Abstract
Inadequate physical activity is linked to many chronic diseases. However, the mechanisms that tie muscle activity to health are unclear. The peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) controls several exercise-related aspects of muscle function. We propose here mechanisms by which this protein controls muscle plasticity, suppresses a broad inflammatory response and mediates the beneficial effects of exercise.
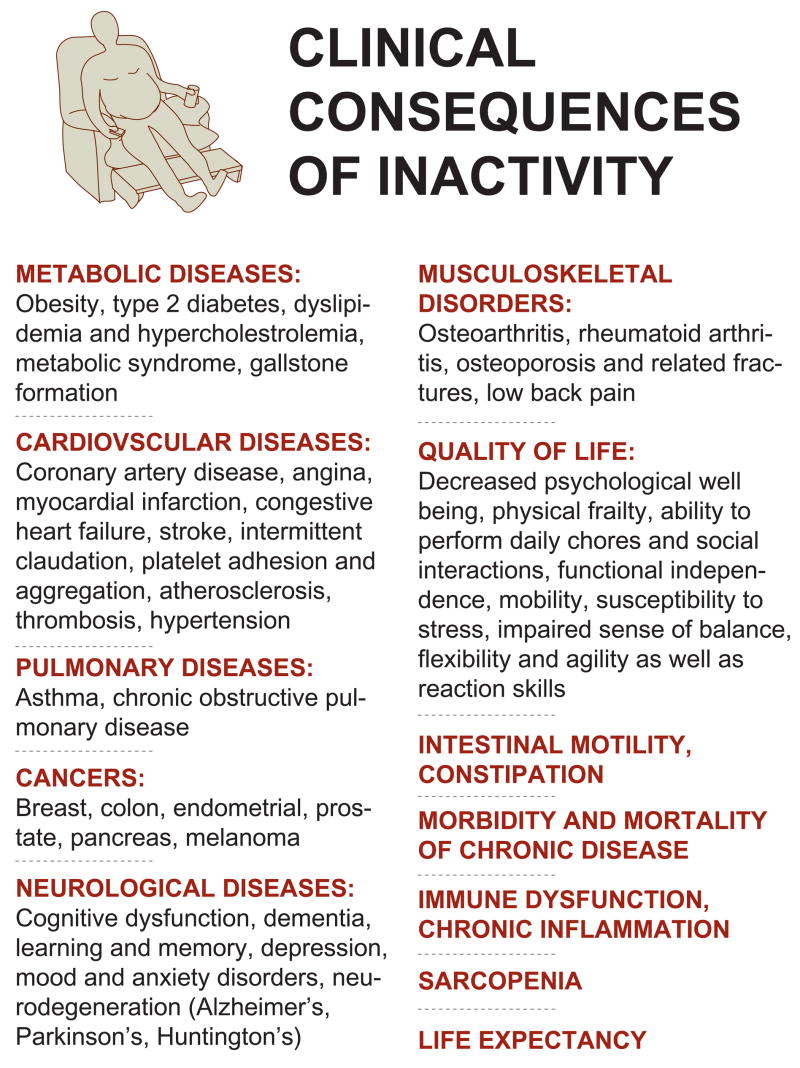
The reduction in physical activity, resulting from shifts in the nature of work and the replacement of muscle with machine in the developed world, has driven a dramatic increase in the incidence of many chronic diseases. In addition to the more obvious consequences associated with reduced activity, such as obesity, cardiovascular diseases, hypertension and type 2 diabetes, lack of sufficient exercise has also been linked to certain important types of cancer, pulmonary diseases, immune dysfunction, musculoskeletal diseases and several types of neurodegenerative disorders1 (Fig. 1). In fact, a sedentary lifestyle is a major risk factor for many chronic pathologies and it has been shown unequivocally that inactivity increases the morbidity and mortality of these diseases2,3. Exercise capacity is a strong predictor of overall mortality, regardless of health and race4. Unfortunately, more than 50% of US adults do not exercise enough to achieve health benefits and 25% of adults shun any form of physical activity in their leisure time (Source: Center for Disease Control and Prevention, www.cdc.gov)5; especially alarming is the rising trend of physical inactivity among young people6. Devastating effects of insufficient physical activity are likewise observed in the elderly7. Decreased muscle function in this population is not only directly linked to sarcopenia and the prevalence of a number of chronic diseases, but contributes enormously to the overall quality of life by diminishing strength, the ability to perform daily chores and social interactions, mobility, cognitive performance and life expectancy7. Even in the early elderly years, changes in physical activity have drastic consequences for health and lifespan. For example, sedentary behaviour in a 70-year-old man reduces the probability of survival to age 90 from 54% to 44%8. In contrast, increasing physical activity is an effective preventative measure for many chronic disorders. Furthermore, exercise is an excellent therapeutic intervention for pathologies such as obesity, type 2 diabetes, neurodegeneration, osteoporosis and sarcopenia1; in terms of efficacy, exercise can rival the effects of drugs that are prescribed for many of these diseases, e.g. type 2 diabetes9.
Figure 1. Clinical consequences of a sedentary lifestyle.
Inactivity is an independent risk factor for a number of chronic diseases regardless of age, gender, race and health.
