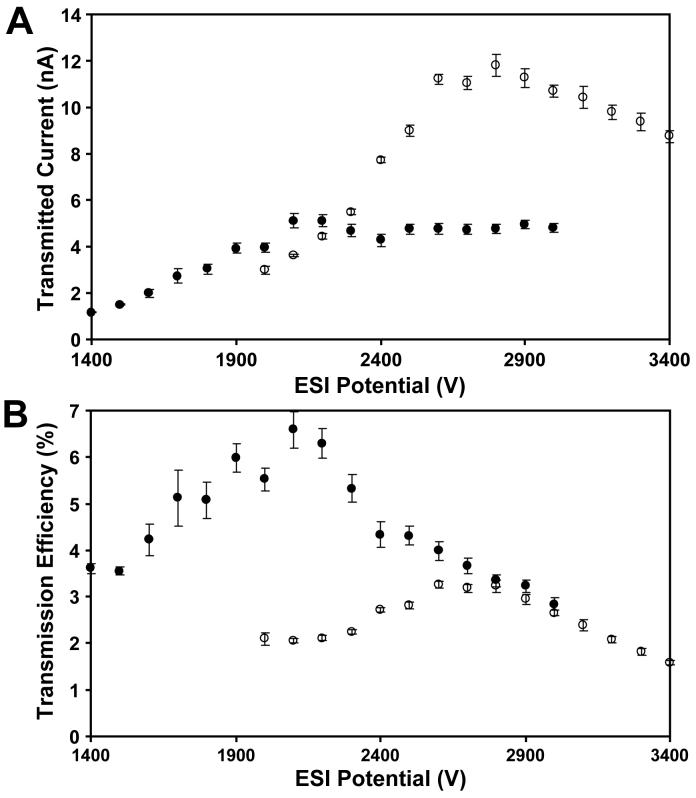
A benchtop ESI current monitoring system was employed that enabled transmitted ion currents to be differentiated from ions and charged droplets lost to the surface of the interface under the same conditions as those used for the MS-based sensitivity experiment. Using the same multicapillary inlet and the emitter–inlet spacing that provided maximum MS sensitivity, transmitted currents were measured for both single and multi-emitters over a range of ESI potentials, as shown in Figure 4A. The maximum transmitted current from the multi-emitter was ∼2.3 times greater than that of the individual emitter. This was very close to the observed ∼2.2-fold average sensitivity increase observed with the mass spectrometer, indicating that under these optimized conditions, MS sensitivity scaled with transmitted ion current. Transmission efficiency, the fraction of the total current that traverses the inlet and is available for analysis, is plotted in Figure 4B, and is shown to decrease ∼twofold when the multi-emitter is used. The decrease in ion transmission efficiency for the multi-emitter is due to the greater ESI current, which increases space charge-driven expansion, and the smaller droplets formed, which suffer larger diffusional losses while being transported through the heated capillary inlet.17 In other words, the very characteristics of the multi-emitters that lead to enhanced ionization efficiencies pose greater challenges for ion transmission. The combined results of Figure 4A and 4B indicate that if ion transmission efficiency had been preserved for the multi-emitter, the MS sensitivity would have increased by a factor of ∼4 simply by dividing the flow among 12 emitters, in addition to the sensitivity enhancement already provided by the multi-capillary inlet and tandem ion funnel. It may be possible to develop a more favorable multi-emitter/inlet from atmospheric pressure combination that reduces transmission losses, but as long as a conductance limiting inlet is employed, it is likely that the multi-emitters will prove difficult for ion transmission, particularly as the number of emitters in an array is expanded.
Figure 4.
Ion transmission evaluation. (A) Currents transmitted through the multi-capillary inlet for a single emitter (●) and an array of 12 emitters (○) using the same solvent and emitter position used for Figure 3. (B) Corresponding ion transmission efficiencies. Error bars for each data point represent standard deviations based on 100 consecutive measurements.
We have recently developed a subambient pressure ionization with nanoelectrospray (SPIN) source,21 in which electrospray takes place inside a ∼30 Torr MS inlet chamber that contains an electrodynamic ion funnel. By electrospraying directly into the ion funnel, which captures and refocuses the entire ion cloud, transmission losses through the capillary interface are essentially eliminated. Combining circular multi-emitters with the SPIN source is projected to allow ionization and transmission efficiency to be simultaneously maximized.
