Abstract
A CD4+ T-cell clone (HC/2G-1) was established by stimulating peripheral blood T cells from a patient with renal cell cancer (RCC) with dendritic cells pre-incubated with the autologous apoptotic renal tumor line in the presence of IFN-α. It recognizes the autologous RCC and most allogeneic RCC lines by IFN-γ release (10 of 11 lines) and lysis (9 of 10 lines), but does not recognize multiple EBV-B cells or fibroblasts. It shows little or no recognition of a panel of melanomas, breast cancers and non-small cell lung cancers. Phenotypically, HC/2G-1 is CD3+CD4+ TCRα/β+, but CD161-CD16-NKG2D-. Tumor recognition by clone HC/2G-1 was not blocked by antibodies to HLA-class I or class II, but was significantly reduced by anti-TCRα/β Ab. Furthermore, tumor recognition was beta-2-microglobulin (B2M)-independent. HC/2G-1 does not utilize a Vα or Vβ described for classical NKT cells, but rather Vα14 and Vβ2.1. Allogeneic T cells co-transfected with mRNAs encoding the α and β chains of the HC/2G-1 TCR recognized renal tumor lines, demonstrating that tumor recognition is TCR mediated. Interestingly, TNF-related apoptosis inducing ligand (TRAIL) appears to play a role in tumor recognition by HC/2G-1 in that reactivity was blocked by anti-TRAIL Ab, and soluble TRAIL could enhance IFN-γ secretion by HC/2G-1 in response to renal tumors. Our findings suggest that clone HC/2G-1 represents a novel type of CD4+ cell that has broad TCR-mediated recognition of a determinant(s) widely expressed by RCC.
Keywords: CD4 T cell, renal cell carcinoma, T cell receptor, MHC, TNF-related apoptosis inducing ligand
Introduction
The anti-tumor immune response is a complex event that can involve many different types of cells. Classical T cells, such as major histocompatibility complex (MHC)-class I restricted CD8+ cytotoxic T lymphocytes (CTL) and MHC-class II restricted CD4+ T cells have always been at the center of this response. CD8+ CTL can directly kill tumor cells by recognizing epitopes presented by MHC molecules on the tumor surface, and from murine models, they are thought to play a major role in anti-tumor immunity(1). CD4+ T cells, in particular, T helper (Th) cells, on the other hand, are thought to be important in modulating the anti-tumor response. CD4 Th cells can activate antigen presenting cells (APC) to present Ag to CD8 CTL, maintain CD8 T cell memory and survival, and secrete cytokines such as IL-2, IFN-γ and TNF-α which can also affect CTL, APC and tumor cells(2-4). Other types of cells with tumor reactivity, such as non-classical CD8 and CD4 T cells, NK cells, and NKT cells, have also been reported and characterized (5-9). Among them, type I NKT cells, typically expressing an invariant T cell receptor (TCR) α chain (Vα24 in human; Vα14 in mice) and β chain (Vβ11 in human; varying in mice) and recognizing CD1d-restricted glycolipid antigens, such as α-galactosylceramide, iGb3 and diacylglycerol, have been shown to inhibit tumor growth in vivo (10,11).
Clear cell renal cell carcinoma (RCC) has been shown to respond to immunotherapy with a 15-20% response rate to IL-2 and a 5-7% cure rate seen in selected patients with metastatic disease(12). However, there has been little further progress in treating metastatic RCC patients with immunotherapy in contrast with another immuno-responsive cancer, melanoma. This is mainly due to a disparity in identifying tumor-reactive T cells recognizing these two cancers. Previously, we developed a method to generate RCC-reactive T cells by stimulating patient peripheral blood lymphocytes (PBL) with dendritic cells (DC) engulfing and presenting apoptotic tumor cells (ATC) in a completely autologous setting (13). Since DCs express both MHC class I and class II molecules on their surface, we were able to generate both MHC-class I and II restricted RCC-specific CD8 and CD4 T cells. The ability to generate such T cells might be exploited by identifying tumor associated antigens, developing vaccines and administering cultured T-cells for therapy in patients with RCC. However, there are no constraints on the nature of the tumor reactivity generated by this in vitro autologous tumor stimulation and there is also the potential for generating non-classical immune cells which recognize tumor. Here we report the generation of such an RCC-reactive CD4 T cell clone (HC/2G-1) that recognizes multiple renal tumors in an MHC-independent fashion with IFN-γ secretion and tumor lysis. This appears to be a novel immune reactivity by function and phenotype, which is largely specific for renal cancer. Tumor recognition is TCR α/β mediated, but MHC- and B2M-independent. Introducing the cloned TCR α and β chains of this clone into allogeneic lymphocytes stimulated with anti-CD3 confers a pattern of tumor reactivity similar to the parental clone. Furthermore, TNF-related apoptosis inducing ligand (TRAIL) plays a role in tumor recognition by HC/2G-1 reactivity, as shown by blocking with anti-TRAIL Ab, and enhanced recognition when renal tumors were pretreated with soluble TRAIL. It is not clear what role this type of T-cell plays in the immune response to human RCC, but it may be the source of novel immunotherapeutic strategies for treating this malignancy.
Materials and Methods
Cell Lines
Tumor lines from RCC patients and Epstein-Barr virus-transformed B (EBV-B) cells were established as described previously. RCC lines were maintained in Dulbecco modified Eagle medium (DMEM; Invitrogen, Gaithersburg, MD) including 10% fetal bovine serum (FBS; Invitrogen), and EBV-B cells were maintained in RPMI 1640 (Invitrogen) including 10% FBS. Tumor lines used as controls in experiments were obtained from Surgery Branch laboratories (National Cancer Institute, Bethesda, MD), and maintained in RPMI 1640 including 10% FBS. Human primary renal epithelial cells were gifts from Drs. Scott Garrett and Donald Sens (University of North Dakota, Grand Forks, ND), or purchased from Cambrex (Rockland, ME).
