Abstract
Objective
We hypothesized that intrauterine infection may lead to placental CRH expression via Toll like receptor signaling.
Methods
In order to test this hypothesis JEG3 cells were stimulated with LPS, chlamydial heat shock protein 60 and IL1. CRH expression was assessed by RT-PCR. The signaling mechanisms involved were examined in transient transfection experiments using β-galactosidase, CRH-luciferase, CRE-luciferase, dominant-negative (DN)-MyD88 and DN-TRIF vectors. Luciferase activity was determined by luciferase assay. β-galactosidase assay was performed to determine transfection efficiency.
Results
LPS, cHSP60 and IL-1 stimulation led to CRH expression in the JEG3 cells. LPS-induced CRH expression was not due to the autocrine effect of LPS-induced IL1 since the supernatant from LPS conditioned JEG3 cells did not induce CRH expression in the naïve cells. DN-MyD88 but not DN-TRIF blocked the LPS-induced CRH expression. cAMP response element (CRE) did not play a role in LPS induced CRH expression.
Conclusion
TLR4 may induce placental CRH expression via MyD88.
Keywords: LPS, placenta, trophoblast, Toll like receptors, signaling, MyD88, preterm delivery
Introduction
Intrauterine infections are thought to play a key role in the etiology of preterm labor and the molecular mechanisms involved are not clearly known19, 1. However the proinflammatory cytokines secreted as part of the maternal-fetal immune response to microbial invasion are thought to play a key role20, 21 2, 3. Toll-like receptors (TLR) are the innate immune system molecules expressed in the female reproductive tract and the placenta and mediate immune responses against microbial antigens26 4. The intracellular domain of TLRs is homologous with the IL-1 receptor, called the Toll-interleukin-1 receptor homology region (TIR) domain, and activates a common intracellular signaling cascade including the myeloid differentiation primary response gene (MyD88) which in turn leads to IKK activation, IκB phosphorylation and degradation26 4. This sequence of events allows NF-κB to move into the nucleus to initiate inflammation associated gene expression. TLR4, with TLR3, shares a MyD88-independent, TRAM/TRIF dependent signaling pathway; which leads to IRF3 activation26 4. TLR2, TLR3- and TLR4-induced IRAK/TRAF6 activation also leads to the activation of mitogen activated protein (MAP) kinases (p38, ERK and c-Jun kinase)27 5. p38 MAP kinase then activates the cyclic AMP (cAMP) response element binding protein (CREB) and c-Jun-kinase; which then activate AP-128 6. The hypothalamic peptide, corticotrophin releasing hormone (CRH) is the main stress hormone and is not detectable in the peripheral blood except during the pregnant state. The source of CRH during pregnancy is the placental trophoblasts, where cyclic AMP (cAMP) activation through G-coupled receptors leads to protein kinase A (PKA) activation, activation of the transcription factor AP-1 and cyclic AMP responsive element (CRE) 7–10 action on the CRH promoter to increase the transcription of CRH gene.
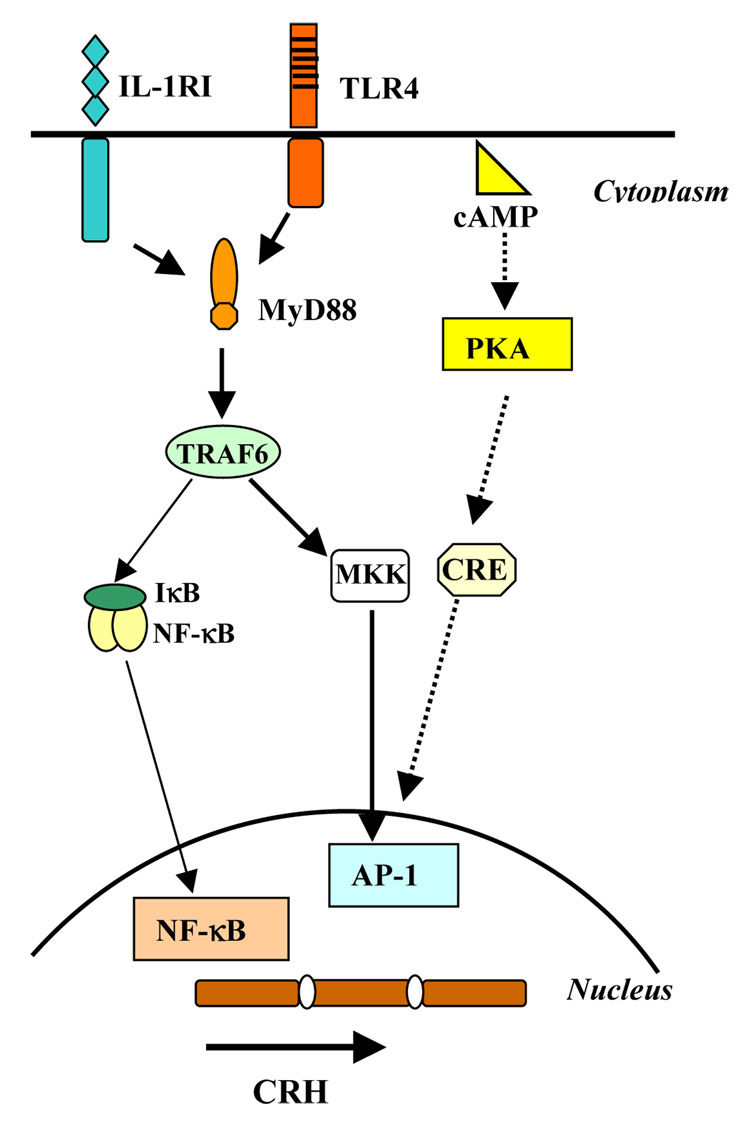
Converging lines of evidence suggest that “placental” CRH plays an important role in coordinating and regulating the physiology of parturition11–17. Studies that conducted serial assessments of CRH over the course of gestation have found that women delivering preterm not only had significantly elevated CRH levels but also a significantly accelerated rate of CRH increase over the course of their gestation, and these elevations were present in some studies as early as 15 weeks gestation17–28. Although both TLR and cAMP stimulation are known to activate the transcription factor AP-1; which mediates CRH expression (Figure 1), currently there are no data on the role of TLR stimulation in trophoblast CRH expression. Based on this background we hypothesized that TLR stimulation via microbial antigens may lead to CRH expression pathway and that this may play a role in the pathogenesis of infection associated preterm delivery.
