Abstract
Objective
S100B is produced by glia of the central and peripheral nervous systems and is considered a marker of neurologic injury in the perinatal period. Indeed, increased neonatal urine S100B concentration is associated with adverse neurological outcomes including intraventricular hemorrhage and hypoxic-ischemic encephalopathy, while elevated adult serum concentrations are associated with infectious diseases/sepsis. The objective of this study was to determine whether amniotic fluid (AF) S100B concentrations change with advancing gestational age and intra-amniotic infection (IAI).
Study Design
S100B concentration was measured in the AF of women in midtrimester, at term, and in pregnancies with preterm labor and intact membranes (PTL) or preterm premature rupture of membranes (PPROM), with and without IAI. Placental pathology was performed and neonatal outcomes were analyzed.
Results
(1) AF S100B concentration did not change during gestation; (2) patients with IAI had significantly higher AF S100B concentration than those without IAI following an episode of PTL or PPROM and; (3) neonates who had morbidity/mortality had had an elevated AF S100B concentration; however, this could be explained by the association with intra-amniotic infection/inflammation. Thus, AF S100B concentration was not an independent predictor of neonatal morbidity or fetal/neonatal death.
Conclusions
An elevated concentration of AF S100B may reflect intra-amniotic infection/inflammation and not necessarily fetal neurologic damage.
Keywords: Chorioamnionitis, fetal inflammation, funisitis, interleukin-6, intra-amniotic infection, neonatal morbidity, parturition, preterm premature rupture of membranes
Introduction
S100 proteins are dimeric, acidic proteins that constitute the largest subfamily of EF-hand proteins [29]. S100B has a very short half-life (25 min) and is eliminated in the urine [20]. Due to its high concentration in astrocytes and oligodendrocytes of the nervous system [31, 40], S100B has been proposed as a marker of cerebral damage in the perinatal period. Evidence in support of this view includes: (1) elevated concentrations of urinary, cord blood, and serum S100B in neonates are associated with neonatal hypoxic-ischemic encephalopathy (HIE) and have been proposed to be an early biochemical indicator of HIE [13, 24, 32]. Indeed, high urine S100B concentrations within three days of birth [12] were found to be predictive of adverse neurologic outcome in asphyxiated full-term infants at 12 months of age; (2) elevated urine and blood S100B concentrations were also demonstrated in term or preterm neonates who subsequently developed intra-ventricular hemorrhage (IVH) [7, 12, 14]. Moreover, urine S100B concentration at birth significantly correlated with the degree of IVH (r2=0.76, P<0.001) ; and (3) an elevated urinary concentration of S100B in neonates with intrauterine growth restriction was associated with adverse short-term neurologic outcome (7 day) [4].
Neonatal death has also been associated with high urine and AF concentrations of S100B. Indeed, the S100B first urine concentration of preterm newborns had a sensitivity of 100% and a specificity of 97.8% for predicting neonatal death, using a cut-off of 12.93 MOM [9]. Furthermore, amniotic fluid S100B concentration was found to be significantly higher in women undergoing mid-gestation genetic amniocentesis who later had spontaneous fetal death before 28 weeks of gestational age [5]. Since S100B is eliminated in the urine [20], it is possible that fetal urine contributes to the elevated S100B concentration in the AF in these pregnancies.
Elevated S100B cerebral spinal fluid (CSF), serum, and plasma concentrations are also associated with infection and inflammation [3, 6, 10, 23, 35]. Neonates with bacterial meningitis have significantly higher median CSF concentrations of S100B than those with septicemia without meningitis. Moreover, the severity of CNS infection is associated with a high CSF S100B concentration as demonstrated by the observation that neonates with bacterial meningitis and encephalitis had the highest median CSF S100B concentration [10]. Extra-cerebral infectious diseases and inflammation are also associated with high serum S100B concentrations. For example, 73% of patients with bacterial meningitis, as well as 25% of patients with bacterial pneumonia or enteritis had serum S100B concentrations [35]. Patients with septic shock also have serum concentrations of S100B equivalent to those described in patients with severe traumatic brain injury [23]. Finally, plasma S100B concentrations in preterm fetal sheep were significantly higher following systemic endotoxin administration, but not saline administration [6].
The objective of this study was to determine whether amniotic fluid concentrations of S100B change with advancing gestational age in normal pregnancy and with intra-amniotic infection in patients with preterm labor and intact membranes and those with preterm premature rupture of membranes.
Materials and methods
Study design
A cross-sectional study was conducted using our clinical database and bank of biological samples. This study included 343 women with singleton pregnancies with no congenital anomalies or karyotype abnormalities in the following groups: (1) mid-trimester (n=74); (2) term not in labor (n=27); (3) term in labor (n=51); (4) preterm labor (PTL) who delivered at term (n=35); (5) preterm labor without intra-amniotic infection (IAI) who delivered preterm (n=49); (6) preterm labor with IAI (n=25); (7) preterm premature rupture of membranes (preterm PROM) without IAI (n=41); and (8) preterm PROM with IAI (n=41). Intra-amniotic infection was defined as a positive amniotic fluid culture for microorganisms. Normal pregnant women at term, with or without labor, had a gestational age of ≥37 weeks and <42 weeks. Preterm labor was characterized by the presence of regular uterine contractions occurring at a frequency of at least 2 every 10 min, with cervical changes at <37 completed weeks of gestation. Rupture of membranes was diagnosed by testing for pooling, nitrazine paper color change, and ferning.
