Abstract
Dementia with Lewy Bodies (DLB) is often characterized by pronounced impairment in visuospatial skills, attention, and executive functions. However, the strength of the phenotypic expression of DLB varies and may be weaker in patients with extensive concomitant Alzheimer’s disease (AD). To determine whether strength of the DLB clinical phenotype impacts cognitive decline, visuospatial and language tests were retrospectively used to predict two-year rate of global cognitive decline in 22 autopsy-confirmed DLB patients (21 with concomitant AD) and 44 autopsy-confirmed “pure” AD patients. Generalized Estimating Equations (GEE) revealed a significant interaction such that poor baseline performances on tests of visuospatial skills were strongly associated with a rapid rate of cognitive decline in DLB but not AD (p < .001). No effect of confrontation naming was found. DLB patients with poor visuospatial skills had fewer neurofibrillary tangles and were more likely to experience visual hallucinations than those with better visuospatial skills. These results suggest that the severity of visuospatial deficits in DLB may identify those facing a particularly malignant disease course and may designate individuals whose clinical syndrome is impacted more by Lewy body formation than AD pathology.
Keywords: Dementia with Lewy bodies, cognitive decline, visuospatial skills, Alzheimer’s disease
Dementia with Lewy bodies (DLB) is now recognized as the second most common cause of dementia in older adults, exceeded only by Alzheimer’s disease [AD; (McKeith et al., 2005)]. DLB shares many clinical features with AD, including the insidious onset of cognitive deficits that gradually worsen over time and ultimately result in complete functional dependence. Comparisons of the rate of cognitive decline in patients with autopsy-confirmed DLB or AD have yielded mixed results with some showing equal rates of cognitive decline (Helmes, Bowler, Merskey, Munoz, & Hachinski, 2003; Heyman et al., 1999; Johnson, Morris, & Galvin, 2005; Stern et al., 2001) and others showing more rapid decline in DLB than in AD (Galasko, Gould, Abramson, & Salmon, 2000; Kraybill et al., 2005; Olichney et al., 1998). Both disorders are marked by substantial individual variation in the progression rate (Helmes et al., 2003; Olichney et al., 1998), but this variability may be more pronounced in patients with DLB than in those with AD (Olichney et al., 1998).
Inconsistent results across studies and marked variation in rate of decline within studies may be related to inclusion of DLB patients whose dementia syndrome is better explained by another process [e.g., extensive AD pathology; (McKeith et al., 2005)]. In addition to the hallmark α-synuclein inclusions (i.e., Lewy bodies) in neocortical, limbic, and subcortical brain regions, the majority of patients with DLB also develop concomitant neurofibrillary tangles (NFT) and neuritic plaques (NP) in the same limbic and neocortical distribution as in AD (Armstrong, Cairns, & Lantos, 1998; Hansen et al., 1990), although fewer NFT are typically found in DLB (Gelb, Oliver, & Gilman, 1999; Hansen et al., 1990). The clinical phenotype associated with DLB appears to be weaker in patients who have extensive NFT (Cormack, Aarsland, Ballard, & Tovee, 2004; Del Ser et al., 2000; Merdes et al., 2003). In patients with Lewy bodies, a clinical syndrome that is strongly characteristic of DLB is associated with greater Lewy body burden (Cormack et al., 2004; Harding, Broe, & Halliday, 2002) and neurochemical disruption (Ballard et al., 2000; Ballard et al., 2002; Perry et al., 1991; Teaktong et al., 2005). Higher Lewy body counts are related to the severity of dementia in DLB (Haroutunian et al., 2000) and a faster rate of cognitive decline in Parkinson’s disease (Aarsland, Perry, Brown, Larsen, & Ballard, 2005). Therefore, patients who exhibit the prototypical clinical syndrome of DLB may decline more rapidly and present with more severe pathology than those who do not.
Visuospatial deficits may be a particularly salient marker of DLB (Tiraboschi et al., 2006). The level of visuospatial impairment found in patients with DLB is disproportionately severe relative to the deficits that they exhibit in other cognitive domains (Aarsland et al., 2003; Hansen et al., 1990; Johnson et al., 2005). Prominent loss of visuospatial abilities is listed among the deficits that compose the cognitive syndrome of DLB (McKeith et al., 2005), and DLB patients consistently exhibit disproportionately severe deficits in visuospatial, visuoperceptual, and construction abilities relative to patients with “pure” AD (Aarsland et al., 2003; Ala, Hughes, Kyrouac, Ghobrial, & Elble, 2001; Galasko, Katzman, Salmon, & Hansen, 1996; Hamilton et al., 2004; Hansen et al., 1990; Johnson et al., 2005; Mori et al., 2000; Salmon et al., 1996). These cognitive indicators of particularly pronounced posterior cortical dysfunction in DLB are paralleled by imaging results showing hypometabolism in parieto-occipital cortex in patients with DLB worse than in those with AD (Higuchi et al., 2000; Imamura et al., 1997; Minoshima et al., 2001). Taken together, these findings suggest that severity of visuospatial dysfunction may be an easily quantifiable marker of those patients in whom the clinical phenotype of DLB is particularly strong and as such may identify the patients at greatest risk for rapid cognitive deterioration due to more extensive Lewy-related pathology.
The present study addressed this hypothesis by examining whether the severity of visuospatial dysfunction at initial presentation predicted subsequent rate of global cognitive decline over a two year period in patients with autopsy-confirmed DLB. Patients with autopsy-confirmed AD were also examined to determine if this relationship was unique to DLB. We hypothesized that performance on measures of visuospatial ability, specifically construction (i.e., Block Design and Clock Drawing Test-copy), would better predict subsequent decline on the DRS for DLB patients compared to AD patients because relatively severe deficits in this domain appear to be indicative of the DLB phenotype. We also examined the relationship between severity of initial language dysfunction (i.e., Boston Naming Test) and subsequent global cognitive decline in both patient groups because some limited success has been achieved in predicting rate of cognitive decline in patients with AD based upon early integrity of language abilities (Berg et al., 1984; Chan, Salmon, Butters, & Johnson, 1995; Chui, Lyness, Sobel, & Schneider, 1994; Mortimer, Ebbitt, Jun, & Finch, 1992). Language is not preferentially affected by DLB (Galasko et al., 1996; Hamilton et al., 2004; Hansen et al., 1990) so no differential predictive utility for this measure was anticipated.
