Abstract
Choline is an essential nutrient that is critical during fetal brain development. Choline deficiency, through disturbing methyl metabolism, may alter DNA methylation and thereby influence neural precursor cell proliferation and apoptosis. This results in long term alterations in brain structure and function, specifically memory function. A recommended dietary intake for choline in humans was set in 1998, and a portion of the choline requirement can be met via endogenous de novo synthesis of phosphatidylcholine catalyzed by phosphatidylethanolamine N-methyltransferase (PEMT) in the liver. Though many foods contain choline, many humans do not get enough in their diets. When deprived of dietary choline, most adult men and postmenopausal women developed signs of organ dysfunction (fatty liver, liver or muscle cell damage). However, only a portion of premenopausal women developed such problems. The difference in requirement occurs because estrogen induces expression of the PEMT gene and allows premenopausal women to make more of their needed choline endogenously. In addition, there is significant variation in the dietary requirement for choline that can be explained by common genetic variants (single nucleotide polymorphisms; SNPs) in genes of choline and folate metabolism. Some of these increase the risk of choline deficiency many fold. These variations in choline requirement could have important implications for brain development.
Keywords: choline, brain development, single nucleotide polymorphism, dietary requirement
Effects of a low choline diet in humans
Choline is a dietary component essential for normal function of all cells (Zeisel, 2006) because it is a major source of methyl-groups in the diet (one of choline’s metabolites, betaine, participates in the methylation of homocysteine to form methionine) and because it is used as a constituent of cell membranes and the neurotransmitter acetylcholine (da Costa et al., 2005; Zeisel, 2006). Choline is important for brain development (Cheng et al., 2008; Craciunescu et al., 2003) and for normal closure of the neural tube (Fisher et al., 2001; Fisher et al., 2002), and pregnant women eating diets low in choline have a 4-fold increased risk of having a baby with a birth defect (Shaw et al., 2004; Shaw et al., 2006). Also, dietary choline deficiency in humans results in fatty liver (Buchman et al., 1995; Zeisel et al., 1991), liver damage (Albright et al., 1996; Albright and Zeisel, 1997; Albright et al., 2005a; Fischer et al., 2007; Zeisel et al., 1991) and muscle damage (da Costa et al., 2004; Fischer et al., 2007). Hepatosteatosis occurs because a lack of phosphatidylcholine limits the export of excess triglyceride from liver in lipoproteins (Yao and Vance, 1988; Yao and Vance, 1989). Liver damage, detected as elevated serum aminotransferases, occurs secondary to hepatocyte apoptosis (Albright et al., 1996; James et al., 1997; Shin et al., 1997). Muscle damage, detected as elevated creatine phosphokinase in blood, occurs because muscle membranes are more fragile and because of induction of apoptosis in myocytes (da Costa et al., 2004). Apoptosis is also induced in lymphocytes during choline deficiency (da Costa et al., 2006b).
In 1998 the U.S. Institute of Medicine’s Food and Nutrition Board established an Adequate Intake (AI) and Tolerable Upper Limit (UL) for choline (Institute of Medicine and National Academy of Sciences USA, 1998).
Choline, Folate And Methionine Metabolism Are Interrelated
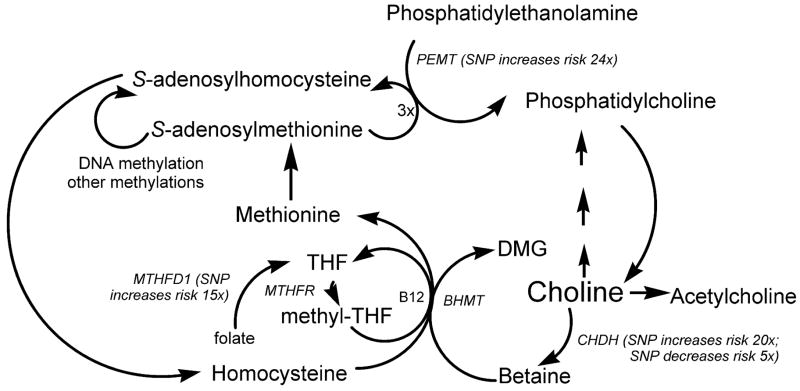
Choline, methionine and folate metabolism are inter-related at the step that homocysteine is methylated to form methionine (Finkelstein, 2000) (Figure 1). There are two parallel pathways, one using methyl-tetrahydrofolate, and one using betaine (derived from choline) that mediate this methylation (Olthof et al., 2003). In the first, catalyzed by methionine synthase, vitamin B12 is a cofactor (Weisberg et al., 2001). Deficiency of folate or B12 (Jacques et al., 2001; Shelnutt et al., 2003), or single nucleotide polymorphisms in the genes for the enzymes involved in this pathway (Jacques et al., 2001; Watkins et al., 2002; Weisberg et al., 2001), can result in elevated plasma homocysteine concentrations. The second pathway is catalyzed by betaine homocysteine methyltransferase (BHMT) (Sunden et al., 1997). Betaine, derived from dietary choline by the action of choline dehydrogenase (CHDH), is the methyl group donor in this reaction and supplemental oral betaine can lower plasma homocysteine concentrations (Steenge et al., 2003; Wendel and Bremer, 1984).
Figure 1.