Reagents
For immunophenotyping, monoclonal antibodies (MoAbs) including FITC-labeled anti-human IgG isotypes, CD3, CD4, CD8, CD16, CD57, CD94, CD244 and TCRγ/δ, PE-labeled anti-human IgG isotypes, CD3, CD4, CD8, TCRα/β, CD56, CD161 NKG2D and TRAIL, and APC-labeled anti-human IgG isotypes, CD3, CD4 and CD8 were purchased from BD Pharmingen (San Jose, CA). PE-labeled anti-Vβ2 Ab was purchased from Beckman Coulter (Miami, FL).
For blocking experiments, W6/32 (pan anti-MHC class I) and IVA12 (pan anti-MHC class II) were gifts of Dr. Paul Robbins (National Cancer Institute). Purified anti-human TCRα/β (Clone T10B9.1A-31), anti-human CD4, and anti-human TRAIL Abs were purchased from BD Pharmingen.
Granzyme B ELISA kit was purchased from Abcam Inc. (Cambridge, MA). Soluble TRAIL (Apo2L; aa114-281) was purchased from Biomol International L.P. (Plymouth Meeting, PA).
In vitro stimulation, cloning and expansion of RCC-specific T cells
To establish RCC-specific T cells, CD8 and/or CD4 enriched T cells (Miltenyi Biotec Inc, Auburn, CA) were stimulated with Day 6 dendritic cells (DCs) co-cultured with UV irradiated autologous tumor cells, as described previously with some modifications (13,14). Briefly, CD14+ were isolated from patient peripheral blood mononuclear cells (PBMCs) using CD14 microbeads according to manufacturer’s instructions (Miltenyi Biotec Inc), and cultured in RPMI 1640, supplemented with 10% human serum (HS; Valley Biomedical Inc. Winchester, VA), GM-CSF (1000U/ml; Peprotech, Rocky Hill, NJ) and IL-4 (1000U/ml; Peprotech) for 6 days to generate monocyte-derived DCs. To stimulate T cells, Day 6 DC were co-cultured with UV-irradiated tumor cells (UVB; 312mm; Spectroline, Westbury, NY) at 1:1 ratio in RPMI 1640 + 10%HS in the presence of GM-CSF, IL-4 and IFN-α (1000U/ml; Pierce Biotechnology, Inc. Rockford, IL) overnight in 96-well round-bottom plates. The next day, the DC-tumor co-culture plates were replenished with fresh RPMI 1640 +10%HS + GM-CSF + IL4. CD8 enriched T cells (Miltenyi CD8+ cell isolation kit II) and CD4 enriched, CD25 depleted T cells (Miltenyi CD4+ cell isolation kit II and CD25 microbeads) were isolated and added to DC-tumor co-culture in RPMI +10%HS + IL-2 (120IU/ml) + CD40L (500ng/ml; Immunex, Seattle, WA). Seven days later, T cells were re-stimulated once by transferring them to a second identically prepared DC-tumor culture. Testing for IFN-γ production in response to autologous EBV-B or RCC occurred 7 days after restimulation. Criteria for a positive microwell were an IFN-γ concentration that was at least 100pg/ml and twice that of a co-culture with autologous EBV-B cells. T cell clones were derived from positive microwell cultures by diluting T cells to 1, 3 or 10 cells per well and co-culturing with 5 × 104 irradiated PBMCs (3000 ∼ 4000 cGy) from 3 allogeneic donors in RPMI 1640 medium containing 10% HS, 300IU/ml IL-2 and 30ng/ml OKT-3 (Ortho-McNeil Pharmaceuticals, Raritan, NJ). Further expansion of T cell clones was performed when they show reactivity against autologous tumor as measured by IFN-γ secretion.
Cell lysis assay
A standard 4-hour 51Cr-release assay was performed to test the cytotoxicity of T cells against tumors. Briefly, target cells were labeled for 1 h at 37°C with 51Cr (200 μCi; GE Healthcare Life Sciences; Piscataway, NJ). Labeled target cells were then washed three times and plated in triplicate at a concentration of 1x 104 per well in 96-well round-bottom plates. Effector cells were prepared and added to target cells at various E:T ratio. After 4-hour incubation, supernatants were harvested and counted on a Wallac 1470 Wizard automatic gamma counter (PerkinElmer, Wellesley, MA). Maximum and spontaneous releases were determined by adding 2% SDS or medium to target cells, respectively. The percentage of specific lysis was calculated as: (experimental cpm - spontaneous cpm)/(maximum cpm - spontaneous cpm) × 100.
Blocking assay
RCC cells (5 × 104 cells in 100 μl culture medium) were incubated with each blocking MoAb at a concentration of 10μg/ml for 30 min. at 37°C in a flat-bottom 96-well plate. T cells (1 ∼ 5 × 104 cells/well) were then added and incubated with target cells overnight at 37°C. The supernatants were harvested and assayed for IFN-γ concentration by ELISA.