Amniotic fluid was collected by trans-abdominal amniocentesis under Ultrasonographic guidance. Fluid not required for clinical purposes was centrifuged to remove cellular and particulate matter. Aliquots of amniotic fluid were stored at −70°C until analysis. A sample of amniotic fluid was transported to the laboratory to be cultured for aerobic and anaerobic species. All samples except those from the midtrimester were also cultured for genital mycoplasmas (Ureaplasma urealyticum and Mycoplasma hominis). Amniotic fluid white blood cell (WBC) count, Gram stain, and glucose concentrations were used in the management of patients with preterm labor with intact membranes and preterm PROM. Pregnant patients were enrolled between October 1990 and October 2000 at Hutzel Hospital, Detroit, Michigan and Sotero del Rio Hospital, Puente Alto, Chile. The collection of samples for research was approved by the Institutional Review Boards of Wayne State University, Pennsylvania Hospital, and Sotero del Rio Hospital, respectively, as well as, the National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services.
Many of these samples have been previously employed to study the biology of inflammation, hemostasis, angiogenesis regulation, and growth factor concentrations.
S100B and IL-6 Immunoassays
Amniotic fluid S100B and IL-6 were measured with commercially available enzyme-linked immunosorbent assays. Immunoassay kits for S100B were obtained from BioVendor, LLC (Chandler, NC, USA) and IL-6 assays were purchased from R&D Systems (Minneapolis, MN, USA). Both S100B and IL-6 assays were specifically validated for use with human amniotic fluid in our laboratory prior to the conduction of this study. Validation included spike and recovery experiments, which produced parallel curves indicating that amniotic fluid constituents did not interfere with antigen-antibody binding in these assay systems. The concentrations of S100B or IL-6 in amniotic fluid samples were determined by interpolation from individual standard curves composed of human S100B or IL-6. The calculated inter-assay coefficients of variation (CV) for S100B and IL-6 immunoassays in our laboratory were 6.32% and 9.02%, respectively; while, calculated intra-assay CVs for S100B and IL-6 were 2.65% and 7.24%, respectively. The detection limits (sensitivities) were calculated to be 23.30 pg/mL for S100B and 2.28 pg/mL for IL-6 assays.
Diagnosis of neonatal morbidity, funisitis and chorioamnionitis
Respiratory distress syndrome (RDS), bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), neonatal sepsis, and IVH were diagnosed according to the definitions previously described in detail [38, 39]. Placental pathology was available for 58 of the 74 patients who delivered preterm with intact membranes and for 77 of the 82 patients with preterm PROM. Funisitis was diagnosed as the presence of neutrophil infiltration into the umbilical vessel walls or Wharton's jelly, and histologic chorioamnionitis was defined as the presence of acute inflammatory changes on examination of a membrane roll and chorionic plate of the placenta with the use of previously published criteria [38, 39].
Statistical Analysis
The Shapiro-Wilk test was used to evaluate the distribution of data. Amniotic fluid S100B and IL-6 concentrations were not normally distributed. Therefore, the Mann-Whitney U-test was used for comparison of continuous variables and the Kruskal Wallis test with post hoc analysis was employed for multiple comparisons. Spearman's rho was utilized for nonparametric correlations. Receiver operating characteristic (ROC) curve analysis was performed to determine the diagnostic value of S100B AF concentration for determination of IAI. Logistic regression was used to determine if the AF concentration of S100B was an independent explanatory variable for a composite of neonatal morbidity (including RDS, BPD, NEC, IVH, and sepsis), IVH alone, and fetal/neonatal death after controlling for gestational at delivery. The statistical package employed was SPSS 12 (SPSS Inc, Chicago, IL, USA). A p value of <0.05 was considered statistically significant.
Results
Table I displays the demographic and clinical characteristics of the study groups. The amniotic fluid S100B concentration (median and range) by study group is displayed in Table II. Of note, the AF concentration of S100B did not significantly change with advancing gestational age [(median and range: midtrimester 23.4 (23.3−182.8) vs. term 23.7 pg/ml (23.3−370.9); p=0.76)]. There was no significant difference in AF concentration of S100B between patients with labor at term and no labor [median and range: 27.0 pg/ml (23.3−370.9) vs. 23.3 pg/ml (23.3−140.5); p=0.20].
Table 1.
Demographic and clinical characteristics of the study groups.
| Midtrimester (n=74) | Term not in Labor (n=27) | Term in Labor (n=51) | Preterm labor delivered at term (n=35) | Preterm labor delivered preterm (n=49) | Preterm labor with IAI (n=25) | PPROM without IAI (n=41) | PPROM with IAI (n=41) | |
|---|---|---|---|---|---|---|---|---|
| Maternal age (years) | 36.5 (24−42) | 28 (17−40) | 23 (16−37) | 23 (16 − 38) | 23.5 (15−44) | 23 (16−32) | 25.0 (15−37) | 29.0 (17−39) |
| Nulliparity (%) | 16.2 (12/74) | 21.7 (5/23) | 46.0 (23/50) | 22.9 (8/35) | 39.6 (19/48) | 52 (13/25) | 34.1 (14/41) | 22.0 (9/41) |
| Race (%) | ||||||||
| African-American | 91.9 (68/74) | - | - | 88.6 (31/35) | 79.6 (39/49) | 96.0 (24/25) | 80.5 (33/41) | 90.2 (37/41) |
| Caucasian | 4.1 (3/74) | - | - | 8.6 (3/35) | 10.2 (5/49) | 4.0 (1/25) | 17.1 (7/41) | 7.3 (3/41) |
| Hispanic | - | 100 (27/27) | 100 (51/51) | 2.9 (1/35) | 2.0 (1/49) | - | 2.4 (1/41) | - |
| Other | 4.1 (3/74) | - | - | - | 8.2 (4/49) | - | - | 2.4 (1/41) |
| Gestational age at delivery (weeks)* | 39.0 (38.0−40.0) | 39.3 (38.5−40.0) | 39.2 (38.0−40.0) | 38.0 (37.0−40.0) | 27.4 (24.1−31.0) | 26.6 (21.5−29.9) | 31.0 (28.0−33.0) | 32.0 (31.0−34.0) |
| Birthweight (g) | 3345 (2689 − 4277) | 3380 (2810−4530) | 3250 (2540−4440) | 2930 (1899−3960) | 1588.0 (148−3300) | 1077.0 (220−2420) | 1880 (560−3200) | 1580.0 (198−2381) |
Values are expressed as percentage (number), median (range), or
median (inter-quartile range). Data regarding parity was missing for 6 patients.
