Abstract
The central importance of calcium clearance proteins, and their regulators, in the modulation of myocardial contractility and intracellular Ca2+ concentration ([Ca2+]i) has long been established. Key players identified include the Na+-Ca2+ exchanger, the Na+-K+ ATPase, the sarco(endo)plasmic reticulum Ca2+-ATPase and associated phospholamban. Gene-targeted and transgenic murine models have been critical in the elucidation of their function. The study of these proteins in the regulation of contractile parameters in vascular smooth muscle, on the other hand, is less well studied. More recently, gene-targeted and transgenic models have expanded our knowledge of Ca2+ clearance proteins and their role in both tonic and phasic smooth muscle contractility. In this review, we will briefly treat the mechanisms which underlie Ca2+ clearance in smooth muscle. These will be addressed in light of studies using gene-modified mouse models, the results of which will be compared and contrasted with those in the cardiomyocyte. The recently identified human mutations in phospholamban, which lead to dilated cardiomyopathy, are also present in vascular and other smooth muscle. Given the importance of these Ca2+ clearance systems to modulation of smooth muscle, it is likely that mutations will also lead to smooth muscle pathology.
Ca2+ CLEARANCE AND REGULATION OF [Ca2+]i
Ca2+ homeostasis is central to the regulation of smooth muscle function. It is well established that [Ca2+]i plays an essential role in the activation of myosin light chain kinase, which phosphorylates myosin, thereby activating the actin-myosin interaction (for review see [1]). It has also be estimated that Ca2+ influx under basal conditions is 16 μmole/liter per minute, more than 2 orders of magnitude greater than the resting [Ca2+]i. Thus Ca2+ clearance from the cytosol is critical to the maintenance of a quiescent baseline and is a major factor in modulation of Ca2+ homeostasis and thus contractile force. The plasma membrane Ca2+ ATPase (PMCA), sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA), Na+-Ca2+-exchanger (NCX) and mitochondria all function to some extent in this process (for reviews see [2, 3]). Figure 1 shows a schematic illustration of these Ca2+ clearance pathways. Also of importance to smooth muscle Ca2+ clearance are phospholamban (PLN), an endogenous inhibitor of SERCA, and the Na+-K+ ATPase (NKA), which couples to NCX, and facilitates the extrusion of Ca2+ via the forward mode of the exchanger. NCX is generally considered to be a high-capacity exchanger, i.e., low affinity for Ca2+ (Kd≈1 μM), but high turnover [4-7]. SERCA and PMCA, on the other hand, have a higher affinity for Ca2+ (Kd≈0.1-0.3 μM) [6, 8-11], but lower turnover than NCX. For smooth muscle, the relative contribution of each to Ca2+ clearance is dependent on conditions and smooth muscle type, but in general, NCX accounts for about 60%, while PMCA and SERCA facilitate about 20-30% each. Mitochondrial Ca2+-uptake can also be a factor under certain conditions, but its apparent affinity is thought to be relatively low (∼10-20 μM, [12]). Recent evidence, however, suggest mitochondria may play some role as both as a buffer and/or regulator of Ca2+ clearance [13, 14].
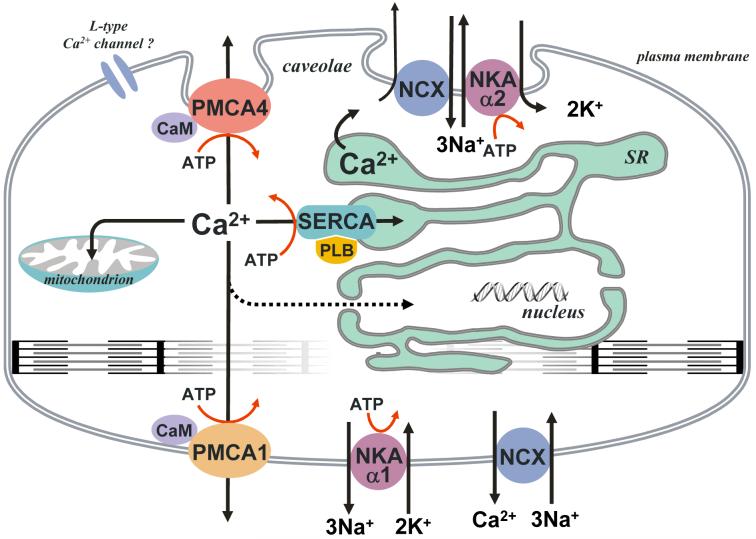
Fig. 1.
Schematic overview of the Ca2+-clearance systems associated with smooth muscle. These include the plasma membrane Ca2+ ATPase isoforms (PMCA1 & 4), the plasma membrane Na+-Ca2+ exchanger (NCX) coupled to the Na+-K+ ATPase (α-isoforms 1 & 2) and the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA isoforms 2a & 2b). Mitochondria are also included, though their role in Ca2+-clearance is not known with certainty. Adapted from Ishida and Paul [2].
Much recent interest has been directed toward the role of caveolae, and their corresponding subsarcolemmal compartments, in smooth muscle Ca2+ handling. Caveolae [Ca2+] may, at least transiently, differ from that of the general extracellular milieu (for review see [15, 16]). Localized to regions of the plasma membrane closely associated with the peripheral sarco(endo)plasmic reticulum (SR), these vesicular membrane structures have been suggested to be a source of Ca2+ that can be recycled to and from the SR, and therefore have been implicated in excitation-contraction coupling. Localization of PMCA, NCX and voltage dependent calcium channels (VDCC) to this compartment has been firmly established in smooth muscle ([15, 17], Fig. 1). Colocalization of these Ca2+-handling proteins with caveolae suggest a role for Ca2+ extrusion and regulation in this subsarcolemmal compartment. Indeed, it is hypothesized that both caveolae, via L-type Ca2+ channels, and SR, via ryanodine receptors (RyRs), can supply the subsarcolemmal space with Ca2+, while either NCX or PMCA are involved in its extrusion from caveolae. The SERCA pump, on the other hand, could be used to sequester such Ca2+ into the SR pool. While the NKA has been observed in association with caveolae in cardiac muscle [18], there is currently no direct evidence for its colocalization in the caveolae of smooth muscle. The colocalization of NCX and the α2-isoform of NKA in smooth muscle ([19, 20], Fig. 1), however, suggests that such an association may indeed exist. The structure of the SR itself can also be a factor in this interaction complicating this picture. Whether it is a single structure or multiple vesicles has long been debated with the most recent evidence favoring a single entity [21]
The importance of Ca2+ handling in cardiac performance has long been established, with [Ca2+]i known to be a critical determinant of contractility. Binding to troponin-C, Ca2+ initiates a conformational change, which ultimately relieves the inhibition of the actin-myosin interaction [22]. Furthermore, it is long established that, as in smooth muscle, PMCA, NCX and SERCA all contribute to some extent in the process of Ca2+ removal from the cytoplasm, with PLN and NKA playing regulatory and facilitory roles, respectively. Extensive effort has been expended into the study of these proteins, and great emphasis has been directed toward associations between altered Ca2+ handling and cardiac disease. Nowhere is this better exhibited than in the study of PLN [23]. Studies utilizing both murine models and naturally occurring mutations in humans have assigned great importance to the role of PLN in both the normo- and pathophysiology of the heart.