Inactivity, inflammation and chronic disease
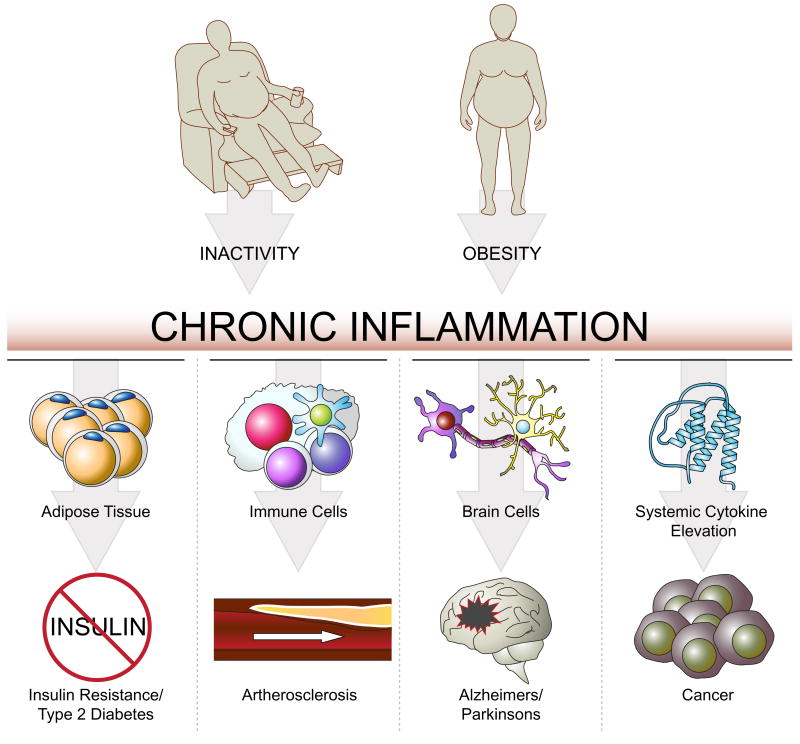
Many chronic diseases have been found to be associated with a sterile, persistent, low-grade inflammation (Fig. 2). For example, the development of insulin resistance and type 2 diabetes tissue is closely correlated with immune cell infiltration and inflammation in white adipose tissue10. In cardiovascular diseases, activated immune cells and inflammation play a major role, particularly in the etiology of atherosclerosis11,12. Importantly, tumor initiation, promotion, and progression is stimulated by systemic elevation of pro-inflammatory cytokines13.
Figure 2. Inflammation and chronic diseases.
A persistent, low-grade inflammatory state of different tissues is linked to the development of many chronic diseases.
A number of neurodegenerative diseases are linked to a local inflammatory response in the brain (neuroinflammation). For example, neuroinflammation influences activation of glia cells and subsequent release of pro-inflammatory cytokines such as tumor necrosis factor α (TNFα); these are thought to promote the death of dopaminergic neurons in the substantia nigra and thereby contribute to the pathology of Parkinson’s disease14,15. Similarly, interleukin-1β (IL-1β), TNF-related apoptosis-inducing ligand (TRAIL) and other cytokines have been postulated to be involved in the etiology of Alzheimer’s disease16, as has amyloid-β, itself exhibiting pro-inflammatory effects17. It is important to note that in addition to the neuroinflammation found in many neurodegenerative disorders, systemic inflammation further exacerbates these diseases and promotes the progression of neurodegeneration18.
Physical activity, inflammation and immunity are tightly linked in an interesting and complex way19. Regular, moderate exercise reduces systemic inflammation20. The mediators of this beneficial effect of exercise are unclear; however, several candidate mechanisms have been identified. First, exercise increases the release of epinephrine, cortisol, growth hormone, prolactin and other factors that have immunomodulatory effects21. Furthermore, exercise results in decreased expression of Toll-like receptor on monocytes suggested to be involved in mediating whole body inflammation22. In contrast to the reduction of chronic inflammation by regular, moderate exercise, prolonged, high intensity training results in increased systemic inflammation and elevated risk of infection20. In fact, subsequent to this type of exercise, athletes exhibit a transient exercise-induced immunodepression23.
The recent discovery of “myokines”, cytokines produced and secreted from skeletal muscle, analogous to “adipokines” made from fat tissue, shed light on this bivalent association between exercise and inflammation19. The first myokine to be described was interleukin-6 (IL-6); similar factors synthesized and secreted upon contraction of muscle fibers include IL-8 and IL-1524. In addition to these muscle-derived cytokines, increased IL-1 receptor antagonist (IL-1ra), IL-10 and TNFα are found in the circulation after exercise24. However, systemic elevation of TNFα is restricted to physical activity of extremely high intensity and therefore could be responsible for the elevated inflammatory state upon prolonged, intense exercise.
Once released transiently into the blood stream, myokines mediate some of the systemic and beneficial effects of exercise in non-muscle tissue, e.g. modulation of hepatic glucose production through IL-6. Some of these cytokines are clearly pro- (e.g. IL-1, TNFα) or anti-inflammatory (e.g. IL-10, IL-1ra). Paradoxically, both pro- and anti-inflammatory effects have been attributed to others25. For example, chronically elevated serum IL-6 levels have a predictive value for obesity and type 2 diabetes. In addition, chronically elevated levels of systemic IL-6, IL-8, IL-10, IL-1 and TNFα have been linked to the development of many diseases associated with inflammation including cancer, and other age-associated diseases, such as sarcopenia, neurodegeneration and depression10,11,13,26–28. Finally, chronic elevation of IL-6 and TNFα results in skeletal muscle atrophy and inhibition of muscle regeneration, respectively29,30. Thus, the transient fluctuations of myokines following physical activity might contribute to the beneficial effects of exercise on organs other than muscle in a hormone-like fashion, whereas chronic elevation of many of these same molecules is almost certainly pro-inflammatory and detrimental. Obviously, then, the increase of IL-6 and other cytokines secreted from muscle in exercise and their subsequent return to basal levels must be tightly regulated.