Figure 1. TLR4 and cAMP signaling pathways to activate AP-1.
TLR and cAMP signaling both activate AP-1.
MATERIALS AND METHODS
Cells Lines and Reagents
JEG3 human trophoblast cell lines were obtained from American Type Tissue Culture Collection (Manaaas, VA) and cultured in MEM (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum, 10 mM HEPES, 1 mM sodium pyruvate and 100 nM of penicillin/streptomycin (Invitrogen Life Technologies). In culture, over time, the cells adhere to the plate and form syncytial clumps as described by Tuan RS et al29. Ultrapure LPS was obtained from Sigma-Aldrich (cat # L2637; St. Louis, MO) and used according to the manufacturer’s instructions. Recombinant human Interleukin (IL)1β was obtained from BioVision (Mountain View, CA; cat# 4128-10). The recombinant Chlamydia heat shock protein 60 (cHSP60) was obtained from Dr. Richard P. Morrison)30. 8-bromo cyclic AMP (cAMP) was used as the positive control for CRH induction (Promega, Madison, WI; PR-V6421). Fugene6 transfection reagent was purchased and used according to the manufacturers protocols (Roche Diagnostics Inc).
Expression Vectors
The CRH-luciferase vector, pGL3-CRH 663, was characterized and described previously7. Dominant negative cDNA constructs of MyD88 and IRAK have been characterized and described previously31. The pC-DNA3 empty vector and pCMV-β-galactosidase vectors have been reported31. The CRE-luciferase vector was characterized by Giebler et al32.
Transfection of JEG3 Cells
JEG3 cells were plated at a concentration of 50,000 cells/well in 24-well plates and cultured in MCDB-131 with 10% serum overnight. Cells were co-transfected the following day with FuGene6 Transfection Reagent following the manufacturer’s instructions. The Roche Fugene6 transfection system is routinely used by our laboratory and others, and has not been shown to affect the cell viability or induce cytokine production in the transfected cells. The reporter genes CRH-Luciferase (0.5µg) and either empty vector or dominant negative mutants of MyD88 and TRIF were transfected into the JEG3 cells. Reporter gene CRE-luciferase (0.5µg) was transfected to assess the effect of TLR stimulation on CRE expression. pCMV-β-galactosidase cDNA (0.1µg) was transfected to normalize the results for transfection efficiency as described earlier31. Cells were transfected for 24 hours and then stimulated with various concentrations of LPS, cHSP60 or IL1β for 5 hrs or 24 hrs. Cells were then lysed and luciferase activity was measured with a Promega kit (Promega, Madison, WI) and a luminometer. β-galactosidase activity determined by calorimetric method31 is a well established and accepted method to assess the transfection efficiency. The cells transfected with β-galactosidase are considered to be cotransfected with the vector in question. The luciferase data is normalized for transfection efficiency by dividing the luciferase measurement with the galactosidase measurement.
RT-PCR
In order to assess whether microbial antigen stimulation induces CRH expression, we stimulated culture-differentiated JEG3 syncytiotrophoblast cells with LPS, cHSP60 and IL1β and assessed CRH expression by RT-PCR. To amplify CRH cDNA, primers 5′-AAGAAAAAGAGAGTGGGAACAGTAAAGA-3′ and 5′-CACTCGCTTCCCAGGCG-3′ were used to obtain a product of the expected size of 124 bp. Human β-actin expression was assessed as the loading control.