Common genetic polymorphisms in choline and folate metabolism The pathways described are all present in the liver, with other tissues having one or more of these pathways. Each of the genes indicated in italics have single nucleotide polymorphisms that are described in the text. Some of these increase dietary choline requirements (effect on observed risk of choline deficiency (see text) noted next to gene name). PEMT = phosphatidylethanolamine-N-methyltransferase; CHDH = choline dehydrogenase; BHMT = betaine homocysteine methyltransferase; MTHFR = methylene tetrahydrofolate reductase; MTHFD1 = methylene tetrahydrofolate dehydrogenase; DMG = dimethylglycine; THF = tetrahydrofolate; SNP = single nucleotide polymorphism.
Perturbing metabolism of one of the methyl-donors results in compensatory changes in the other methyl-donors due to the intermingling of these metabolic pathways (Kim et al., 1995; Selhub et al., 1991; Varela-Moreiras et al., 1992). Rats treated with the anti-folate, methotrexate, had diminished pools of choline metabolites in liver (Pomfret et al., 1990; Selhub et al., 1991). Rats ingesting a choline-deficient diet had diminished tissue concentrations of methionine and S-adenosylmethionine (Zeisel et al., 1989) and doubled plasma homocysteine concentrations (Varela-Moreiras et al., 1995). We recently reported that humans who are choline deficient, even when fed adequate amounts of folic acid, had diminished capacity to methylate homocysteine and developed elevated homocysteine concentrations in plasma after a methionine loading test (da Costa et al., 2005).
Dietary Intake And Endogenous Synthesis Of Choline
Excellent sources of dietary choline include liver, eggs and wheat germ (Zeisel et al., 2003a; Zeisel et al., 2003b) (also see http://www.nal.usda.gov/fnic/foodcomp/Data/Choline/Choline.html). In foods, choline is found free and esterified; these are likely to be substantially equivalent to one another because liver converts much of the ingested water soluble forms to phosphatidylcholine. It is not clear whether normal dietary patterns deliver the recommended amounts of choline for all people. Shaw and colleagues, studying pregnant women in California, observed intakes of choline in 25% of the population that were less than those needed to prevent birth defects in their fetuses (Shaw et al., 2004; Shaw et al., 2006). We noted that approximately 10% of subjects required at least 850 mg/day choline in the diet (about 2x the recommended adequate intake) to avoid fatty liver, liver damage or muscle damage (Fischer et al., 2007).
The only source of choline other than diet is from the de novo biosynthesis of phosphatidylcholine catalyzed by phosphatidylethanolamine-N-methyltransferase (PEMT) in liver. This enzyme uses S-adenosylmethionine as a methyl donor and forms a new choline moiety (Blusztajn et al., 1985). When fed a diet deficient in choline, Pemt -/- mice developed fatty liver, severe liver damage and died; a choline supplemented diet prevented this (Walkey et al., 1998) and reversed hepatic damage if begun early enough (Waite et al., 2002). The PEMT pathway is not just a minor pathway that backs up the cytidine diphosphocholine pathway for phosphatidylcholine biosynthesis. Pemt -/- mice have lower choline pools in liver despite being fed sufficient or supplemental amounts of dietary choline (Zhu et al., 2003), suggesting that choline production by PEMT is a significant source of choline relative to dietary intake. When Pemt is deleted in mice, plasma homocysteine concentrations fall 50% and, when it is over expressed, plasma homocysteine concentrations increase 40% (Jacobs et al., 2005; Shields et al., 2005), demonstrating that PEMT activity is a very major consumer of S-adenosylmethionine (and thereby a producer of homocysteine).
Estrogen Response Elements and the requirement for choline
Premenopausal women, relative to males and postmenopausal women, are resistant to developing organ dysfunction when fed a low choline diet (Fischer et al., 2007). The classic actions of estrogen occur through its receptors ERα and ERβ which bind as homodimers or heterodimers to estrogen response elements (EREs) in the promoters of many estrogen-responsive genes (Walter et al., 1985). The consensus ERE (PuGGTCAnnnTGACCPy) (Walter et al., 1985) and some imperfect ERE half site motifs (ERE1/2) bind with ERα and ERβ (Agarwal et al., 2002; Lopez et al., 2002; Xie et al., 1999). There are multiple EREs in the promoter region(s) of the PEMT gene (Resseguie et al., 2007) and estrogen caused a marked up-regulation in PEMT mRNA expression and enzyme activity in human hepatocytes (Resseguie et al., 2007). Thus, premenopausal women have an enhanced capacity for de novo biosynthesis of choline moiety. During pregnancy, estradiol concentration rises from approximately 1 nM to 60 nM at term (Adeyemo and Jeyakumar, 1993; Sarda and Gorwill, 1976), suggesting that capacity for endogenous synthesis of choline is highest during the period when females need to support fetal development.