Method
Participants
Patients with autopsy-confirmed DLB or AD who had participated in the University of California, San Diego (UCSD) Alzheimer's Disease Research Center (ADRC) longitudinal project from 1985 to 2002 were included in this study. Eligible participants met the following inclusion criteria: 1) autopsy revealed no significant pathological process (e.g., hippocampal sclerosis, metabolic encephalopathy, or infarct with a clinical history of stroke) other than DLB or AD; 2) the patient was diagnosed with dementia by two senior staff neurologists at the initial ADRC evaluation; 3) the patient scored at least 14 on the Mini-Mental State Exam (MMSE) at the initial evaluation and completed at least two more annual evaluations; and 4) the patient experienced no debilitating medical (e.g., cancer) or psychiatric (e.g., depression) illness during the two-year evaluation period. From a series of 70 patients with pathologically-confirmed DLB, these criteria yielded 22 patients. These 22 patients did not differ from the larger sample with respect to age or education; however, at baseline they were less functionally impaired, had higher MMSE total scores, and lived longer following the baseline evaluation than those DLB patients who did not meet the inclusion criteria. Dementia preceded or accompanied motor signs of parkinsonism in all cases. Each DLB patient was matched on the basis of age, education, and initial MMSE score to two patients with pathologically-confirmed AD. In the event that more than one AD patient was an appropriate match for a DLB patient, the selected patient was randomly chosen.
As expected from the matching procedure, the DLB and AD groups did not differ in age, years of education, or initial MMSE score (see Table 1). There was a higher ratio of men to women in the DLB group than in the AD group, but this difference did not reach statistical significance. Although the groups were not specifically matched for estimated duration of illness or interval between baseline testing and death, they did not differ significantly in this regard. On average, DLB patients died 3.7 ± 3.7 years and AD patients died 3.8 ± 2.4 years after the third evaluation (t = 0.14; p > 0.8; Cohen’s d = −0.03). There were no group differences in caregiver ratings of baseline functional capacity as measured by the Pfeffer Outpatient Disability Scale. The majority of patients died before anticholinesterase medications were widely used so only 14% (3/22) of the DLB patients and 11% (5/44) of the AD patients were taking a cholinesterase inhibitor during the two-year evaluation period.
Table 1.
Baseline demographic characteristics and clinical data [mean (standard deviation)] of patients with Dementia with Lewy bodies (DLB) or Alzheimer's disease (AD).
| DLB | AD | t-test | ||
|---|---|---|---|---|
| n = 22 | n = 44 | |||
| Mean (SD) | Mean (SD) | p-value | Cohen’s d | |
| Age | 73.4 (6.2) | 72.0 (5.6) | > 0.3 | 0.20 |
| Education | 15.0 (3.0) | 14.7 (2.7) | > 0.7 | 0.09 |
| Mini-Mental State Exam (MMSE) | 22.6 (3.2) | 22.6 (3.8) | > 0.9 | 0.01 |
| Sex (men : women) | 14 : 8 | 20 : 24 | > 0.1† | |
| Estimated disease onset to test interval (yrs) | 4.0 (1.7) | 4.1 (2.0) | > 0.8 | 0.08 |
| Test to death interval (yrs) | 5.8 (3.7) | 6.0 (2.4) | > 0.8 | 0.01 |
| Pfeffer Outpatient Disability Scale (0–20) | 11.4 (4.0) | 12.3 (4.3) | > 0.9 | 0.22 |
| Extrapyramidal signs (frequency) | 8/22 | 10/44 | > 0.1† | |
| Visual hallucinations (frequency)* | 2/18 | 2/44 | > 0.3† |
Visual hallucinations were not systematically assessed in the original UCSD ADRC longitudinal study protocol.
Chi-square analysis
Participants and/or their caregivers had provided written informed consent for participation in the longitudinal study after it was explained to them. Informed consent for autopsy was obtained at the time of death from the next of kin.
Procedure
As part of their participation in the ADRC longitudinal project, each patient received a baseline and two subsequent annual evaluations (i.e., each evaluation separated by approximately 12 months) that included comprehensive medical, neurological, neuropsychiatric, and neuropsychological assessments. A trained psychometrist administered the neuropsychological tests to the participants in a quiet, well-lit room. Specific measures of global cognitive function (i.e., DRS), visuospatial ability, specifically construction (i.e., Block Design Test, Clock Drawing Test – Copy), and language (i.e., Boston Naming Test – 30-Item Version) were selected from the larger test battery that is used to assess participants in the ADRC.
Dementia Rating Scale (DRS)
The DRS is a standardized, 144-point mental status examination with subscales for Attention (37 points), Initiation and Perseveration (37 points), Conceptualization (39 points), Construction (6 points), and Memory (25 points). The DRS was administered according to the standardized instructions with the exception that every item was administered to every participant (Mattis, 1988). Total score achieved on the test was the dependent variable.
Block Design Test
The Block Design subtest from the Wechsler Intelligence Test for Children-Revised (WISC-R) is a widely used test of visuospatial abilities, specifically visuoconstruction (Capruso, Hamsher, & Benton, 2006; Lezak, Howieson, Loring, Hannay, & Fischer, 2004), requiring assembly of a series of visual designs using patterned blocks. Each design must be constructed as quickly as possible. The children’s version of the test was used because its level of difficulty is more appropriate for patients with dementia who become frustrated by the more challenging adult version. Raw scores range from 0 to 62 with higher scores indicative of better performance.
Clock Drawing Test - Copy (CDT-Copy)
The Clock Drawing Test-copy condition requires participants to copy a model of the face of a clock with the hands set to show a time of 10 past 11; thus, the task is similar to other drawing tests (e.g., Rey-Osterreith Complex Figure). No semantic knowledge of a clock is necessary for successful performance. Drawings are scored on a 0 to 3 point scale with higher scores indicative of a more accurate rendition. For the purposes of the present study, CDT-Copy scores were dichotomized to reflect intact (3 points) or impaired (< 3 points) performance.
Boston Naming Test (BNT) - 30-Item Version
The BNT requires participants to name objects that are pictured as black-and-white line drawings. The test was administered according to standardized instructions for the 60-item version with the exception that only even-numbered or odd-numbered items were presented. If an item could not be spontaneously named, a semantic cue was provided. The total number of correct responses provided spontaneously and after semantic cues was the measure of interest. Possible scores range from 0 to 30 with higher scores indicative of better performance.