CONCLUSIONS
We have developed circular nanoESI emitter arrays for reducing inter-emitter electric field inhomogeneities. The new design enables greater flexibility for emitter positioning and ensures that all of the emitters can operate optimally at a given electric potential. Using a triple quadrupole mass spectrometer modified with a multi-capillary inlet, the sensitivity for a mixture of peptides achieved with a circular array of 12 emitters produced a 2.2-fold gain compared to a single emitter, and greater gains are anticipated from further refinement of the inlet design and from the use of increased numbers of emitters. In this regard, a benchtop ESI current profiler was used to determine that the enhanced ionization efficiency afforded by the multi-emitters was partially offset by a decrease in transmission efficiency through the capillary interface. We also plan to combine the circular emitter arrays with the recently developed SPIN source,21 in which the electrospray array functions at a pressure where the ion funnel can effectively focus ions and mitigate the need for a conductance limiting aperture. We anticipate this approach will allow analyte ions to be efficiently ionized and transferred to the MS, even at the higher liquid flow rates encountered with robust LC systems.
ACKNOWLEDGEMENTS
Portions of this research were supported by the U.S. Department of Energy (DOE) Office of Biological and Environmental Research, the NIH National Center for Research Resources (RR018522), and the National Institute of Allergy and Infectious Diseases NIH/DHHS through interagency agreement Y1-AI-4894-01NIH. This research was performed in the Environmental Molecular Sciences Laboratory, a U.S. DOE national scientific user facility located at the Pacific Northwest National Laboratory (PNNL) in Richland, Washington. PNNL is a multiprogram national laboratory operated by Battelle for the DOE under Contract No. DE-AC05-76RLO 1830.
REFERENCES
- 1.Geromanos S, Freckleton G, Tempst P. Anal. Chem. 2000;72:777–790. doi: 10.1021/ac991071n. [DOI] [PubMed] [Google Scholar]
- 2.Kelly RT, Page JS, Tang K, Smith RD. Anal. Chem. 2007;79:4192–4198. doi: 10.1021/ac062417e. [DOI] [PubMed] [Google Scholar]
- 3.Kelly RT, Page JS, Zhao R, Qian W-J, Mottaz HM, Tang K, Smith RD. Anal. Chem. 2008;80:143–149. doi: 10.1021/ac701647s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang K, Lin Y, Matson DW, Kim T, Smith RD. Anal. Chem. 2001;73:1658–1663. doi: 10.1021/ac001191r. [DOI] [PubMed] [Google Scholar]
- 5.Kim W, Guo M, Yang P, Wang D. Anal. Chem. 2007;79:3703–3707. doi: 10.1021/ac070010j. [DOI] [PubMed] [Google Scholar]
- 6.Hogan CJ, Yun KM, Chen D-R, Lenggoro IW, Biswas P, Okuyama K. Colloids and Surfaces A-Physicochem. Eng. Aspects. 2007;311:67–76. [Google Scholar]
- 7.Jaworek A. J. Mater. Sci. 2007;42:266–297. [Google Scholar]
- 8.Gamero-Castaño M. J. Propul. Power. 2004;20:736–741. [Google Scholar]
- 9.Velásquez-García LF, Akinwande AI, Martínez-Sánchez M. Journal of Microelectromechanical Systems. 2006;15:1260–1271. [Google Scholar]
- 10.Velásquez-García LF, Akinwande AI, Martínez-Sánchez M. Journal of Microelectromechanical Systems. 2006;15:1272–1280. [Google Scholar]
- 11.Rulison AJ, Flagan RC. Rev. Sci. Instrum. 1993;64:683–686. [Google Scholar]
- 12.Regele JD, Papac MJ, Rickard MJA, Dunn-Rankin D. J. Aerosol Sci. 2002;33:1471–1479. [Google Scholar]
- 13.Bocanegra R, Galán D, Márquez M, Loscertales IG, Barrero A. J. Aerosol Sci. 2005;36:1387–1399. [Google Scholar]
- 14.Deng W, Klemic JF, Li X, Reed MA, Gomez A. J. Aerosol Sci. 2006;37:696–714. [Google Scholar]
- 15.Si BQT, Byun D, Lee S. J. Aerosol Sci. 2007;38:924–934. [Google Scholar]
- 16.Deng W, Gomez A. J. Aerosol Sci. 2007;38:1062–1078. [Google Scholar]
- 17.Page JS, Kelly RT, Tang K, Smith RD. J. Am. Soc. Mass Spectrom. 2007;18:1582–1590. doi: 10.1016/j.jasms.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Kelly RT, Page JS, Luo Q, Moore RJ, Orton DJ, Tang K, Smith RD. Anal. Chem. 2006;78:7796–7801. doi: 10.1021/ac061133r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibrahim Y, Tang K, Tolmachev AV, Shvartsburg AA, Smith RD. J. Am. Soc. Mass Spectr. 2006;17:1299–1305. doi: 10.1016/j.jasms.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marginean I, Kelly RT, Page JS, Tang K, Smith RD. Anal. Chem. 2007;79:8030–8036. doi: 10.1021/ac0707986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page JS, Tang K, Kelly RT, Smith RD. Anal. Chem. 2008;80:1800–1805. doi: 10.1021/ac702354b. [DOI] [PMC free article] [PubMed] [Google Scholar]