GENE-TARGETED AND TRANSGENIC STUDIES OF SMOOTH MUSCLE AND ASSOCIATED NON-MUSCLE TISSUES
The role of Ca2+ handling proteins and their associated regulators in the modulation of smooth muscle tone has long been a subject of great interest to investigators. With the advent of novel gene-targeted and transgenic murine models, the last decade has seen intensified efforts to elucidate the specific functions of Ca2+ clearance proteins. Specifically, we will here review those studies that have addressed the functions of PMCA, SERCA, NCX, PLN and NKA, including a brief comparison to those results from the investigation of cardiomyocytes.
Plasma Membrane Ca2+ATPase: Isoforms And Tissue Specific Functions
PMCA, a calmodulin-dependent calcium ATPase, is a ubiquitous transport protein that acts to extrude Ca2+ across the plasmalemma [8, 24-26]. To date, the existence of four PMCA isoforms has been established, with further variability arising from alternative splicing (for review see [27]). PMCA1 and PMCA4 are both ubiquitously expressed, and, importantly, are the only isoforms currently reported in smooth muscle [28]. PMCA2 and PMCA3 exhibit cell-specific patterns of expression. The expression of PMCA1, 2 and 4 has been reported in the myocardium. Much knowledge has been gained regarding the biochemical properties and expression patterns of these various isoforms, but the lack of specific inhibitors has limited studies on their physiological significance.
In the myocardium, Neyses and colleagues [29] utilized a transgenic rat model carrying human PMCA4 cDNA under the control of the ventricle-specific myosin light chain-2 promoter. Based on these data, they suggested little relevance for PMCA in the beat-to-beat regulation of contraction-relaxation in the adult rat heart. This is supported by the fact that the fast Ca2+ transients were unchanged in the transgenic tissue. Its role instead appeared to involve the regulation of myocardial growth via the modulation of caveolar signal transduction. This is complemented by the finding that PMCA colocalizes with caveolin 3 in cardiomyocytes. Additionally, an increased rate of synthesis of total protein in these models when incubated with 2% fetal calf serum (FCS) or phenylephrine (PE) was reported. These results suggest a role for the sarcolemmal calcium pump in the modulation of caveolar signaling, possibly through the modification of subcellular Ca2+ pools. Undoubtedly, as the fields of caveolar and PMCA research continue to expand, such mechanisms will be either confirmed or refuted.
In contrast to its role in cardiac myocytes, PMCA was shown to be a major player in bladder [30, 31] and uterine [32] smooth muscle contractility. Utilizing Pmca1+/-, Pmca4-/-, and Pmca1+/-×Pmca4-/- mice, Paul and colleagues [30] observed a significant prolongation of the half-time for force development to potassium chloride (KCl) in these gene-targeted bladders, as compared to controls. As the ablation of one Pmca1 allele did not significantly reduce total PMCA protein levels, the observed abnormalities of contractility appeared to be due to the elimination of one or both Pmca4 alleles. These results suggested that the ablation of the Pmca4 allele(s) may limit depolarization-induced Ca2+ influx. One mechanism proposed by Paul and colleagues for this phenomenon involves the stimulation of NCX-mediated Ca2+ extrusion via an increased near membrane [Ca2+] in PMCA gene-ablated smooth muscles. This proposition is supported by the observation that the inhibition of NCX in Pmca4-/- and Pmca1+/-×Pmca4-/- bladders results in a significantly shortened contraction half-time, when compared to non-NCX inhibited transgenic models. Based on the increase of relaxation times to PMCA in these gene-altered mice, and further increases with CPA inhibition of SERCA, Paul and coworkers calculated that PMCA and SERCA each contribute 20-25% to relaxation, with NCX responsible for the rest. In uterine smooth muscle, similar data obtained using a PMCA4 KO mouse concluded that PMCA accounts for at least ∼65% of relaxation [33]. Taken together, these data are indicative of a role for PMCA4 in both excitation-contraction coupling and Ca2+ extrusion/relaxation in bladder and uterine smooth muscle.
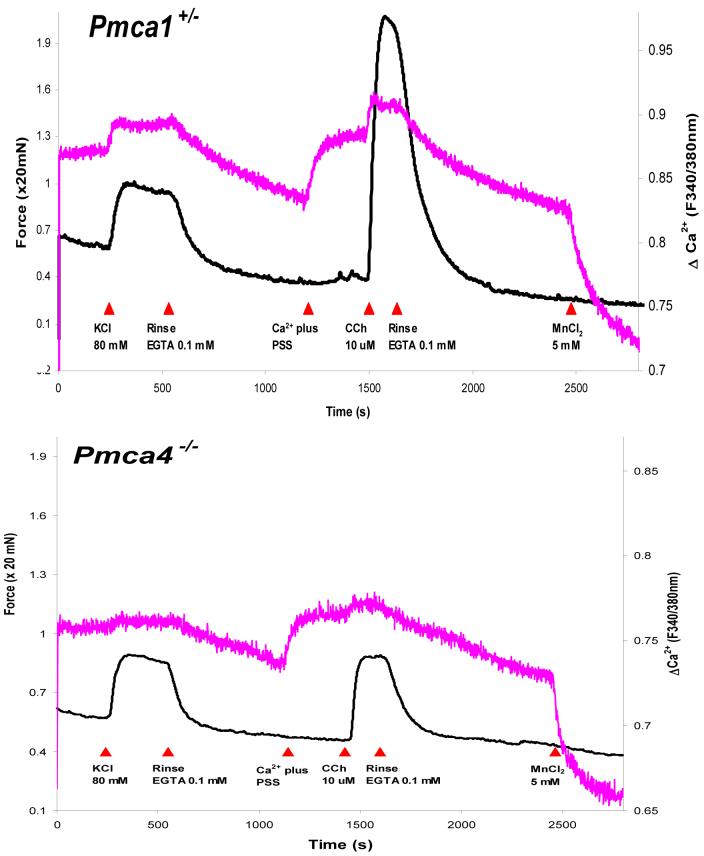
Paul and colleagues [31] further elucidated the specific roles of the PMCA1 and PMCA4 isoforms in bladder smooth muscle. Pmca1+/- bladders exhibited higher [Ca2+]i and force responses (Fig. 2) to both KCl and carbachol (CCh) stimulation upon comparison to wild-type (WT) controls. Pmca4-/- responses to CCh, on the other hand, were significantly suppressed (Fig. 2) when compared to wild-type bladders. Peak tension and [Ca2+]i measurements for Pmca4-/- bladders in response to KCl were similar to wild-type bladders. The data suggested a major house-keeping role for PMCA1, while receptor signaling modulation appears to be the function of PMCA4. Such a role for PMCA1 is supported by the observation that the Pmca1-/- genotype is embryonically lethal [34]. Of particular interest is the function of PMCA4, in light of the finding that this isoform is localized to caveolae ([35], Fig. 1). Altered caveolar Ca2+ dynamics are suggested by prolonged half-times of responses to CCh and their relaxation in Pmca4-/- and Pmca1+/-×Pmca4-/- bladders [30]. Paul and colleagues suggest several potential mechanisms for the suppressed responses to CCh. One such mechanism involves the activation of Ca2+-activated K+ channels via an increased [Ca2+] in PMCA4-associated sub-cellular compartments, while yet another suggests that a high [Ca2+] in a sarcoplasmic reticulum (SR)-associated subsarcolemmal space may lead to a hyperloaded SR, suppressing store-operated Ca2+ entry. Sorting between these and other mechanisms will require future experimentation.