Table 2.
Amniotic fluid S100B concentration by study group.
| Midtrimester (n=74) | Term not in Labor (n=27) | Term in Labor (n=51) | Preterm labor delivered at term (n=35) | Preterm labor delivered preterm (n=49) | Preterm labor with IAI (n=25) | PPROM without IAI (n=41) | PPROM with IAI (n=41) | |
|---|---|---|---|---|---|---|---|---|
| S100B (pg/ml) |
23.4 (23.3−182.8) |
23.3 (23.3−140.5) |
27.0 (23.3−370.9) |
51.0 (23.3−130.0) |
99.0 (23.3−4731.9) |
1133.6 (23.3−29221.2) |
49.0 (23.3−17683.7) |
98.3 (23.3−19922.5) |
Values expressed as median and range.
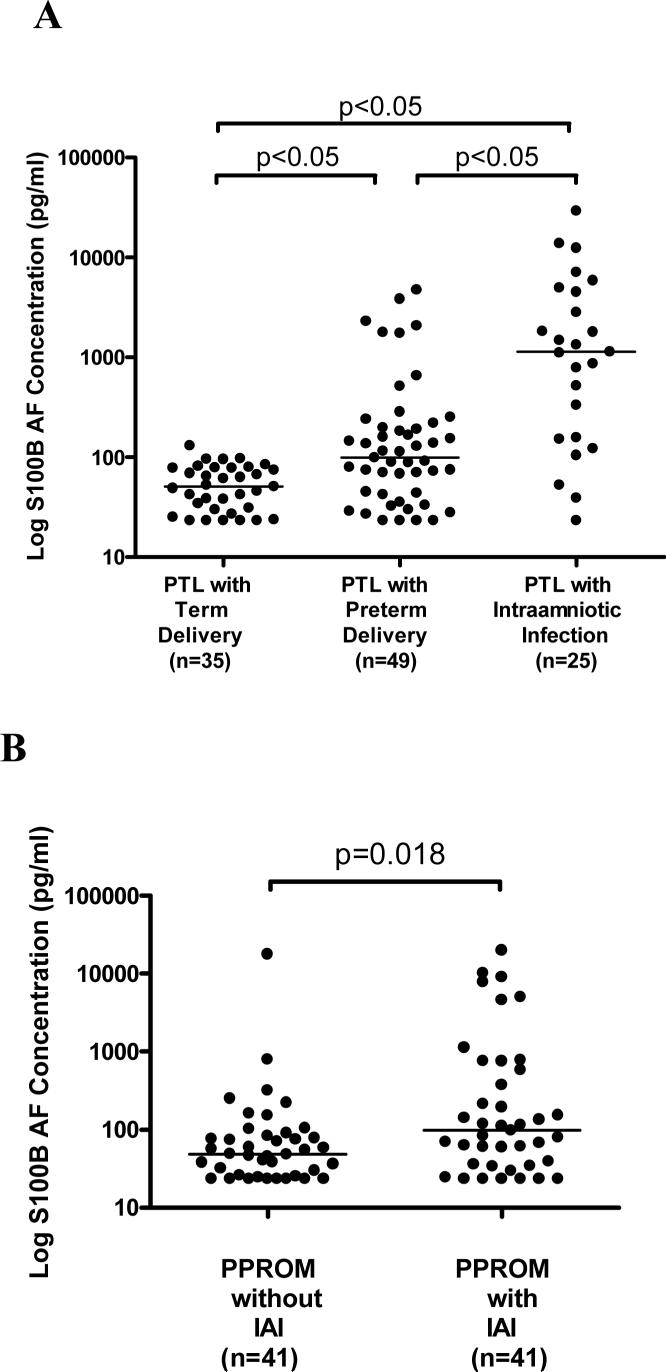
Among patients with preterm labor and intact membranes, those with IAI had significantly higher S100B AF concentration [median and range: 1133.6 pg/ml (23.3−29,221.2)] than those who delivered preterm without IAI [median and range: 99.0 pg/ml (23.3−4731.9); p<0.05] and those who delivered at term [median and range: 51.0 pg/ml (23.3−130.0); p<0.05] (Figure 1A). Similarly, the AF S100B concentration was significantly higher in patients with preterm PROM and IAI than in those without IAI [median and range: 98.3 pg/ml (23.3−19,922.5) vs. 49.0 pg/ml (23.3−17,683.7); p=0.018] (Figure 1B). ROC curve analysis revealed that the sensitivity and specificity of S100B AF concentration for predicting IAI following an episode of preterm labor with intact membranes using a cutoff point of 308.23 were 72% and 90.5%, respectively.
Figure 1.