Pregnancy and lactation are times when demand for choline is especially high. Large amounts of choline are delivered to the fetus across the placenta, where choline transport systems pump it against a concentration gradient (Sweiry and Yudilevich, 1985; Sweiry et al., 1986) and deplete maternal plasma choline in humans (McMahon and Farrell, 1985). Plasma or serum choline concentrations are 6–7-fold higher in the fetus and newborn than they are in the adult (Ozarda et al., 2002; Zeisel and Wurtman, 1981). High levels of choline circulating in the neonate presumably ensure enhanced availability of choline to tissues. It is interesting that despite enhanced capacity to synthesize choline, the demand for this nutrient is so high that stores are depleted during pregnancy. Pregnant rats had diminished total liver choline compounds compared to non-mated controls and become as sensitive to choline-deficient diets as were male rats (Zeisel et al., 1995). Because milk contains a great deal of choline, lactation further increases maternal demand for choline resulting in further depletion of tissue stores (Holmes-McNary et al., 1996; Zeisel et al., 1995). These observations suggest that women depend on high rates of PEMT activity, as well as on dietary intake of choline to sustain normal pregnancy. Pemt −/− mice abort pregnancies around 9–10 days gestation unless fed supplemental choline (personal observation; (Zhu et al., 2004)). Choline nutriture during pregnancy is especially important because it influences brain development in the fetus (Albright et al., 1998; Albright et al., 1999a; Albright et al., 1999b; Albright et al., 2001; Albright et al., 2003; Albright et al., 2005b; Craciunescu et al., 2003; Meck and Williams, 1997; Meck et al., 1988; Meck and Williams, 2003; Mellott et al., 2004; Niculescu et al., 2006; Pyapali et al., 1998) and because it is important for maintaining normal plasma homocysteine concentrations during pregnancy (Velzing-Aarts et al., 2005). High maternal homocysteine concentrations are associated with increased incidence of birth defects (Hobbs et al., 2005). A better understanding of genetic polymorphisms that cause variation in dietary choline requirements might be important for identifying women at greater risk for choline deficiency during pregnancy.
Gene polymorphisms and dietary choline requirements
Though premenopausal women should be resistant to choline deficiency, a significant portion of them (45%) develop organ dysfunction when deprived of choline (Fischer et al., 2007). Genetic variation likely underlies these differences in dietary requirements. Several metabolic pathways influence how much choline is required from diet, and single nucleotide polymorphisms (SNPs) in specific genes influence the efficiency of these pathways. Specifically, some polymorphisms in the folate pathways limit the availability of methyltetrahydrofolate and thereby increase use of choline as a methyl donor; polymorphisms in the PEMT gene alter endogenous synthesis of choline; and polymorphisms in other genes of choline metabolism influence dietary requirements by changing the utilization of choline moiety.
We developed a clinical methodology for phenotyping individuals with respect to their susceptibility to developing organ dysfunction when fed a low choline diet (Busby et al., 2004; da Costa et al., 2004; da Costa et al., 2005; Fischer et al., 2007). In a repeated measure within subject study design with graded repletion, adult men and women (pre- and post-menopausal) ages 18–70 were admitted to the General Clinical Research Center and fed a standard diet containing a known amount of choline (550 mg/70kg/d; baseline). On day 11 subjects were placed on a diet containing <50 mg choline/day for up to 42 days. Blood and urine were collected to measure various experimental parameters of dietary choline status and markers of organ dysfunction and liver fat were assessed. If at some point during the depletion period, functional markers indicated organ dysfunction associated with choline deficiency, subjects were switched to a diet containing choline until repleted.
Folate SNPs
We examined whether major genetic variants of folate metabolism modified the susceptibility of these subjects to choline deficiency (Kohlmeier et al., 2005). Premenopausal women who were carriers of the very common 5,10-methylenetetrahydrofolate dehydrogenase-G1958A (MTHFD1; rs2236225) gene allele were more than 15x as likely as non-carriers to develop signs of choline deficiency (p<0.0001) on the low-choline diet. Sixty-three percent of our study population had at least one allele for this SNP. The MTHFD1 G1958A polymorphism alters the delicately balanced flux between 5,10-methylene tetrahydrofolate and 10-formyl tetrahydrofolate and thereby influences the availability of 5-methyl THF for homocysteine remethylation (Horne, 2003). This increases demand for choline as a methyl-group donor. It is of interest that the risk of having a child with a neural tube defect increases in mothers with the G1958A SNP in MTHFD1 (Brody et al., 2002). We did not have sufficient power in the study to detect any effects of other folate metabolism SNPs (C677T and A1298C polymorphisms of the 5,10-methylene tetrahydrofolate reductase gene and the A80C polymorphism of the reduced folate carrier 1 gene) (Kohlmeier et al., 2005).
Choline SNPs
As noted earlier, PEMT encodes for a protein responsible for endogenous formation of choline. We identified an SNP in the promoter region of the PEMT gene (rs12325817) for which 18 of 23 carriers of the C allele (78%) developed organ dysfunction when fed a low choline diet (odds ratio 25, P=0.002) (da Costa et al., 2006a). Given the sexual differences in the effect of PEMT rs12325817, it is possible that this SNP alters the estrogen responsiveness of the promoter. The frequency of this variant allele was 0.74. A SNP in the PEMT coding region (rs7946) results in a 30% loss of function and is associated with increased risk for nonalcoholic fatty liver disease (Dong et al., 2007; Song et al., 2005) but we did not have the power in this study to identify any association with susceptibility to choline deficiency (da Costa et al., 2006a). The first of two SNPs in the coding region of the choline dehydrogenase gene (CHDH; rs9001) had a protective effect on susceptibility to choline deficiency, while a second CHDH variant (rs12676) was associated with increased susceptibility to choline deficiency (da Costa et al., 2006a). We did not have the power in this study to identify any association of a SNP in the betaine:homocysteine methyltransferase gene (BHMT; rs3733890) with susceptibility to choline deficiency (da Costa et al., 2006a).