Fig. 2.
Experimental records showing the Ca2+ signal (upper trace in each panel) and simultaneously recorded isometric force (lower trace in each panel) of bladder smooth muscle from Pmca1+/- and Pmca4-/- mice. While KCl contractures are similar, the deletion of one allele of PMCA1 (upper panel) resulted in both Ca2+ and force responses to carbachol (CCh) that were greater, whereas, the loss of PMCA4 (lower panel) resulted in lower responses, than that of the wild-type (not shown). Adapted from Liu et al.[31].
Supporting a role for PMCA4 in the regulation of caveolar signal transduction, experiments in transgenic mice overexpressing human PMCA4b (hPMCA4b) targeted to vascular smooth muscle suggested that PMCA4b regulates vascular tone via inhibition of nitric oxide synthase I (neuronal (n)NOS) [36, 37]. These studies revealed an elevated blood pressure in transgenic models versus controls. Neyses and coworkers observed an increased maximum contraction to KCl in de-endothelialized aortic rings of the transgenic mice compared to controls [36], while Husain and coworkers observed enhanced sensitivity to phenylephrine and prostaglandin F2α in the hPMCA4b-overexpressing mice [37]. Husain also reported that the effect of NOS inhibitors was limited to those arteries from control mice, and that the transgenic aortic smooth muscle cells (SMCs) exhibited a significantly reduced level of cGMP in comparison to control aortic SMCs. These findings, in conjunction with the observation by Schuh et al that PMCA4 and nNOS co-immunoprecipitate [36], suggest that PMCA4 regulates vascular tone via inhibition of nNOS. Although a significant decrease in the resting global [Ca2+]i was not observed [37] it is suggested by both groups that the down-regulation of NO production may occur via the reduction of [Ca2+] in a microdomain associated with both PMCA4 and nNOS. Importantly, nNOS, like PMCA4, has been found to associate with caveolae in smooth muscle tissue [15]. Based upon the colocalization of both these proteins to the caveolae of smooth muscle, it appears likely that the proposed microdomains might correspond to the subsarcolemmal compartments of these surface microvesicles.
Na+-Ca2+ Exchanger, NKA and Ca2+ Clearance: Function Via Colocalization
The mammalian NCX family, a set of bidirectional enzymes that act to transport Ca2+ ions across the plasma membrane in exchange for 2-3 Na+ ions, has been found to consist of at least three isoforms, NCX1-3, which, in turn, give rise to various splice variants (for review see [38]). While the heart solely expresses NCX1.1, the vascular smooth muscle predominantly expresses NCX1.3 and NCX1.7 [39]. Both the membrane potential and transmembrane gradients of Na+ and Ca2+ may act to modulate NCX.
NKA is critical to the establishment of both the membrane potential and Na+ gradient. It is composed of an αβ dimer, with multiple isoforms of the α- and β-subunits currently identified (for review see [40]). Four known isoforms of the catalytic α-subunit exist [40, 41], but only the α1 and α2 subunits are associated with adult murine aortic smooth [42] and cardiac [43] muscle. As opposed to humans, in which both the α1- and α2-isoforms are similarly sensitive to inhibition by ouabain, both mice and rats exhibit an α2 subunit that is much more sensitive to ouabain inhibition when compared to the α1 subunit [41]). Studies of cultured vascular smooth muscle [42] and astrocytes [20] have shown distinct isoform-specific distribution patterns, with the α1-isoform uniformly distributed across the plasmalemma and the α2-isoform localizing to membrane microdomains. Furthermore, the colocalization of NCX, NKA and the SR in smooth muscle has been established [19], with much interest given to the localization of NCX and the SR to those same microdomains found to include NKA α2 subunits ([20, 44], Fig. 1). Such findings have led Blaustein and colleagues to propose a model in which the α2-isoform modulates [Ca2+] via NCX in a microdomain, which, through communications with SERCA, regulates SR Ca2+ loading and contractility (for review see [4]). The α1-isoform, on the other hand, might very well fulfill a “housekeeping” function [20, 45]). Gene-targeted and transgenic models have further improved our understanding of both the NCX and NKA enzymes. A discussion of the utilization of such models, of the interactions between NCX and NKA, and their roles in the modulation of Ca2+ homeostasis and contractility in the heart and smooth muscle follows.
These interactions have been most extensively studied in cardiac muscle. The Na+-Ca2+ exchanger is a dominant player in Ca2+ efflux from cardiomyocytes under physiological conditions, playing a role in the beat-to-beat regulation of intracellular Ca2+ (for review see [46]). It is thus surprising that NCX1 cardiac-specific knockout mice live to adulthood, displaying normal Ca2+ transients, relaxation kinetics and responses to isoproterenol, with only a modestly reduced cardiac function at 7 to 8 weeks [47]. Such Ca2+ dynamics are maintained via a decreased inward Ca2+ current through voltage-dependent L-type channels and an abbreviated action potential in NCX1 knockout mice. By effectively reducing the amount of Ca2+ influx, the cardiomyocyte reduces the amount of necessary Ca2+ efflux, such that the sarcolemmal calcium pump is now sufficient to maintain Ca2+ homeostasis.
Additional information on the role that NCX plays in both cardiac normo- and pathophysiology was gained from studies involving a transgenic mouse model overexpressing canine cardiac NCX under the control of the α-myosin heavy chain promoter. The data, reviewed by Reuter and Philipson [46], suggest a role for NCX in both Ca2+ influx and efflux in transgenic cardiomyocytes. Various studies have suggested that the overexpression of NCX accelerates the rate of removal of Ca2+ from the cytosol, while field stimulation of NCX-overexpressing cardiomyocytes exhibits an increased rate of contraction. An augmented Ca2+ influx is suggested by the presence of a normal basal [Ca2+]i, a normal [Ca2+]i transient amplitude and a normal or increased SR Ca2+ content in the face of increased Ca2+ extrusion via NCX. Enhanced reverse Na+-Ca2+ exchange could certainly be one such mechanism leading to an increased Ca2+ influx. Indeed, existing experimental evidence, briefly reviewed by Reuter and Philipson, support such a role.
Studies of mice heterozygous for either the NKA α1- or α2-isoform have shown a critical role for this enzyme in the regulation of Ca2+ clearance, and thus cardiac contractility, with both isoforms performing distinct functional roles. Evidence suggesting a non-specific “housekeeping” function for α1-NKA in cardiomyocytes can be extrapolated from what Juhaszova and Blaustein [44] refer to as an α1 pattern that is “uniformly distributed in the PM of guinea pig and rat cardiac myocytes.” Experiments conducted by Lingrel and colleagues [48] showed that heterozygous α2-hearts are hypercontractile, while those hearts heterozygous for α1 are hypocontractile. A 40% reduction in the α1-isoform level was observed in the α1+/--hearts, while a 50% reduction in the α2-isoform level was observed in the α2+/--hearts. Importantly, it was also discovered that α2-isoform levels were increased by ∼50% in α1+/--hearts as compared to wild-type, while α1- isoform levels in α2+/--hearts were similar to those in wild-type hearts. Further experiments showed that the inhibition of the α2-isoform in α1+/--hearts with low concentrations of ouabain partially relieved the depressed cardiac function [48, 49]. These data, alongside the isoform-specific distribution patterns described by Blaustein and colleagues, led Lingrel and coworkers [48] to suggest a “functional compartmentalization model” as the basis for distinct roles for the α1- and α2-isoforms in the mouse heart.