Statistical Analysis
Experiments were set up in triplicate or quadruplicate in a plate. The experiments were repeated on at least three separate occasions. Student t-test was used to compare the means between media and each treatment group. p-value < 0.05 was reported as statistically significant
Results
TLR4 ligands induce CRH expression in the JEG3 cells
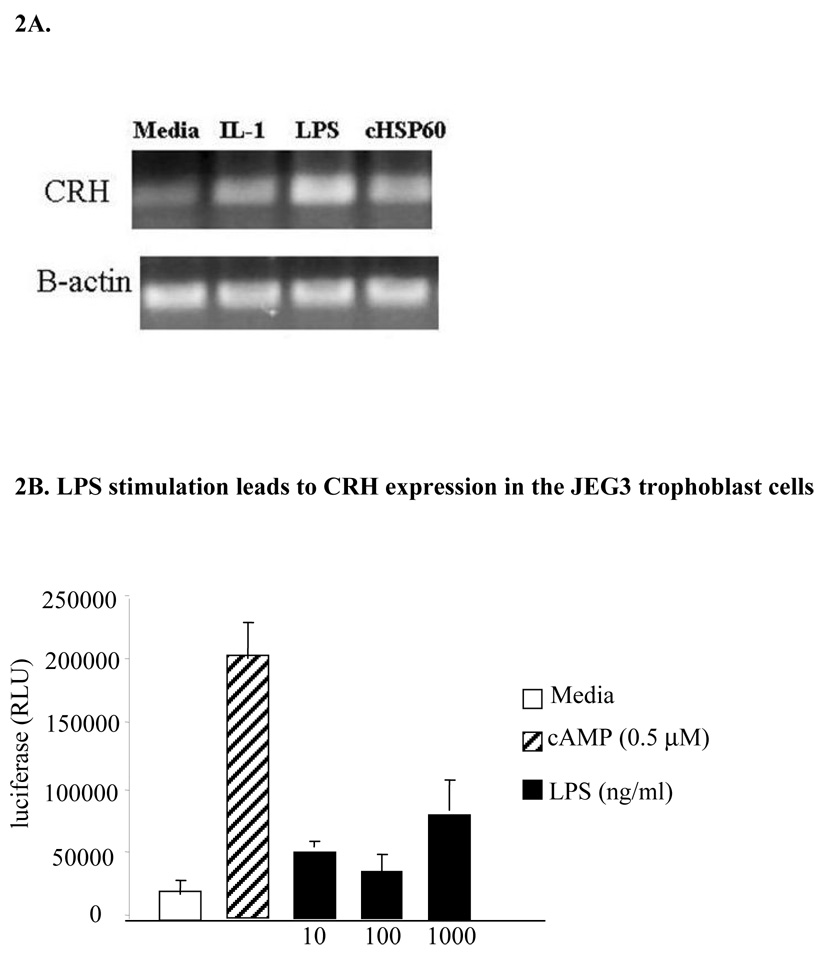
We stimulated culture differentiated JEG3 cells with LPS (1 µg/ml), chlamydial heat shock protein (cHSP) 60 (5 µg/ml) and IL-1β (100 ng/ml) for 5 hours. CRH expression was determined by using RT-PCR. We observed that stimulation with TLR4 ligands and IL1 induced CRH expression (Figure 2A).
Figure 2. LPS stimulation leads to CRH expression in the JEG3 trophoblast cells.
LPS, cHSP60 and IL1 stimulation of JEG3 cells induces CRH expression as assessed by RT-PCR (2A) and in JEG3 cells transiently transfected with a CRH-luciferase construct, stimulated with LPS for 24 hours, CRH promoter activation assessed by luciferase assay (2B). Test well luciferase levels were compared with those observed in the media treated control group. (*p< 0.05 as compared to media treated control).
We stimulated JEG3 cells transiently expressing CRH-luciferase and B-galactosidase reporter constructs with various concentrations of LPS for 5 hrs or 24 hrs. The luciferase level was measured to assess CRH expression. We observed that LPS stimulation induced CRH promoter activation in the JEG3 cells in a concentration dependent manner at 24 hr (Figure 2B) but not at 5 hr (data not shown).