Isolation of TCR α and β chains and vector construction
Total RNA from T cell clone HC/2G-1 was purified from T cells using an RNeasy mini kit (Qiagen, Valencia, CA). Primers that are complimentary to the 3′ end of the coding sequences were synthesized (Operon Technologies, Huntsville, AL) to make full-length cDNAs of TCR α and β chains. These primers were: Cα (TCAGCTGGACCACAGCCGCAGC), Cβ1 (TCAGAAATCCTTTCTCTTGACCATG) and Cβ2 (CTAGCCTCTGGAATCCTTTCTCTTG). 5′ RACE reaction was performed by SMART RACE cDNA amplification kit (Clontech, Mountain View, CA) following the manufacturer’s instructions. The RACE cDNAs (∼1kb) were obtained with Cα and Cβ1 primers and then inserted into the pCR2.1 vector by TA cloning (Invitrogen). The sequences of HC/2G-1 TCR α and β chains can be found at www.ncbi.nlm.nih.gov/Genbank/ (EF101778; EF101779).
In vitro transcription and electroporation
In vitro transcription of TCR α and β chains was performed using mMESSAGE mMACHINE ULTRA according to manufacturer’s recommendations (Applied Biosystem, Foster City, CA). The RNA was purified using the RNAeasy mini kit (Qiagen, Valencia, CA). Electroporation of mRNAs encoding the TCR α and β chains was performed as described previously (15). In summary, PBMC from allogeneic donors were stimulated with 50 ng/ml soluble OKT3 for 3 days, washed, and resuspended in OPTI-MEM medium (Invitrogen) at 2.5 × 107/ml. Fifty to 200 μl of suspended PBMC were mixed with mRNAs in varying amounts and transferred to pre-chilled 2-mm cuvettes (Harvard Apparatus BTX, Holliston, MA). Electroporation was performed at 400V/500 μSec using an ECM830 Electro Square Wave Porator (Harvard Apparatus BTX). Following electroporation, the cells were transferred to fresh RPMI + 10%HS and incubated at 37°C. Two hours after incubation, TCR-transfected cells were used for co-culture assay. Expression of Vβ2 was used to determine transfection efficiency.
CD107a mobilization assay
CD107a expression by HC/2G-1 was performed as described previously (16). Briefly, HC/2G-1 (1 × 105) were co-cultured with renal tumors (4 × 105) in a round-bottom 96-well plate for 2hrs at 37°C, 5% CO2 in the presence of FITC-labeled CD107a or isotype controls. After incubation, cells were stained with PE-labeled anti-CD3 Ab, and then washed and analyzed by flow cytometry.
Cell viability assay
To determine sensitivity to soluble TRAIL, cells were plated in triplicate in 96-well plates at 5000 per well in 100μl of RPMI +10% FBS, incubated for 24 hours, and then treated with soluble TRAIL in various concentrations (10, 30, 100, 300, and 1000 ng/ml). 72 hours later, MTT assay (ATCC, Manassas, VA) was performed following manufacturer’s protocol.
Results
T cell clone HC/2G-1 recognizes multiple renal tumors irrespective of HLA type
To generate renal cancer carcinoma (RCC)-specific T cells, CD3 enriched T cells from a RCC patient were stimulated with autologous DCs which had been co-cultured with autologous UV-irradiated tumor cells in the presence of IFN-α. One microwell, HC/2G, showing reactivity to autologous RCC (RCC #1, Figure 1A), was cloned and expanded for further characterization. Clone HC/2G-1, derived from HC/2G, was tested against a panel of allogeneic renal tumor lines, EBV-B cell lines, melanoma lines, and other cell lines including breast cancer and non-small cell lung cancer, fibroblasts, and normal renal epithelium. This showed that clone HC/2G-1 was highly reactive to the autologous RCC (RCC #1) and 9 of 10 HLA-mismatched renal tumors by IFN-γ secretion (Figure 1B). Only one renal tumor, RCC #11 was not recognized by HC/2G-1. One of nine melanoma lines (888 mel) was strongly recognized and there was also weak recognition of another melanoma line (526 mel), a breast cancer line (MDA386), a non-small cell lung cancer line (H2228), and an esophageal adenocarcinoma line (BE-3). EBV-B cell lines generated from the PBL of each of the RCC patients who were the sources of the recognized tumor lines were not significantly recognized. Six allogeneic fibroblast lines were not recognized. While there was weak reactivity against two purchased (but uncharacterized) renal epithelial cells (HRE1 and HRE2) by HC/2G-1, no reactivity was seen against three renal epithelial cells raised from primary NCI nephrectomy specimens using published methodology (17). HC/2G-1 lytic function was also tested in chromium release assay, and only RCC #11 was not lysed among 9 RCC lines (Figure 1C). In the same assay, autologous EBV-B cells, Daudi, K562 and 4 allogeneic normal renal epithelial lines were not lysed. Lysis was highly correlated with IFN-γ secretion in the same experiment (data not shown, R2 = 0.7982; P=0.000003 by regression analysis).
Figure 1.