Epigenetics and the effects of choline
The effects of choline on neural tube closure and on brain development likely are mediated by changes in the expression of genes. Dietary choline deficiency decreases S-adenosylmethionine concentrations in tissues (Shivapurkar and Poirier, 1983; Zeisel et al., 1989), with resulting hypomethylation of DNA (Locker et al., 1986; Tsujiuchi et al., 1999). DNA methylation occurs at cytosine bases that are followed by a guanosine (5′-CpG-3′ sites) (Holliday and Grigg, 1993) and influences many cellular events, including gene transcription, genomic imprinting and genomic stability (Jaenisch, 1997; Jones and Gonzalgo, 1997; Robertson and Wolffe, 2000). In mammals, about 60 to 80% of CpG sites in DNA are methylated, while most CpGs within CpG islands are not (Jeltsch, 2002). When this modification occurs in promoter regions, gene expression is altered (Bird, 1986); increased methylation is associated with gene silencing or reduced gene expression (Jeltsch, 2002). In choline deficient cells in culture, and in fetal rodent brains from mothers fed choline deficient diets, methylation of the CDKN3 gene promoter is decreased, resulting in over expression of this gene which inhibits cell proliferation (Niculescu et al., 2004; Niculescu et al., 2005). This change in gene promoter methylation likely alters neurogenesis in the hippocampus for life – prenatal choline supplementation in rats resulted in increased neurogenesis that was still detectable at 7 months of age (Glenn et al., 2007). There are other examples where maternal diets high in methyl groups had permanent effects on their offspring. Feeding pregnant Pseudoagouti Avy/a mouse dams a choline methyl-supplemented diet altered epigenetic regulation of agouti expression in their offspring, as indicated by increased agouti/black mottling of their coats (Cooney et al., 2002; Wolff et al., 1998). In another example, there was increased DNA methylation of the fetal gene axin fused (Axin(Fu)) after methyl donor supplementation of female mice before and during pregnancy which reduced by 50% the incidence of tail kinking in Axin(Fu)/+ offspring (Waterland et al., 2006). It is clear that the dietary manipulation of methyl donors (either deficiency or supplementation) can have a profound impact upon gene expression and, by consequence, upon the homeostatic mechanisms that ensure the normal function of physiological processes.
Summary
Elucidating the human dietary choline requirement requires understanding of 1-carbon and choline metabolism as well as of genetic variations that influence these pathways; this process provides one of the best examples of the importance of nutrigenomics. Common genetic polymorphisms have major effects on the dietary requirement for choline. The only source for this nutrient, other than diet is endogenous biosynthesis mediated by PEMT, whose expression is induced by estrogen via estrogen response elements in the promoter of this gene. The availability of choline, in turn, influences DNA methylation, and this modulates gene expression. These observations have important implications: organ dysfunction or increased risk for birth defects may occur only when a combination of inadequate diet, SNPs and/or low estrogen status is present. It may be that mothers eating low choline diets but who have no SNPs in genes of methyl metabolism have good fetal outcome, and that mothers having such SNPS but eating high choline diets also have good fetal outcome. Perhaps, only mothers eating a low choline and having such SNPs are at risk for having a baby with a birth defect. This would require a very different study design than the simple comparison of choline control and supplemented groups.
Acknowledgments
This work was funded by grants from the National Institutes of Health (AG09525, DK55865). Support for this work was also provided by grants from the NIH to the UNC Clinical Nutrition Research Unit (DK56350), the UNC General Clinical Research Center (RR00046) and the Center for Environmental Health and Susceptibility (ES10126).
References
- Adeyemo O, Jeyakumar H. Plasma progesterone, estradiol-17 beta and testosterone in maternal and cord blood, and maternal human chorionic gonadotropin at parturition. Afr J Med Med Sci. 1993;22:55–60. [PubMed] [Google Scholar]
- Agarwal A, Yeung WS, Lee KF. Cloning and characterization of the human oviduct-specific glycoprotein (HuOGP) gene promoter. Mol Hum Reprod. 2002;8:167–75. doi: 10.1093/molehr/8.2.167. [DOI] [PubMed] [Google Scholar]
- Albright CD, Lui R, Bethea TC, da Costa K-A, Salganik RI, Zeisel SH. Choline deficiency induces apoptosis in SV40-immortalized CWSV-1 rat hepatocytes in culture. FASEB J. 1996;10:510–516. doi: 10.1096/fasebj.10.4.8647350. [DOI] [PubMed] [Google Scholar]
- Albright CD, Zeisel SH. Choline deficiency causes increased localization of TGFβ1 signaling proteins and apoptosis in rat liver. Pathobiology. 1997;65:264–270. doi: 10.1159/000164137. [DOI] [PubMed] [Google Scholar]
- Albright CD, Tsai AY, Mar M-H, Zeisel SH. Choline availability modulates the expression of TGFβ1 and cytoskeletal proteins in the hippocampus of developing rat brain. Neurochem Res. 1998;23:751–758. doi: 10.1023/a:1022411510636. [DOI] [PubMed] [Google Scholar]