Effects of endurance and strength training
Distinct exercise regimens are useful for the prevention and treatment of different pathologies. For example, endurance training improves cardiovascular parameters31, strength training reduces sarcopenia32, and the combination of both training regiments was recently reported to be the most beneficial paradigm for type 2 diabetic patients33. For other diseases, the optimal training form remains to be defined. It is clear that sedentary behaviour increases the risk for developing certain types of cancer1,34. Likewise, the type of exercise that confers the greatest protection against neurodegenerative diseases is unknown. Interestingly, moderate exercise (e.g. walking) is sufficient to reduce the risk of developing dementia, as shown in a prospective study with persons 65 years of age or older35.
Mechanistically, resistance training and endurance exercise activate distinct signalling pathways and result in specific adaptations of skeletal muscle. A significant proportion of the capacity for adaptations of skeletal muscle is determined by the relative number and cross-sectional area of different muscle fiber types within a particular muscle bed36. Endurance is improved with increased numbers of type I and type IIa fibers and endurance training increases the number of these fibers. Type I and type IIa fibers are red in appearance and are characterized by a high number of mitochondria, elevated myoglobin and vascularization, and express a specific set of myofibrillar proteins. As a result, they display resistance to fatigue and slow contractions with low peak force generation37. The main source of ATP is the oxidative phosphorylation of glucose and fatty acids. In contrast, the white type IIb fibers (type IIx fibers in humans) have a relatively low number of mitochondria and mainly use anaerobic phosphocreatine and glucose metabolism to generate ATP. These fibers fatigue rapidly, but are able to generate fast contractions with a high peak force37. When stimulated by strength training, type IIb muscle fibers can undergo substantial hypertrophy38.
Regulation and role of the PGC-1 coactivators in skeletal muscle physiology
Contraction of skeletal muscle is initiated by motor neuron-induced calcium signalling. The adaptation of muscle fibers to endurance vs. strength training is mediated by different firing patterns of their respective motor neurons39. Type I and IIa-specific gene expression patterns are achieved by frequent bursts of sarcoplasmic calcium with low amplitude, as seen in endurance training. Strength training results in intermittent rises in sarcoplasmic calcium with high amplitude; this promotes transcription of the genes that mediate a type IIb-specific response and fiber hypertrophy39. Elevated sarcoplasmic calcium, in turn, activates the protein phosphatase calcineurin A (CnA) and the calcium/calmodulin-dependent protein kinases (CaMK), which then alter the phosphorylation state of multiple transcription factors and coactivators40.
This heightened calcium signalling activates several important transcription factors: cAMP responsive element binding protein (CREB), the myocyte enhancer factors 2 (MEF2C and MEF2D) and the nuclear factor of activated T cells (NFAT). This results in altered expression of exercise-regulated muscle genes, particularly the powerful transcriptional coactivator PGC-1α41. Accordingly, PGC-1α expression is rapidly induced by these proteins following a single bout of endurance exercise in vivo42. When physical activity is stopped, PGC-1α mRNA and protein levels quickly revert to the pre-exercised quantity42. In acute bouts of exercise, it is likely that increased expression of PGC-1α is primarily a mechanism for modulating metabolic fluxes in skeletal muscle as a response to decreased ATP and altered fuel demands43. The multi-faceted interaction of PGC-1α with the AMP-activated protein kinase (AMPK) is likely to play a major role in this process44. In contrast, PGC-1α is found at an elevated level in chronically exercised skeletal muscle, even between individual bouts of exercise, when compared to untrained muscle45. This reflects short term vs. long term adaptation of skeletal muscle to endurance exercise.
Thus, changes in muscle plasticity induced by chronic exercise, for example fiber-type switching towards the more oxidative and high endurance type IIa and type I fibers, correlate with an increased basal expression of PGC-1α45. Furthermore, higher levels of PGC-1α are found in oxidative fibers compared to glycolytic fibers, even in a rested state46. Transgenic elevation of PGC-1α in the skeletal muscle of animals up to the levels seen in type I fibers results in a stable and robust fiber-type switch towards both type IIa and type I oxidative fibers46. Individual muscle fibers from these mice are more fatigue resistant, compared to fibers from wild type animals, and transgenic animals perform better in endurance exercise indicating that chronic elevation of PGC-1α mediates many, if not all of the phenotypic changes seen in endurance-trained muscle46,47. The fiber switch promoted by PGC-1α is characterized by increased mitochondrial density and function, increased oxidative metabolism, elevated expression of myofibrillar proteins characteristic of type I and type IIa muscle fibers and a switch in substrate fuel usage46,48. Conversely, animals with PGC-1α specifically ablated from skeletal muscle exhibit a higher number of glycolytic muscle fibers and have a reduced endurance exercise capacity49. Taken together, it is clear that PGC-1α is a key mediator of many of the known beneficial effects of physical activity on skeletal muscle physiology50,51.
Protective effects of PGC-1α in muscle biology: Suppression of chronic inflammation and muscle catabolism
One of the most important effects of exercise in human health is to prevent muscle catabolism and muscle wasting. Limb immobilization, prolonged hospitalization and various muscular dystrophies are conditions where developing an exercised muscle phenotype by the patient would improve the disease and the overall quality of life - but these patients often cannot train effectively. Several studies indicate that PGC-1α prevents protein catabolism and muscle wasting in a number of different contexts. Denervation-induced muscle atrophy, Duchenne muscular dystrophy and muscle damage via treatment with statin drugs are all greatly ameliorated when PGC-1α levels are maintained or elevated52–54.