Amniotic fluid concentration of S100B according to pregnancy outcome and AF culture results. A) Among patients who delivered preterm, those with intra-amniotic infection had significantly higher S100B AF concentration [median: 1133.6 pg/ml (23.3−29,221.2)] than those who delivered preterm without IAI [median: 99.0 pg/ml (23.3−4731.9); p<0.05] or those who delivered at term [median: 51.0 pg/ml (23.3−130.0); p<0.05]. B) Similarly, the AF S100B concentration was significantly higher in patients with preterm PROM and IAI than in those without IAI [median and range: 98.3 pg/ml (23.3−19,922.5) vs. 49.0 pg/ml (23.3−17,683.7); p=0.018].
The amniotic fluid S100B concentration correlated significantly with amniotic fluid IL-6 concentration in patients with preterm delivery following preterm labor with intact membranes (r = 0.74; p < 0.001), as well as, in patients with preterm PROM (r = 0.52; p < 0.001). Patients without IAI, but with intra-amniotic inflammation, defined as amniotic fluid IL-6 concentration of >2.6 ng/ml [37], had a significantly higher S100B concentration than those without inflammation regardless of membrane status (Table 3). Moreover, patients with chorioamnionitis also had a significantly higher median S100B AF concentration than those without chorioamnionitis regardless of membrane status (Table 3). Finally, patients with preterm labor and intact membranes with funisitis, the histopathologic hallmark of the fetal inflammatory response syndrome [27], had a significantly higher median S100B AF concentration than those without funisitis. In contrast, there was no significant difference in the median AF S100B concentration between patients with preterm PROM with or without funisitis (Table3).
Table 3.
Amniotic fluid S100B concentration with or without inflammation in patients with preterm labor and intact membranes or preterm PROM.
| Preterm Labor and Intact Membranes | |||
| No | Yes | p value | |
| Intra-amniotic Inflammation (AF IL-6 > 2.6 ng/ml) | 68.3 (23.3−284.2) n=27 |
190.3 (43.9−4737.9) n=22 |
<0.001 |
| Histologic Chorioamnionitis | 79.2 (23.3−7,125.3) n=23 |
788.4 (27.9−29,221.2) n=35 |
<0.001 |
| Funisitis | 115.8 (23.3−13,842.2) n=37 |
865.9 (33.1−29,221.2) n=21 |
0.003 |
| Preterm Premature Rupture of Membranes | |||
| Intra-amniotic Inflammation (AF IL-6 > 2.6 ng/ml) | 45.9 (23.3−318.5) n=32 |
151.4 (25.2−17,683.7) n=9 |
0.018 |
| Histologic Chorioamnionitis | 45.9 (23.3−10,164.3) n=32 |
82.8 (23.3−19,922.5) n=45 |
0.018 |
| Funisitis | 58.9 (23.3−10,164.3) n=44 |
82.8 (23.3−19,922.5) n=33 |
0.15 |
* AF S100B pg/ml (median and range)
Short-term neonatal outcomes were analyzed for patients with preterm labor and intact membranes. Amniotic fluid S100B concentrations were significantly higher in pregnancies with neonates who subsequently had complications of respiratory distress syndrome, bronchopulmonary dysplasia, necrotizing enterocolitis, and proven sepsis (Table 4). Furthermore among the cohort which underwent neurosonography, S100B concentration was significantly higher in pregnancies with neonates that developed intraventricular hemorrhage than those that did not [median and range: 1305.1 pg/ml (122.1−29,221.2) vs. median and range: 113.7 pg/ml (23.3−13,842.2); p=0.005] (Figure 2A). S100B concentration did not significantly correlate with intraventricular hemorrhage grade in this small subset of patients (r=0.33; p=0.35; n=10). After excluding these 10 patients from the analysis, however, S100B AF concentration remained significantly higher in patients with PTL with IAI than those who delivered preterm without IAI [median and range: 865.9 pg/ml (23.3−13,842.2) vs. 91.3 pg/ml (23.3−4731.9); p=0.002], suggesting that the primary association is with intra-amniotic infection and inflammation (Figure 2B). The relationship between gestational age and S100B with a composite of neonatal morbidity, including RDS, BPD, NEC, IVH, and sepsis was investigated using logistic regression analysis of all patients following an episode of preterm labor with intact membranes. Gestational age at birth was a significant predictor of composite neonatal morbidity (OR 0.71, 95% CI 0.61−0.81, p<0.001); while S100B was not an independent predictor (p=0.39). Logistic regression analysis was also used to investigate the relationship between gestational age at birth, mode of delivery, and S100B with IVH. Gestational age at delivery was the only explanatory variable for the occurrence of IVH (OR 0.77, 95% CI 0.60−0.99, p=0.04), while mode of delivery (p=0.97) and the AF S100B concentration (p=0.075) were not.
Table 4.
Amniotic fluid S100B concentration with or without adverse neonatal outcomes.
| No | Yes | P-value | |
|---|---|---|---|
| Respiratory distress syndrome | 70.4 (23.3−191.4) n=16 |
157.5 (23.3−29,221.2) n=33 |
0.001 |
| Bronchopulmonary dysplasia | 83.9 (23.3−13,842.2) n=38 |
864.8 (122.1−29,221.2) n=8 |
0.001 |
| Necrotizing enterocolitis | 88.6 (23.3−13,842.2) n=43 |
4399.3 (197.5−29,221.2) n=4 |
0.003 |
| Proven sepsis | 109.0 (23.3−13,842.2) n=38 |
1476.5 (253.1−29,221.2) n=3 |
0.03 |
| Death | 118.9 (23.3−29,221.2) n=58 |
1100.6 (43.9−5,858.8) n=16 |
0.002 |
* AF S100B pg/ml (median and range)
Figure 2.