- Albright CD, Friedrich CB, Brown EC, Mar MH, Zeisel SH. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Res Dev Brain Res. 1999a;115:123–9. doi: 10.1016/s0165-3806(99)00057-7. [DOI] [PubMed] [Google Scholar]
- Albright CD, Tsai AY, Friedrich CB, Mar MH, Zeisel SH. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res Dev Brain Res. 1999b;113:13–20. doi: 10.1016/s0165-3806(98)00183-7. [DOI] [PubMed] [Google Scholar]
- Albright CD, Mar MH, Friedrich CB, Brown EC, Zeisel SH. Maternal choline availability alters the localization of p15Ink4B and p27Kip1 cyclin-dependent kinase inhibitors in the developing fetal rat brain hippocampus. Dev Neurosci. 2001;23:100–6. doi: 10.1159/000048701. [DOI] [PubMed] [Google Scholar]
- Albright CD, Siwek DF, Craciunescu CN, Mar MH, Kowall NW, Williams CL, Zeisel SH. Choline availability during embryonic development alters the localization of calretinin in developing and aging mouse hippocampus. Nutr Neurosci. 2003;6:129–34. doi: 10.1080/1028415031000084418. [DOI] [PubMed] [Google Scholar]
- Albright CD, da Costa KA, Craciunescu CN, Klem E, Mar MH, Zeisel SH. Regulation of choline deficiency apoptosis by epidermal growth factor in CWSV-1 rat hepatocytes. Cell Physiol Biochem. 2005a;15:59–68. doi: 10.1159/000083653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright CD, Mar MH, Craciunescu CN, Song J, Zeisel SH. Maternal dietary choline availability alters the balance of netrin-1 and DCC neuronal migration proteins in fetal mouse brain hippocampus. Brain Res Dev Brain Res. 2005b;159:149–54. doi: 10.1016/j.devbrainres.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–13. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK, Zeisel SH, Wurtman RJ. Developmental changes in the activity of phosphatidylethanolamine N-methyltransferases in rat brain. Biochem J. 1985;232:505–11. doi: 10.1042/bj2320505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody LC, Conley M, Cox C, Kirke PN, McKeever MP, Mills JL, Molloy AM, O’Leary VB, Parle-McDermott A, Scott JM, Swanson DA. A polymorphism, R653Q, in the trifunctional enzyme methylenetetrahydrofolate dehydrogenase/methenyltetrahydrofolate cyclohydrolase/formyltetrahydrofolate synthetase is a maternal genetic risk factor for neural tube defects: report of the Birth Defects Research Group. Am J Hum Genet. 2002;71:1207–15. doi: 10.1086/344213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A, Dubin M, Moukarzel A, Jenden D, Roch M, Rice K, Gornbein J, Ament M. Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology. 1995;22:1399–1403. [PubMed] [Google Scholar]
- Busby MG, Fischer L, Da Costa KA, Thompson D, Mar MH, Zeisel SH. Choline-and betaine-defined diets for use in clinical research and for the management of trimethylaminuria. J Am Diet Assoc. 2004;104:1836–45. doi: 10.1016/j.jada.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Cheng RK, MacDonald CJ, Williams CL, Meck WH. Prenatal choline supplementation alters the timing, emotion, and memory performance (TEMP) of adult male and female rats as indexed by differential reinforcement of low-rate schedule behavior. Learn Mem. 2008;15:153–62. doi: 10.1101/lm.729408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132:2393S–2400S. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- Craciunescu CN, Albright CD, Mar MH, Song J, Zeisel SH. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J Nutr. 2003;133:3614–8. doi: 10.1093/jn/133.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa KA, Badea M, Fischer LM, Zeisel SH. Elevated serum creatine phosphokinase in choline-deficient humans: mechanistic studies in C2C12 mouse myoblasts. Am J Clin Nutr. 2004;80:163–170. doi: 10.1093/ajcn/80.1.163. [DOI] [PubMed] [Google Scholar]
- da Costa KA, Gaffney CE, Fischer LM, Zeisel SH. Choline deficiency in mice and humans is associated with increased plasma homocysteine concentration after a methionine load. Am J Clin Nutr. 2005;81:440–444. doi: 10.1093/ajcn.81.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa KA, Kozyreva OG, Song J, Galanko JA, Fischer LM, Zeisel SH. Common genetic polymorphisms affect the human requirement for the nutrient choline. Faseb J. 2006a;20:1336–1344. doi: 10.1096/fj.06-5734com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa KA, Niculescu MD, Craciunescu CN, Fischer LM, Zeisel SH. Choline deficiency increases lymphocyte apoptosis and DNA damage in humans. Am J Clin Nutr. 2006b;84:88–94. doi: 10.1093/ajcn/84.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Wang J, Li C, Hirose A, Nozaki Y, Takahashi M, Ono M, Akisawa N, Iwasaki S, Saibara T, Onishi S. The phosphatidylethanolamine N-methyltransferase gene V175M single nucleotide polymorphism confers the susceptibility to NASH in Japanese population. J Hepatol. 2007;46:915–20. doi: 10.1016/j.jhep.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Finkelstein JD. Pathways and regulation of homocysteine metabolism in mammals. Semin Thromb Hemost. 2000;26:219–25. doi: 10.1055/s-2000-8466. [DOI] [PubMed] [Google Scholar]