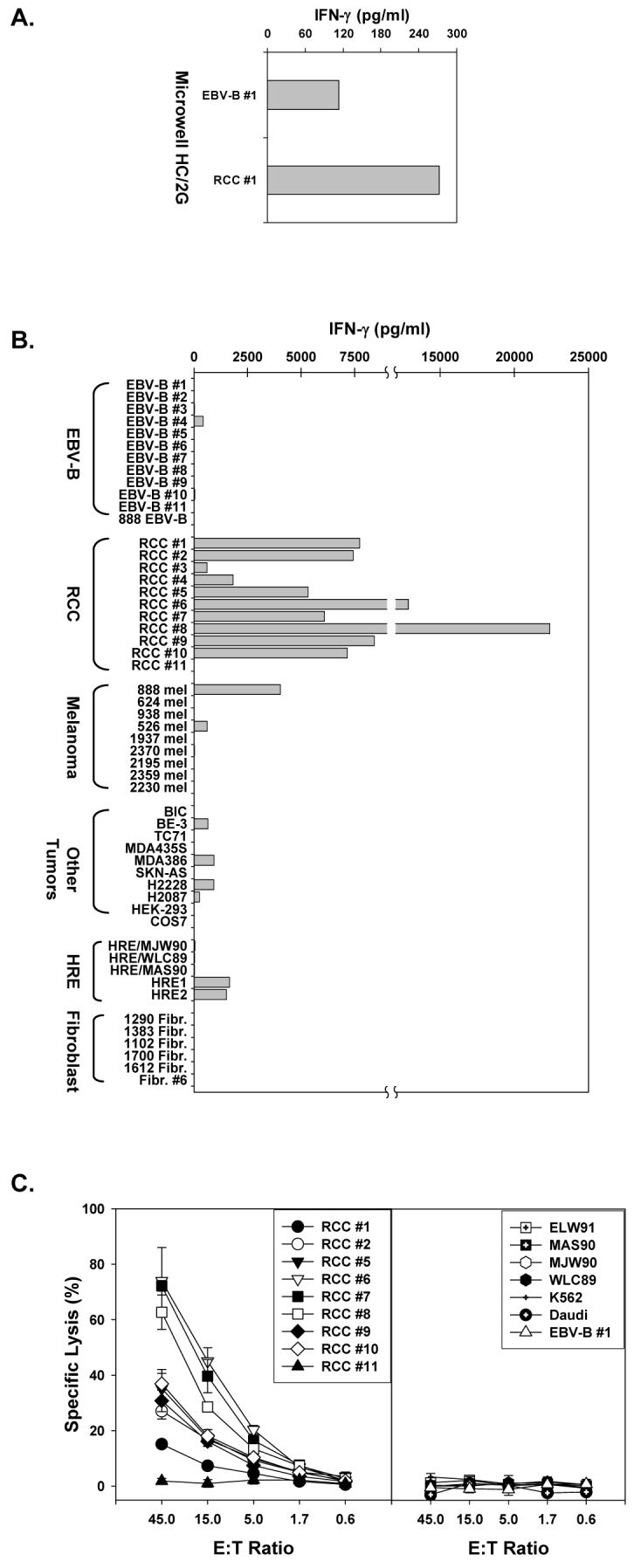
T cell clone HC/2G-1 recognition of renal tumors by IFN-γ secretion and lysis. A. Bulk microwell HC/2G reactivity against autologous tumor by IFN-γ secretion. CD8+ and CD4+CD25- cells (2.5 × 104/well each) were isolated from PBL of a patient with RCC and stimulated twice with autologous DC co-cultured with UV-irradiated tumor cells in the presence of IFN-α. Fourteen days later, stimulated T cells were tested against autologous EBV-B (EBV-B #1) and renal tumor (RCC #1) by IFN-γ secretion. B. Testing of a T cell clone, HC/2G-1, isolated from microwell HC/2G by limiting dilution. HC/2G-1 (5 × 104/well) was co-cultured with autologous renal tumor RCC #1 and a panel of allogeneic renal tumors (RCC #2- #11), their matched EBV-B cell lines, melanoma and other tumor lines, normal human renal epithelial (HRE) lines and fibroblast lines overnight and tested for IFN-γ secretion by ELISA. C. T cell clone HC/2G-1 lyses autologous RCC #1 and allogeneic renal tumors. HC/2G-1 was co-cultured with 51Cr-labeled target cells at different E:T ratio in triplicate for 4 hrs at 37°C. ELW91, MAS90, MJW90 and WLC89 are normal HRE lines.
Characteristics of clone HC/2G-1
Phenotypically, clone HC/2G-1 expressed CD3 and CD4, but did not express CD16, CD161, CD94, NKG2D, or CD244 on its surface (Figure 2A). It did not express CD8. Approximately 5 and 9 percent of HC/2G-1 expressed some degree of CD56 and CD57, respectively, compared to isotype controls. The reactivity of HC/2G-1 was not due to a CD56+ contaminant, because HC/2G-1 cells depleted of CD56+ cells with microbeads showed the same reactivity against renal tumor cells as unseparated HC/2G-1 (data not shown).
Figure 2.

The reactivity of HC/2G-1 is TCR-dependent, but MHC-, CD4- and B2M independent. A. Immunophenotype of HC/2G-1. HC/2G-1 was stained with FITC-, PE- and APC-labeled MoAbs, and analyzed by FACSCalibur. B. Anti-TCR Ab reduces the reactivity of HC/2G-1. HC/2G-1 was co-cultured with RCC#1 in the presence of anti-HLA class I, HLA class II, TCRα/β and CD4 Abs overnight, and tested for IFN-γ secretion. Control T cells include a RCC-reactive HLA-B44 restricted CTL clone (MW/5H-5) and an HLA-class II restricted CD4 T cell clone (HC/10C-3) co-cultured with their autologous tumors. C. The reactivity of HC/2G-1 is B2M independent. Left: HLA class I and II expression on RCC #12, RCC #12 transduced with CIITA and RCC #12 transduced with both CIITA and B2M. Tumor cells were stained with FITC-labeled anti-HLA class I (W6/32) or PE-labeled anti-HLA DR MoAb and analyzed by FACSCalibur. Open: isotype control; Shaded: anti-HLA class I or HLA DR. Right: HC/2G-1 was co-cultured with RCC #12, RCC#12/CIITA, and RCC #12/CIITA/B2M overnight, and tested for IFN-γ secretion. RCC#8, RCC#6 and RCC #11 were included as controls. D. CD1d expression on RCC tumor lines. RCC tumor lines were stained with PE-labeled anti-CD1d Ab or isotype control and analyzed by FACSCalibur. Open: isotype control; Shaded: CD1d. MOLT4 was used as a positive control cell line.