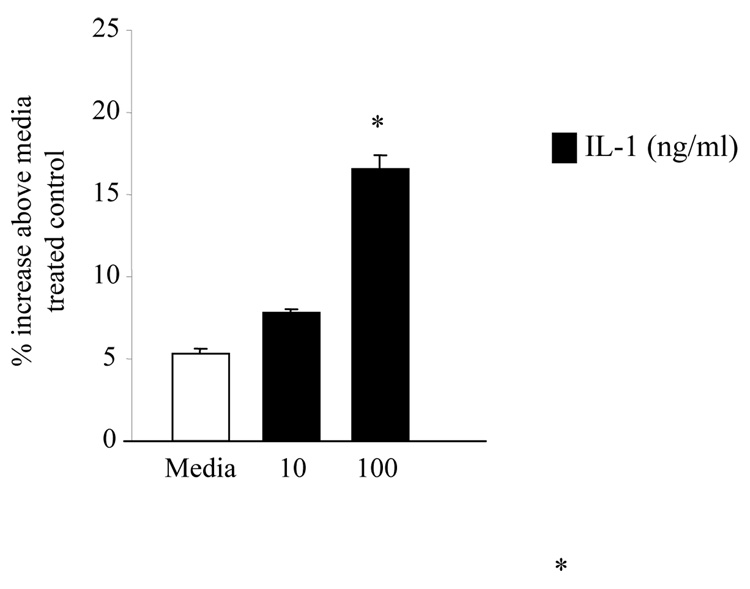
IL1 stimulation is known to induce CRH expression in the hypothalamus33–35 and in the human endometrial cells36, 37. We hypothesized that IL1 may induce CRH expression in the trophoblast cells as well. In order to test this hypothesis we stimulated JEG3 cells transiently expressing CRH-luciferase and B-galactosidase with various concentrations of IL1β (10–1000 ng/ml) and assessed the luciferase activity at 24 hours. IL1 induced the CRH promoter activation in JEG3 cells in a dose dependent fashion (Figure 3).
Figure 3. IL1 stimulation induces CRH expression in JEG3 cells (* p<0.05 above media treated wells.
IL-1β stimulation of JEG3 cells transiently transfected with CRH-luciferase cDNA induced CRH expression at 24 hours. % increase above the media treated wells was calculated by subtracting the test well luciferase value from control well luciferase value, and dividing the result by the control well luciferase value and by multiplying with 100. (*p< 0.05 as compared to the media treated cells).
LPS-induced CRH expression is not due to the autocrine effect of cytokines
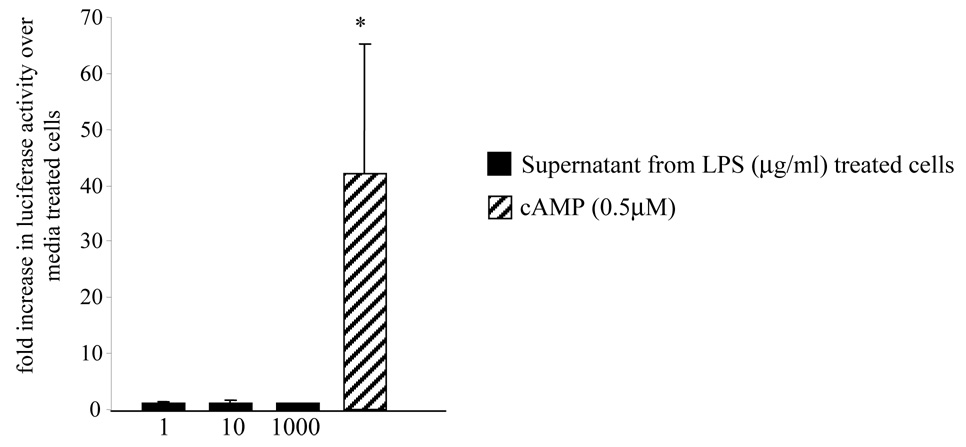
LPS stimulation is known to induce cytokine expression, including IL1, in trophoblast cells38. We then assessed whether the LPS effect in inducing CRH expression in trophoblasts is secondary to the autocrine effect of the cytokines produced. In order to test this we stimulated non-transfected JEG3 cells with various concentrations of LPS (1–1000 ng/ml) for 24 hours. We isolated the supernatant and stimulated naive JEG3 cells expressing CRH-luciferase vector with this supernatant for 24 hours. Luciferase activity was measured by a luminometer to assess the CRH promoter activation. No increase in CRH promoter activation was observed (Figure 4).
Figure 4. Treatment with the supernatant obtained from LPS-stimulated cells does not induce CRH expression in the JEG3 cells.