- Fischer LM, daCosta K, Kwock L, Stewart P, Lu T-S, Stabler S, Allen R, Zeisel S. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr. 2007;85:1275–1285. doi: 10.1093/ajcn/85.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MC, Zeisel SH, Mar MH, Sadler TW. Inhibitors of choline uptake and metabolism cause developmental abnormalities in neurulating mouse embryos. Teratology. 2001;64:114–22. doi: 10.1002/tera.1053. [DOI] [PubMed] [Google Scholar]
- Fisher MC, Zeisel SH, Mar MH, Sadler TW. Perturbations in choline metabolism cause neural tube defects in mouse embryos in vitro. Faseb J. 2002;16:619–21. doi: 10.1096/fj.01-0564fje. [DOI] [PubMed] [Google Scholar]
- Glenn MJ, Gibson EM, Kirby ED, Mellott TJ, Blusztajn JK, Williams CL. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. Eur J Neurosci. 2007;25:2473–2482. doi: 10.1111/j.1460-9568.2007.05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs CA, Cleves MA, Melnyk S, Zhao W, James SJ. Congenital heart defects and abnormal maternal biomarkers of methionine and homocysteine metabolism. Am J Clin Nutr. 2005;81:147–53. doi: 10.1093/ajcn/81.1.147. [DOI] [PubMed] [Google Scholar]
- Holliday R, Grigg GW. DNA methylation and mutation. Mutat Res. 1993;285:61–7. doi: 10.1016/0027-5107(93)90052-h. [DOI] [PubMed] [Google Scholar]
- Holmes-McNary M, Cheng WL, Mar MH, Fussell S, Zeisel SH. Choline and choline esters in human and rat milk and infant formulas. Am J Clin Nutr. 1996;64:572–576. doi: 10.1093/ajcn/64.4.572. [DOI] [PubMed] [Google Scholar]
- Horne DW. Neither methionine nor nitrous oxide inactivation of methionine synthase affect the concentration of 5,10-methylenetetrahydrofolate in rat liver. J Nutr. 2003;133:476–8. doi: 10.1093/jn/133.2.476. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine, National Academy of Sciences USA. Dietary reference intakes for folate, thiamin, riboflavin, niacin, vitamin B12, panthothenic acid, biotin, and choline. Vol. 1. National Academy Press; Washington D.C.: 1998. Choline; pp. 390–422. [Google Scholar]
- Jacobs RL, Stead LM, Devlin C, Tabas I, Brosnan ME, Brosnan JT, Vance DE. Physiological regulation of phospholipid methylation alters plasma homocysteine in mice. J Biol Chem. 2005;280:28299–28305. doi: 10.1074/jbc.M501971200. [DOI] [PubMed] [Google Scholar]
- Jacques PF, Bostom AG, Wilson PW, Rich S, Rosenberg IH, Selhub J. Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am J Clin Nutr. 2001;73:613–21. doi: 10.1093/ajcn/73.3.613. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. DNA methylation and imprinting: why bother? Trends Genet. 1997;13:323–9. doi: 10.1016/s0168-9525(97)01180-3. [DOI] [PubMed] [Google Scholar]
- James SJ, Miller BJ, Basnakian AG, Pogribny IP, Pogribna M, Muskhelishvili L. Apoptosis and proliferation under conditions of deoxynucleotide pool imbalance in liver of folate/methyl deficient rats. Carcinogenesis. 1997;18:287–93. doi: 10.1093/carcin/18.2.287. [DOI] [PubMed] [Google Scholar]
- Jeltsch A. Beyond Watson and Crick: DNA Methylation and Molecular Enzymology of DNA Methyltransferases. Chembiochem. 2002;3:382. doi: 10.1002/1439-7633(20020402)3:4<274::AID-CBIC274>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Jones PA, Gonzalgo ML. Altered DNA methylation and genome instability: a new pathway to cancer? Proc Natl Acad Sci U S A. 1997;94:2103–5. doi: 10.1073/pnas.94.6.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-I, Miller JW, da Costa K-A, Nadeau M, Smith D, Selhub J, Zeisel SH, Mason JB. Folate deficiency causes secondary depletion of choline and phosphocholine in liver. J Nutr. 1995;124:2197–2203. doi: 10.1093/jn/124.11.2197. [DOI] [PubMed] [Google Scholar]
- Kohlmeier M, da Costa KA, Fischer LM, Zeisel SH. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc Natl Acad Sci U S A. 2005;102:16025–30. doi: 10.1073/pnas.0504285102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker J, Reddy TV, Lombardi B. DNA methylation and hepatocarcinogenesis in rats fed a choline devoid diet. Carcinogenesis. 1986;7:1309–1312. doi: 10.1093/carcin/7.8.1309. [DOI] [PubMed] [Google Scholar]
- Lopez D, Sanchez MD, Shea-Eaton W, McLean MP. Estrogen activates the high-density lipoprotein receptor gene via binding to estrogen response elements and interaction with sterol regulatory element binding protein-1A. Endocrinology. 2002;143:2155–68. doi: 10.1210/endo.143.6.8855. [DOI] [PubMed] [Google Scholar]
- McMahon KE, Farrell PM. Measurement of free choline concentrations in maternal and neonatal blood by micropyrolysis gas chromatography. Clin Chim Acta. 1985;149:1–12. doi: 10.1016/0009-8981(85)90267-0. [DOI] [PubMed] [Google Scholar]
- Meck W, Williams C. Perinatal choline supplementation increases the threshold for chunking in spatial memory. Neuroreport. 1997;8:3053–3059. doi: 10.1097/00001756-199709290-00010. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev Psychobiol. 1988;21:339–353. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–99. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Mellott TJ, Williams CL, Meck WH, Blusztajn JK. Prenatal choline supplementation advances hippocampal development and enhances MAPK and CREB activation. Faseb J. 2004;18:545–7. doi: 10.1096/fj.03-0877fje. [DOI] [PubMed] [Google Scholar]