According to the “functional compartmentalization model”, the colocalization of the NKA α2- isoform with NCX in a local, functional compartment in close proximity to the sarco/endoplasmic reticulum would allow the α2-isoform to regulate NCX activity, intracellular Ca2+ concentrations and cardiac contractility. Experiments utilizing both fura-2 AM and fluo-3 loaded cells revealed that [Ca2+]i transients were increased in the α2+/--hearts, supporting this idea [48, 50]. Furthermore, the discovery that NCX current is reduced in α1+/--myocytes, but increased in α2+/--myocytes, coupled with the finding that [Ca2+]i transients are decreased in fluo-3 loaded α1+/--myocytes, lends further support for the α2-NKA as the isoform-specific regulator of Ca2+ signaling during cardiac contraction [50]). Recent evidence supports the localization of the α2-isoform with t-tubules in cardiac muscle [51]. Heiny and colleagues [52, 53] reported that α1 expression is nearly constant, but that α2 increases postnatally at the time of t-tubule development in mouse hind limb. These data plus functional evidence [54] would indicate that separate functions for the α1- and α2-isoforms are not limited to cardiac muscle.
There is also a fair literature on the distribution of NCX in smooth muscle, implicating, as in cardiac muscle, a role for this protein in Ca2+ homeostasis and contractility. A recent study of smooth muscle by Kanaide and colleagues [55] has sought to elucidate the functional role of NCX in Ca2+ homeostasis through the tissue-specific overexpression of this protein. They overexpressed canine NCX1.3 in a mouse model utilizing the human smooth muscle α-actin promoter. As it has been previously reported [56] that the phosphorylation of NCX1 by PKA increases its activity, the effects of NCX1.3 overexpression on forskolin-induced relaxation were investigated. Forskolin-induced decreases in [Ca2+]i and tension were greater in aortas from the transgenic mice when compared with those from the wild-type controls [55]. They observed decreases in the transgenic model that were greatly inhibited in the presence of low Na+ PSS or SEA0400, both inhibitors of NCX, implicating a role for the Na+-Ca2+ exchanger in these forskolin-induced phenomena. These observations by Kanaide and co-workers lend support to the hypothesis that cAMP-mediated relaxation pathways function at least partially through an increased activity of the forward mode of NCX.
Just as the NKA α2-isoform has shown to be a critical player in the control of cardiac contractility, so has it established itself as a modulator of vascular smooth muscle tone. Utilizing α1+/--, α2-/-- and α2+/--mice, Paul and colleagues [42] found the α2-/--aortae to be more sensitive to receptor-mediated stimulation than wild-type aortae, while also exhibiting a faster rate of force development. The α2-/--aortae were also less sensitive to relaxation by either A- or G-kinase pathway activation. The contractility values for the α1+/--aortae, on the other hand, were identical to those of the wild-types. The α2+/--aortic contractility values generally fell between those of α2-/-- and wild-type aortae. These data, alongside the finding that the α1-isoform assumes a uniform distribution across smooth muscle cells isolated from wild-type aorta, while the α2-isoform assumes a more localized pattern, is consistent with Blaustein’s hypothesis discussed above. If the α2-isoform does indeed modulate SR function via colocalization with NCX and SERCA, its absence in α2-/--aortae would lead to the inhibition or reversal of Na+-Ca2+ exchange, ultimately causing an increase in Ca2+ content in the subsarcolemmal compartment. This could, in turn, result in greater SR loading, which might explain the increased sensitivity to receptor-mediated stimulation and the faster rate of force development. Such a “hyperloaded” SR might also explain the observed decreased sensitivity to agonist-stimulated relaxation, as it is long established that SERCA can be inhibited by elevated SR Ca2+.
An alternative hypothesis based on a direct role for NKA in signaling has been proposed by Xie and colleagues [57]. The increased contractility observed with low levels of ouabain, which, in the murine model, bind only to the α2-isoform, is proposed to be related not only to altered pumping, but also to the activation of Src kinase, ultimately leading to the generation of inositol triphosphate (IP3), sensitization of the IP3 receptor and SR Ca2+ release. Thus, ouabain inhibition of NKA could favor both SR Ca2+ store enhancement and depletion. Recent modeling in vascular smooth muscle cells comparing these alternatives [58] indicates that ouabain can lead to enhanced [Ca2+]i transients when its predominant effect is inhibition of α2 NKA, leading to enhanced SR Ca2+ loading Further complicating matters is a recent study on cultured aortic smooth muscle cells from 18 day fetal α2-/- mice indicating that the SR Ca2+ load is similar to the control, wild-type mice [59]. Altered PMCA activity and capacitative Ca2+ entry were potential compensatory pathways affecting Ca2+ handling in these cultured cells. As future studies further elucidate the state of the SR Ca2+ load in the presence of altered α2 NKA function, the true relationship between these physiologic variables will unquestionably be revealed.
Of particular interest to any discussion of smooth muscle tone is the subject of blood pressure. As this review deals generally with the mouse model, in which the baseline blood pressure often depends on background, it should be noted that hyper- and hypotension are here defined in relation to the WT mouse. Two recent studies have addressed the role of the NKA in the regulation of this critical physiologic parameter. The first of these studies, conducted by Blaustein and colleagues [60], found α2+/--mice to be hypertensive, while α1+/--mice, like the wild-type controls, were normotensive. An increased myogenic tone was also observed in isolated, pressurized arteries from α2+/--mice, as compared to wild-type controls, but not in those from α1+/--mice. These investigators suggested that the hypertension observed in the α2+/--mice might result from this elevated myogenic tone. As the pharmacologic NCX inhibitors SEA0400 and KB-R7943 both blocked the augmentation of myogenic tone in α2+/- arteries, one might suspect that NCX is a major player. If one is to adopt Blaustein’s hypothesis, a decrease in the α2-Na+ pump activity could lead to an increase in myogenic tone via reverse NCX activity. Further studies will be necessary to either confirm or refute such a relationship. Ultimately, the α2-isoform of the arterial myocyte appears to be a long-term regulator of blood pressure; indeed, this is an exciting discovery.
The second study addressing the role of NKA in the regulation of blood pressure was undertaken by Paul and colleagues [61]. Utilizing mice carrying the transgene for either the α1- or α2-isoform of NKA, and expressing this gene under the control of the smooth muscle specific α-actin promoter, SMP8, two important discoveries were made. The first of these was that smooth muscle displays a coordinate expression of the α-isoforms. That is, both α-isoforms were increased to a similar degree at both the protein and mRNA levels, regardless of which transgene was being expressed. The second finding was that the mice carrying the α2-transgene (α2sm+) were hypotensive, while the mice carrying the α1-transgene (α2sm+) were hypotensive, while the mice carrying the α1-transgene (α1sm+) were normotensive. While both transgenic lines displayed increases in smooth muscle α1- and α2-isoform levels, these increases were greater in the α2sm+ line, suggesting that the observed decrease in blood pressure is dependent on the extent of the increase in α-isoform expression. It is important to note that increases in the total NKA activity were concurrent with the increases in smooth muscle α-isoform levels.
