Summary
For adults with high-risk or recurrent acute lymphoblastic leukemia (ALL) who lack a suitable sibling donor, the decision between autologous (Auto) and unrelated donor (URD) hematopoietic stem cell transplantation (HSCT) is difficult due to variable risks of relapse and treatment-related mortality (TRM). We analyzed data from two transplant registries to determine outcomes between Auto and URD HSCT for 260 adult ALL patients in first (CR1) or second (CR2) complete remission. All patients received a myeloablative conditioning regimen. The median follow-up was 77 (range 12-170) months. TRM at 1 year post-transplant was significantly higher with URD HSCT; however, there were minimal differences in TRM according to disease status. Relapse was higher with Auto HSCT and was increased in patients transplanted in CR2. Five year leukemia-free (37% vs. 39%) and overall (38% vs. 39%) survival rates were similar for Auto HSCT vs. URD HSCT in CR1. There were trends favoring URD HSCT in CR2. The long follow-up in this analysis demonstrated that either Auto or URD HSCT can result in long-term leukemia free and overall survival for adult ALL patients. The optimal time (CR1 vs. CR2) and technique to perform HSCT remains an important clinical question for adult ALL patients.
Keywords: acute lymphoblastic leukemia (ALL), adult, autologous HSCT, unrelated donor HSCT
INTRODUCTION
The overall prognosis for adults with acute lymphoblastic leukemia (ALL) with either high-risk features at diagnosis or with disease that recurs after an initial remission is grave.1-3 There have been several reports suggesting that adults with high-risk ALL in first complete remission or recurrent ALL are best treated with allogeneic hematopoietic stem cell transplantation (HSCT) using bone marrow or blood stem cells from a histocompatible (i.e. HLA-matched) sibling donor.4-9 For adult ALL patients with high-risk features in first complete remission, HLA-matched sibling allogeneic HSCT can yield extended disease-free and overall survival.4,7,9 Similarly, for adult patients with recurrent ALL, there have been single institution reports also suggesting that allogeneic HSCT can improve long-term survival as compared to conventional therapy.10-13
Unfortunately only a minority of adult ALL patients have a suitable, HLA-matched sibling donor. For those patients lacking a HLA-matched sibling donor, HSCT with autologous hematopoietic stem cells14, an unrelated donor (URD) marrow15, or cord blood16 are potential options. Several factors and scenarios arise in the choice between these potential stem cell sources, primarily the relative risks and benefits associated with each procedure.17-19 Autologous HSCT is associated with relatively low treatment-related mortality (TRM)18, but a significantly higher risk of relapse.19 In contrast, allogeneic HSCT from an URD may be delayed until a suitable donor is identified20 and is associated with a significantly higher rate of TRM from complications such as graft failure, graft-versus-host disease (GVHD) and prolonged immunodeficiency.21 However, allogeneic HSCT from URD has been observed to have a significantly lower rate of relapse18 that is attributed to an anti-leukemic effect mediated by T-cells within the allograft. The second factor is the timing of each procedure, as the clinical decision is whether transplantation should be recommended to the high-risk or even standard risk adult ALL patient while in first complete remission (CR) or be reserved until relapse. Although data suggest that survival may be improved with allogeneic HSCT in first CR, a proportion of patients may be cured with conventional therapy alone, and therefore the use of either allogeneic or autologous HSCT is controversial.22
The use of URD HSCT and autologous HSCT for the treatment of ALL in adults has not been compared in any prospective randomized study. There also are limited long-term data on the efficacy of these two procedures. We had previously performed an analysis to determine toxicities and outcome of patients with ALL who underwent either URD HSCT or autologous HSCT and were reported to the National Marrow Donor Program and the Autologous Blood and Marrow Transplant Registry.23 However, the data set of the prior analysis contained both adult and pediatric patients. We performed this analysis, with extended followup, to specifically examine the longterm outcome of adults with ALL in first or second CR, to compare autologous HSCT and allogeneic HSCT from URD using data from these two international bone marrow transplantation registries. The aims of this retrospective analysis were to determine the engraftment, TRM, relapse, and, most importantly, survival using these two treatment options for adult ALL patients. These data provide the long-term follow-up on the treatment of adult ALL with either unrelated donor or autologous bone marrow transplantation.
PATIENTS AND METHODS
Two patient data sets were used for this analysis; the first included URD transplants facilitated through the National Marrow Donor Program (NMDP) and the second, autologous HSCT, with data reported to the Center for International Blood and Marrow Transplant Research (CIBMTR). Analyses were restricted to adults over age 18 years who received transplantation in either first CR (CR1) or second CR (CR2) prior to December 31, 1998. All patients received a myeloablative conditioning regimen. The individual data sets were compiled and verified through the multi-center data collection and audit processes of the NMDP and CIBMTR, respectively.