Naïve JEG3 cells transfected with CRH-luciferase vector were stimulated with supernatant obtained from 24 hour-LPS stimulated JEG3 cells. cAMP was used as the positive control. Luciferase activity was measured to assess the CRH expression. (* p< 0.05 as compared to media treated controls)
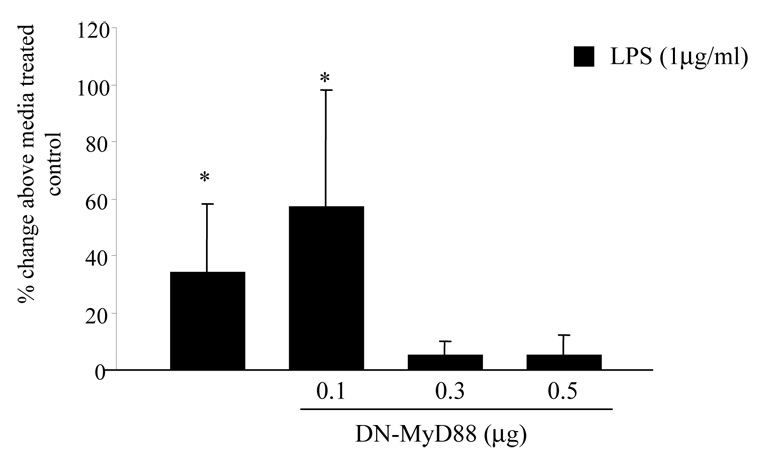
DN-MyD88 blocks LPS-induced CRH expression in JEG3 cells
MyD88 is the common adapter molecule for Toll like receptors and IL1. Next we assessed whether MyD88 plays a role in LPS- and IL1β-induced CRH expression in the trophoblasts. We co-transfected the JEG3 cells with CRH-luciferase and B-galactosidase constructs and either empty vector (pcDNA3) or various concentrations of dominant negative(DN)-MyD88 as described under Methods. The cells were treated with LPS (1 µg/ml) for 24 hours. Luciferase activity was measured to assess the CRH expression. We observed that LPS stimulation induced significant CRH expression in the wells transfected with empty vector or 0.1 µM of DN-MyD88 as assessed by increased luciferase activity above that seen in control wells treated with media. Higher concentrations of DN-MyD88 blocked the LPS-induced CRH promoter activation in a dose dependent manner (Figure 5).
Figure 5. DN-MyD88 blocks the LPS-induced CRH expression in the JEG3 cells.
JEG3 cells transfected with CRH-luciferase and β-galactosidase expression vectors were cotransfected with either dominant negative, nonsignaling MyD88 cDNA or empty vector to keep intra-cellular DNA content the same. The cells were stimulated with either media or LPS and luciferase activity was measured to assess CRH expression. (*p<;0.05 as compared to media treated control cells transfected with empty vector)
In parallel experiments the JEG3 cells were transfected with dominant-negative TRIF and stimulated with LPS (1µg/ml) for 24 hours. We observed that inhibition of TRIF signaling did not block the LPS-induced CRH promoter activation (data not shown).
cAMP response element (CRE) does not mediate LPS-induced CRH expression in the JEG3 cells
The cAMP stimulation of CRH promoter activity in transfected primary cultures of human placental cells was shown to be mediated by specific nuclear proteins interacting with the CRE contained within the CRH promoter region9, 10. We assessed whether LPS-induced CRH expression is solely mediated via the CRE by transiently transfecting the JEG3 cells with a CRE-luciferase construct and measuring the luciferase expression upon LPS and cAMP stimulation. As expected cAMP stimulation induced CRE promoter activation in the trophoblasts; however LPS did not have any effect.
COMMENTS
Here we show that stimulation of IL1β and TLR4 signaling induces CRH expression in a trophoblast cell line, in a delayed fashion and that MyD88 mediates the LPS-induced CRH expression. cAMP but not LPS stimulation induces the cyclic AMP response element (CRE) activation in the trophoblasts. In humans, placental CRH plays a central role in the maternal-placental and fetal neuroendocrine system in pregnancy, and placental CRH has been proposed to regulate a “placental clock” that determines or alters the timing of onset of parturition11. In this model, CRH is produced by the placenta and promotes fetal cortisol and dehydroepiandrosterone sulphate (DHEAS) production by the fetal adrenal gland. These steroids return via the umbilical circulation to the placenta, where cortisol promotes further CRH secretion (the positive feedback circuit), and DHEA-S serves as the immediate precursor for the production of estriol (E3) influencing the state of uterine activity. Plasma concentrations of placental CRH are low during the first trimester. As a result of the positive feedback loop, they rise exponentially from mid-gestation to term reaching concentrations that are 1000-fold greater than those found in non-pregnant women. Once established, this positive feedback loop is progressively amplified and drives the fetal–placental unit towards the outcomes of fetal maturation and delivery39.
Although infection is thought to play a major role in the pathogenesis of preterm delivery, currently there are no data on the role of infection on placental CRH expression. Here we show that although less robust than cAMP, exposure to microbial antigens and IL1β induces CRH expression in the placenta.
A variety of endogenous biochemical agents stimulate CRH release from the hypothalamus and endometrium including interleukin-1, angiotensin II, oxytocin, arginine vasopressin, norepinephrine, epinephrine, and acetylcholine33–37. These ligands act on various cells through cAMP-dependent protein kinase signaling pathways and turn on targeted genes by trans-activation through a consensus DNA sequence, defined as the cAMP regulatory element (CRE), in the promoter region of responsive genes40. CRE-binding protein (CREB) a member of the bZIP or leucine zipper family of transcription factors41, is phosphorylated by several protein kinases42, and modulates gene transcription in response to ligand stimulation of the cAMP pathways43–46. A CRE has been identified in the human CRH promoter region47.