No differences in force-concentration relations to KCl or PE stimulation were observed in either denuded or endothelium-intact α1sm+-aortae. Similarly, the half-times of force development and relaxation to KCl or PE in α1sm+-aortae also revealed no significant differences when compared to those for wild-type tissues. Given that no change in blood pressure was observed for α1sm+-aortae, this appears reasonable. The data for the α2sm+-aortae, in contrast, appear rather counterintuitive.
As is typical for transgenic studies, more questions are raised than answered. No differences in contractile response to KCl or PE were observed in endothelium-intact α2sm+-aortae, while endothelium denuded α2sm+-aortae actually displayed denuded moderately increased force responses to both KCl and PE stimulation when compared to those observed for wild-type tissues. Such increases in force would appear to be the exact opposite of the expected response, as one might predict an augmented Ca2+ extrusion via increased NKA-NCX coupling. While no statistically significant differences were observed between endothelium-intact wild-type and α2sm+-aortae, endothelium-denuded α2sm+-aortae exhibited a shorter half-time to relaxation from KCl-induced contraction when compared to wild-type tissues. Such an observation appears sensible in light of increased NKA activity, and might provide a clue as to why α2sm+-mice are hypotensive.
Further complicating matters are recent studies on protein expression in these mice. As previously discussed, in the α2sm+-aortae and antra, not only was the α2-isoform protein increased, but also to a similar degree, the α1-isoform, which was not in the inserted transgene [61]. Even more surprising, was that other Ca2+ clearance proteins, PMCA, NCX, and SERCA, but not contractile associated proteins actin and myosin regulatory light chain, were increased to a similar extent [62]. These data suggest that Ca2+ clearance is so critical to the cell, that all its elements may be coordinately regulated as a single unit. This most recent evidence poses an alternative explanation to the functional studies, in that the level of expression was considerably higher in the α2sm+ than the α1sm+ mice. Thus the observed effects in the α2sm+ tissues may also be related to the greater expression of a number of Ca2+ clearance proteins. NKA-NCX are clearly important players in Ca2+ clearance, homeostasis and, consequently, contractility and blood pressure regulation. The effects of the α-isoforms in NKA’s role in Ca2+ clearance appear to be distinct but mechanism(s) are yet to be fully resolved.
Sarco(Endo)Plasmic Reticulum Ca2+-ATPase: A Critical Role for SR Filling
SERCA Ca2+ pumps, members of the P-type superfamily of ion transport ATPases, are encoded by three homologous genes, SERCA1-3, and are responsible for the sequestration of cytosolic Ca2+ into the SR and/or ER (for review see [63]). The SERCA1 gene encodes SERCA1a and SERCA1b, two alternatively spliced transcripts (for review see [64]), which are found only in fast-twitch skeletal muscle. The SERCA2 gene encodes SERCA2a and SERCA2b, again, two alternatively spliced transcripts [65]. SERCA2a is expressed primarily in cardiac and slow-twitch skeletal muscle, while SERCA2b is expressed ubiquitously, and is generally regarded as an essential housekeeping pump [66-68]. Importantly, SERCA2b is a major SR Ca2+ pump of most smooth muscle tissue [69]. SERCA2b is endogenously expressed in the heart, but present in much smaller amounts than SERCA2a. SERCA3, like SERCA1, has a more limited tissue distribution and cell-type specificity than SERCA2, with endothelial cells [70-72], platelets, mast cells, lymphocytes [73] and epithelial cells of the trachea [74], intestine [66] and salivary glands [75] expressing this intracellular Ca2+ pump.
Two independent mouse lines that overexpress the SERCA2a protein 1.2- and 1.5-fold have demonstrated a critical role for this pump in both cardiac Ca2+ homeostasis and contractility (for review see [64]). These models have faster rates of Ca2+ decline along with increased rates of shortening and relengthening in isolated cardiomyocytes. A 29% increase in the SR Ca2+ content of caffeine-sensitive stores was also observed. In vivo cardiac catheterization revealed that the maximal rates of contraction and relaxation were increased. Elevated parameters of contraction and relaxation have additionally been observed in transgenic rats overexpressing SERCA2a [76]. A transgenic mouse model with elevated cardiac levels of SERCA2b has also been important in elucidating the roles of SERCA. Augmented cardiac function suggested that SERCA2b plays a role in SR Ca2+ transport on a beat-to-beat basis [77].
A SERCA2 gene-targeted mouse, in which both SERCA2a and SERCA2b are knocked out, has also demonstrated the importance of SERCA2 in such cardiac parameters. Heterozygous mice exhibited a 35% reduction in both SERCA2a protein levels and the maximal velocity of SR Ca2+ uptake [64]. Furthermore, the peak amplitude of Ca2+ transients in isolated cardiomyocytes was reduced by more than 30%, while decreased rates of cell shortening and relengthening were observed. In vivo cardiovascular function was assessed via transducers in the left ventricle and the right femoral artery, revealing reductions in heart rate, mean arterial pressure, systolic ventricular pressure and the absolute values of contraction and relaxation. In contrast to the heterozygous mice, which are alive and reproduce well, the disruption of both copies of the SERCA2 gene is embryonically lethal. All in all, such transgenic studies suggest a direct correlation between the SERCA protein level and the contractile status of the heart.
Unlike the depressed cardiac function observed in the SERCA2 gene-targeted mouse model, no alterations of smooth muscle contractility are observed in either the tonic aorta or the phasic portal vein [78]. Study of the aorta revealed no differences in the concentration-force relations for KCl and PE when compared to wild-type values. Maximum force and sensitivity were additionally not found to differ. Furthermore, the relaxation to acetylcholine (ACh), sodium nitroprusside (SNP) or forskolin were all unaffected. Studies of the phasic portal vein revealed unaltered spontaneous mechanical and Ach-enhanced activity. A role for SERCA in the regulation of smooth muscle [Ca2+]i and contractility, largely based on the use of the inhibitors thapsigargin [79] and cyclopiazonic acid [30, 80], has been long established. This would suggest that the observed 40% reduction in SERCA2 protein level might be indicative of significant SERCA2 reserves or compensation via upregulation of the remaining gene copy. Ultimately, such discrepancies between the gene-targeted and pharmacologically inhibited models will need to be resolved via further experimentation.
SERCA3 is not endogenous to smooth muscle, however, this Ca2+ pump does play a critical role in both endo- and epithelium-dependent relaxation of smooth muscle tissue [74, 81]. Shull and colleagues [81] demonstrated that aortic smooth muscle from SERCA3-deficient mice exhibits a significantly reduced response to ACh-induced, endothelium-dependent relaxation when compared to wild-type aortae. Paul and colleagues showed that SERCA3-deficient trachea demonstrated a slower rate of relaxation to the epithelial-dependent substance P, while exhibiting faster rates of relaxation to the epithelial-dependent ATP [74].