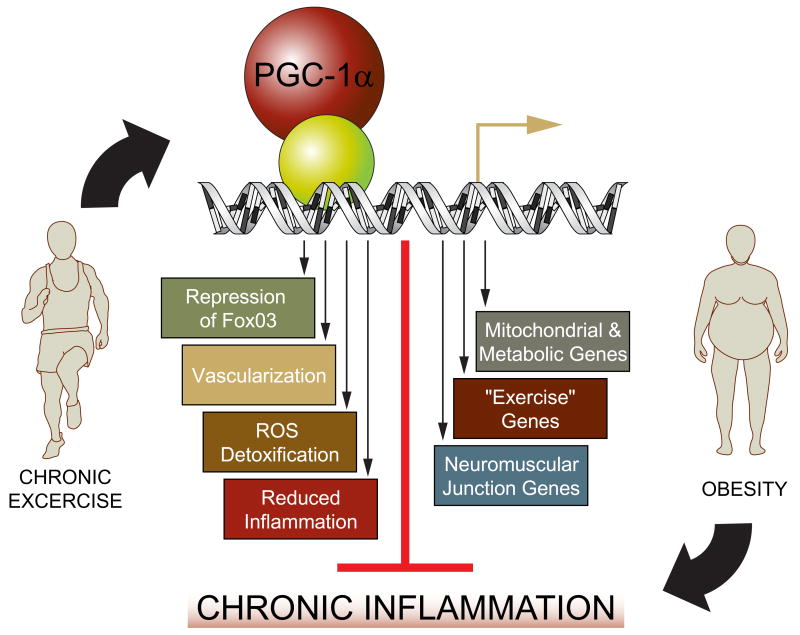
The precise mechanisms by which PGC-1α mediates these beneficial effects are not yet clear, but several possibilities exist (Fig. 3). Elevation of mitochondrial and other metabolic genes, and the resulting correction of the energy crisis associated with muscular dystrophies44,55 are obvious and plausible mechanisms. In addition, reduction of atrophy-specific gene transcription by inhibition of FoxO3 activity54, increase in the gene program for protein synthesis54,56, and stabilization of the postsynaptic side of the neuromuscular junction (NMJ)53 also are likely to contribute to the anti-muscle wasting effect of PGC-1α. In particular, regulation of genes that encode the post-synaptic NMJ by PGC-1α has the potential to ameliorate the pathologies of neuromuscular diseases with decreased NMJ functionality, even those based on primary defects in the motor neuron.
Figure 3. Protective effect of PGC-1α on skeletal muscle.
The relative level of PGC-1α in skeletal muscle is determined by physical activity. PGC-1α, in turn, controls muscle fiber adaptation to exercise and confers a number of beneficial changes. As a result, a reduction of systemic inflammation is observed in exercised individuals, possibly mediated through elevation of PGC-1α. In contrast, inactivity, and thus low skeletal muscle PGC-1α, results in a chronic inflammatory state and thereby causes serious pathological consequences. This inactivity-driven systemic inflammation is further exacerbated by obesity.
A key observation with potential relevance to a much broader set of chronic diseases arose from detailed studies of the animals with muscle-specific ablation of PGC-1α49,57. These showed that loss of PGC-1α specifically in muscle causes a transcriptional induction in muscle for many genes that can be part of local or systemic inflammation49,57. In particular, increased expression of inflammatory marker genes such as IL-6, TNFα, suppressor of cytokine signalling 1 (SOCS1), SOCS3 and CD68 was observed in skeletal muscle of muscle-specific PGC-1α knockout animals in vivo49,57. Mice heterozygous for PGC-1α showed a smaller but significantly elevation expression of many of these same pro-inflammatory genes57. In both cases, chronic elevation of circulating IL-6 was observed57. Primary muscle cells with a genetic ablation of PGC-1α exhibit higher levels of TNF-α and IL-6 mRNA than wild type myotubes57 and elevated IL-6 protein was observed in the culture medium of the PGC-1α knockout cells compared to control cells57. Conversely, adenoviral expression of PGC-1α in C2C12 myotubes in culture reduced the expression of TNFα and IL-6 mRNA57. These data strongly suggest that at least part of the circulating pro-inflammatory cytokines in vivo with ablation of PGC-1α is originating from the muscle cells themselves. Of course, amplification of this program may well involve subsequent recruitment to muscle of immune cells that are “specialists” at amplifying a pro-inflammatory response.
Importantly, mice with a heterozygous mutation in PGC-1α in muscle have a reduction in mRNA expression of this coactivator that is quantitatively comparable to the transcriptional dysregulation of 36% observed in muscle of type 2 diabetic humans compared to healthy volunteers58,59. Furthermore, the drop in PGC-1α expression in muscle-specific heterozygous animals57 and skeletal muscle of type 2 diabetic patients59, respectively, corresponds quantitatively to the decreased expression of PGC-1α in an inactive vs. an active muscle in mice54. Although the establishment of causality between these reduced expression of PGC-1α and the expression of pro-inflammatory genes is not possible in humans, these patients do also exhibit increased transcription of pro-inflammatory genes such as IL-6 and TNFα in skeletal muscle, as well as elevated IL-6 serum concentration57. Thus, the reduction of PGC-1α mRNA in skeletal muscle of type 2 diabetics is likely to be closely linked to the chronic, low-grade inflammation present in these patients. Finally, the lower PGC-1α levels observed in the muscle of pre-diabetic individuals likely contributes to increases in systemic IL-6, a strong predictor for the development of type 2 diabetes60. Indeed, skeletal muscle PGC-1α levels correlate inversely with expression of IL-6 and TNFα in individuals with normal glucose tolerance and in type 2 diabetic patients57. In contrast, body mass index, fasting glucose and fasting insulin levels exhibit no significant correlation to these inflammatory markers in this population57. Taken together, these finding also strongly suggest a causal relationship between the increases in PGC-1α expression observed in human skeletal muscle following physical activity and the reduction of cytokine release from skeletal muscle known to occur with moderate exercise. Conversely, the effects of loss of even one allele of the PGC-1α gene in mice, which stimulates the expression of a broad program of cytokine expression and release, strongly suggests that something very similar is occurring in humans who engage in chronically sedentary behavior. Accordingly, PGC-1α muscle-specific knockout animals exhibit decreased exercise capacity and a fiber-type switch towards glycolytic muscle fibers49.