In contrast to the hypothalamus, the mechanisms that regulate CRH expression in human placenta remain unclear. Scatena and Adler have shown that cAMP stimulates CRH promoter activity in human choriocarcinoma cell lines (BeWo and JEG-3) transfected with human and mouse CRH gene constructs48. Using primary cultures of human placental cells, Smith and colleagues9, 10 have shown that incubation with the adenylate cyclase activator forskolin or 8-bromo-cAMP increased human CRH promoter activity and endogenous CRH peptide. Here we show that microbial antigen stimulation of JEG3 trophoblast cells induces CRH expression.
Trophoblasts release proinflammatory cytokines such as IL1β upon LPS stimulation38. Although we observed that IL1β induces CRH expression in the trophoblasts, our data suggest that LPS-induced CRH promoter activation is a direct effect and not due to the autocrine effect of IL1β-released from the trophoblasts.
The human CRH gene has a highly conserved classic CRE within its proximal promoter8. Specific nuclear proteins interacting with the CRE are thought to mediate cAMP induced CRH expression in the primary cultures of human placental cells9. Here we show that cAMP but not LPS stimulation of JEG3 trophoblasts leads to CRE activation. These data suggest that LPS-induced CRH expression is not mediated via CRE in the JEG3 cells. We have not tested the role of AP-1 in the LPS effect. In our experiments inhibition of MyD88 signaling blocked the LPS-induced CRH expression. This is anticipated since MyD88 is the common adaptor molecule that mediates the TLR signaling. Interestingly TRIF, another adaptor molecule that plays a role in LPS-induced delayed NF-κB activation and interferon expression, did not participate in the LPS-induced CRH expression in trophoblasts.
Our data suggest that infection with Gram-negative bacteria or exposure to LPS may induce placental CRH expression; which may contribute to the pathogenesis of preterm delivery. However LPS induced CRH expression was significantly lower compared to cAMP-induced CRH.
Strengths of the study include the analyses at the molecular level linking exposure to microbial antigens to placental CRH release. Limitations of the study are that this is an in vitro study and inclusive in the use of trophoblast cell lines for transfection experiments. However transfection of primary trophoblasts is not a well established technique and the use of this in vitro-cell line system gives us a better understanding of the potential mechanisms involved; which can then be tested in vivo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported by NIH NCRR GCRC Grant (M01-RR00425) to OE and March of Dimes grant (#6-FY06-329) to OE and RS
References
- 1.Goldenberg RL, Culhane JF. Infection as a cause of preterm birth. Clin Perinatol. 2003;30:677–700. doi: 10.1016/s0095-5108(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, Berry SM. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 4.Patni S, Flynn P, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA. An introduction to Toll-like receptors and their possible role in the initiation of labour. BJOG. 2007;114:1326–1334. doi: 10.1111/j.1471-0528.2007.01488.x. [DOI] [PubMed] [Google Scholar]
- 5.Dunne A, O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2003;2003:re3. doi: 10.1126/stke.2003.171.re3. [DOI] [PubMed] [Google Scholar]
- 6.Minden A, Karin M. Regulation and function of the JNK subgroup of MAP kinases. Biochim Biophys Acta. 1997;1333:F85–F104. doi: 10.1016/s0304-419x(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 7.Cheng YH, Nicholson RC, King B, Chan EC, Fitter JT, Smith R. Corticotropin-releasing hormone gene expression in primary placental cells is modulated by cyclic adenosine 3′,5′-monophosphate. J Clin Endocrinol Metab. 2000;85:1239–1244. doi: 10.1210/jcem.85.3.6420. [DOI] [PubMed] [Google Scholar]
- 8.Cheng YH, Nicholson RC, King B, Chan EC, Fitter JT, Smith R. Glucocorticoid stimulation of corticotropin-releasing hormone gene expression requires a cyclic adenosine 3′,5′-monophosphate regulatory element in human primary placental cytotrophoblast cells. J Clin Endocrinol Metab. 2000;85:1937–1945. doi: 10.1210/jcem.85.5.6552. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson RC, King BR, Smith R. Complex regulatory interactions control CRH gene expression. Front Biosci. 2004;9:32–39. doi: 10.2741/1204. [DOI] [PubMed] [Google Scholar]