Phospholamban: From the Bench to the Bedside
Phospholamban, a 52 amino acid phosphoprotein, acts as a critical modulator of SR Ca2+ pump activity (for reviews see [82, 83]). Unlike the other proteins that we have reviewed, PLN is a single copy gene with no known isoforms. In its unphosphorylated form, the PLN monomer inhibits SERCA, while phosphorylation of PLN-Ser16 via PKA or PLN-Thr17 via CaMKII relieves such inhibition, increasing the apparent affinity of the pump for Ca2+. Phospholamban exists in a dynamic equilibrium between the monomeric and pentameric form in the SR membrane. The monomeric form is a potent inhibitor of SERCA, while phosphorylation of the PLN monomer acts to reduce the net charge of the cytoplasmic domain, which, in turn, causes the monomers to self-associate into a less inhibitory pentameric structure. Such an equilibrium allows for the modulation of the SERCA:PLN ratio, an important determinant of contractility in both cardiac [82, 83] and smooth muscle tissues [84].
Critical to the establishment of phospholamban as a primary modulator of Ca2+ homeostasis and contractility in the heart have been studies utilizing PLN-deficient mice and mice carrying PLN or modified PLN transgenes with a cardiac specific promoter (for reviews see [23, 85, 86]). Utilizing PLN-targeted mice, with either a 60% or 100% reduction in protein levels, negative linear correlations have been shown between the relative levels of PLN in the heart and the following: (1) the apparent affinity of SERCA2a for Ca2+, (2) the contractile parameters of isolated cardiomyocytes and (3) the rates of contraction and relaxation in both isolated heart preparations and intact animals. These mice displayed a hyperdynamic cardiac function that was maintained throughout the aging process. Hypercontractile function existed in the absence of morphological and histological abnormalities, and the life span did not differ from that of wild-type mice. Importantly, these changes in contractility accompanied increases in both the amplitude and rates of the [Ca2+]i transient. This reflected a larger SR Ca2+ store and rate of Ca2+ uptake in the PLN-null animals. Basal contractile parameters in the hearts of PLN-deficient mice were only minimally stimulated by the application of β-agonists, suggesting a role for the phosphoprotein at the intersection of β-adrenergic and Ca2+ signaling pathways in the heart.
In contrast to the hypercontractile cardiac phenotype in PLN-deficient mice, transgenic mice exhibiting a two-fold or four-fold cardiac-specific overexpression of wild-type PLN presented with depressed cardiac contractile parameters. Again, these changes in contractile function were accompanied by altered Ca2+ kinetics. In this case, isolated cardiomyocytes exhibited diminished and prolonged Ca2+ transients that were associated with a significant decrease in the apparent affinity of SERCA for Ca2+. This depressed cardiac phenotype was relieved by stimulation with isoproterenol, a β-agonist. Transgenic mice exhibiting a two-fold overexpression of PLN in their hearts displayed no phenotypical alterations, but those mice exhibiting a four-fold overexpression of PLN ultimately displayed progressive remodeling, overt heart failure and premature mortality.
Cardiac-specific overexpression of superinhibitory forms of mutant PLN in a mouse model provided further important information (for reviews see [23, 85, 86]). Utilizing L37A, I40A, N27A and V49G mutants, all transgenic models displayed marked decreases in contractility, Ca2+ transient kinetics and the apparent affinity of SERCA2a for Ca2+. The L37A and I40A mutants exerted their superinhibitory effects via an increase in the concentration of the monomeric PLN, and, accordingly, the depression of the myocyte Ca2+ kinetics and mechanics. The effects could be relieved by the addition of isoproterenol. These mice displayed significant left-ventricular hypertrophy, although their life span was not shortened. The N27A and V49G mutants, on the other hand, exerted their superinhibitory effects via an increased affinity for SERCA, and, subsequently, the depression of the Ca2+ kinetics and mechanics. Such phenotypes, however, could not be fully relieved by the addition of isoproterenol. Both transgenic models developed myocardial hypertrophy, with those overexpressing the N27A mutation on the PLN-deficient background progressing to dilated cardiomyopathy and expiring after less than one year. Transgenic males overexpressing the V49G mutation also experienced a progression to dilated cardiomyopathy, and expired at six months of age. Clearly, such findings illustrate the importance of the β-adrenergic pathway, and consequently the phosphorylation of PLN, in normal cardiac homeostasis.
The field of phospholamban research, strengthened by these classic transgenic studies, has taken on important clinical relevance based on landmark studies, which identified novel PLN mutations in human patients with hereditary dilated cardiomyopathy. Clinically, and in light of the studies utilizing the PLN-deficient mouse, the T116G mutation, identified by Kranias and colleagues [87], is quite interesting. Generating an L39stop substitution, this point mutation provides researchers with a model analagous to the null mouse, as no detectable PLN immunoreactivity was detected. Interestingly, as the PLN-KO mice were asymptomatic with a hypercontractile cardiac function, patients homozygous for the T116G mutation presented with dilated cardiomyopathy and heart failure, with cardiac transplantation necessitated at a young age.
The PLN-R14Del mutation, identified in studies by both Kranias [88] and McNally[89], as well as the PLN-R9C mutation, discovered and studied by Seidman and colleagues [90], would prove interesting clinically and in light of the superinhibitory PLN-expressing mice described above. Through both in vivo and in vitro studies, affected individuals, all heterozygous for the defect, were found to posses a superinhibitory PLN protein [89, 90]. While individuals afflicted with the R9C mutation progress to heart failure within 5 to 10 years after symptom onset and succumb at an average age of 25.1 ± 12.7 years [90], individuals possesing the R14Del mutation typically exhibit a milder phenotype, with cardiac dysfunction often occuring later in life [88, 89]. Importantly, however, all three mutations share a common theme, in that they confer an altered Ca2+ homeostasis [88, 90]. Such studies certainly show the central importance of Ca2+ handling in the every day functioning of the heart.
Similar to their role in understanding cardiac PLN function, transgenic mouse models also solidified the role of PLN in vascular smooth muscle function. Both SERCA and PLN levels are significantly lower in smooth muscle, and the PLN:SERCA ratio can vary widely, at least in terms of the reported mRNA levels in a study of several smooth muscle tissues of the pig [91]. This probably reflects the lower rates of contractile cycles and consequent Ca2+ cycling than cardiac muscle. In terms of function, PLN has been, in terms of smooth muscle tissues, perhaps the most widely studied utilizing the Ca2+ clearance related transgenic mouse models. Since there are no known isoforms of PLN, a mutation would affect all PLN-containing tissues. While the cardiac effects to date are the most evident of the human PLN mutations, there may be smooth muscle effects that potentially could be identified prior to cardiac failure. Thus we will briefly review the effects of PLN in a number of smooth muscle tissues.
Investigation of aortic smooth muscle contractility in the PLN-deficient mouse by Paul and colleagues [92] showed that force responses of the PLN-KO aortae were less sensitive to KCl or PE stimulation than wild-type tissues. Importantly, these differences were abolished upon treatment with cyclopiazonic acid (CPA), an inhibitor of the SR Ca2+ pump, pointing to the SR as the source of this shift. Consistent with disinhibition of SERCA and concomitant increased SR Ca2+ loading, the magnitude of the rapid phase of PE-induced contraction was twice as great in PLN-KO aortae and relaxation was faster in 7 of 11 arteries upon washout of KCl. Alterations in cardiac contractility of the PLN-KO mouse were unlikely to contribute to the altered aortic function, as no gross histological changes were observed in the aortae. Changes in Ca2+ homeostatic mechanisms, such as an upregulation of plasma membrane Ca2+ channels or downregulation of PMCA activity, also seem quite unlikely, as the functional elimination of SR activity eliminated any differences in sensitivity between the PLN-KO and wild-type aortae.