The molecular mechanisms that link PGC-1α and inflammatory gene expression in muscle are unknown, but they may reflect the role of PGC-1α in the control of reactive oxygen species (ROS). It has previously been shown that PGC-1α has a powerful suppressive effect on ROS production, in parallel to its effects in elevating mitochondrial respiration. This occurs through the PGC-1α-mediated expression of genes involved in ROS detoxification, as well as the expression of uncoupling proteins that can attenuate ROS production61,62. In fact, increased oxidative stress and inflammation are well-known to go hand in hand in many skeletal muscle-associated diseases63. Specifically, ROS have been shown to induce pro-inflammatory cytokine production in skeletal muscle64. Thus, the decreased expression of the anti-ROS genes in muscle-specific PGC-1α knockouts57 are very likely to make a substantial contribution to the increases seen in cytokine expression. Obviously though, there may be additional, more directs effects of PGC-1α on the expression of genes with either pro- or anti-inflammatory action.
Analysis of muscle-specific PGC-1α knockout animals revealed that dysregulation of PGC-1α in skeletal muscle does not cause insulin resistance in this tissue per se, but precipitates abnormal whole body glucose and insulin homeostasis due to reduced insulin levels and abnormal pancreatic islet morphology. This unexpected, distal signalling apparently results from a noxious cross-talk between skeletal muscle and pancreatic β-cells in these animals57. Elevation of systemic inflammation is one likely mechanism by which skeletal muscle with dysregulated PGC-1α expression modulates β-cell function: elevation of IL-6 in the blood of PGC-1α muscle-specific knockout animals correlates with the ability of IL-6 to suppress glucose-stimulated insulin secretion in isolated islets57. These data indicate unambiguously that the levels of skeletal muscle PGC-1α can powerfully influence the function of pancreatic islets; inescapably, these same data also suggest that muscle PGC-1α levels likely affect the structure and functions of other tissues and organs too.
Systemic effects of exercise and PGC-1α
We suggest here that the decrease in PGC-1α gene expression in skeletal muscle due to sedentary behavior can set off a low level but chronic pro-inflammatory response that impacts many other tissues negatively. As noted above, many if not most chronic diseases of aging, including heart disease, cancer and neurodegeneration are associated with chronic inflammation. In many cases that association has been shown to be causal in defined experimental systems. The suppression of chronic inflammation in muscle via exercise-mediated induction of PGC-1α gene expression would be expected to lower the frequency and/or severity of these very same disorders. In terms of clinical data, exercise has many neurological benefits, most notably improvement of learning and memory, protection against neurodegeneration and amelioration of depression as well as other mood disorders17. Cancer of the colon, breast, prostate, endometrium, pancreas and skin all exhibit an increased incidence in inactive individuals, compared to those who exercise1,65. Thus, multi-organ health and plasticity as a result of exercise might be strongly influenced by altered systemic inflammation controlled by skeletal muscle PGC-1α activity.
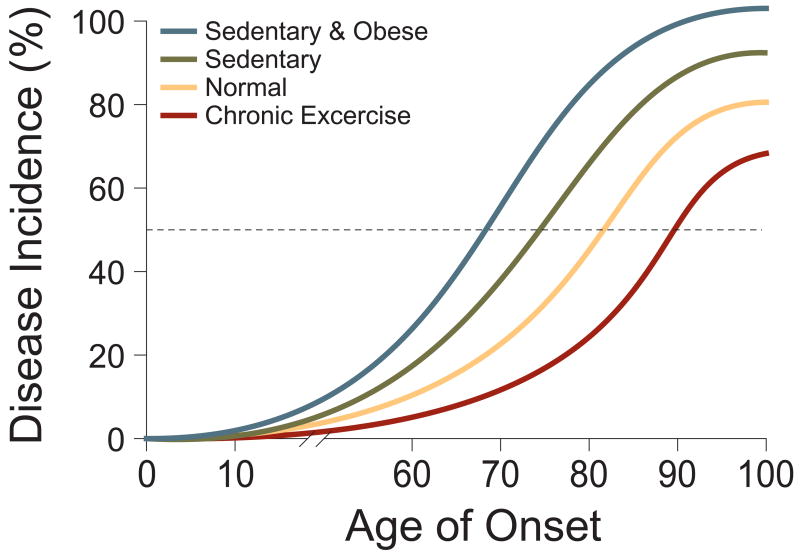
It is important to note that it is very unlikely that any of the chronic diseases being discussed here are caused by reduced PGC-1α alone. Rather, these are multi-factorial diseases requiring multiple hits to yield full-blown disease. The multi-hit nature of human cancer now serves as useful conceptual model for most chronic diseases. These multiple insults may originate in the genetic heritage of an individual patient, may be acquired due to spontaneous somatic mutations, and may also be brought about by environmental or lifestyle factors. Thus, variables such as sedentary behavior and reduced PGC-1α levels in muscle may be viewed, on a population basis, as shifting the likelihood of disease to the left on a standard plot of incidence vs. age, compared to individuals with average exercise/physical activity (Fig 4). For any given individual, it is difficult or impossible to know what impact sedentary behaviour had on their risk for cancer or brain disease, for example. But that it does have an impact on the total population is a certainty. Conversely, populations with above average physical activity and thus increased PGC-1α levels experience a reduced incidence of disease, relative to those with average activity and PGC-1α.