Patients were categorized by karyotype as high-risk, normal, or other. A high-risk karyotype was defined as possessing t(4;11) or 11q32, t(9;22), t(8;14), t(1;19) or hypodiploidy. Patients were considered evaluable for engraftment if they survived at least 21 days. Engraftment was defined as the time to recovery of greater than 500 neutrophils per μL for three consecutive measurements. Treatment-related mortality is defined as death in continuous complete remission. For analyses of leukemia-free survival (LFS), failures were clinical or hematologic relapses or deaths from any cause; patients alive and in CR were censored at the time of last follow-up. For analyses of overall survival (OS), failure was death from any cause; surviving patients were censored at the date of last contact. Relapse was defined as clinical or hematologic recurrence.
Patient-, disease-, and transplant-related variables for the patient cohorts were described and compared across stem cell source groups (autologous vs. URD) using the chi-square statistic for categorical variables and the Kruskal-Wallis test for continuous variables. Probabilities of TRM and relapse were calculated using cumulative incidence curves to accommodate competing risks. Univariate probabilities of LFS and OS were calculated using the Kaplan-Meier estimator. Estimates of standard error for the survival function were calculated by Greenwood’s formula and 95% confidence intervals (CI) were constructed using log-transformed intervals. Multivariate models were built using a stepwise forward selection technique, using a p-value of 0.05 or less as the criterion for inclusion in the final model. The primary objective was to compare outcomes according to stem cell source; this variable was included in all models. All possible risk factors were checked for proportional hazards using a time-dependent covariate approach. Factors found to have non-proportional hazards were adjusted for in subsequent analyses using time-dependent effects. Since stem cell source had highly non-proportional hazards for LFS and OS, point-wise adjusted LFS probabilities and OS probabilities at five years were estimated and compared between URD and autologous transplants, after controlling for other significant variables. There were no significant interactions between stem cell source and any other variables. However, because of the modest sample size and lack of power to detect such interactions, univariate subgroup analyses were performed comparing Kaplan-Meier estimates of LFS and OS at five years between URD and autologous transplants separately by disease status (CR1 vs. CR2). All p values are two-sided. Analyses were completed with the use of PROC PHREG in SAS software, version 9.1 (SAS Institute).
RESULTS
Patient Characteristics
The comparison included 260 adults ALL patients (Table 1) who underwent either autologous HSCT (N = 101) or URD HSCT (N = 159). The two groups were relatively well matched by sex and age. More patients who received autologous HSCT were in CR1 than patients who received URD HSCT (63% vs. 48%; p = 0.014), and accordingly their median time from diagnosis to transplant was shorter than for URD transplant recipients (6.2 months vs. 7.5 months; p = 0.048). Fewer autologous HSCT used total body irradiation in the conditioning regimen (51% versus 91%; p < 0.0001). Forty-five percent of patients undergoing autologous HSCT received purged autografts; 31% of patients receiving URD HSCT received T-cell depleted allografts. Fifteen percent of patients receiving URD HSCT had a mismatch at either a class I or class II allele with their respective donor. When analyzed for the time when transplants were performed, 75% of autologous HSCT occurred between 1989 and 1995 versus 42% of URD transplants (p < 0.0001). The median follow-up in surviving patients was 77 months (range: 12 – 170 months).
Table 1.
Patient Characteristics
| Autologous HSCT | URD HSCT | p-Value | |
|---|---|---|---|
| Number | 101 | 159 | ----- |
| Age | 28.0 years (18 -51) | 27.4 years (18 – 51) | 0.58 |
| Sex (Male%/Female %) | 67/33 | 62/38 | 0.352 |
| Disease Status at HSCT: | CR1 = 64 (63%) | CR1 = 76 (48%) | 0.014 |
| CR2 = 37 (37%) | CR2 = 83 (52%) | ||
| Time from Dx to HSCT (1CR): | 6.2 months (2 - 40) | 7.5 months (4 – 30) | 0.048 |
| Duration of 1CR (2CR): | 31 months (6-111) | 19 months (6-115) | 0.33 |
| Disease Lineage: | |||
| B-cell | 53 (52%) | 66 (42%) | 0.032 |
| T-cell | 21 (21%) | 25 (16%) | |
| Unclassified | 27 (27%) | 68 (43%) | |
| WBC >= 50,000/uL at Dx: | 24 (14%) | 34 (21%) | 0.036 |
| < 50,000/uL | 69 (68%) | 83 (52%) | |
| Missing | 18 (18%) | 42 (26%) | |
| Karyotype: | |||
| High-risk | 17 (17%) | 44 (28%) | 0.066 |
| Normal | 24 (24%) | 21 (13%) | |
| Other | 16 (16%) | 23 (14%) | |
| Unknown | 44 (44%) | 71 (45%) | |
| Recipient CMV Seropositive: | 45 (45%) | 59 (37%) | 0.232 |
| Conditioning: | |||
| TBI + Cy +/- other | 52 (51%) | 144 (91%) | <0.0001 |
| Bu + Cy +/- other | 40 (40%) | 15 (9%) | |
| Other or unspecified | 9 (9%) | ------ | |
| HLA-matching (A, B, DRB1) | |||
| Class I and II match | ------ | 136 (86%) | |
| Class I mismatch | ------ | 2 (1%) | |
| Class II mismatch | ------ | 18 (11%) | |
| Class I and II mismatch | ------ | 1 (1%) | |
| Missing | ------ | 2 (1%) | |
| Graft Manipulation: | |||
| T-cell depletion | ------ | 31 (19%) | |
| Purged ex-vivo | 45 (45%) | ------ | |
| Date of HSCT: | |||
| 1989 – 1995 | 76 (75%) | 42 (42%) | <0.0001 |
| 1996 – 1998 | 25 (25%) | 93 (58%) | |
| Median follow-up (months) | 74 (12-141) | 84 (36-170) |
Legend: HSCT = hematopoietic stem cell transplant; URD = unrelated donor; CR1 = first complete remission; CR2 = second complete remission; WBC = white blood cell count; Dx = diagnosis; CMV = cytomegalovirus. TBI = total body irradiation; Cy = cyclophosphamide; Bu = busulfan; HLA = human leukocyte antigen.