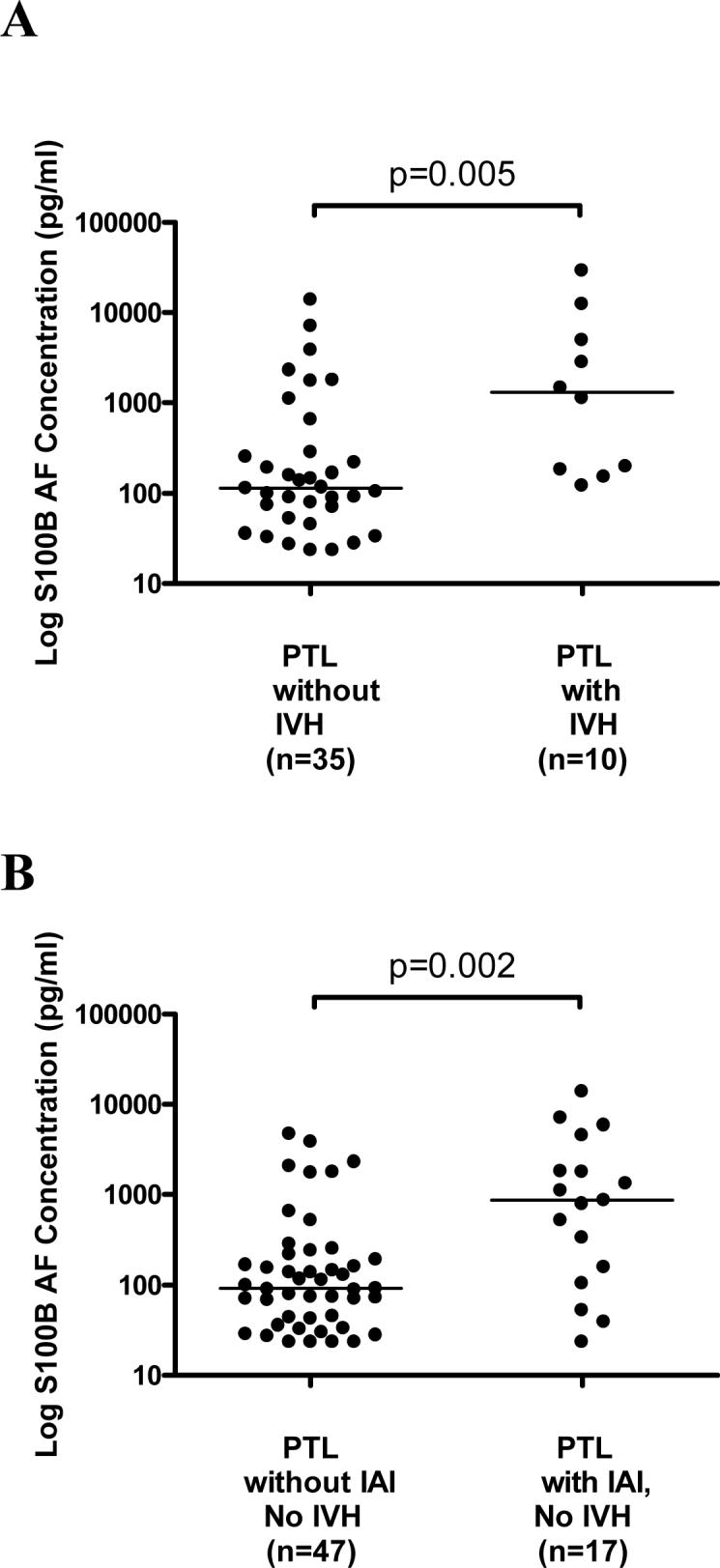
Amniotic fluid concentration of S100B in patients in preterm labor with intact membranes who subsequently delivered preterm neonates who underwent neurosonography. There was a significant difference in the AF S100B concentration between patients with PTL with and without IVH [median: 1305.1 pg/ml (122.1−29,221.2) vs. median: 113.7 pg/ml (23.3−13,842.2); p=0.005]. After excluding these 10 patients from the analysis, however, S100B AF concentration remained significantly higher in patients with PTL with IAI than those who delivered preterm without IAI [median and range: 865.9 pg/ml (23.3−13,842.2) vs. 91.3 pg/ml (23.3−4731.9); p=0.002].
Finally, S100B concentration was significantly higher in patients with preterm labor and intact membranes with subsequent intrapartum or neonatal death than those alive at discharge (Table 4). A multivariate logistic regression analysis revealed that these deaths were related to gestational age at delivery (OR 0.35, 95% CI 0.19−0.62, p<0.001). AF S100B concentration was not an independent predictor of fetal or neonatal death (p=0.81).
Discussion
Principal findings of the study
Preterm parturition following preterm labor with intact membranes and intra-amniotic infection/inflammation is associated with a significantly higher median AF concentration of S100B, even after excluding patients whose neonates subsequently had overt adverse neurologic outcome, i.e., intra-ventricular hemorrhage. This observation suggests that an elevation in the AF concentration of S100B may reflect infection/inflammation and not necessarily fetal neurologic damage. Furthermore, the AF concentration of S100B is not an independent predictor of composite neonatal morbidity (including RDS, BPD, IVH, NEC, and neonatal sepsis), IVH alone, or fetal/neonatal death.
Amniotic fluid concentration of S100B does not change with advancing gestational age
Our results that AF S100B concentration did not change with gestational age are novel. Prior reports indicated that the correlation of S100B concentration decreased with gestational age in the cord blood, urine, and saliva of neonates [8, 11, 15]. However, this may reflect that these biological samples were obtained from patients with complications of pregnancy leading to preterm delivery. Such samples can not be obtained in preterm gestations without complications.