Neuropathologic Examination
Autopsy was performed within 12 hours of death using a protocol previously detailed (Terry, Peck, DeTeresa, Schechter, & Horoupian, 1981). Briefly, the left hemibrain was fixed by immersion in 10% formalin for 5–7 days. Paraffin-embedded blocks from mid-frontal, rostral superior temporal, and inferior parietal neocortex, anterior cingulate gyrus, posterior cingulate gyrus, hippocampus, entorhinal cortex, basal ganglia/substantia innominata, mesencephalon, and pons were cut at 7 µm thickness for hematoxylin-eosin (H & E) and thioflavin-S counts. Total plaques, neuritic plaques, NFT, and Lewy body counts were determined by the same examiner (L.A.H) using the same criteria. A modified Braak stage was obtained for each case using previously detailed methods (Hansen & Samuel, 1997). Briefly, the modified Braak stage involves counting the number of NFT in at least five neuron clusters in layer two of the entorhinal cortex and then averaging the results. Cases with modified Braak stage I to IV have fewer than 18 tangles on average in layer two of the entorhinal cortex and sparse neocortical tangles. Cases assigned to modified Braak stage V have moderate numbers of tangles in at least two neocortical sections, and in modified Braak stage VI, all neocortical areas assessed have at least moderate numbers of tangles.
The DLB cases met consensus criteria for the pathologic diagnosis of DLB based on hematoxylin-eosin (H & E) staining and antiubiquitin immunostaining (McKeith et al., 1996), and anti-α-synuclein immunostaining (McKeith et al., 2005). Cases were only construed as DLB if Lewy bodies were found in the locus ceruleus, substantia nigra, and/or nucleus basalis of Meynert, as well as in the neocortex. Because all cases categorized as DLB had neocortical as well as brainstem Lewy bodies, they would all fall into either the limbic (transitional) or neocortical categories proposed in the 1996 consensus guidelines for the pathologic diagnosis of DLB (McKeith et al., 1996). Further, all DLB cases were neocortical stage 5 or 6 according to the proposed Lewy-body based staging of brain pathology related to sporadic Parkinson’s disease (Braak et al., 2003). Cases were not classified as DLB if Lewy bodies were only found in the amygdala (McKeith et al., 2005).
The neuropathologic diagnosis of AD was based on both NIA-Reagan (1997) and Consortium to Establish a Registry for Alzheimer’s Disease [CERAD; (Mirra et al., 1991)] criteria. Of the DLB patients, the likelihood that dementia was caused by AD was high in 36% (8/22), intermediate in 27% (6/22), and low in 27% (6/22) according to NIA-Reagan criteria. Based on CERAD criteria, the majority of DLB patients also had definite [68% (15/22)] or probable [27% (6/22)] AD. Overall, 95% of the DLB patients had concomitant AD by either NIA-Reagan or CERAD criteria and would conform to what Hansen and colleagues (1990) called Lewy Body Variant of AD. One DLB patient did not meet either NIA-Reagan or CERAD criteria for AD and would conform to what Hansen and colleagues (1990) previously called Diffuse Lewy Body Disease. The majority (91%) of the AD patients met both NIA-Reagan criteria for high likelihood that dementia is caused by AD and CERAD criteria for definite AD. The remaining four patients met criteria either for “high likelihood” or for definite AD. None of the AD cases had Lewy bodies recognized in the neocortex or pigmented brainstem nuclei where they are readily apparent with H & E stain and where they would appear prior to neocortical involvement, except in “amygdala-only” cases (Braak et al., 2003; Rub et al., 2002). The modified Braak stage of the patients with DLB (mean = 3.5 ± 2.1) was significantly lower than that of the patients with AD (mean = 5.6 ± 0.6; Mann-Whitney U = 183.5, p < 0.001).
Statistical Analysis
Group differences in scores achieved on the DRS and the three neuropsychological tests at the baseline evaluation were evaluated with Student’s t-tests, or with non-parametric Mann-Whitney U tests when assumptions for parametric statistics were not met. To reduce the likelihood of Type I error, an alpha level of .01 was adopted for statistical significance in the GEE models. A more liberal alpha level of .05 was adopted for the exploratory analyses that examined characteristics of the DLB patients to reduce the likelihood of Type II error.
Group differences in the rate of progression on the DRS as a function of Baseline scores on the neuropsychological tests were analyzed using the Generalized Estimating Equations (GEE) approach (Liang & Zeger, 1986). GEE is an extension of the General Linear Model and is used to analyze longitudinal, repeated measures data in which multiple observations from the same subject are likely to be correlated. The GEE method accounts for the correlation among observations from the same participant and is robust to misspecification of the unknown correlation structure among the participants’ follow-up measurements. Thus, it provides more efficient and less biased regression parameters than ordinary least square regression methods. The GEE analyses were carried out using the R statistical package (2005).
Three separate GEE models, each with an unspecified correlation structure, were fit to examine the relationship between rate of decline on the DRS and Baseline scores on the Block Design subtest, CDT-Copy, and BNT, respectively. In each model, DRS scores (at Baseline, Evaluation 1, and Evaluation 2) served as the dependent variable and Group (DLB v. AD), Time (Time 1: interval between Baseline and Evaluation 1; Time 2: interval between Baseline and Evaluation 2), and Baseline neuropsychological test score (Block Design, Clock copy, or BNT) were entered as independent variables. Time was treated as a factor rather than as a continuous variable because inspection of the DRS data suggested that decline over time was not linear and because patients were reevaluated at distinct one-year intervals. Each model initially included age, sex, education, and frequency of baseline visual hallucinations and extrapyramidal signs, but these variables were excluded from the final models because their contributions were non-significant. All two-way and three-way interactions were initially included in each model, but were not included in final models when statistically non-significant. A significant three-way interaction indicates that the relationship between the rate of decline on the DRS and Baseline neuropsychological test score is different in the DLB and AD patient groups. The neuropsychological test scores were initially entered as continuous variables; however, the complex three-way interactions between Group, Time, and Baseline cognitive test score cannot be easily represented graphically. Therefore, the models were also computed by splitting performance on the neuropsychological tests at the median, and graphic presentation was facilitated by dividing each group into high and low performers on each test before plotting DRS scores as a function of time. Because the significance of the categorical test models did not differ from the continuous test models, only the categorical test models are presented.
Results
Baseline Cognitive Test Performance
The DLB and AD patient groups did not differ significantly in Baseline scores achieved on the Mattis DRS, the Block Design subtest, the CDT-Copy, or the BNT (see Table 2).
Table 2.