Engraftment
By day +30 post-transplant, neutrophil recovery was significantly more likely after URD HSCT than autologous HSCT (p < 0.0001). By day +100 nearly all patients had neutrophil counts in excess of 500/μL, and the differences were no longer significant.
Treatment-Related Mortality
The incidence of TRM was higher after URD HSCT at all measured time points, as compared to autologous HSCT (Figure 1). Early (day +100) TRM was 28% vs. 5% (p < 0.001) for recipients of URD HSCT and autologous HSCT, respectively (Table 2). TRM rates were 43% vs. 8% (p < 0.0001) and 45% vs. 9% (p < 0.0001), at 1 year and 2 years post-transplant, respectively. However, the incidence of TRM at one year was not significantly different whether patients were transplanted in CR1 as compared to CR2 (URD: 45% vs. 41%; autologous: 5% vs. 6%). The majority of treatment-related deaths occurred prior to six months post-transplant, and the risk of treatment-related deaths was less than 5% beyond two years post-transplant for both autologous and URD HSCT. As predicted, multivariate analysis showed a significantly higher relative risk (RR) of treatment-related mortality after URD HSCT as compared to autologous HSCT (RR = 4.49, 95% Confidence Interval [CI]: 2.43, 8.29; p < 0.001). The RR for TRM for URD HSCT was increased for recipients over the age of 30 years (RR = 2.57; CI: 1.66, 3.97; p < 0.001) and lower for recipients who were sero-positive for cytomegalovirus (RR = 0.61; CI: 0.39, 0.96; p = 0.033).
Figure 1.
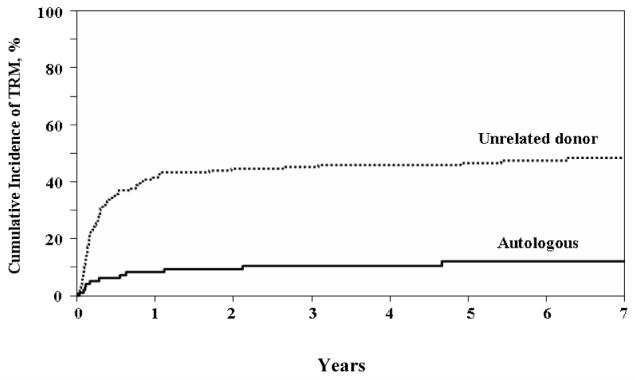
Cumulative Incidence of Treatment-related Mortality (TRM)
Table 2.
Univariate probabilities of outcomes by donor type and disease status.
| Unrelated Donor |
Autologous |
|||||
|---|---|---|---|---|---|---|
| All patients (n = 159) |
CR1 (n = 76) |
CR2 (n = 83) |
All patients (n = 101) |
CR1 (n = 64) |
CR2 (n = 37) |
|
| TRM | ||||||
| @ 100 days | 28% | 23% | 32% | 5% | 5% | 6% |
| @ 1 year | 43% | 45% | 41% | 8% | 9% | 6% |
| @ 2 years | 45% | 47% | 43% | 9% | 12% | |
| Relapse | ||||||
| @ 1 year | 13% | 5% | 21% | 49% | 38% | 69% |
| @ 2 years | 18% | 12% | 23% | 54% | 41% | 75% |
| @ 3 years | 20% | 15% | 25% | 58% | 45% | 81% |
| @ 5 years | 21% | 15% | 26% | 58% | 45% | 81% |
| Leukemia-Free Survival | ||||||
| @ 1 year | 43% | 49% | 38% | 42% | 52% | 24% |
| @ 2 years | 37% | 41% | 33% | 37% | 47% | 19% |
| @ 3 years | 35% | 39% | 31% | 31% | 42% | 14% |
| @ 5 years | 33% | 37% | 29% | 29% | 39% | 14% |
| Overall Survival | ||||||
| @ 1 year | 47% | 51% | 42% | 52% | 59% | 41% |
| @ 2 years | 40% | 45% | 36% | 41% | 51% | 24% |
| @ 3 years | 37% | 42% | 33% | 37% | 48% | 19% |
| @ 5 years | 34% | 38% | 30% | 29% | 39% | 14% |
Legend: CR1 = first complete remission; CR2 = second complete remission; TRM = Treatment-related mortality.