- 10.King BR, Smith R, Nicholson RC. Novel glucocorticoid and cAMP interactions on the CRH gene promoter. Mol Cell Endocrinol. 2002;194:19–28. doi: 10.1016/s0303-7207(02)00218-6. [DOI] [PubMed] [Google Scholar]
- 11.McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1:460–463. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- 12.McLean M, Smith R. Corticotrophin-releasing hormone and human parturition. Reproduction. 2001;121:493–501. doi: 10.1530/rep.0.1210493. [DOI] [PubMed] [Google Scholar]
- 13.Petraglia F, Florio P, Nappi C, Genazzani AR. Peptide signaling in human placenta and membranes: autocrine, paracrine, and endocrine mechanisms. Endocr Rev. 1996;17:156–186. doi: 10.1210/edrv-17-2-156. [DOI] [PubMed] [Google Scholar]
- 14.Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- 15.Grammatopoulos DK, Hillhouse EW. Role of corticotropin-releasing hormone in onset of labour. Lancet. 1999;54:1546–1549. doi: 10.1016/S0140-6736(99)03418-2. [DOI] [PubMed] [Google Scholar]
- 16.Karalis K, Majzoub JA. Regulation of placental corticotropin-releasing hormone by steroids. Possible implications in labor initiation. Ann N Y Acad Sci. 1995;771:551–555. doi: 10.1111/j.1749-6632.1995.tb44709.x. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe CD, Patel SP, Linton EA, Campbell EA, Anderson J, Dornhorst A, Lowry PJ, Jones MT. Plasma corticotrophin-releasing factor (CRF) in abnormal pregnancy. Br J Obstet Gynaecol. 1988;95:1003–1006. doi: 10.1111/j.1471-0528.1988.tb06504.x. [DOI] [PubMed] [Google Scholar]
- 18.Warren WB, Patrick SL, Goland RS. Elevated maternal plasma corticotropin-releasing hormone levels in pregnancies complicated by preterm labor. Am J Obstet Gynecol. 1992:1198–1204. doi: 10.1016/s0002-9378(11)90606-1. discussion 1204-7. [DOI] [PubMed] [Google Scholar]
- 19.Wadhwa PD, Porto M, Garite TJ, Chicz-DeMet A, Sandman CA. Maternal corticotrophin-releasing hormone levels in the early third trimester predict length of gestation in human pregnancy. Am J Obstet Gynecol. 1998;179:1079–1085. doi: 10.1016/s0002-9378(98)70219-4. [DOI] [PubMed] [Google Scholar]
- 20.Petraglia F, Aguzzoli L, Florio P, Baumann P, Genazzani AD, Di Carlo C, Romero R. Maternal plasma and placental immunoreactive corticotrophin-releasing factor concentrations in infection-associated term and pre-term delivery. Placenta. 1995;16:157–164. doi: 10.1016/0143-4004(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 21.Kurki T, Laatikainen T, Salminen-Lappalainen K, Ylikorkala O. Maternal plasma corticotrophin-releasing hormone--elevated in preterm labour but unaffected by indomethacin or nylidrin. Br J Obstet Gynaecol. 1991;98:685–691. doi: 10.1111/j.1471-0528.1991.tb13456.x. [DOI] [PubMed] [Google Scholar]
- 22.Korebrits C, Ramirez MM, Watson L, Brinkman E, Bocking AD, Challis JR. Maternal corticotropin-releasing hormone is increased with impending preterm birth. J Clin Endocrinol Metab. 1998;83:1585–1591. doi: 10.1210/jcem.83.5.4804. [DOI] [PubMed] [Google Scholar]
- 23.Holzman C, Jetton J, Siler-Khodr T, Fisher R, Rip T. Second trimester corticotrophin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstet Gynecol. 2001;97:657–663. doi: 10.1016/s0029-7844(00)01209-6. [DOI] [PubMed] [Google Scholar]
- 24.Hobel CJ, Arora CP, Korst LM. Corticotrophin-releasing hormone and CRH-binding protein. Differences between patients at risk for preterm birth and hypertension. Ann N Y Acad Sci. 1999;897:54–65. doi: 10.1111/j.1749-6632.1999.tb07878.x. [DOI] [PubMed] [Google Scholar]
- 25.Erickson K, Thorsen P, Chrousos G, Grigoriadis DE, Khongsaly O, McGregor J, Schulkin J. Preterm birth: associated neuroendocrine, medical, and behavioral risk factors. J Clin Endocrinol Metab. 2001;86:2544–2552. doi: 10.1210/jcem.86.6.7607. [DOI] [PubMed] [Google Scholar]
- 26.Campbell EA, Linton EA, Wolfe CD, Scraggs PR, Jones MT, Lowry PJ. Plasma corticotropin-releasing hormone concentrations during pregnancy and parturition. J Clin Endocrinol Metab. 1987;64:1054–1059. doi: 10.1210/jcem-64-5-1054. [DOI] [PubMed] [Google Scholar]
- 27.Smith R, Mesiano S, McGrath S. Hormone trajectories leading to human birth. Regul Pept. 2002;108:159–164. doi: 10.1016/s0167-0115(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 28.Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, Sandman CA. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191:1063–1069. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 29.Tuan RS, Moore CJ, Brittingham JW, Kirwin JJ, Akins RE, Wong M. In vitro study of placental trophoblast calcium uptake using JEG-3 human choriocarcinoma cells. J Cell Sci. 1991;98:333–342. doi: 10.1242/jcs.98.3.333. [DOI] [PubMed] [Google Scholar]