Cognitive test scores [mean (standard deviation)] of patients with Dementia with Lewy bodies (DLB) or Alzheimer's disease (AD) at baseline.
| DLB | AD | t-test | Effect Size | |
|---|---|---|---|---|
| n = 22 | n = 44 | |||
| Mean (SD) | Mean (SD) | p-value | Cohen’s d | |
| Mattis Dementia Rating Scale (0–144) | 109.5 (11.4) | 114.4 (15.4) | > 0.1 | 0.36 |
| WISC-R Block Design Test (0–62) | 15.1 (13.9) | 19.8 (14.1) | > 0.2 | 0.32 |
| Clock Drawing Test - Copy (0–3) | 2.2 (1.0) | 2.4 (0.8) | > 0.4* | 0.22 |
| Boston Naming Test (0–30) | 21.1 (5.7) | 22.1 (5.5) | > 0.4 | 0.18 |
Analyzed with Mann-Whitney nonparametric test.
DLB: n= 16; AD: n = 35
Rate of Global Cognitive Decline
There were no significant group differences in the number of days between the baseline visit and first follow up visit (DLB: mean = 400.3 ± 70.3 days, median = 383 days, range = 292; AD: mean = 414.5 ± 62.4 days, median = 403 days, range = 364; p > 0.4) or between the baseline visit and second follow up visit (DLB: mean = 778.5 ± 77.8 days, median = 769 days, range = 297; AD: mean = 806.7 ± 74.0 days, median = 783.5 days, range = 355; p > 0.2). The rate of decline on the Mattis DRS across the three evaluations is shown for the DLB and AD patients in Figure 1. Patients with DLB lost an average of 17.0 points (SD = 24.2; median = 7.5), and patients with AD an average of 7.9 points (SD = 11.6; median = 7.0), between the Baseline evaluation and Evaluation 1 (i.e., over 1 year). The DLB and AD patients lost an average of 39.3 points (SD = 35.1; median = 27.0) and 23.9 points (SD = 24.7; median = 20.0), respectively, between the Baseline evaluation and Evaluation 2 (i.e., over two years). A GEE analysis indicated a significant effect of Time 1 (z = −4.6; p < 0.001) and Time 2 (z = −6.6; p < 0.001), but no significant Group effect (z = −1.5; p < 0.13) or Group X Time 1 (z = −1.7; p < 0.09) or Group X Time 2 (z = −1.9; p < 0.06) interaction effect, although these indices of differential decline approached significance. Thus, the groups did not decline at a significantly different rate.
Figure 1.
Mean Mattis Dementia Rating Scale (DRS) total scores at Baseline, Evaluation 1, and Evaluation 2 in patients with Dementia with Lewy bodies (DLB) or Alzheimer’s disease (AD). Standard error bars are included.
Prediction of Rate of Global Cognitive Decline
The β coefficients and their standard error (S.E.) from the three GEE models that best fit the relationship between Baseline performance on each neuropsychological test measure and dementia progression are included as SUPPLEMENTARY DATA. Significant two-way interactions and main effects were interpreted in the context of significant three-way interactions. To aid in the visual inspection of the data in Figure 2, the patient groups were divided into subgroups achieving high and low scores based on a split at the median score (in the combined AD and DLB group) for each test. The results did not differ when the Baseline cognitive measures were treated as continuous versus categorical variables; therefore, to maintain continuity with the figures, only details from the categorical analyses are presented.
Figure 2.
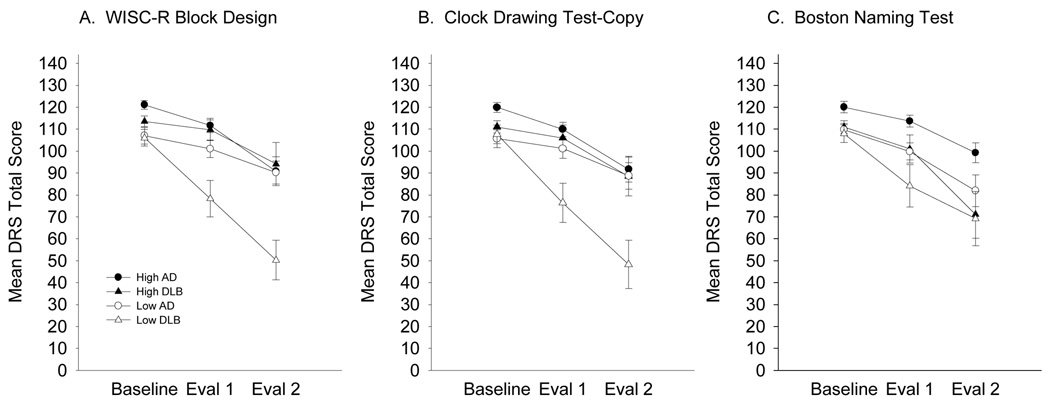
Mean two-year decline on the Mattis Dementia Rating Scale (DRS) as a function of median cognitive test performance at baseline in patients with Dementia with Lewy bodies (DLB: triangles) or Alzheimer’s disease (AD: circles). To graphically represent the complex three-way interactions, patient groups are differentiated by performances above (high: closed symbols) or below (low: open symbols) the overall group median of each cognitive test. Panel A. Wechsler Intelligence Scale for Children-Revised (WISC-R) Block Design score: median = 20. Panel B. Clock Drawing Test—copy: high = 3, low < 3. Panel C. 30-item Boston Naming Test: median = 23. Standard error bars are included.
Visuospatial Abilities
The GEE analysis for the Block Design subtest revealed significant main effects of Test (z = 3.4, p = 0.001), Time 1 (z = −2.7, p < 0.01), and Time 2 (z = −4.6, p < 0.001), significant Group X Time 1 (z = −2.9, p < 0.01) and Group X Time 2 (z = −4.5, p < 0.001) interaction effects, and significant Group X Test X Time 1 (z = 3.1, p < 0.01) and Group X Test X Time 2 (z = 3.4, p < 0.001) interaction effects. The significant three-way interactions indicate that Baseline performance on the Block Design subtest predicts the subsequent rate of decline on the DRS in DLB patients, but not in patients with AD. As Figure 2 (Panel A) shows, DLB patients with poor performance on the Block Design subtest (i.e., below a median score of 20) declined more rapidly over one year and two years than those with better performance. This relationship was not evident in patients with AD.