Relapse
The incidence of leukemia recurrence was significantly less after URD HSCT than after autologous HSCT (Figure 2). The incidence of relapse changes very little beyond three and up to 7 years post-transplant both for recipients of URD and autologous HSCT (Table 2). At three years post-transplant the incidence of relapse for recipients of URD HSCT was 20% as compared to 58% for recipients of autologous (p < 0.0001). For patients receiving transplants while in CR1, the incidence of relapse at three years post-transplant was lower for URD recipients as compared to autologous recipients (15% vs. 45%; p < 0.0001). For patients receiving transplants while in CR2, the incidence of relapse at three years post-transplant was also lower for URD recipients as compared to autologous recipients (25% vs. 81%; p < 0.0001). The multivariate adjusted RR of relapse after URD HSCT was 0.32 (CI: 0.2, 0.5; p < 0.001) In addition, the Cox model identified transplantation in CR1 within six months from diagnosis (RR = 1.89; CI: 1.0, 3.57; p = 0.049) and transplantation in CR2 (RR = 1.73; CI; 1.10, 2.94; p = 0.044) as variables significantly associated with increased risk of relapse for both URD and autologous HSCT.
Figure 2.
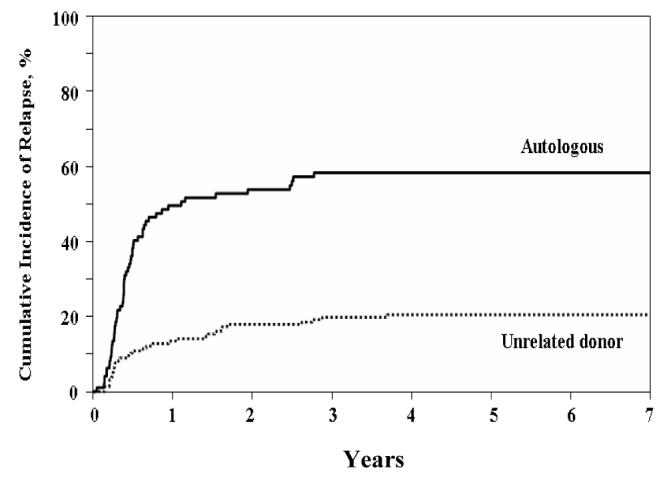
Cumulative Incidence of Relapse
Survival
Leukemia-free survival at five years following transplantation for patients who received an URD HSCT was 33% as compared to 29% (p = 0.5) after autologous HSCT (Figure 3A). At five years following transplantation, LFS for patients transplanted in CR1 was 37% with an URD vs. 39% with an autograft (p = 0.8). For patients who underwent transplantation in CR2, the LFS at 5 years with an URD was 29% as compared to 14% with an autograft (p = 0.04). However, after adjusting for age (≤ 30 years vs. > 30 years), no significant differences in 5 year LFS (p = 0.118) were noted for patients who underwent either URD or autologous HSCT in CR2.
Figure 3.
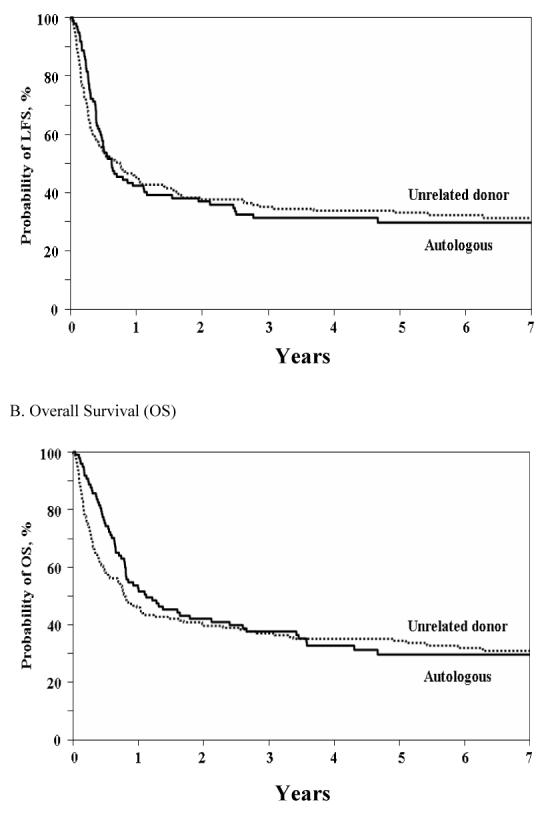
Kaplan-Meier Curves of Leukemia-free and Overall Survival A. Leukemia-free Survival (LFS) B. Overall Survival (OS)
Overall survival at five years following transplantation (Figure 3B) for patients transplanted with URD HSCT was not statistically different from recipients of autologous HSCT (34% vs. 29%; p = 0.46). Similarly at five years following transplantation (Figure 3B), OS for patients transplanted in CR1 was 38% with an URD as compared to 39% with an autograft (p = 0.91). When OS was compared in analyses adjusted for disease status, and timing of transplant in CR1 (Figure 4), OS at 5 years was 34.5% with URD HSCT versus 29% with autologous HSCT (p = 0.38). For patients who underwent transplantation in CR2, the OS with an URD was superior at 30% as compared to 14% with an autograft (p = 0.03). However, similar to analysis of LFS, adjusted analyses showed only a marginal difference in 5 year OS (p = 0.069) for patients who underwent either URD or autologous HSCT in CR2.