Figure 4. Inactivity and obesity are independent risk factors in the etiology of chronic diseases.
A theoretical depiction of how sedentary lifestyle and obesity lower the threshold for age of onset and disease incidence. Together, inactivity and obesity worsen the relative risk for developing chronic diseases.
Obesity: A most dangerous co-conspirator
Sedentary behavior often coexists with and is a contributing factor in the development of obesity1,66,67. Conversely, obese individuals are less likely to exercise66,67. Furthermore, inactivity and obesity are independent risk factors for many of the same chronic diseases. In fact, inactivity worsens the prevalence of chronic diseases in individuals regardless of their BMI. Thus, being an independent risk factor, inadequate physical activity exacerbates the detrimental effects of obesity1. In keeping with the mechanisms discussed above, we predict a negative interaction between the lack of exercise and obesity in the specific molecular programs discussed here. Obesity has been strongly associated with the expression of a pro-inflammatory program of gene expression including TNFα, IL-1 and IL-610. More precisely, it has been known for more than a decade that adipose tissue, in the context of obesity, begins to secrete elevated levels of these “adipokines”68,69. This is true in both rodents and humans70,71. Moreover, a functional role for TNFα and other adipokines has been shown in the insulin-resistance of obese mice10. If we assume that there is a quantitative threshold of cytokines required both chronically and systemically to bring about pathology in other (non-adipose, non-muscle) tissues, then inactivity combined with obesity is much more likely to reach such a threshold (Fig 4). Furthermore, if the age of onset of a particular disease, or the extent of disease is dose-responsive with respect to the levels of systemic pro-inflammatory molecules, obesity with sedentary behavior would be expected to bring about earlier and/or more severe disease72. Lastly, we do not know the full array of specific cytokines necessary to contribute to the onset of any particular disease, but obesity and sedentary behavior may interact in ways that are not just quantitative. Indeed, they may bring together a particular combination of adipokines/myokines that act synergistically in the causation of disease. How big are the synergistic effects of obesity and sedentary behavior in humans? Like other modifiable factors such as smoking, diabetes and hypertension, obesity is predicted to reduce life expectancy between 1 to 5 years. In contrast, physical activity is estimated to add up to 5 years. Importantly, a composite lifestyle of healthy behaviours has been proposed to potentially add 10 years to the average life expectancy8,73.
Conclusion and perspectives: testing the Hypothesis
It is obvious from the hypotheses presented here that many aspects of the physiological and pathophysiological effects of modulating PGC-1α in skeletal muscle and other organs remain enigmatic. Even less is known about the functions and therapeutic potential of the other two members of this gene family, PGC-1β and PGC-1-related coactivator (PRC). Similar to PGC-1α muscle-specific transgenic animals, ectopic expression of PGC-1β increases endurance exercise capacity; however, different mechanisms seem to control the exercise-like phenotype in these two animal models74. Alterations in the amount and/or activity of PGC-1α protein in muscle are likely to have future applications in the prevention and treatment of a number of diseases. Amelioration of disuse-induced muscle atrophy54, DMD53 and statin-mediated muscle wasting52 through PGC-1α has already been described in animal models. The potential role of skeletal muscle PGC-1α as a modulator of non-muscular diseases, as discussed here, is not known but these ideas are readily testable in experimental animal models. It is hypothesized here that mice lacking one or both copies of the PGC-1α gene specifically in skeletal muscle will be more susceptible to cancer, heart and brain disease. For example, mutant mice can be treated with chemical carcinogens that cause cancer of the breast, colon and other tissues and the rate of tumor formation and progression can be determined quantitatively. Likewise, the same mutant strains of mice can be given challenges that induce heart failure, or certain kinds of neurodegeneration that model Parkinson’s disease or Alzheimer’s disease, and the rates of disease incidence and progression can be carefully monitored.
Determination of the proper exercise regimens to protect from diseases linked to muscle-based inflammation will be important. But chemical modulation of the PGC-1α pathway in skeletal muscle is also of obvious significance. That PGC-1α gene expression can be modulated by drugs and drug-like compounds has been demonstrated56,75,76. In addition, a number of transcription factors that complex with PGC-1α in controlling skeletal muscle gene transcription are already known50,51 and may represent therapeutic targets. Co-activation of the estrogen-related receptor α (ERRα, official nomenclature NR3B1) specifies PGC-1α to induce the same mitochondrial oxidative phosphorylation genes that are dysregulated in muscle of type 2 diabetic patients56. Pharmacological disruption of the interaction between these two proteins provokes a metabolic phenotype in cultured muscle cells resembling that of type 2 diabetic muscle in vivo56. These findings provide a lead as to how selectivity in targeting PGC-1α could be achieved77. If successful, therapeutic modulation of PGC-1α has a huge clinical potential for muscle wasting, sarcopenia, type 2 diabetes, muscular dystrophies and other very serious non-muscular chronic diseases.