- Niculescu MD, Yamamuro Y, Zeisel SH. Choline availability modulates human neuroblastoma cell proliferation and alters the methylation of the promoter region of the cyclin-dependent kinase inhibitor 3 gene. J Neurochem. 2004 doi: 10.1111/j.1471-4159.2004.02414.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu MD, Craciunescu CN, Zeisel SH. Gene expression profiling of choline-deprived neural precursor cells isolated from mouse brain. Brain Res Mol Brain Res. 2005;134:309–22. doi: 10.1016/j.molbrainres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. Faseb J. 2006;20:43–9. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olthof MR, van Vliet T, Boelsma E, Verhoef P. Low dose betaine supplementation leads to immediate and long term lowering of plasma homocysteine in healthy men and women. J Nutr. 2003;133:4135–8. doi: 10.1093/jn/133.12.4135. [DOI] [PubMed] [Google Scholar]
- Ozarda IY, Uncu G, Ulus IH. Free and phospholipid-bound choline concentrations in serum during pregnancy, after delivery and in newborns. Arch Physiol Biochem. 2002;110:393–399. doi: 10.1076/apab.110.5.393.11832. [DOI] [PubMed] [Google Scholar]
- Pomfret EA, da Costa K, Zeisel SH. Effects of choline deficiency and methotrexate treatment upon rat liver. J Nutr Biochem. 1990;1:533–541. doi: 10.1016/0955-2863(90)90039-n. [DOI] [PubMed] [Google Scholar]
- Pyapali G, Turner D, Williams C, Meck W, Swartzwelder HS. Prenatal choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats. J Neurophysiol. 1998;79:1790–1796. doi: 10.1152/jn.1998.79.4.1790. [DOI] [PubMed] [Google Scholar]
- Resseguie M, Song J, Niculescu M, da Costa K, Randall T, Zeisel S. Phosphatidylethanolamine n-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 2007 doi: 10.1096/fj.07-8227com. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KD, Wolffe AP. DNA methylation in health and disease. Nat Rev Genet. 2000;1:11–9. doi: 10.1038/35049533. [DOI] [PubMed] [Google Scholar]
- Sarda IR, Gorwill RH. Hormonal studies in pregnancy. I. Total unconjugated estrogens in maternal peripheral vein, cord vein, and cord artery serum at delivery. Am J Obstet Gynecol. 1976;124:234–8. [PubMed] [Google Scholar]
- Selhub J, Seyoum E, Pomfret EA, Zeisel SH. Effects of choline deficiency and methotrexate treatment upon liver folate content and distribution. Cancer Res. 1991;51:16–21. [PubMed] [Google Scholar]
- Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004;160:102–9. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Carmichael SL, Laurent C, Rasmussen SA. Maternal nutrient intakes and risk of orofacial clefts. Epidemiology. 2006;17:285–91. doi: 10.1097/01.ede.0000208348.30012.35. [DOI] [PubMed] [Google Scholar]
- Shelnutt KP, Kauwell GP, Chapman CM, Gregory JF, 3rd, Maneval DR, Browdy AA, Theriaque DW, Bailey LB. Folate status response to controlled folate intake is affected by the methylenetetrahydrofolate reductase 677C-->T polymorphism in young women. J Nutr. 2003;133:4107–11. doi: 10.1093/jn/133.12.4107. [DOI] [PubMed] [Google Scholar]
- Shields DJ, Lingrell S, Agellon LB, Brosnan JT, Vance DE. Localization-independent regulation of homocysteine secretion by phosphatidylethanolamine N-methyltransferase. J Biol Chem. 2005 doi: 10.1074/jbc.M504658200. [DOI] [PubMed] [Google Scholar]
- Shin OH, Mar MH, Albright CD, Citarella MT, daCosta KA, Zeisel SH. Methyl-group donors cannot prevent apoptotic death of rat hepatocytes induced by choline-deficiency. J Cell Biochem. 1997;64:196–208. doi: 10.1002/(sici)1097-4644(199702)64:2<196::aid-jcb3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Shivapurkar N, Poirier LA. Tissue levels of S-adenosylmethionine and S-adenosylhomocysteine in rats fed methyl-deficient, amino acid-defined diets for one to five weeks. Carcinogenesis. 1983;4:1051–1057. doi: 10.1093/carcin/4.8.1051. [DOI] [PubMed] [Google Scholar]
- Song J, da Costa KA, Fischer L, Kohlmeier M, Kwock L, Wang S, Zeisel SH. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD) Faseb J. 2005 doi: 10.1096/fj.04-3580com. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenge GR, Verhoef P, Katan MB. Betaine supplementation lowers plasma homocysteine in healthy men and women. J Nutr. 2003;133:1291–5. doi: 10.1093/jn/133.5.1291. [DOI] [PubMed] [Google Scholar]
- Sunden S, Renduchintala M, Park E, Miklasz S, Garrow T. Betaine-Homocysteine methyltransferase expression in porcine and human tissues and chromosomal localization of the human gene. Arch Biochem Biophys. 1997;345:171–174. doi: 10.1006/abbi.1997.0246. [DOI] [PubMed] [Google Scholar]
- Sweiry JH, Yudilevich DL. Characterization of choline transport at maternal and fetal interfaces of the perfused guinea-pig placenta. J Physiol. 1985;366:251–266. doi: 10.1113/jphysiol.1985.sp015795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweiry JH, Page KR, Dacke CG, Abramovich DR, Yudilevich DL. Evidence of saturable uptake mechanisms at maternal and fetal sides of the perfused human placenta by rapid paired-tracer dilution: studies with calcium and choline. J Devel Physiol. 1986;8:435–445. [PubMed] [Google Scholar]