Figure 4.
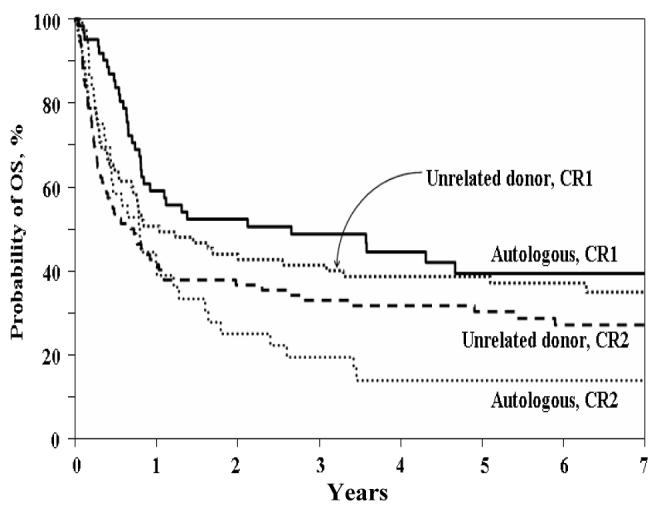
Probability of Overall Survivor (OS) by Donor and Remission Status
DISCUSSION
The clinical question of when and whether to include HSCT in the management of adult ALL remains problematic, and the difficulty is increased when the patient does not have a suitable HLA-matched sibling donor. In this situation the question is further complicated by choice and availability of donor cells, extreme variations in toxicities relative to the specific type of and timing of transplantation.24 The final and most important question is do either of these treatments potentially result in superior long-term survival free of leukemic recurrence. This analysis directly addresses these questions, and in particular provides important information on long-term leukemia-free and overall survival. The analyses are limited by the problems inherent to all registry analyses, primarily the change in care over time, in including improved supportive care measures and HLA-typing. However, when the results are examined focusing on treatment-related mortality and relapse, they are similar to those of recent reports with much shorter follow-up.8,25,26 The significant advantage of this data set is that it provides long-term follow-up, out to seven years, for both autologous and URD HSCT, and may aid both physicians and patients in their clinical decision-making.
The analyses demonstrate that for patients in first CR who lack a suitable HLA-matched sibling or unrelated donor, the option of autologous HSCT can result in prolonged and sustained leukemia-free and overall survival. These results were similar to those adult ALL patients in CR1 who underwent transplant with a well matched unrelated donor. The results of this analysis resemble those from a prior registry analysis on patients with acute myelogenous leukemia in early remission undergoing either autologous or URD HSCT.27
For patients in second CR, there is a suggestion that HSCT from unrelated donor is preferable over an autologous HSCT. However, the assumption that the patient will have a choice of either an unrelated donor or autologous HSCT in CR2 may be only speculative.11,28 Autografts are limited by the requirement for stable CR and suitable graft collection. A well-matched URD is available for a large fraction of patients29, but less so for ethnic and racial minorities. Additionally, the risks of recurrence may limit URD HSCT unless the donor can be procured quickly, before subsequent relapse may occur. However, if a patient achieves a second CR and an unrelated donor can be identified rapidly, URD HSCT results in long-term leukemia-free survival for nearly a third of patients.
A major question that remains is whether to perform either autologous or URD HSCT in CR1 or wait until CR2 to perform an URD HSCT. Major determining factors in this decision are the risk features of the leukemia versus the expected treatment-related mortality. In contrast to previous reports, we observed similar incidence of TRM regardless of whether patients were transplanted in CR1 or CR2. It has been presumed that the incidence of TRM is increased in CR2 due to advanced disease, increased exposure to cytotoxic agents, and older age. Our observation may be of help when weighing the risk of HSCT in CR1 or CR2, especially for patients considering URD HSCT utilizing a myeloablative conditioning regimen.