Acknowledgments
We thank Eric Smith for assistance with graphics as well as our colleagues and the members of our labs for comments on the manuscript, in particular Susan Loffredo, Jennifer Estall, Zoltan Arany, Goran Hansson, Serge Summermatter, Marco Toigo and Urs A. Meyer. C.H. is supported by the University Research Priority Program “Integrative Human Physiology” of the University of Zurich, an SNSF-Professorship of the Swiss National Science Foundation and the Muscular Dystrophy Association USA. B.M.S. is supported by several grants from the US National Institutes of Health.
Footnotes
Competing Interests statement. The authors declare no competing financial interests.
References
- 1.Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J Appl Physiol. 2002;93(1):3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- 2.Erikssen G, et al. Changes in physical fitness and changes in mortality. Lancet. 1998;352(9130):759–762. doi: 10.1016/S0140-6736(98)02268-5. [DOI] [PubMed] [Google Scholar]
- 3.Hu FB, et al. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351(26):2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 4.Kokkinos P, et al. Exercise capacity and mortality in black and white men. Circulation. 2008;117(5):614–622. doi: 10.1161/CIRCULATIONAHA.107.734764. [DOI] [PubMed] [Google Scholar]
- 5.Booth FW, Lees SJ. Fundamental questions about genes, inactivity, and chronic diseases. Physiol Genomics. 2007;28(2):146–157. doi: 10.1152/physiolgenomics.00174.2006. [DOI] [PubMed] [Google Scholar]
- 6.McCracken M, Jiles R, Blanck HM. Health behaviors of the young adult U.S. population: behavioral risk factor surveillance system, 2003. Preventing chronic disease. 2007;4(2):A25. [PMC free article] [PubMed] [Google Scholar]
- 7.Hollmann W, Struder HK, Tagarakis CV, King G. Physical activity and the elderly. Eur J Cardiovasc Prev Rehabil. 2007;14(6):730–739. doi: 10.1097/HJR.0b013e32828622f9. [DOI] [PubMed] [Google Scholar]
- 8.Yates LB, et al. Exceptional longevity in men: modifiable factors associated with survival and function to age 90 years. Arch Intern Med. 2008;168(3):284–290. doi: 10.1001/archinternmed.2007.77. [DOI] [PubMed] [Google Scholar]
- 9.Knowler WC, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 11.Haffner SM. The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol. 2006;97(2A):3A–11A. doi: 10.1016/j.amjcard.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Matter CM, Handschin C. RANTES (regulated on activation, normal T cell expressed and secreted), inflammation, obesity, and the metabolic syndrome. Circulation. 2007;115(8):946–948. doi: 10.1161/CIRCULATIONAHA.106.685230. [DOI] [PubMed] [Google Scholar]
- 13.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117(5):1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tansey MG, et al. Neuroinflammation in Parkinson's disease: is there sufficient evidence for mechanism-based interventional therapy? Front Biosci. 2008;13:709–717. doi: 10.2741/2713. [DOI] [PubMed] [Google Scholar]
- 15.Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson's disease. Br J Pharmacol. 2007;150(8):963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zipp F, Aktas O. The brain as a target of inflammation: common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci. 2006;29(9):518–527. doi: 10.1016/j.tins.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7(2):161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 19.Febbraio MA. Exercise and inflammation. J Appl Physiol. 2007;103(1):376–377. doi: 10.1152/japplphysiol.00414.2007. [DOI] [PubMed] [Google Scholar]
- 20.Gleeson M. Immune function in sport and exercise. J Appl Physiol. 2007;103(2):693–699. doi: 10.1152/japplphysiol.00008.2007. [DOI] [PubMed] [Google Scholar]
- 21.Nieman DC. Current perspective on exercise immunology. Curr Sports Med Rep. 2003;2(5):239–242. doi: 10.1249/00149619-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Gleeson M, McFarlin B, Flynn M. Exercise and Toll-like receptors. Exerc Immunol Rev. 2006;12:34–53. [PubMed] [Google Scholar]
- 23.Gleeson M, Nieman DC, Pedersen BK. Exercise, nutrition and immune function. J Sports Sci. 2004;22(1):115–125. doi: 10.1080/0264041031000140590. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103(3):1093–1098. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 25.Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54(Suppl 2):S114–124. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]
- 26.Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006;236(1):13–23. doi: 10.1016/j.canlet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Bremmer MA, et al. Inflammatory markers in late-life depression: Results from a population-based study. J Affect Disord. doi: 10.1016/j.jad.2007.07.002. epub ahead of print (2007) [DOI] [PubMed] [Google Scholar]
- 28.Roubenoff R. Physical activity, inflammation, and muscle loss. Nutr Rev. 2007;65(12 Pt 2):S208–212. doi: 10.1111/j.1753-4887.2007.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 29.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98(3):911–917. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 30.Coletti D, et al. Tumor necrosis factor-alpha gene transfer induces cachexia and inhibits muscle regeneration. Genesis. 2005;43(3):120–128. doi: 10.1002/gene.20160. [DOI] [PubMed] [Google Scholar]
- 31.Manson JE, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999;341(9):650–658. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- 32.Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clinical nutrition (Edinburgh, Scotland) 2007;26(4):389–399. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Sigal RJ, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Annals of internal medicine. 2007;147(6):357–369. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 34.Zhou JR, Blackburn GL, Walker WA. Symposium introduction: metabolic syndrome and the onset of cancer. Am J Clin Nutr. 2007;86(3):s817–819. doi: 10.1093/ajcn/86.3.817S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larson EB, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Annals of internal medicine. 2006;144(2):73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 36.Pette D. Historical Perspectives: plasticity of mammalian skeletal muscle. J Appl Physiol. 2001;90(3):1119–1124. doi: 10.1152/jappl.2001.90.3.1119. [DOI] [PubMed] [Google Scholar]