Intra-amniotic infection is associated with a higher amniotic fluid S100B
The findings that preterm patients with IAI had a significantly higher S100B AF concentration than those without IAI regardless of membrane status are novel, and indicate that an elevation in AF concentration of S100B may reflect infection/inflammation. A growing body of evidence indicates that a high serum or plasma concentration of S100B is associated with systemic infection/inflammation including: 1) serum concentrations of S100B are elevated in bacterial sepsis, as well as cerebral infection [23]; 2) patients with an initial serum S100B concentration >0.50 ng/mL also had elevated serum interleukin-8 and polymorphonuclear elastase plasma concentrations [23]; 3) patients with severe sepsis and septic shock had an elevated serum S100B concentration, particularly those who died early (within 4 days of intensive care unit admission) [25]; 4) S100B concentration was the strongest independent predictor of intensive care unit survival [25]; and 5) systemic endotoxin administration to preterm fetal sheep resulted in significantly higher S100B plasma concentrations post-administration compared with saline treatment [6].
Although ROC curve analysis revealed a high sensitivity and specificity of S100B AF concentration for predicting IAI, amniotic fluid interleukin-6 or MMP-8 concentration determination remains the most sensitive test for IAI [16, 22]. Furthermore, S100B is not better than a rapid matrix metalloproteinase-8 bedside test (sensitivity 83% and specificity 95%) [26].
Higher amniotic fluid S100B associated with adverse neonatal outcomes is related to gestational age
A significantly higher AF concentration of S100B is associated with adverse neonatal outcomes including acute and chronic lung disease, necrotizing enterocolitis, neonatal sepsis, as well as IVH and death. The AF concentration of S100B, however, was not an independent predictor of composite neonatal morbidity (including RDS, BPD, IVH, NEC, and neonatal sepsis), IVH, or fetal/neonatal death, but may reflect infection/inflammation which leads to earlier preterm delivery.
What are the possible sources of S100B in the amniotic fluid?
S100B protein is found in the placenta as determined by immunohistochemistry [21, 36] and by Western blot analysis [21]. However, the specificity of the polyclonal antibodies used in these studies remains unclear. It is possible that S100P, a placental protein having approximately 50% sequence homology with S100B, may have cross reacted with the polyclonal antibodies [2]. More compelling evidence that fetal membranes produce S100B was reported in patients with preeclampsia [34]. S100B mRNA expression in the amnion of these patients was measured by RT-PCR using sequence-specific primers for S100B. Amnion S100B mRNA expression was significantly higher than that of amnion from uncomplicated term pregnancies (p<0.05). Furthermore, the AF S100B protein concentration in patients with preeclampsia, as measured by ELISA, was significantly higher than that of uncomplicated term pregnancies (p<0.05). This study demonstrates that the amnion could be a source of S100B contributing to the AF concentration which must be taken into consideration.
The conventional view is that the main source of S100B is neuronal tissue. However, S100B is also found in adipocytes, melanocytes, and chondrocytes, albeit in lower concentrations [18, 19]. Indeed, high serum S100B concentrations have been reported in trauma patients without head injuries, particularly those with large fractures or abdominal injury [1, 30]. Another study demonstrated increased concentrations of serum S100B in a cohort of critically ill patients without brain injury [28]. These patients had various diagnoses, such as acute respiratory distress syndrome, trauma, acute pancreatitis, hemorrhagic-, cardiogenic-, and septic shock. Thus, it is possible that fetal tissues other than neural tissue may contribute to the AF concentration of S100B.
The systemic inflammatory response syndrome (SIRS) in critically ill patients may contribute to the elevated serum S100B in adults. Tsao et al. proposed that SIRS may affect the tone of systemic and cerebral vasculature increasing the permeability of the blood-brain barrier facilitating diffusion of S100B [33]. Moreover, there is experimental evidence that bacteria can increase the permeability of the blood-brain barrier as demonstrated in a mouse model of sepsis where disruption of brain microvascular vessels was confirmed by transmission electron microscopy. Indeed, intravenous injection of tumor necrosis factor-α results in increased permeability of the blood-brain barrier which is inhibited by anti-tumor necrosis factor-α antibody [33]. Thus, increased fetal plasma pro-inflammatory cytokines produced during the fetal counterpart of SIRS, the fetal inflammatory response syndrome [17], may also lead to the disruption of the fetal blood brain barrier permeability facilitating release of S100B from the fetal central nervous system without concomitant neurological damage.
Strengths of the study
The strengths of the study include: (1) a large number of patients per group (25−74); (2) consistent results of a higher AF concentration of S100B with several parameters of infection and inflammation in patients who delivered preterm following both preterm labor with intact membranes or preterm PROM; and (3) multivariate logistic regression analysis to determine whether AF concentration of S100B was an independent explanatory variable for neonatal morbidity or fetal/neonatal death.
Limitations/future research directions
Since the amnion is a possible source contributing to the AF S100B concentration, the extent to which the amnion produces S100B and whether or not its production is up-regulated by microbial products should be explored. Prospective studies are required to determine if a high AF S100B concentration in the midtrimester or at the time of the clinical presentation of preterm labor or preterm PROM is associated with adverse long-term neurologic outcome.
Conclusions
Patients who deliver preterm following preterm labor with intact membranes or preterm PROM who have intra-amniotic infection/inflammation have a higher AF concentration of S100B than those without intra-amniotic infection/inflammation.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS. The authors wish to acknowledge the contributions of the Nursing staff of the Perinatology Research Branch and Detroit Medical Center: Ms Nancy Hauff, Ms Sandy Field, Ms Lorraine Nikita, Ms Vicky Ineson, Ms Mahbubeh Mahmoudieh, Ms Julie McKinley, Ms Sue Rehel, Ms Shannon Donegan, Ms Linda Bouey, Ms Carolyn Sudz, Ms Sylvia Warren, Ms Gail Barley, Ms Denise Bayoneto, Ms Judy Kerman, and Ms Barbara Steffy. We would also like to thank Pat Schoff and Valerie Richardson for their help with their graphics support and expertise.