These data represent extended, long-term follow-up on adult ALL patients receiving either autologous or URD HSCT. It is remarkable that LFS and OS were nearly identical for these two treatments for patient in CR1, despite major differences in both relapse and treatment-related mortality. The data suggest that that the use of an autologous HSCT is still a reasonable option if a patient does not have an HLA-matched sibling. The data also provide the impetus to re-address the issue of autologous HSCT as part of the treatment of ALL in CR1. If TRM were reduced without significantly compromising the anti-leukemic effect associated with URD HSCT, it could be considered as a preferred treatment in both CR1 and CR2. Despite better HLA matching and improved donor selection30, nearly half of adult patients undergoing URD transplantation for ALL die of transplant-associated complications and only one-third of those transplanted in CR1 and one-quarter of those in CR2 survive disease-free.31 The use of high resolution HLA typing and matching at more than 6 HLA loci, specifically including HLA-C, has been demonstrated to improve outcome after URD HSCT32; however, these requirements also decrease the pool of acceptable unrelated donors. If an 8 of 8 HLA-matched unrelated donor is readily available, this may be considered the preferable stem cell source over autologous stem cells. In addition, there is increasing data that the use non-myeloablative and reduced-intensity conditioning regimens may decrease early mortality associated with URD HSCT.33,34 However, there are minimal data on the efficacy of reduced-intensity URD HSCT in adult ALL.35-37 In contrast, the probability of decreasing the relatively high rates of relapse after autografting is less optimistic.38 Additional study will be needed to determine which patients having a defined mix of risk features will be best served by one or the other of these two transplant choices. However, both autologous and URD HSCT offer adult ALL patients options which can result in long-term leukemia-free survival.
Acknowledgments
This research has been supported by funding from the National Marrow Donor Program, the Health Resources and Services administration #240-97-0036 and the Office of Naval Research N00014-96-2-0016 and N00014-99-2-0006 to the National Marrow Donor Program. The views expressed in this article do not reflect the official policy or position of the National Cancer Institute, the Department of the Navy, the Department of Defense, or the U.S. Government.
REFERENCES
- 1.Jabbour EJ, Faderl S, Kantarjian HM. Adult acute lymphoblastic leukemia. Mayo Clin Proc. 2005;80:1517–1527. doi: 10.4065/80.11.1517. [DOI] [PubMed] [Google Scholar]
- 2.Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM, et al. ECOG; MRC/NCRI Adult Leukemia Working Party: Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106:3760–3767. doi: 10.1182/blood-2005-04-1623. [DOI] [PubMed] [Google Scholar]
- 3.Larson RA. Recent clinical trials in acute lymphocytic leukemia by the Cancer and Leukemia Group B. Hematol Oncol Clin North Am. 2000;14:1367–1379. doi: 10.1016/s0889-8588(05)70191-x. [DOI] [PubMed] [Google Scholar]
- 4.Chao NJ, Forman SJ, Schmidt GM, Snyder DS, Amylon MD, Konrad PN, et al. Allogeneic bone marrow transplantation for high-risk acute lymphoblastic leukemia during first complete remission. Blood. 1991;78:1923–1927. [PubMed] [Google Scholar]
- 5.Oh H, Gale RP, Zhang MJ, Ino T, Murakami H, Ohno R, et al. Chemotherapy vs HLA-identical sibling bone marrow transplants for adults with acute lymphoblastic leukemia in first remission. Bone Marrow Transplant. 1998;22:253–257. doi: 10.1038/sj.bmt.1701316. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M-J, Hoelzer D, Horowitz M, Gale RP, Messerer D, Klein JP, et al. Acute Lymphoblastic Leukemia Working Committee Long-term follow-up of adults with acute lymphoblastic leukemia in first remission treated with chemotherapy or bone marrow transplantation. Ann Int Med. 1995;123:428–431. doi: 10.7326/0003-4819-123-6-199509150-00006. [DOI] [PubMed] [Google Scholar]
- 7.Hunault M, Harousseau JL, Delain M, Truchan-Graczyk M, Cahn JY, Witz F, et al. GOELAMS (Groupe Ouest-Est des Leucemies Airgues et Maladies du Sang) Group Better outcome of adult acute lymphoblastic leukemia after early genoidentical allogeneic bone marrow transplantation (BMT) than after late high-dose therapy and autologous BMT: a GOELAMS trial. Blood. 2004;104:3028–3037. doi: 10.1182/blood-2003-10-3560. [DOI] [PubMed] [Google Scholar]