Characterization of HC/2G-1 TCR expression by surface staining with fluorescence-labeled monoclonal antibodies (MoAb) showed surface expression of TCRα/β but not TCR γ/δ (Figure 2A). Blocking MoAb against HLA class I, HLA class II, TCRα/β, and CD4 were evaluated in the recognition of autologous tumor by HC/2G-1. Anti-TCRα/β MoAb blocked reactivity, and a small effect compared to positive controls was seen with anti-HLA class I MoAb, but no effect was seen with anti-HLA class II and anti-CD4 MoAbs (Figure 2B). This result suggests that HC/2G-1 recognition of renal tumor is mediated by the α/β TCR, but is not classically MHC-class I or class II restricted (as supported by its broad alloreactivity versus RCCs). The minor effect of the anti-HLA class I MoAb suggested that a related presenting molecule may have a role, as is seen for some NKT cells. Therefore, we investigated if HC/2G-1 recognition was dependent on B2M by testing HC/2G-1 against a renal tumor cell line (RCC #12) that is naturally B2M deficient and does not express HLA class I on its surface unless B2M is retrovirally introduced (Figure 2C). The B2M-deficient RCC line was well recognized by HC/2G-1, and induction of HLA class I or HLA class II expression by retroviral transduction of B2M or CIITA, respectively, did not affect this recognition (Figure 2C). This observation was also confirmed by testing HC/2G-1 reactivity against two well-recognized renal tumors after silencing B2M gene expression by introducing two B2M-specific short-hairpin RNAs (shRNAs). There were no differences in recognition between the two native RCC lines and their B2M -suppressed variants (data not shown). Furthermore, no recognized RCC tumor expressed CD1d or other CD1 molecules on their surface by flow analysis (Figure 2D and data not shown), which excludes the possible involvement of CD1 molecules in tumor recognition by HC/2G-1.
Tumor recognition of HC/2G-1 is mediated by TCR
To further delineate the role of TCRα/β in tumor recognition by HC/2G-1, we isolated total RNA from HC/2G-1, amplified cDNAs encoding the TCR α and β chains by 5′RACE, and cloned these cDNAs into the pCR2.1 vector. Only a pair of TCR α and β chain were identified, and these were Vα14 and Vβ2.1 (GeneBank ID# EF101778; EF101779). This supports the clonal nature of the HC/2G-1 population. To confirm that tumor recognition by HC/2G-1 is mediated by its α/β TCR, we synthesized mRNAs from the TCR α and β chains by in vitro transcription and electroporated them into allogeneic PBL stimulated with anti-CD3 and IL-2. The expression of the HC/2G-1 TCR after transfection was determined by Vβ2 staining when varying amounts of mRNAs were used for electroporation (Figure 3A). Vβ2 expression increased in T cells transfected with increasing amounts of mRNAs encoding both alpha and beta chain. As shown in Figure 3B, introduction of only the α or β chain alone into stimulated allogeneic T-cells did not result in recognition of RCC #6. Reactivity after transfection with both TCR chains was seen against RCC #6 but not RCC #11 as was also seen with HC/2G-1. We further measured the reactivity of T cells transfected with different amounts of HC/2G-1 TCR α and β mRNAs (Figure 3C). Using 8μg mRNA of each TCR chain per 106 cells resulted in the highest percentage of Vβ2 expression (Figure 3A), and those TCR-transfected T cells recognized multiple renal tumors including RCC #1, 5, 6, 7, 8, and 10. Again, RCC #11, a negative target for HC/2G-1, was not recognized by TCR-transfected T cells. Furthermore, we tested CD8 and CD4 enriched T cell populations independently after transfection with HC/2G-1 TCR mRNAs (Figure 3D). Although TCR-transfected CD8 T cells showed slightly less overall reactivity against renal tumors than TCR-transfected PBL and CD4 T cells, the pattern of induced recognition was similar. The fact that both CD8 and CD4 T cells recognize renal tumors when transfected with HC/2G-1 TCR is in accord with anti-CD4 blocking experiments that showed HC/2G-1 reactivity is CD4 independent.
Figure 3.
Introduction of α and β chains of the HC/2G-1 TCR into anti-CD3-stimulated allogeneic T cells. A. Vβ2 Expression on anti-CD3-stimulated allogeneic T cells electroporated with HC/2G-1 TCR mRNAs. PBL from allogeneic donors were stimulated with OKT-3 (50ng/ml) and IL-2 (300IU/ml) for 2 days, and electroporated with HC/2G-1 TCR α and β mRNAs (2-8μg/106 cells). Electroporated cells were incubated overnight in the presence of IL-2 and Vβ2 expression analyzed by flow cytometry. B. Both TCR alpha and beta chains are required for TCR recognition. OKT-3 stimulated T cells were electroporated with mRNAs of either TCR alpha chain, TCR beta chain or both (4μg each) and co-cultured with RCC #6 overnight and tested for IFN-γ secretion. RCC #11 served as a negative control. C. Allogeneic T cells electroporated with HC/2G-1 TCR mRNAs recognize renal tumors. T cells (1 × 105) electroporated with mRNAs (2-8 μg/106 cells) were co-cultured with renal tumors overnight, and supernatant was harvested and tested for IFN-γ secretion. HC/2G-1 was included in the same assay. D. HC/2G-1 TCR transfection of CD8 and CD4 purified T-cell populations. PBL were separated into CD8 enriched and CD4 enriched cells, and then stimulated by OKT-3 and IL-2. They were then electroporated with HC/2G-1 TCR mRNAs (4μg/ml) and co-cultured with renal tumors overnight, and supernatant was harvested and tested for IFN-γ secretion. RCC#11, HEK293 and 624 mel were served as negative controls in the assay. Vβ2 expression of OKT-3 stimulated PBL, CD8, and CD4 enriched cells were also shown in the figure. The results were representative of more than three allogeneic PBL in independent experiments that gave similar results.