A remarkably similar result was obtained with the CDT-Copy. The GEE analysis with this test revealed significant main effects of Test (z = 3.0, p < 0.01) and Time 2 (z = −3.8, p < 0.001), significant Group X Time 1 (z = −3.2, p = 0.001) and Group X Time 2 (z = −3.9, p < 0.001) interaction effects, and significant Group X Test X Time 1 (z = 3.5, p < 0.001) and Group X Test X Time 2 (z = 3.3, p = 0.001) interaction effects. The significant three-way interaction effects indicate that Baseline CDT-Copy score predicts the rate of subsequent DRS decline in the DLB patients, but not in those with AD. Figure 2 (Panel B) shows that DLB patients with impaired scores on the CDT-Copy (i.e., scores below 3) declined more rapidly on the DRS than those with intact scores over a one year or two year period. Taken together, these results support the hypothesis that performance on tests of construction predicts the rate of subsequent global cognitive decline in patients with DLB, but not in those with AD.
Language
The GEE model with the BNT revealed a significant main effect of Test (z = 3.0, p < 0.01), Time 1 (z = −5.2, p < 0.001), and Time 2 (z = −8.1, p < 0.001), but no significant Group effect and no significant interaction effects. Figure 2 (Panel C) shows that patients with low BNT scores declined more rapidly as measured by the DRS than those with high scores, and this was true for both the DLB and AD patient groups. The subsequent rate of decline on the DRS was not related to Baseline BNT performance in either group.
Correlates of Visuospatial Performance
Because poor initial construction ability was predictive of a precipitous decline in cognition in the DLB group, post-hoc analyses compared DLB patients with relatively severe constructions deficits (Block Design < 20) to those with relatively mild construction deficits (Block Design ≥ 20). Because of the limited sample size and the exploratory nature of these analyses, a more liberal alpha of .05 was adopted. The groups did not differ statistically with respect to age, education, or functional status; however, there was a trend for the severe DLB patients to score lower on the MMSE compared to the mild DLB patients (Table 3; mean difference −2.6; 95% CI −5.3 to .1; p < .06). Importantly, entry of baseline MMSE score into the GEE models as a covariate did not change the relationship between severe visuospatial deficits and a rapid rate of decline. Therefore, it is unlikely that the difference in MMSE score is clinically meaningful.
Table 3.
Demographic and clinical characteristics of Dementia with Lewy bodies (DLB) patients as a function of Block Design (BD) scores.
| DLB | ||||||
|---|---|---|---|---|---|---|
| BD < 20 | BD ≥ 20 | |||||
| n = 12 | n = 10 | |||||
| Mean (SD) | Mean (SD) | p value | Mean Difference | Std. Error Difference | 95% CI | |
| Age | 71.5 (5.0) | 75.6 (7.1) | >0.1 | 0.8 | 1.7 | −2.7 to 4.2 |
| Education | 14.3 (2.7) | 15.7 (3.2) | >0.3 | −1.9 | 0.8 | −3.4 to 0.3 |
| Sex (men : women) | 9 : 3 | 5 : 5 | >0.2† | |||
| Mini-Mental State Exam (MMSE) | 21.4 (2.8) | 24.0 (3.3) | 0.06 | −2.6 | 1.3 | −5.3 to 0.1 |
| Pfeffer Outpatient Disability Scale (0–20) | 11.3 (4.0) | 11.4 (4.2) | >0.9 | −0.1 | 1.8 | −3.8 to 3.6 |
| Modified Braak stage | 2.6 (2.1) | 4.6 (1.6) | < 0.05‡ | −2.0 | 0.8 | −3.7 to −0.4 |
| Baseline extrapyramidal signs (frequency) | 6/12 | 2/10 | >0.2† | |||
| Baseline visual hallucinations (frequency) * | 2/8 | 0/10 | >0.09† | |||
Visual hallucinations were not systematically assessed in the original UCSD ADRC longitudinal study protocol.
Chi-square analysis
Mann-Whitney analysis
Visual hallucinations were not common at baseline in either DLB group with only 11% (2/18) of the patients or caregivers endorsing them (Table 3). Interestingly, of the patients with DLB whose Block Design performance was more severely impaired, 92% (11/12) experienced visual hallucinations during the course of their illness while only 10% (1/10) of the DLB patients with mildly impaired Block Design performance did so (p < 0.001). Importantly, this pattern was unique to DLB. In the AD group, 9.5% (2/21) of the patients with severe visuospatial deficits and 13% (3/23) of the patients with mild deficits experienced visual hallucinations during the course of their illness.
As detailed in Table 3, DLB patients with severe construction deficits were more likely to exhibit lower NFT counts than those with mild deficits. Eighty-three percent (10/12) of the DLB patients with severe construction impairment compared to 30% (3/10) of the DLB patients with mild impairment had modified Braak stages below five. Lower Braak stage was associated with more rapid cognitive loss over two years in the DLB patients even after accounting for the interval until death (adjusted R2 = 0.23; F(2,19) = 4.9; p < 0.05).
Discussion
The results of the present study indicate that the severity of deficits on tests of visuospatial ability, specifically construction, predicts the rate of ensuing global cognitive decline for patients with DLB but not for patients with AD. Specifically, DLB patients who exhibited severe construction deficits at baseline declined rapidly over the subsequent two years. Such a relationship was not apparent in patients with “pure” AD who progressed at a similar rate regardless of severity of their baseline construction impairment nor was it apparent using a measure of language ability (i.e., the Boston Naming Test). The groups were well-matched in terms of demographics and disease course so these factors are not likely to contribute to the observed differences. Age, baseline MMSE score, education, and sex failed to make a meaningful contribution in any of the GEE models.
The relationship between construction deficits and global cognitive decline was remarkably similar whether the measure was complex and timed (the WISC-R Block Design subtest) or relatively simple and untimed (Clock Drawing Test – Copy). Furthermore, exploratory analyses with a subset of the DLB (n = 16) and AD (n = 35) patients found no difference in simple motor speed and accuracy on the Grooved Pegboard test (DLB: mean = 5.6 ± 2.4 seconds/peg; AD: mean 5.6 ± 4.8 seconds/peg, p > .9). When this measure was entered into a GEE model (data not shown), it was not related to a differential rate of decline within or between the groups. Therefore, it is unlikely that group differences in psychomotor speed or executive functions needed to perform the visuoconstruction tests can account for these results.