- 8.Kiehl MG, Kraut L, Schwerdtfeger R, Hertenstein B, Remberger M, Kroeger N, et al. Outcome of allogeneic hematopoietic stem-cell transplantation in adult patients with acute lymphoblastic leukemia: no difference in related compared with unrelated transplant in first complete remission. J Clin Oncol. 2004;22:2816–2825. doi: 10.1200/JCO.2004.07.130. [DOI] [PubMed] [Google Scholar]
- 9.Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol. 2004;22:4075–4086. doi: 10.1200/JCO.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 10.Wingard JR, Piantadosi S, Santos GW, Saral R, Vriesendorp HM, Yeager AM, et al. Allogeneic bone marrow transplantation for patients with high-risk acute lymphoblastic leukemia. J Clin Oncol. 1990;8:820–830. doi: 10.1200/JCO.1990.8.5.820. [DOI] [PubMed] [Google Scholar]
- 11.Weisdorf DJ, Woods WG, Nesbit ME, Jr, Uckun F, Dusenbery K, Kim T, et al. Allogeneic bone marrow transplantation for acute lymphoblastic leukaemia: risk factors and clinical outcome. Br J Haematol. 1994;86:62–69. doi: 10.1111/j.1365-2141.1994.tb03253.x. [DOI] [PubMed] [Google Scholar]
- 12.Giona F, Annino L, Rondelli R, Arcese W, Meloni G, Testi AM, et al. Treatment of adults with acute lymphoblastic leukaemia in first bone marrow relapse: results of the ALL R-87 protocol. Br J Haematol. 1997;97:896–903. doi: 10.1046/j.1365-2141.1997.1102926.x. [DOI] [PubMed] [Google Scholar]
- 13.Camera A, Annino L, Chiurazzi F, Fazi P, Cascavilla N, Fabbiano F, et al. GIMEMA ALL - Rescue 97: a salvage strategy for primary refractory or relapsed adult acute lymphoblastic leukemia. Haematologica. 2004;89:145–153. [PubMed] [Google Scholar]
- 14.Simonsson B, Burnett AK, Prentice HG, Hann IH, Brenner MK, Gibson B, et al. Autologous bone marrow transplantation with monoclonal antibody purged marrow for high risk acute lymphoblastic leukemia. Leukemia. 1989;3:631–636. [PubMed] [Google Scholar]
- 15.Schiller G, Feig SA, Territo M, Wolin M, Lill M, Belin T, et al. Treatment of advanced acute leukaemia with allogeneic bone marrow transplantation from unrelated donors. Br J Haematol. 1994;88:72–78. doi: 10.1111/j.1365-2141.1994.tb04979.x. [DOI] [PubMed] [Google Scholar]
- 16.Cornetta K, Laughlin M, Carter S, Wall D, Weinthal J, Delaney C, et al. Umbilical cord blood transplantation in adults: results of the prospective Cord Blood Transplantation (COBLT) Biol Blood Marrow Transplant. 2005;11:149–160. doi: 10.1016/j.bbmt.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Busca A, Anasetti C, Anderson G, Appelbaum FR, Buckner CD, Doney K, et al. Unrelated donor or autologous marrow transplantation for treatment of acute leukemia. Blood. 1994;83:3077–3084. [PubMed] [Google Scholar]
- 18.Ringden O, Labopin M, Gluckman E, Hows JM, Bradley BA, Kolb HJ, et al. Donor search or autografting in patients with acute leukaemia who lack an HLA-identical sibling? A matched-pair analysis. Acute Leukaemia Working Party of the European Cooperative Group for Blood and Marrow Transplantation (EBMT) and the International Marrow Unrelated Search and Transplant (IMUST) Study. Bone Marrow Transplant. 1997;19:963–968. doi: 10.1038/sj.bmt.1700787. [DOI] [PubMed] [Google Scholar]
- 19.Weisdorf DJ, Billett AL, Hannan P, Ritz J, Sallan SE, Steinbuch M, et al. Autologous versus unrelated donor allogeneic marrow transplantation for acute lymphoblastic leukemia. Blood. 1997;90:2962–2968. [PubMed] [Google Scholar]
- 20.Barker JN, Krepski TP, DeFor TE, Davies SM, Wagner JE, Weisdorf DJ. Searching for unrelated donor hematopoietic stem cells: availability and speed of umbilical cord blood versus bone marrow. Biol Blood Marrow Transplant. 2002;8:257–260. doi: 10.1053/bbmt.2002.v8.pm12064362. [DOI] [PubMed] [Google Scholar]
- 21.Cornelissen JJ, Carston M, Kollman C, King R, Dekker AW, Lowenberg B, et al. Unrelated marrow transplantation for adult patients with poor-risk acute lymphoblastic leukemia: strong graft-versus-leukemia effect and risk factors determining outcome. Blood. 2001;97:1572–1577. doi: 10.1182/blood.v97.6.1572. [DOI] [PubMed] [Google Scholar]
- 22.Gale RP, Park RE, Dubois RW, Herzig GP, Hocking WG, Horowitz MM, et al. Delphi-panel analysis of appropriateness of high-dose therapy and bone marrow transplants in adults with acute lymphoblastic leukemia in first remission. Leuk Res. 1998;22:973–981. doi: 10.1016/s0145-2126(98)00085-x. [DOI] [PubMed] [Google Scholar]
- 23.Weisdorf D, Bishop M, Dharan B, Bolwell B, Cahn JY, Cairo M, et al. Autologous versus allogeneic unrelated donor transplantation for acute lymphoblastic leukemia: comparative toxicity and outcomes. Biol Blood Marrow Transplant. 2002;8:213–220. doi: 10.1053/bbmt.2002.v8.pm12014810. [DOI] [PubMed] [Google Scholar]