- 30.LaVerda D, Albanese LN, Ruther PE, Morrison SG, Morrison RP, Ault KA, Byrne GI. Seroreactivity to Chlamydia trachomatis Hsp10 correlates with severity of human genital tract disease. Infect. Immun. 2000;68:303–309. doi: 10.1128/iai.68.1.303-309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang FX, Kirschning CJ, Mancinelli R, Xu XP, Jin Y, Faure E, Mantovani A, Rothe M, Muzio M, Arditi M. Bacterial lipopolysaccharide activates nuclear factor-kappaB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J. Biol. Chem. 1999;274:7611. doi: 10.1074/jbc.274.12.7611. [DOI] [PubMed] [Google Scholar]
- 32.Giebler HA, Loring JE, van Orden K, Colgin MA, Garrus JE, Escudero KW, Brauweiler A, Nyborg JK. Anchoring of CREB binding protein to the human T-cell leukemia virus type 1 promoter: a molecular mechanism of Tax transactivation. Mol Cell Biol. 1997;17:5156–5164. doi: 10.1128/mcb.17.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sapolsky R, River C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238:522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- 34.Berkenbosch F, Oers J, Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- 35.Assenmacher I, Szafarczyk A, Alonso G, Ixart G, Barbanel G. Physiology of neural pathways affecting CRH secretion. Ann NY Acad Sci. 1987;512:149–161. doi: 10.1111/j.1749-6632.1987.tb24957.x. [DOI] [PubMed] [Google Scholar]
- 36.Petraglia F, Sutton S, Vale W. Neurotransmitters and peptides modulate the release of immunoreactive corticotropin-releasing factor from cultured human placental cells. Am J Obstet Gynecol. 1989;160:247–251. doi: 10.1016/0002-9378(89)90130-0. [DOI] [PubMed] [Google Scholar]
- 37.Makrigiannakis A, Margioris AN, Zoumakis E, Stournaras C, Gravanis A. The transcription of corticotropin-releasing hormone in human endometrial cells is regulated by cytokines. Neuroendocrinology. 1999;70:451–459. doi: 10.1159/000054507. [DOI] [PubMed] [Google Scholar]
- 38.Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, Mor G. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–4296. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 39.Smith R. Parturition. N Engl J Med. 2007;356:271–283. doi: 10.1056/NEJMra061360. [DOI] [PubMed] [Google Scholar]
- 40.Deutsch P, Hoeffler J, Jameson J, Lin J, Habener J. Structural determinants for transcriptional activation by cAMP-responsive DNA elements. J Biol Chem. 1988;263:18466–18472. [PubMed] [Google Scholar]
- 41.Lee C, Yun Y, Poeffler J, Habener J. Cyclic-AMP-responsive transcriptional activation of CREB-327 involves interdependent phosphorylated subdomains. EMBO J. 1990;9:4455–4465. doi: 10.1002/j.1460-2075.1990.tb07896.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Yamamoto K, Gonzalez G, Biggs W, Montminy M. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988;334:494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto K, Gonzalez G, Rivier M, Montminy M. Characterization of a bipartite activator domain in transcription factor CREB. Cell. 1990;60:611–617. doi: 10.1016/0092-8674(90)90664-z. [DOI] [PubMed] [Google Scholar]
- 44.Foulkes N, Borrelli E, Sassone-Corsi P. CREM gene: use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell. 1991;64:739–749. doi: 10.1016/0092-8674(91)90503-q. [DOI] [PubMed] [Google Scholar]
- 45.Liu F, Thompson M, Wagner S, Greenberg M, Green M. Activating transcription factor-1 can mediate Ca2+ and cAMP-inducible transcriptional activation. J Biol Chem. 1993;268:6714–6720. [PubMed] [Google Scholar]
- 46.Rehfuss R, Walton K, Loriaux M, Goodman R. The cAMP-regulated enhancer-binding protein ATF-1 activates transcription in response to cAMP-dependent protein kinase A. J Biol Chem. 1991;266:18431–18434. [PubMed] [Google Scholar]
- 47.Spengler D, Rupprecht R, Van L, Holsboer F. Identification and characterization of a 3′,5′-cyclic adenosine monophosphate-reponsive element in the human corticotrophin-releasing hormone gene promoter. Mol Endocrinol. 1992;6:1931–1941. doi: 10.1210/mend.6.11.1480179. [DOI] [PubMed] [Google Scholar]
- 48.Scatena C, Adler S. Trans-acting factors that dictate the species-specific placental expression of corticotrophin releasing factor genes in choriocarcinoma cell lines. Endocrinology. 1996;137:3000–3008. doi: 10.1210/endo.137.7.8770924. [DOI] [PubMed] [Google Scholar]