HC/2G-1 reactivity is “modulated by” TRAIL
To delineate the effector molecules involved in tumor recognition, we first assessed CD107a mobilization by HC/2G-1. As shown in Figure 4A, CD107a mobilization occurred when HC/2G-1 was stimulated with autologous tumor RCC #1 and allogeneic tumor RCC #6, but not without stimulation or with negative control renal tumor RCC #11. We further tested granzyme B secretion by HC/2G-1 when stimulated with multiple renal tumors, melanomas or EBV-Bs. Granzyme B was produced by HC/2G-1 with all renal tumors except for RCC #11, which significantly correlated with IFN-γ secretion that was done in the same experiment. (Figure 4B and the inset).
Figure 4.
Both granzyme/perforin and TRAIL are involved in HC/2G-1 tumor recognition. A. CD107a mobilization by HC/2G-1. HC/2G-1 (1 × 105) were co-cultured for 2 hours with autologous renal tumor RCC #1, allogeneic renal tumor RCC #6 and RCC #11 in the presence of FITC-labeled anti-CD107a Ab. CD107a expression was analyzed by FACSCalibur. B. Granzyme B secretion by HC/2G-1. HC/2G-1 (1 × 104 per well) was co-cultured with multiple renal tumors, melanomas and EBV-Bs overnight, and the supernatant was harvested and tested for both granzyme B and IFN-γ secretion. Inset: Correlation between Granzyme B secretion and IFN-γ secretion by HC/2G-1 in the same experiment. C. Top: TRAIL expression on HC/2G-1. HC/2G-1 was labeled with PE-labeled anti-TRAIL Ab. Bottom: anti-TRAIL MoAb reduced HC/2G-1 reactivity. HC/2G-1 (1 × 104 per well) was pretreated with anti-TRAIL MoAb for 30 min, and then co-cultured with RCC#1, RCC#6, and RCC#8 overnight. The supernatant was collected and tested for IFN-γ secretion. The negative control was a melanoma-reactive CTL (JKF-6) that also expresses TRAIL on its surface. D. Cytotoxicity of soluble TRAIL (aa114-281). Left: Renal tumors and melanomas were plated in triplicate in 96-well plates at 5000 per well overnight, and then treated with soluble TRAIL in various concentrations (10, 30, 100, 300, and 1000 ng/ml). 72 hours later, MTT assay was performed. Right: Effect of soluble TRAIL on HC/2G-1 tumor recognition. Renal tumors and melanomas were pre-treated with or without soluble TRAIL (100ng/ml) for 2 hrs, and then co-cultured with HC/2G-1 (1 × 104) overnight. The supernatant was collected and tested for IFN-γ secretion. Inset: Correlation between soluble TRAIL (100ng/ml)-induced cytotoxicity and IFN-γ secretion. All data were representative of more than three independent experiments that gave similar results.
Since several reports have shown that CD4+ T cells could mediate cytotoxicity against targets through a TRAIL mediated pathway (18,19), we explored the possibility of TRAIL involvement in HC/2G-1 tumor recognition. By staining HC/2G-1 with anti-TRAIL MoAb, we found that there is a consistent low level of TRAIL expression on its surface (Figure 2C top). Addition of anti-TRAIL Ab to HC/2G-1 reduced its reactivity by 50%∼100% when co-cultured with renal tumors (Figure 2C bottom), which was not seen in a control melanoma-reactive CTL, JKF6. We further tested whether renal tumors were sensitive to soluble TRAIL (aa114-281) by MTT assay. As shown in Figure 4D (left), both renal tumors and melanomas were resistant to soluble TRAIL treatment, except for one renal tumor line, RCC#7. Surprisingly, when we pre-incubated renal tumors with soluble TRAIL, we detected an enhanced IFN-γ secretion by HC/2G-1 against all renal tumors including RCC #11 (Figure 4D right), while minimal effect was seen in melanomas. Enhancement of immune recognition by HC/2G-1 did not correlate with TRAIL-induced cytotoxicity, as demonstrated in Figure 4D (right, the inset).
Discussion
A diverse array of tumor-reactive CD4 lymphoid cells has been described in mouse and man, including classical MHC-class II restricted T cells, type I and II NKT cells, and NK cells (7,10,20,21). In the present study, we have identified a novel CD4+ T cell clone that is distinct from these relatively well-characterized populations in its phenotype and function. This CD3+CD4+ T cell clone possesses broad reactivity against RCCs with cytokine release, tumor lysis, CD107a mobilization and granzyme/perforin release. However, it differs from classical T cells, in that tumor recognition is not restricted by MHC class I or class II. It is not an NK cell, as it expresses CD3 and TCRα/β, does not express any NK cell markers such as CD16, CD94, CD244 and NKG2D (22,23), and does not lyse NK-sensitive target cells, such as K562 (21). It also differs from type I NKT cells, since it does not express CD161, and its TCR usage is Vα14, Vβ2.1, whereas type I NKT cells use an invariant TCR Vα24 and Vβ11 in humans (21). Furthermore, no sensitive RCC target line expresses CD1d on its surface (Figure 2D). Perhaps its most striking characteristic is the strong bias of HC/2G-1 towards recognition of RCC as opposed to other tumors. In addition, HC/2G-1 appears to discriminate well between RCC and normal tissues. Although two unvalidated commercial renal epithelial lines showed weak recognition, four others, procured from NCI nephrectomy specimens and generated by well-documented methods (17), were not recognized.