Paul and colleagues [70] also reported that the endothelium-dependent relaxation to ACh was blunted in aortae of PLN-KO mice when compared to wild-type controls. Furthermore, the fact that SNP-mediated relaxation in both denuded and endothelium-intact aortae was not affected by the targeted ablation of PLN indicated that this attenuated response was not the result of decreased smooth muscle sensitivity to nitric oxide (NO). The endothelium-dependent component of vascular relaxation to forskolin was also attenuated in PLN-deficient aortae, suggesting that the targeted ablation of PLN induces a loss of endothelium-dependent A-kinase vasorelaxation. Such results, indicative of a role for PLN in the endothelium itself, were accompanied by both RT-PCR and Western blot analyses showing, for the first time, the presence of this regulatory protein in endothelial tissue.
As the mobilization of SR Ca2+ stores was previously shown to be critical for the contraction of bladder smooth muscle [93], one might predict PLN to play an integral role in its regulation. Indeed, CCh stimulation of PLN-deficient bladder exhibited significant attenuation of the maximal increases in [Ca2+]i and force when compared to wild-type controls [94]. Furthermore, the EC50 values for CCh-induced contraction of the PLN-KO bladder were increased in comparison to those for wild-type bladder. As was observed in the study of PLN-deficient aortae, the functional inhibition of SERCA with CPA eliminated these differences, again localizing the observed effects to the SR.
Although targeted to the SR, one could not assert whether these effects were specific to PLN. The generation of mice (PLN-SMOE) carrying a transgene with PLN cDNA driven by the smooth muscle-specific SMP8 α-actin promoter circumvented this roadblock [94]. Importantly, Western blot analysis revealed an approximately 8-fold overexpression of PLN in the presence of a 12-fold reduction of SERCA, providing investigators with a model in which the PLN:SERCA ratio had been increased. The relations between [Ca2+]i and force as a function of CCh stimulation in PLN-SMOE bladders were significantly shifted leftward compared to wild-type controls. Moreover, CPA elicited a leftward shift in the wild-type but had little effect on the PLN-SMOE tissues. This suggests that the SR Ca2+ uptake in PLN-SMOE bladders was already substantially inhibited. These observations helped localize the observed effects of the PLN-KO and PLN-SMOE bladders to PLN.
A brief consideration of the transient data generated for both the PLN-KO and PLN-SMOE bladders is appropriate, although the interpretation of such data is not as clear as that for the steady state parameters. CCh-induced maximal increases in [Ca2+]i and force were attenuated in PLN-KO bladders compared to wild-type controls, suggesting a more rapid Ca2+ removal upon washout [94]. The observations of Paul and colleagues, however, showed that the decrease in [Ca2+]i, if anything, was slower in the PLN-deficient bladder, suggesting that other rate-limiting steps may be involved. It is also of interest to note that the rise in [Ca2+]i was also slower, although not significantly, in the PLN-KO bladder. This observation is at odds with the classic cardiac data. For the PLN-SMOE bladders, the t1/2 values for the [Ca2+]i rise and fall times were greater than those for the wild-type controls, consistent with PLN inhibition of SERCA. The t1/2 for the decrease in force upon washout of CCh, however, showed no difference, suggesting that the uptake of Ca2+ via SERCA was not the rate-limiting step for mechanical relaxation in the bladder. Ultimately, dephosphorylation of myosin light chains is necessary for relaxation and may be a rate limiting step in some smooth muscle tissues. Time courses are also complicated by the wide range of Ca2+ affinities of the Ca2+ clearance systems. The resolution of such discrepancies between the observed steady state and transient parameters will require further experimentation.
Liggett and colleagues [95], primarily interested in the effects of persistent β2-adrenergic receptor (β2AR) activation on the gene expression of airway smooth muscle cells, would also find the PLN-KO mouse to be of great value. These investigators discovered that cultured airway smooth muscle cells obtained from transgenic mice overexpressing β2AR exhibited a 60% decrease in PLN in comparison to cultured WT cells. The PLN-deficient mouse was utilized to elucidate the physiological effects of decreased PLN in airway smooth muscle. Interestingly, they reported that PLN-KO mice showed a markedly different response to methacholine, a bronchoconstrictor, when compared to the wild-type model. Although the baseline airway resistance did not differ between the wild-type and PLN-KO mice, their data indicate that reducing the level of PLN decreases both the sensitivity and maximal responsiveness to this Gq-coupled M3-muscarinic agonist.
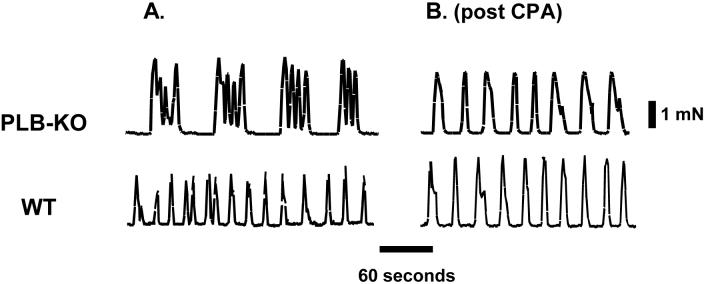
To this point, the role of PLN in tonic smooth muscle has only been considered. A study by Paul and colleagues [96], however, investigated the effects of PLN gene-ablation in the phasic smooth muscle of the portal vein. As the rapid contraction/relaxation cycles of phasic smooth muscle demand a quicker rate of intracellular Ca2+ cycling, one might expect PLN to play a crucial role in such tissue. The basal frequency of the spontaneous mechanical activity was decreased in PLN-KO portal vein in comparison to wild-type tissue (Fig. 3A). Treatment with CPA did not affect the spontaneous activity of the wild-type portal vein (Fig. 3B). In contrast, CPA erased these differences between the PLN-KO and wild-type tissues, effectively localizing the observed effects to the SR. Furthermore, both the quiescent (off) and mechanically active (on) periods of portal vein were greater in the PLN-KO model. The rates of force development and relaxation were also enhanced in the PLN-KO portal vein. CPA treatment eliminated the differences in “on” and “off” times. Again, compensatory mechanisms appear unlikely, as the pharmacological removal of SR function via CPA abolished the differences between PLN-KO and wild-type tissues.
Fig. 3.
Experimental record showing the pattern of altered spontaneous mechanical activity in mouse portal vein from PLN gene-ablated mice (PLB-KO, upper panel) and wild-type mice (WT, lower panel), before (A) and after (B), cyclopiazonic acid (CPA) treatment. Adapted from Sutliff et al. [96]
Quite interestingly, despite phospholamban’s apparent major role in the regulation of basal phasic activity in smooth muscle of the portal vein, the data suggest that PLN plays only a small role, if any, in the modulation of agonist-mediated responses [96]. While the sensitivity of the PLN-KO portal vein to ACh stimulation was similar to the wild-type, the PLN-KO developed larger forces. The effects of CPA on these differences, however, were only moderate. In addition, the relaxation to isoproterenol was not altered by either CPA in the wild-type portal vein or PLN gene ablation. These observations suggest that processes other than SR uptake modulated by PLN are dominant in the responses to PKA activation in portal vein.