These results suggest that DLB patients who exhibit a strong clinical phenotype of the disease (quantified as early, severe visuospatial deficits) experience a more malignant disease course than those who do not. It is important to note that unlike the visuospatial measures, early expression of extrapyramidal signs or visual hallucinations failed to make a meaningful contribution to the prediction models. The mechanism underlying the relationship between construction deficits and global cognitive decline is unknown but may relate to cortical deterioration specific to the disease. Pronounced deficits on tests such as the WISC-R Block Design and Clock Drawing Test are associated with posterior cortical damage (Goodglass & Kaplan, 1983; Warrington, James, & Maciejewski, 1986). Despite the virtual absence of Lewy bodies in the occipital cortex (Gomez-Tortosa et al., 1999; Harding et al., 2002), DLB patients exhibit reduced blood flow in visual association cortex compared to patients with AD (Firbank, Colloby, Burn, McKeith, & O'Brien, 2003), and also exhibit significant reductions in glucose metabolism (Albin et al., 1996; Higuchi et al., 2000; Imamura et al., 1997; Ishii et al., 1998; Minoshima et al., 2001) and blood flow (Lobotesis et al., 2001) in the primary visual cortex. Thus, early evidence of impaired visuospatial ability in DLB may serve as a marker for the integrity of the visual association cortices and may signal their susceptibility to further degeneration.
In addition to rapid progression of dementia, DLB patients with early, severe visuospatial deficits experienced a greater incidence of visual hallucinations than their counterparts with less severe visuospatial deficits, a finding that has been reported previously (Mori et al., 2000; Mosimann & McKeith, 2003). There was a remarkably strong relationship between severe visuospatial deficits and the presence of visual hallucinations in the DLB patients that was not evident in the AD patients. Harding and colleagues (2002) found higher densities of Lewy bodies in parahippocampal and inferior temporal cortices in DLB patients who developed well-formed visual hallucinations early in their disease course, and increasing density of Lewy bodies may be related to more severe dementia (Aarsland et al., 2005; Haroutunian et al., 2000). The relationship between visuospatial deficits and distribution and density of Lewy bodies has not been examined. However, DLB patients with more severe visuospatial deficits carried less NFT burden, suggesting that a process related to the formation of Lewy bodies rather than AD pathology contributed to their clinical syndrome. These results also bolster the contention that the DLB phenotype is strongest in individuals who have minimal NFT (Cormack et al., 2004; Del Ser et al., 2000; McKeith et al., 2005; Merdes et al., 2003).
There was a trend towards a faster rate of decline in the DLB patients compared to the AD patients, consistent with previous studies (Galasko et al., 2000; Kraybill et al., 2005; Olichney et al., 1998). The DLB patients lost, on average, nine more points on the DRS over a one-year period, and 16 more points over a two-year period, than patients with AD. The trend towards greater disparity in the rate of decline between DLB and AD patients as the observation period increased suggests that group differences in rate of progression may have become apparent if additional follow-up evaluations had been available. Consistent with this notion, previous studies that demonstrated a difference in rate of decline followed patients for more than two years (Galasko et al., 2000; Olichney et al., 1998). These studies also included larger numbers of patients than the relatively small sample size in the present study and this may have enhanced their ability to observe a true difference in rate of decline. However, there is a wide variation in rates of cognitive decline in DLB and AD, and many patients overlap. It may be necessary to dissect the relative contributions of underlying pathological mechanisms to understand dementia progression more clearly.
It should also be noted that the current sample of DLB patients did not show the disproportionately severe visuospatial deficit compared to patients with AD that has been previously reported (Ala et al., 2001; Hamilton et al., 2004, Galasko et al., 1996; Johnson et al., 2005; Salmon et al., 1996). Although the DLB patients scored worse than the AD patients on the Block Design subtest and the CDT-Copy at baseline, these differences did not reach statistical significance. The failure to observe such a difference is likely related to the criteria used to select the DLB sample. Unlike previous studies, the DLB and AD patients included in the present study had to complete two annual evaluations beyond baseline and this may have excluded DLB patients with particularly rapid decline. If DLB patients with extremely rapid decline were those most likely to have severe visuospatial deficits, as seen in a case study (Armstrong et al., 1991), the present sample would be skewed towards those with better baseline visuospatial deficits (i.e., more similar to those of patients with AD). In fact, even after equating the groups on baseline MMSE score, our subsample of DLB patients tended to perform better on the Block Design test and the Clock Drawing Test (p’s = .08) than those DLB patients who were excluded from the current study because they were not followed for two additional years.
In summary, the present results are the first to demonstrate that a cognitive marker of visuospatial ability predicts subsequent rate of global cognitive decline in patients with DLB. Although these results require replication in a larger sample of patients, they suggest that DLB patients with prominent visuospatial dysfunction will suffer a particularly malignant course of cognitive deterioration. The relationship between visuospatial dysfunction and subsequent cognitive decline appears to be unique to DLB, a finding that is bolstered by the inclusion of autopsy-verified AD patients in the present study. The mechanism underlying this relationship remains unknown. However, visuospatial deficits may indicate the extent or severity of neuronal damage due to Lewy body pathology and may signal the vulnerability of the association cortex to further deterioration. Future research that directly examines the integrity of the posterior association cortex in patients with DLB through functional or structural imaging could help to determine if pathological change within this region is the underlying anatomical marker for the rate of subsequent global cognitive decline.