Our data prove that tumor recognition by HC/2G-1 is TCR α/β mediated. First, anti-TCRα/β MoAb blocking reduced HC/2G-1 reactivity against autologous tumor; second, when allogeneic T cells were transfected with TCR mRNAs encoding the TCR α and β chains from HC/2G-1, these T cells acquired the RCC recognition pattern of parental HC/2G-1. This was true for CD4+ or CD8+ recipient populations. Neither MHC-class II on tumor, nor CD4 on T cells seems to participate in tumor recognition by HC/2G-1. Another interesting observation is that HC/2G-1 reactivity is B2M independent. It recognizes RCC #12 despite B2M deficiency, and B2M gene expression silencing with shRNA in other RCC lines did not affect tumor recognition (data not shown). This finding argues against the possibility of MHC class Ib molecules such as HLA-E, -F, -G, or -H participating as potential restriction elements, since B2M is required for stability of these molecules on the cell surface (24). Other B2M -dependent molecules, such as CD1d are also not seen on recognized RCC lines and cannot be restriction elements for HC/2G-1. Broad recognition of RCC lines via an α/β TCR suggests an antigen or epitope presented by a relatively non-polymorphic MHC-like presenting entity, but so far, the basis of HC/2G-1 recognition remains unidentified. We are currently making modifications in the CDR2 and CDR3 regions of the HC/2G-1 TCR to determine if these structures are involved in TCR-Ag interaction.
Our data also suggest that HC/2G-1 recognition is modulated by TRAIL. It constitutively expresses surface TRAIL, and blocking antibody to TRAIL resulted in reduced tumor recognition. Previous studies have demonstrated that CD4+ T cells can express TRAIL (18,19,25). These studies also suggest that CD4+ T cells can exhibit cytotoxicity to renal tumors through both TRAIL and granzyme/perforin pathways. Yet for HC/2G-1, TRAIL augments both target lysis and cytokine release. Target cell apoptosis and destruction might be expected to reduce cytokine release by removing antigenic stimulation. Furthermore, we see no positive or negative relationship between RCC susceptibility to soluble TRAIL and the ability to stimulate HC/2G-1. These findings imply that TRAIL on HC/2G-1 may not simply be an effector molecule in addition to granzyme/perforin. One possible explanation is that TRAIL is a co-stimulatory molecule, as demonstrated by Chou AH et al. (26), wherein cross-linking TRAIL on CD4+ T cells with immobilized death receptor 4 (DR4) enhanced proliferation and IFN-γ production by CD4 T cells. However, in their study, soluble TRAIL reduced T-cell stimulation by competing for DR4 whereas we found the opposite. Another possibility is that TRAIL may induce changes in renal tumors such as up-regulating the unidentified restriction element or its presenting molecule, but knowing its restriction element and Ag is needed to study these possibilities. Nonetheless, the involvement of TRAIL may give us a tool to identify populations similar to HC/2G-1 by examining CD3+TRAIL+ cells from different RCC patients, and this study is currently underway.
Recently, the concept of creating tumor-reactive T-cells by genetic introduction of a tumor-reactive TCR into PBL and using those in adoptive therapy was validated in a small clinical study (27). Two of 17 metastatic melanoma patients treated with T-cells retrovirally engineered to express a TCR which recognized the MART-1 melanoma-associated antigen had clinical responses that have persisted for more than a year. Their responses were associated with survival of receptor-expressing transferred T-cells in their blood for weeks to months after transfer. Therefore, finding new TCRs which confer immune recognition of non-melanoma tumors may allow expanded application of adoptive T-cell transfer. The HC/2G-1 TCR is an attractive candidate and has several advantages. It is not constrained by MHC haplotype, and its antigen is expressed by nearly all RCCs tested. Its MHC independent nature should circumvent immune-escape mechanisms in which tumors lose MHC or B2M (28). Current laboratory studies are underway to identify the antigen recognized by HC/2G-1. Because RCC#11 is the only RCC not recognized by HC/2G-1, using RCC#11 as target cells for cDNA library screening may help in understanding the molecular mechanism of HC/2G-1 reactivity. Unfortunately the patient from whom HC/2G-1 was obtained succumbed prior to any systemic therapy, so its anti-tumor efficacy cannot be evaluated directly. Yet new approaches utilizing its cloned TCR may allow us to ascertain whether this novel anti-tumor reactivity can benefit other patients with renal carcinoma.
Acknowledgment
The authors thank Drs. Steven A Rosenberg and Paul Robbins for thoughtful discussions. We also thank Drs. Takashi Inozume, Yangbing Zhao, Mark E Dudley, Cyril Cohen, and Rick Morgan for providing technical support.