- Tsujiuchi T, Tsutsumi M, Sasaki Y, Takahama M, Konishi Y. Hypomethylation of CpG sites and c-myc gene overexpression in hepatocellular carcinomas, but not hyperplastic nodules, induced by a choline-deficient L-amino acid-defined diet in rats. Jpn J Cancer Res. 1999;90:909–13. doi: 10.1111/j.1349-7006.1999.tb00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Moreiras G, Selhub J, da Costa K, Zeisel SH. Effect of chronic choline deficiency in rats on liver folate content and distribution. J Nutr Biochem. 1992;3:519–522. [Google Scholar]
- Varela-Moreiras G, Ragel C, Perez de Miguelsanz J. Choline deficiency and methotrexate treatment induces marked but reversible changes in hepatic folate concentrations, serum homocysteine and DNA methylation rates in rats. J Amer Coll Nutr. 1995;14:480–485. doi: 10.1080/07315724.1995.10718539. [DOI] [PubMed] [Google Scholar]
- Velzing-Aarts FV, Holm PI, Fokkema MR, van der Dijs FP, Ueland PM, Muskiet FA. Plasma choline and betaine and their relation to plasma homocysteine in normal pregnancy. Am J Clin Nutr. 2005;81:1383–9. doi: 10.1093/ajcn/81.6.1383. [DOI] [PubMed] [Google Scholar]
- Waite KA, Cabilio NR, Vance DE. Choline deficiency-induced liver damage is reversible in Pemt(-/-) mice. J Nutr. 2002;132:68–71. doi: 10.1093/jn/132.1.68. [DOI] [PubMed] [Google Scholar]
- Walkey CJ, Yu L, Agellon LB, Vance DE. Biochemical and evolutionary significance of phospholipid methylation. J Biol Chem. 1998;273:27043–6. doi: 10.1074/jbc.273.42.27043. [DOI] [PubMed] [Google Scholar]
- Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M, et al. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci U S A. 1985;82:7889–93. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterland RA, Dolinoy DC, Lin JR, Smith CA, Shi X, Tahiliani KG. Maternal methyl supplements increase offspring DNA methylation at Axin fused. Genesis. 2006;44:401–406. doi: 10.1002/dvg.20230. [DOI] [PubMed] [Google Scholar]
- Watkins D, Ru M, Hwang HY, Kim CD, Murray A, Philip NS, Kim W, Legakis H, Wai T, Hilton JF, Ge B, Dore C, Hosack A, Wilson A, Gravel RA, Shane B, Hudson TJ, Rosenblatt DS. Hyperhomocysteinemia due to methionine synthase deficiency, cblG: structure of the MTR gene, genotype diversity, and recognition of a common mutation, P1173L. Am J Hum Genet. 2002;71:143–53. doi: 10.1086/341354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg IS, Jacques PF, Selhub J, Bostom AG, Chen Z, Curtis Ellison R, Eckfeldt JH, Rozen R. The 1298A-->C polymorphism in methylenetetrahydrofolate reductase (MTHFR): in vitro expression and association with homocysteine. Atherosclerosis. 2001;156:409–15. doi: 10.1016/s0021-9150(00)00671-7. [DOI] [PubMed] [Google Scholar]
- Wendel U, Bremer H. Betaine in the treatment of homocystinuria due to 5,10-methylenetetrahydrofolate reductase deficiency. Eur J Pediatr. 1984;142:147–150. doi: 10.1007/BF00445602. [DOI] [PubMed] [Google Scholar]
- Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. Faseb J. 1998;12:949–57. [PubMed] [Google Scholar]
- Xie T, Ho SL, Ramsden D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol Pharmacol. 1999;56:31–8. doi: 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]
- Yao ZM, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem. 1988;263:2998–3004. [PubMed] [Google Scholar]
- Yao ZM, Vance DE. Head group specificity in the requirement of phosphatidylcholine biosynthesis for very low density lipoprotein secretion from cultured hepatocytes. J Biol Chem. 1989;264:11373–11380. [PubMed] [Google Scholar]
- Zeisel SH, Wurtman RJ. Developmental changes in rat blood choline concentration. Biochem J. 1981;198:565–570. doi: 10.1042/bj1980565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, Zola T, daCosta K, Pomfret EA. Effect of choline deficiency on S-adenosylmethionine and methionine concentrations in rat liver. Biochem J. 1989;259:725–729. doi: 10.1042/bj2590725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, daCosta K-A, Franklin PD, Alexander EA, Lamont JT, Sheard NF, Beiser A. Choline, an essential nutrient for humans. FASEB J. 1991;5:2093–2098. [PubMed] [Google Scholar]
- Zeisel SH, Mar M-H, Zhou Z-W, da Costa K-A. Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J Nutr. 1995;125:3049–3054. doi: 10.1093/jn/125.12.3049. [DOI] [PubMed] [Google Scholar]
- Zeisel SH, Mar M-H, Howe JC, Holden JM. Erratum: Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003a;133:1302–1307. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]; J Nutr. 133:2918–2919. [Google Scholar]
- Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003b;133:1302–7. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–50. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Song J, Mar MH, Edwards LJ, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) knockout mice have hepatic steatosis and abnormal hepatic choline metabolite concentrations despite ingesting a recommended dietary intake of choline. Biochem J. 2003;370:987–93. doi: 10.1042/BJ20021523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Mar MH, Song J, Zeisel SH. Deletion of the Pemt gene increases progenitor cell mitosis, DNA and protein methylation and decreases calretinin expression in embryonic day 17 mouse hippocampus. Brain Res Dev Brain Res. 2004;149:121–9. doi: 10.1016/j.devbrainres.2004.01.004. [DOI] [PubMed] [Google Scholar]