- 24.Marks DI, Aversa F, Lazarus HM. Alternative donor transplants for adult acute lymphoblastic leukaemia: a comparison of the three major options. Bone Marrow Transplant. 2006;38:467–475. doi: 10.1038/sj.bmt.1705464. [DOI] [PubMed] [Google Scholar]
- 25.Ribera JM, Oriol A, Bethencourt C, Parody R, Hernandez-Rivas JM, Moreno MJ, et al. PETHEMA Group, Spain Comparison of intensive chemotherapy, allogeneic or autologous stem cell transplantation as post-remission treatment for adult patients with high-risk acute lymphoblastic leukemia. Results of the PETHEMA ALL-93 trial. Haematologica. 2005;90:1346–1356. [PubMed] [Google Scholar]
- 26.Dhedin N, Dombret H, Thomas X, Lheritier V, Boiron JM, Rigal-Huguet F, et al. Autologous stem cell transplantation in adults with acute lymphoblastic leukemia in first complete remission: analysis of the LALA-85, -87 and -94 trials. Leukemia. 2006;20:336–344. doi: 10.1038/sj.leu.2404065. [DOI] [PubMed] [Google Scholar]
- 27.Lazarus HM, Pérez WS, Klein JP, Kollman C, Bate-Boyle B, Bredeson CN, Gale RP, et al. Autotransplantation versus HLA-matched unrelated donor transplantation for acute myeloid leukaemia: a retrospective analysis from the Center for International Blood and Marrow Transplant Research. Br J Haemato. 2006;132:755–769. doi: 10.1111/j.1365-2141.2005.05947.x. [DOI] [PubMed] [Google Scholar]
- 28.Thomas DA, Kantarjian H, Smith TL, Koller C, Cortes J, O’Brien S, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer. 1999;86:1216–1230. doi: 10.1002/(sici)1097-0142(19991001)86:7<1216::aid-cncr17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 29.Dodson KL, Coppo PA, Confer DL. The National Marrow Donor Program: improving access to hematopoietic stem cell transplantation. Clin Transpl. 1999:121–127. [PubMed] [Google Scholar]
- 30.Noreen HJ, Yu N, Setterholm M, Ohashi M, Baisch J, Endres R, et al. Validation of DNA-based HLA-A and HLA-B testing of volunteers for a bone marrow registry through parallel testing with serology. Tissue Antigens. 2001;57:221–229. doi: 10.1034/j.1399-0039.2001.057003221.x. [DOI] [PubMed] [Google Scholar]
- 31.Anasetti C, Petersdorf EW, Martin PJ, Woolfrey A, Hansen JA. Trends in transplantation of hematopoietic stem cells from unrelated donors. Curr Opin Hematol. 2001;8:337–341. doi: 10.1097/00062752-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Flomenberg N, Baxter-Lowe LA, Confer D, Fernandez-Vina M, Filipovich A, Horowitz M, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104:1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 33.Lazarus HM, Vogelsang GB, Rowe JM. Prevention and treatment of acute graft-versus-host disease: the old and the new. A report from the Eastern Cooperative Oncology Group (ECOG) Bone Marrow Transplant. 1997;19:577–600. doi: 10.1038/sj.bmt.1700710. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan KM, Dykewicz CA, Longworth DL, Boeckh M, Baden LR, Rubin RH, et al. Preventing opportunistic infections after hematopoietic stem cell transplantation: the centers for disease control and prevention, Infectious Diseases Society of America, and American Society for Blood and Marrow Transplantation practice guidelines and beyond. Hematology Am Soc Hematol Educ Program. 2001:392–421. doi: 10.1182/asheducation-2001.1.392. [DOI] [PubMed] [Google Scholar]
- 35.Chakraverty R, Peggs K, Chopra R, Milligan DW, Kottaridis PD, Verfuerth S, et al. Limiting transplantation-related mortality following unrelated donor stem cell transplantation by using a nonmyeloablative conditioning regimen. Blood. 2002;99:1071–1078. doi: 10.1182/blood.v99.3.1071. [DOI] [PubMed] [Google Scholar]
- 36.Maris MB, Niederwieser D, Sandmaier BM, Storer B, Stuart M, Maloney D, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 37.Martino R, Giralt S, Caballero MD, et al. Allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning in acute lymphoblastic leukemia: a feasibility study. Haematologica. 2003;88:555–560. [PubMed] [Google Scholar]
- 38.Powles R, Sirohi B, Treleaven J, et al. The role of posttransplantation maintenance chemotherapy in improving the outcome of autotransplantation in adult acute lymphoblastic leukemia. Blood. 2002;100:1641–1647. doi: 10.1182/blood-2002-03-0776. [DOI] [PubMed] [Google Scholar]
- 38.Shin HJ, Chung JS, Cho GJ. Imatinib interim therapy between chemotherapeutic cycles and in vivo purging prior to autologous stem cell transplantation, followed by maintenance therapy is a feasible treatment strategy in Philadelphia chromosome-positive acute lymphoblastic leukemia. Bone Marrow Transplant. 2005;36:917–918. doi: 10.1038/sj.bmt.1705144. [DOI] [PubMed] [Google Scholar]