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- 1.Palmer DC, Balasubramaniam S, Hanada K, Wrzesinski C, Yu Z, Farid S, Theoret MR, Hwang LN, Klebanoff CA, Gattinoni L, Goldstein AL, Yang JC, Restifo NP. Vaccine-stimulated, adoptively transferred CD8+ T cells traffic indiscriminately and ubiquitously while mediating specific tumor destruction. J.Immunol. 2004;173:7209–7216. doi: 10.4049/jimmunol.173.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol.Immunother. 2005;54:721–728. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 4.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 5.Housseau F, Bright RK, Simonis T, Nishimura MI, Topalian SL. Recognition of a shared human prostate cancer-associated antigen by nonclassical MHC-restricted CD8+ T cells. J.Immunol. 1999;163:6330–6337. [PubMed] [Google Scholar]
- 6.Barnd DL, Lan MS, Metzgar RS, Finn OJ. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc.Natl.Acad.Sci.U.S.A. 1989;86:7159–7163. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smyth MJ, Wallace ME, Nutt SL, Yagita H, Godfrey DI, Hayakawa Y. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. J.Exp.Med. 2005;201:1973–1985. doi: 10.1084/jem.20042280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magarian-Blander J, Ciborowski P, Hsia S, Watkins SC, Finn OJ. Intercellular and intracellular events following the MHC-unrestricted TCR recognition of a tumor-specific peptide epitope on the epithelial antigen MUC1. J.Immunol. 1998;160:3111–3120. [PubMed] [Google Scholar]
- 9.Rohrlich PS, Fazilleau N, Ginhoux F, Firat H, Michel F, Cochet M, Laham N, Roth MP, Pascolo S, Nato F, Coppin H, Charneau P, Danos O, Acuto O, Ehrlich R, Kanellopoulos J, Lemonnier FA. Direct recognition by alphabeta cytolytic T cells of Hfe, a MHC class Ib molecule without antigen-presenting function. Proc.Natl.Acad.Sci.U.S.A. 2005;102:12855–12860. doi: 10.1073/pnas.0502309102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, Hayakawa Y, Godfrey DI, Smyth MJ. Differential antitumor immunity mediated by NKT cell subsets in vivo. J.Exp.Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu K, Idoyaga J, Charalambous A, Fujii S, Bonito A, Mordoh J, Wainstok R, Bai XF, Liu Y, Steinman RM. Innate NKT lymphocytes confer superior adaptive immunity via tumor-capturing dendritic cells. J.Exp.Med. 2005;202:1507–1516. doi: 10.1084/jem.20050956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, Seipp CA, Rogers-Freezer L, Morton KE, White DE, Liewehr DJ, Merino MJ, Rosenberg SA. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J.Clin.Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang QJ, Hanada K, Perry-Lalley D, Bettinotti MP, Karpova T, Khong HT, Yang JC. Generating renal cancer-reactive T cells using dendritic cells (DCs) to present autologous tumor. J.Immunother. 2005;28:551–559. doi: 10.1097/01.cji.0000175495.13476.1f. [DOI] [PubMed] [Google Scholar]
- 14.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat.Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Zheng Z, Cohen CJ, Gattinoni L, Palmer DC, Restifo NP, Rosenberg SA, Morgan RA. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol.Ther. 2006;13:151–159. doi: 10.1016/j.ymthe.2005.07.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, Lee PP. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat.Med. 2003;9:1377–1382. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 17.Detrisac CJ, Sens MA, Garvin AJ, Spicer SS, Sens DA. Tissue culture of human kidney epithelial cells of proximal tubule origin. Kidney Int. 1984;25:383–390. doi: 10.1038/ki.1984.28. [DOI] [PubMed] [Google Scholar]
- 18.Kayagaki N, Yamaguchi N, Nakayama M, Kawasaki A, Akiba H, Okumura K, Yagita H. Involvement of TNF-related apoptosis-inducing ligand in human CD4+ T cell-mediated cytotoxicity. J.Immunol. 1999;162:2639–2647. [PubMed] [Google Scholar]
- 19.Sato K, Niessner A, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J.Exp.Med. 2006;203:239–250. doi: 10.1084/jem.20051062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang RF. The role of MHC class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends Immunol. 2001;22:269–276. doi: 10.1016/s1471-4906(01)01896-8. [DOI] [PubMed] [Google Scholar]
- 21.Papamichail M, Perez SA, Gritzapis AD, Baxevanis CN. Natural killer lymphocytes: biology, development, and function. Cancer Immunol.Immunother. 2004;53:176–186. doi: 10.1007/s00262-003-0478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanier LL. NK cell receptors. Annu.Rev.Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 23.Li P, Morris DL, Willcox BE, Steinle A, Spies T, Strong RK. Complex structure of the activating immunoreceptor NKG2D and its MHC class I-like ligand MICA. Nat.Immunol. 2001;2:443–451. doi: 10.1038/87757. [DOI] [PubMed] [Google Scholar]
- 24.Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat.Rev.Immunol. 2005;5:459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- 25.Kayagaki N, Yamaguchi N, Nakayama M, Eto H, Okumura K, Yagita H. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: A novel mechanism for the antitumor effects of type I IFNs. J.Exp.Med. 1999;189:1451–1460. doi: 10.1084/jem.189.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou AH, Tsai HF, Lin LL, Hsieh SL, Hsu PI, Hsu PN. Enhanced proliferation and increased IFN-gamma production in T cells by signal transduced through TNF-related apoptosis-inducing ligand. J.Immunol. 2001;167:1347–1352. doi: 10.4049/jimmunol.167.3.1347. [DOI] [PubMed] [Google Scholar]
- 27.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khong HT, Wang QJ, Rosenberg SA. Identification of multiple antigens recognized by tumor-infiltrating lymphocytes from a single patient: tumor escape by antigen loss and loss of MHC expression. J.Immunother. 2004;27:184–190. doi: 10.1097/00002371-200405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]