- 37.Flück M, Hoppeler H. Molecular basis of skeletal muscle plasticity--from gene to form and function. Rev Physiol Biochem Pharmacol. 2003;146:159–216. doi: 10.1007/s10254-002-0004-7. [DOI] [PubMed] [Google Scholar]
- 38.Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37(10):1974–1984. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 39.Chin ER, et al. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12(16):2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berchtold MW, Brinkmeier H, Muntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev. 2000;80(3):1215–1265. doi: 10.1152/physrev.2000.80.3.1215. [DOI] [PubMed] [Google Scholar]
- 41.Puigserver P, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 42.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546(Pt 3):851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hood DA, Irrcher I, Ljubicic V, Joseph AM. Coordination of metabolic plasticity in skeletal muscle. The Journal of experimental biology. 2006;209(Pt 12):2265–2275. doi: 10.1242/jeb.02182. [DOI] [PubMed] [Google Scholar]
- 44.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104(29):12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russell AP, et al. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes. 2003;52(12):2874–2881. doi: 10.2337/diabetes.52.12.2874. [DOI] [PubMed] [Google Scholar]
- 46.Lin J, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 47.Calvo JA, et al. Muscle-specific expression of PPAR{gamma} coactivator-1{alpha} improves exercise performance and increases peak oxygen uptake. J Appl Physiol. 2008;104(5):1304–1312. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- 48.Wende AR, et al. A Role for the Transcriptional Coactivator PGC-1{alpha} in Muscle Refueling. J Biol Chem. 2007;282(50):36642–36651. doi: 10.1074/jbc.M707006200. [DOI] [PubMed] [Google Scholar]
- 49.Handschin C, et al. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem. 2007;282(41):30014–30021. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- 50.Handschin C, Spiegelman BM. PGC-1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 51.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1(6):361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 52.Hanai JI, et al. The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity. J Clin Invest. 2007;117(12):3940–3951. doi: 10.1172/JCI32741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Handschin C, et al. PGC-1alpha regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev. 2007;21(7):770–783. doi: 10.1101/gad.1525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandri M, et al. PGC-1{alpha} protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A. 2006;103(44):16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu Z, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 56.Mootha VK, et al. Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci U S A. 2004;101(17):6570–6575. doi: 10.1073/pnas.0401401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Handschin C, et al. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest. 2007;117(11):3463–3474. doi: 10.1172/JCI31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mootha VK, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 59.Patti ME, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100(14):8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alexandraki K, et al. Inflammatory process in type 2 diabetes: The role of cytokines. Ann N Y Acad Sci. 2006;1084:89–117. doi: 10.1196/annals.1372.039. [DOI] [PubMed] [Google Scholar]
- 61.St-Pierre J, et al. Suppression of Reactive Oxygen Species and Neurodegeneration by the PGC-1 Transcriptional Coactivators. Cell. 2006;127(2):397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 62.Valle I, et al. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovascular research. 2005;66(3):562–573. doi: 10.1016/j.cardiores.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 63.Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve. 2007;35(4):411–429. doi: 10.1002/mus.20743. [DOI] [PubMed] [Google Scholar]
- 64.Ji LL. Modulation of skeletal muscle antioxidant defense by exercise: Role of redox signaling. Free radical biology & medicine. 2008;44(2):142–152. doi: 10.1016/j.freeradbiomed.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 65.Brown WJ, Burton NW, Rowan PJ. Updating the evidence on physical activity and health in women. Am J Prev Med. 2007;33(5):404–411. doi: 10.1016/j.amepre.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 66.Perusse L, Bouchard C. Genotype-environment interaction in human obesity. Nutr Rev. 1999;57(5 Pt 2):S31–37. doi: 10.1111/j.1753-4887.1999.tb01785.x. discussion S37–38. [DOI] [PubMed] [Google Scholar]
- 67.Rippe JM, Hess S. The role of physical activity in the prevention and management of obesity. J Am Diet Assoc. 1998;98(10 Suppl 2):S31–38. doi: 10.1016/s0002-8223(98)00708-1. [DOI] [PubMed] [Google Scholar]
- 68.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43(11):1271–1278. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 69.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 70.Hofmann C, et al. Altered gene expression for tumor necrosis factor-alpha and its receptors during drug and dietary modulation of insulin resistance. Endocrinology. 1994;134(1):264–270. doi: 10.1210/endo.134.1.8275942. [DOI] [PubMed] [Google Scholar]
- 71.Hotamisligil GS, et al. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95(5):2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56(11):2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 73.Fraser GE, Shavlik DJ. Ten years of life: Is it a matter of choice? Arch Intern Med. 2001;161(13):1645–1652. doi: 10.1001/archinte.161.13.1645. [DOI] [PubMed] [Google Scholar]
- 74.Arany Z, et al. The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 2007;5(1):35–46. doi: 10.1016/j.cmet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 75.Wagner BK, et al. Large-scale chemical dissection of mitochondrial function. Nat Biotechnol. 2008;26(3):343–351. doi: 10.1038/nbt1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arany Z, et al. Gene expression-based screening identifies microtubule inhibitors as inducers of PGC-1alpha and oxidative phosphorylation. Proc Natl Acad Sci U S A. 2008;105(12):4721–4726. doi: 10.1073/pnas.0800979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Handschin C, Mootha VK. Estrogen-related receptor alpha (ERRalpha): A novel target in type 2 diabetes. Drug Discov Today Ther Strateg. 2005;2(2):151–156. [Google Scholar]