Supplementary Material
Acknowledgment
The results of this study were presented by J.M.H. at the July, 2004, meeting of the Alzheimer’s Association in Philadelphia, PA. The study was supported by NIH grants NS049298, AG12963 and AG05131. We thank Mary Tran and the participants and staff of the UCSD Alzheimer’s Disease Research Center.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/neu/
References
- Aarsland D, Litvan I, Salmon D, Galasko D, Wentzel-Larsen T, Larsen JP. Performance on the dementia rating scale in Parkinson's disease with dementia and dementia with Lewy bodies: comparison with progressive supranuclear palsy and Alzheimer's disease. Journal of Neurology, Neurosurgery, & Psychiatry. 2003;74(9):1215–1220. doi: 10.1136/jnnp.74.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsland D, Perry R, Brown A, Larsen JP, Ballard C. Neuropathology of dementia in Parkinson's disease: a prospective, community-based study. Annals of Neurology. 2005;58(5):773–776. doi: 10.1002/ana.20635. [DOI] [PubMed] [Google Scholar]
- Ala TA, Hughes LF, Kyrouac GA, Ghobrial MW, Elble RJ. Pentagon copying is more impaired in dementia with Lewy bodies than in Alzheimer's disease. Journal of Neurology, Neurosurgery, & Psychiatry. 2001;70:483–488. doi: 10.1136/jnnp.70.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Minoshima S, D'Amato CJ, Frey KA, Kuhl DA, Sima AAF. Fluoro-deoxyglucose positron emission tomography in diffuse Lewy body disease. Neurology. 1996;47:462–466. doi: 10.1212/wnl.47.2.462. [DOI] [PubMed] [Google Scholar]
- Armstrong RA, Cairns NJ, Lantos PL. The spatial patterns of Lewy bodies, senile plaques, and neurofibrillary tangles in dementia with Lewy bodies. Experimental Neurology. 1998;150(1):122–127. doi: 10.1006/exnr.1997.6761. [DOI] [PubMed] [Google Scholar]
- Armstrong TP, Hansen LA, Salmon DP, Masliah E, Pay M, Kunin JM, et al. Rapidly progressive dementia in a patient with the Lewy body variant of Alzheimer's disease. Neurology. 1991;41(8):1178–1180. doi: 10.1212/wnl.41.8.1178. [DOI] [PubMed] [Google Scholar]
- Ballard C, Piggott M, Johnson M, Cairns N, Perry R, McKeith I, et al. Delusions associated with elevated muscarinic binding in dementia with Lewy bodies. Annals of Neurology. 2000;48(6):868–876. [PubMed] [Google Scholar]
- Ballard CG, Court JA, Piggott M, Johnson M, O'Brien J, McKeith I, et al. Disturbances of consciousness in dementia with Lewy bodies associated with alteration in nicotinic receptor binding in the temporal cortex. Conscious Cognition. 2002;11(3):461–474. doi: 10.1016/s1053-8100(02)00013-2. [DOI] [PubMed] [Google Scholar]
- Berg L, Danziger WL, Storandt M, Coben LA, Gado M, Hughes CP, et al. Predictive features in mild senile dementia of the Alzheimer type. Neurology. 1984;34(5):563–569. doi: 10.1212/wnl.34.5.563. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiology of Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Capruso DX, Hamsher Kd, Benton AL. Clinical Evaluation of Visual Perception and Constructional Ability. In: Snyder PD, Robins DL, editors. Clinical neuropsychology: A pocket handbook for assessment. 2nd ed. Washington, DC, US: American Psychological Association; 2006. pp. 547–571. [Google Scholar]
- Chan AS, Salmon DP, Butters N, Johnson SA. Semantic network abnormality predicts rate of cognitive decline in patients with probable Alzheimer's disease. Journal of the International Neuropsychological Society. 1995;1(3):297–303. doi: 10.1017/s1355617700000291. [DOI] [PubMed] [Google Scholar]
- Chui HC, Lyness SA, Sobel E, Schneider LS. Extrapyramidal signs and psychiatric symptoms predict faster cognitive decline in Alzheimer's disease. Archives of Neurology. 1994;51(7):676–681. doi: 10.1001/archneur.1994.00540190056015. [DOI] [PubMed] [Google Scholar]
- Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiology of Aging. 1997;18(4 Suppl):S1–S2. [PubMed] [Google Scholar]
- Cormack F, Aarsland D, Ballard C, Tovee MJ. Pentagon drawing and neuropsychological performance in Dementia with Lewy Bodies, Alzheimer's disease, Parkinson's disease and Parkinson's disease with dementia. International Journal of Geriatric Psychiatry. 2004;19(4):371–377. doi: 10.1002/gps.1094. [DOI] [PubMed] [Google Scholar]
- Del Ser T, McKeith I, Anand R, Cicin-Sain A, Ferrara R, Spiegel R. Dementia with lewy bodies: findings from an international multicentre study. International Journal of Geriatric Psychiatry. 2000;15(11):1034–1045. doi: 10.1002/1099-1166(200011)15:11<1034::aid-gps231>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Firbank MJ, Colloby SJ, Burn DJ, McKeith IG, O'Brien JT. Regional cerebral blood flow in Parkinson's disease with and without dementia. Neuroimage. 2003;20(2):1309–1319. doi: 10.1016/S1053-8119(03)00364-1. [DOI] [PubMed] [Google Scholar]
- Galasko D, Katzman R, Salmon DP, Hansen L. Clinical and neuropathological findings in Lewy body dementias. Brain and Cognition. 1996;31(2):166–175. doi: 10.1006/brcg.1996.0040. [DOI] [PubMed] [Google Scholar]
- Galasko DR, Gould RL, Abramson IS, Salmon DP. Measuring cognitive change in a cohort of patients with Alzheimer's disease. Statistics in Medicine. 2000;19(11–12):1421–1432. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1421::aid-sim434>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Archives of Neurology. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- Gomez-Tortosa E, Newell K, Irizarry MC, Albert M, Growdon JH, Hyman BT. Clinical and quantitative pathologic correlates of dementia with Lewy bodies. Neurology. 1999;53(6):1284–1291. doi: 10.1212/wnl.53.6.1284. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Assessment of aphasia and related disorders. 2nd ed. Philadelphia: Lea and Febiger. Distributed by Psychological Assessment Resources, Odessa, FL; 1983. [Google Scholar]
- Hamilton JM, Salmon DP, Galasko D, Delis DC, Hansen LA, Masliah E, et al. A comparison of episodic memory deficits in neuropathologically-confirmed Dementia with Lewy bodies and Alzheimer's disease. Journal of the International Neuropsychological Society. 2004;10(5):689–697. doi: 10.1017/S1355617704105043. [DOI] [PubMed] [Google Scholar]
- Hansen L, Salmon D, Galasko DR, Masliah E, Katzman R, DeTeresa R, et al. The Lewy body variant of Alzheimer's disease: A clinical and pathologic entity. Neurology. 1990;40:1–8. doi: 10.1212/wnl.40.1.1. [DOI] [PubMed] [Google Scholar]