Reference List
- 1.Anderson RE, Hansson LO, Nilsson O, jlai-Merzoug R, Settergren G. High serum S100B levels for trauma patients without head injuries. Neurosurgery. 2001;48:1255–1258. doi: 10.1097/00006123-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Becker T, Gerke V, Kube E, Weber K. S100P, a novel Ca(2+)-binding protein from human placenta. cDNA cloning, recombinant protein expression and Ca2+ binding properties. Eur J Biochem. 1992 Jul 15;207:541–547. doi: 10.1111/j.1432-1033.1992.tb17080.x. 1992. [DOI] [PubMed] [Google Scholar]
- 3.Bertsch T, Casarin W, Kretschmar M, Zimmer W, Walter S, Sommer C, et al. Protein S-100B: a serum marker for ischemic and infectious injury of cerebral tissue. Clin Chem Lab Med. 2001;39:319–323. doi: 10.1515/CCLM.2001.050. [DOI] [PubMed] [Google Scholar]
- 4.Florio P, Marinoni E, Di IR, Bashir M, Ciotti S, Sacchi R, et al. Urinary S100B protein concentrations are increased in intrauterine growth-retarded newborns. Pediatrics. 2006;118:e747–e754. doi: 10.1542/peds.2005-2875. [DOI] [PubMed] [Google Scholar]
- 5.Florio P, Michetti F, Bruschettini M, Lituania M, Bruschettini P, Severi FM, et al. Amniotic fluid S100B protein in mid-gestation and intrauterine fetal death. Lancet. 2004;364:270–272. doi: 10.1016/S0140-6736(04)16677-4. [DOI] [PubMed] [Google Scholar]
- 6.Garnier Y, Berger R, Alm S, von Duering MU, Coumans AB, Michetti F, et al. Systemic endotoxin administration results in increased S100B protein blood levels and periventricular brain white matter injury in the preterm fetal sheep. Eur J Obstet Gynecol Reprod Biol. 2006;124:15–22. doi: 10.1016/j.ejogrb.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Gazzolo D, Bruschettini M, Lituania M, Serra G, Bonacci W, Michetti F. Increased urinary S100B protein as an early indicator of intraventricular hemorrhage in preterm infants: correlation with the grade of hemorrhage. Clin Chem. 2001;47:1836–1838. [PubMed] [Google Scholar]
- 8.Gazzolo D, Bruschettini M, Lituania M, Serra G, Gandullia E, Michetti F. S100b protein concentrations in urine are correlated with gestational age in healthy preterm and term newborns. Clin Chem. 2001;47:1132–1133. [PubMed] [Google Scholar]
- 9.Gazzolo D, Florio P, Ciotti S, Marinoni E, Di IR, Bruschettini M, et al. S100B protein in urine of preterm newborns with ominous outcome. Pediatr Res. 2005;58:1170–1174. doi: 10.1203/01.pdr.0000185131.22985.30. [DOI] [PubMed] [Google Scholar]
- 10.Gazzolo D, Grutzfeld D, Michetti F, Toesca A, Lituania M, Bruschettini M, et al. Increased S100B in cerebrospinal fluid of infants with bacterial meningitis: relationship to brain damage and routine cerebrospinal fluid findings. Clin Chem. 2004;50:941–944. doi: 10.1373/clinchem.2003.021048. [DOI] [PubMed] [Google Scholar]
- 11.Gazzolo D, Lituania M, Bruschettini M, Ciotti S, Sacchi R, Serra G, et al. S100B protein levels in saliva: correlation with gestational age in normal term and preterm newborns. Clin Biochem. 2005;38:229–233. doi: 10.1016/j.clinbiochem.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Gazzolo D, Marinoni E, Di IR, Bruschettini M, Kornacka M, Lituania M, et al. Measurement of urinary S100B protein concentrations for the early identification of brain damage in asphyxiated full-term infants. Arch Pediatr Adolesc Med. 2003;157:1163–1168. doi: 10.1001/archpedi.157.12.1163. [DOI] [PubMed] [Google Scholar]
- 13.Gazzolo D, Marinoni E, Di IR, Bruschettini M, Kornacka M, Lituania M, et al. Urinary S100B protein measurements: A tool for the early identification of hypoxic-ischemic encephalopathy in asphyxiated full-term infants. Crit Care Med. 2004;32:131–136. doi: 10.1097/01.CCM.0000104116.91462.CD. [DOI] [PubMed] [Google Scholar]
- 14.Gazzolo D, Vinesi P, Bartocci M, Geloso MC, Bonacci W, Serra G, et al. Elevated S100 blood level as an early indicator of intraventricular hemorrhage in preterm infants. Correlation with cerebral Doppler velocimetry. J Neurol Sci. 1999;170:32–35. doi: 10.1016/s0022-510x(99)00194-x. [DOI] [PubMed] [Google Scholar]
- 15.Gazzolo D, Vinesi P, Marinoni E, Di IR, Marras M, Lituania M, et al. S100B protein concentrations in cord blood: correlations with gestational age in term and preterm deliveries. Clin Chem. 2000;46:998–1000. [PubMed] [Google Scholar]
- 16.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol. 1994;32:200–210. doi: 10.1111/j.1600-0897.1994.tb01115.x. 1994 Oct;32(3) [DOI] [PubMed] [Google Scholar]
- 17.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. 1998 Jul;179(1):194- [DOI] [PubMed] [Google Scholar]
- 18.Haimoto H, Hosoda S, Kato K. Differential distribution of immunoreactive S100-alpha and S100-beta proteins in normal nonnervous human tissues. Lab Invest. 1987;57:489–498. [PubMed] [Google Scholar]