- Hansen L, Samuel W. Criteria for Alzheimer's disease and the nosology of Dementia with Lewy bodies. Neurology. 1997;48:126–132. doi: 10.1212/wnl.48.1.126. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Broe GA, Halliday GM. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain. 2002;125(Pt2):391–403. doi: 10.1093/brain/awf033. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Serby M, Dushyant P, Purohit DP, Perl DP, Marin D, et al. Contributions of Lewy body inclusions to dementia in patients with and without Alzheimer's disease neuropathological conditions. Archives of Neurology. 2000;57:1145–1150. doi: 10.1001/archneur.57.8.1145. [DOI] [PubMed] [Google Scholar]
- Helmes E, Bowler JV, Merskey H, Munoz DG, Hachinski VC. Rates of cognitive decline in Alzheimer's disease and dementia with Lewy bodies. Dementia and Geriatric Cognitive Disorders. 2003;15(2):67–71. doi: 10.1159/000067969. [DOI] [PubMed] [Google Scholar]
- Heyman A, Fillenbaum GG, Gearing M, Mirra SS, Welsh-Bohmer KA, Peterson B, et al. Comparison of Lewy body variant of Alzheimer's disease with pure Alzheimer's disease: Consortium to establish a registry for Alzheimer's disease, part XIX. Neurology. 1999;52(9):1839–1844. doi: 10.1212/wnl.52.9.1839. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Tashiro M, Arai H, Okamura N, Hara S, Higuchi S, et al. Glucose hypometabolism and neuropathological correlates in brains of dementia with Lewy bodies. Experimental Neurology. 2000;162(2):247–256. doi: 10.1006/exnr.2000.7342. [DOI] [PubMed] [Google Scholar]
- Imamura T, Ishii K, Sasaki M, Kitagaki H, Yamaji S, Hirono N, et al. Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer's disease: a comparative study using positron emission tomography. Neuroscience Letters. 1997;235(1–2):49–52. doi: 10.1016/s0304-3940(97)00713-1. [DOI] [PubMed] [Google Scholar]
- Ishii K, Imamura T, Sasaki M, Yamaji S, Sakamoto S, Kitagaki H, et al. Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer's disease. Neurology. 1998;51(1):125–130. doi: 10.1212/wnl.51.1.125. [DOI] [PubMed] [Google Scholar]
- Johnson DK, Morris JC, Galvin JE. Verbal and visuospatial deficits in dementia with Lewy bodies. Neurology. 2005;65(8):1232–1238. doi: 10.1212/01.wnl.0000180964.60708.c2. [DOI] [PubMed] [Google Scholar]
- Kraybill ML, Larson EB, Tsuang DW, Teri L, McCormick WC, Bowen JD, et al. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology. 2005;64(12):2069–2073. doi: 10.1212/01.WNL.0000165987.89198.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological assessment. 4th ed. New York, US: Oxford University Press; 2004. [Google Scholar]
- Liang K, Zeger S. Longitudinal data analysis using general linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- Lobotesis K, Fenwick JD, Phipps A, Ryman A, Swann A, Ballard C, et al. Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology. 2001;56(5):643–649. doi: 10.1212/wnl.56.5.643. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale: Professional manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- Merdes AR, Hansen LA, Jeste DV, Galasko D, Hofstetter CR, Ho GJ, et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology. 2003;60(10):1586–1590. doi: 10.1212/01.wnl.0000065889.42856.f2. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Foster NL, Sima A, Frey KA, Albin RL, Kuhl DE. Alzheimer's disease versus dementia with Lewy bodies: Cerebral metabolic distinction with autopsy confirmation. Annals of Neurology. 2001;50(3):358–365. doi: 10.1002/ana.1133. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mori E, Shimomura T, Fujimori M, Hirono N, Imamura T, Hashimoto M, et al. Visuoperceptual impairment in dementia with Lewy bodies. Archives of Neurology. 2000;57(4):489–493. doi: 10.1001/archneur.57.4.489. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, Ebbitt B, Jun SP, Finch MD. Predictors of cognitive and functional progression in patients with probable Alzheimer's disease. Neurology. 1992;42(9):1689–1696. doi: 10.1212/wnl.42.9.1689. [DOI] [PubMed] [Google Scholar]
- Mosimann UP, McKeith IG. Dementia with Lewy bodies--diagnosis and treatment. Swiss Medical Weekly. 2003;133(9–10):131–142. doi: 10.4414/smw.2003.10028. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Galasko D, Salmon DP, Hofstetter CR, Hansen LA, Katzman R, et al. Cognitive decline is faster in Lewy body variant than in Alzheimer's disease. Neurology. 1998;51(2):351–357. doi: 10.1212/wnl.51.2.351. [DOI] [PubMed] [Google Scholar]
- Perry EK, McKeith I, Thompson P, Marshall E, Kerwin J, Jabeen S, et al. Topography, extent, and clinical relevance of neurochemical deficits in dementia of Lewy body type, Parkinson's disease, and Alzheimer's disease. Annals of the New York Academy of Science. 1991;640:197–202. doi: 10.1111/j.1749-6632.1991.tb00217.x. [DOI] [PubMed] [Google Scholar]
- R: A language and environment for statistical computing. The R Foundation for Statistical Computing. 2005 [Google Scholar]
- Rub U, Del Tredici K, Schultz C, Ghebremedhin E, de Vos RA, Jansen SE, et al. Parkinson's disease: the thalamic components of the limbic loop are severely impaired by alpha-synuclein immunopositive inclusion body pathology. Neurobiological Aging. 2002;23(2):245–254. doi: 10.1016/s0197-4580(01)00269-x. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Galasko D, Hansen LA, Masliah E, Butters N, Thal LJ, et al. Neuropsychological deficits associated with diffuse Lewy body disease. Brain and Cognition. 1996;31(2):148–165. doi: 10.1006/brcg.1996.0039. [DOI] [PubMed] [Google Scholar]
- Stern Y, Jacobs D, Goldman J, Gomez-Tortosa E, Hyman BT, Liu Y, et al. An investigation of clinical correlates of Lewy bodies in autopsy-proven Alzheimer disease. Archives of Neurology. 2001;58(3):460–465. doi: 10.1001/archneur.58.3.460. [DOI] [PubMed] [Google Scholar]
- Teaktong T, Piggott MA, McKeith IG, Perry RH, Ballard CG, Perry EK. Muscarinic M2 and M4 receptors in anterior cingulate cortex: relation to neuropsychiatric symptoms in dementia with Lewy bodies. Behavioural Brain Research. 2005;161(2):299–305. doi: 10.1016/j.bbr.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Terry RD, Peck A, DeTeresa R, Schechter R, Horoupian DS. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Annals of Neurology. 1981;10(2):184–192. doi: 10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]
- Tiraboschi P, Salmon DP, Hansen LA, Hofstetter RC, Thal LJ, Corey-Bloom J. What best differentiates Lewy body from Alzheimer's disease in early-stage dementia? Brain. 2006;129(Pt 3):729–735. doi: 10.1093/brain/awh725. [DOI] [PubMed] [Google Scholar]
- Warrington EK, James M, Maciejewski C. The WAIS as a lateralizing and localizing diagnostic instrument. Neuropsychologia. 1986;24:223–239. doi: 10.1016/0028-3932(86)90055-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.