- 19.Jonsson H. S100B and cardiac surgery: possibilities and limitations. Restor Neurol Neurosci. 2003;21:151–157. [PubMed] [Google Scholar]
- 20.Jonsson H, Johnsson P, Hoglund P, Alling C, Blomquist S. Elimination of S100B and renal function after cardiac surgery. J Cardiothorac Vasc Anesth. 2000;14:698–701. doi: 10.1053/jcan.2000.18444. [DOI] [PubMed] [Google Scholar]
- 21.Marinoni E, Di IR, Gazzolo D, Lucchini C, Michetti F, Corvino V, et al. Ontogenetic localization and distribution of S-100beta protein in human placental tissues. Obstet Gynecol. 2002;99:1093–1099. doi: 10.1016/s0029-7844(02)01996-8. [DOI] [PubMed] [Google Scholar]
- 22.Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183:94–99. doi: 10.1067/mob.2000.105344. [DOI] [PubMed] [Google Scholar]
- 23.Mussack T, Briegel J, Schelling G, Biberthaler P, Jochum M. Effect of stress doses of hydrocortisone on S-100B vs. interleukin-8 and polymorphonuclear elastase levels in human septic shock. Clin Chem Lab Med. 2005;43:259–268. doi: 10.1515/CCLM.2005.044. [DOI] [PubMed] [Google Scholar]
- 24.Nagdyman N, Komen W, Ko HK, Muller C, Obladen M. Early biochemical indicators of hypoxic-ischemic encephalopathy after birth asphyxia. Pediatr Res. 2001;49:502–506. doi: 10.1203/00006450-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen DN, Spapen H, Su F, Schiettecatte J, Shi L, Hachimi-Idrissi S, et al. Elevated serum levels of S-100beta protein and neuron-specific enolase are associated with brain injury in patients with severe sepsis and septic shock. Crit Care Med. 2006;34:1967–1974. doi: 10.1097/01.CCM.0000217218.51381.49. [DOI] [PubMed] [Google Scholar]
- 26.Nien JK, Yoon BH, Espinoza J, Kusanovic JP, Erez O, Soto E, et al. A rapid MMP-8 bedside test for the detection of intra-amniotic inflammation identifies patients at risk for imminent preterm delivery. Am J Obstet Gynecol. 2006;195:1025–1030. doi: 10.1016/j.ajog.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 27.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 28.Routsi C, Stamataki E, Nanas S, Psachoulia C, Stathopoulos A, Koroneos A, et al. Increased levels of serum S100B protein in critically ill patients without brain injury. Shock. 2006;26:20–24. doi: 10.1097/01.shk.0000209546.06801.d7. [DOI] [PubMed] [Google Scholar]
- 29.Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and -independent interactions of the S100 protein family. Biochem J. 2006;396:201–214. doi: 10.1042/BJ20060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savola O, Pyhtinen J, Leino TK, Siitonen S, Niemela O, Hillbom M. Effects of head and extracranial injuries on serum protein S100B levels in trauma patients. J Trauma. 2004;56:1229–1234. doi: 10.1097/01.ta.0000096644.08735.72. [DOI] [PubMed] [Google Scholar]
- 31.Schafer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci. 1996;21:134–140. doi: 10.1016/s0968-0004(96)80167-8. [DOI] [PubMed] [Google Scholar]
- 32.Thorngren-Jerneck K, Alling C, Herbst A, mer-Wahlin I, Marsal K. S100 protein in serum as a prognostic marker for cerebral injury in term newborn infants with hypoxic ischemic encephalopathy. Pediatr Res. 2004;55:406–412. doi: 10.1203/01.PDR.0000106806.75086.D3. [DOI] [PubMed] [Google Scholar]
- 33.Tsao N, Hsu HP, Wu CM, Liu CC, Lei HY. Tumour necrosis factor-alpha causes an increase in blood-brain barrier permeability during sepsis. J Med Microbiol. 2001;50:812–821. doi: 10.1099/0022-1317-50-9-812. [DOI] [PubMed] [Google Scholar]
- 34.Tskitishvili E, Komoto Y, Temma-Asano K, Hayashi S, Kinugasa Y, Tsubouchi H, et al. S100B protein expression in the amnion and amniotic fluid in pregnancies complicated by pre-eclampsia. Mol Hum Reprod. 2006;12:755–761. doi: 10.1093/molehr/gal083. [DOI] [PubMed] [Google Scholar]
- 35.Unden J, Christensson B, Bellner J, Alling C, Romner B. Serum S100B levels in patients with cerebral and extracerebral infectious disease. Scand J Infect Dis. 2004;36:10–13. doi: 10.1080/00365540310017294. [DOI] [PubMed] [Google Scholar]
- 36.Wijnberger LD, Nikkels PG, van Dongen AJ, Noorlander CW, Mulder EJ, Schrama LH, et al. Expression in the placenta of neuronal markers for perinatal brain damage. Pediatr Res. 2002;51:492–496. doi: 10.1203/00006450-200204000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 38.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, et al. Fetal exposure to an intraamniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–681. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 39.Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183:1124–1129. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 40.Zimmer DB, Cornwall EH, Landar A, Song W. The S100 protein family: history, function, and expression. Brain Res Bull. 1995;37:417–429. doi: 10.1016/0361-9230(95)00040-2. [DOI] [PubMed] [Google Scholar]