The role of PLN in the regulation of Ca2+ homeostasis and contractility in gastric smooth muscle, including the antrum, yet another phasic tissue, has recently been investigated by Perrino and colleagues [97-100]. Utilizing the PLN-KO mouse model, these investigators observed increases in both force development and in the frequency of spontaneous phasic contractions in antral smooth muscle [99]. Associated with this increased phasic contractile activity were more rapid kinetics of contraction and decay. Furthermore, caffeine-induced relaxation was attenuated in the PLN-KO antra when compared to the wild-type controls. These data suggest that PLN plays a major role in the changes in basal tone of both wild-type and PLN-KO antral smooth muscle. Additionally, an elevated frequency of intracellular Ca2+ waves in the basal state of PLN-KO antral smooth muscle indicates altered intracellular Ca2+ homeostasis.
In general, the ablation of PLN alters contractility in a wide range of smooth muscle types. At present, two major hypotheses have been proposed to explain this phenomenon. One is dependent on activation of Ca2+-activated potassium (KCa) channels, and the other on an increased rate of cytosolic Ca2+ sequestration into the SR.
Nelson and colleagues [101] argue for a Ca2+ spark-driven mechanism of decreased contractility in smooth muscle. This is in contrast to ventricular myocytes, for which Ca2+ sparks are associated with increased contractility [102, 103]. Ca2+ sparks are transient elevations in Ca2+ concentration in a small subsarcolemmal region of the cell, that are caused by the activation of a small number of ryanodine receptors. In cardiomyocytes, these sparks result in an elevated [Ca2+]i and contractile state, as the localized Ca2+ release events work to amplify the existing calcium-induced calcium release events. Ultimately, these Ca2+ sparks sum to generate the [Ca2+]i transient [104].
In contrast, in smooth muscle, Ca2+ “sparks” are proposed to lead to an enhanced relaxation [101]. Sparks in smooth muscle can activate large-conductance KCa (BK) channels in the plasma membrane, causing the membrane to hyperpolarize. Such hyperpolarization closes voltage-dependent Ca2+ channels, leading to a lower [Ca2+]i and relaxation.
Nelson and colleagues [105] reported that the frequency of sparks is elevated in smooth muscle cells from the cerebral arteries of PLN-KO mice. Interestingly, however, the amplitude of these sparks did not differ from those in wild-type controls. This is in contrast to cardiac myocytes [103] and nonvascular smooth muscle [106], in which elevated SR Ca2+ loads resulted in increased Ca2+ spark amplitudes. In line with the observed elevation in spark frequency, it was also reported that PLN-KO myocytes exhibited an elevated BK current frequency [105]. These currents were of similar amplitude to those of wild-type cells. This observation contrasts with increased BK current amplitudes observed in nonvascular smooth muscle in which the SR Ca2+ load was increased [106]. Ultimately, as proposed by Nelson and coworkers, an increased SR Ca2+ load, attributable to increased SERCA activity via the ablation or phosphorylation of PLN, induces an increased frequency of both Ca2+ release events and BK currents, thereby hyperpolarizing the arterial myocytes and dilating the vessel [105]. In light of the above observations, as well as previous data showing that an elevated level of Ca2+ in the SR increases the open probability of the ryanodine channels (for review see [107]), this hypothesis appears reasonable.
Several investigations have supported this mechanism for Ca2+ spark modulation of smooth muscle contractility. Pharmacological studies utilizing iberiotoxin, a KCa channel inhibitor, were found to blunt the observed caffeine-induced relaxation in the smooth muscle of the gastric fundus [97], while studies with both iberiotoxin and apamin, a small-conductance KCa channel inhibitor, were found to reduce the observed sodium nitroprusside-induced relaxation [98]. SNP [98, 100, 108], a nitric oxide donor, and caffeine [97, 99] have been implicated in relaxation of gastric smooth muscle through a pathway involving the phosphorylation of PLN. The reported hyperpolarization of the fundus smooth muscle membrane upon treatment with either SNP [98] or caffeine [97] further supports the proposed connection of PLN to Ca2+ spark mediated relaxation. Additionally, the study of the PLN gene-ablated portal vein revealed a membrane potential that was somewhat more hyperpolarized (-66 vs -61 mV) than that of wild-type tissue, also suggesting a potential role for Ca2+ sparks in the observed modulation of smooth muscle contractility [96].
The second theory proposed for the altered contractility in PLN-deficient mice suggests a direct role for an increased rate of cytosolic Ca2+ sequestration into the SR. This enhanced removal of Ca2+ from the cytosol would effectively lower the [Ca2+]i, in turn leading to a lesser degree of contraction. Such a role is supported by data from several independent studies. One compelling argument from the work of Paul and colleagues [92, 94] is that the suppression of force was also observed upon depolarization with KCl. Under these conditions, Ca2+ sparks would not be anticipated to play a role. A seemingly logical objection to this would be that Ca2+ uptake by the SR could be saturated and suppression of force lost with prolonged contractions. However, there is evidence suggesting that the SR can be vectorially unloaded via a compartmented NCX and NKA [109], as originally suggested by van Breemen and colleagues [109, 110]. Furthermore, it should be noted that although the smooth muscle of PLN-KO portal vein displayed a significantly greater degree of membrane hyperpolarization than wild-type tissue, the application of charybdotoxin, a KCa channel inhibitor, had little effect on the spontaneous activity of the mouse portal vein, and these effects did not differ between PLN-deficient and wild-type tissues [96]. Such observations suggest a dominant role for increased SR Ca2+ uptake in the observed phenotype.
Further supporting a role for augmented cytosolic Ca2+ sequestration into the SR are studies conducted by Perrino and colleagues [97, 98, 100]. Utilizing murine gastric antrum smooth muscle, these investigators found that the use of KCa channel blockers failed to attenuate the inhibitory effects of SNP on contractile activity [100]. Additionally, the caffeine- [97] and SNP- [98] mediated relaxations of the gastric fundus smooth muscle were not fully inhibited by KCa channel inhibitors, suggesting a role for enhanced SR Ca2+ sequestration in the observed relaxation. Ultimately, both an indirect role for augmented SR Ca2+ sequestration in the modulation of Ca2+ sparks, as well as a direct role for enhanced cytosolic Ca2+ removal in the reduction of [Ca2+]i, will likely prove to be relevant in the observed phenotypes of PLN-deficient smooth muscle.
PLN, as the product of a single copy gene with no known splice variants, is identical in both smooth and cardiac muscle. In light of the discovery of novel R14Del, R9C and L39stop PLN human mutations, which are characterized by inherited dilated cardiomyopathy and heart failure, it is of interest to speculate as to the potential modifications of smooth muscle attributable to these mutants. Because PLN is the most intensely studied of the Ca2+ clearance proteins in gene-altered mouse models, reasonable extrapolation may be drawn to potential disease at the level of affected organs. Our speculative predictions, along with a summary of the findings from PLN gene-altered mice, are presented in Table 1.
Table 1.
Smooth Muscle in PLN Transgenics and Predicted Concomitant Organ Level Effects
| Smooth Muscle | Experimental Observations | Predicted Organ Level Effects | |
|---|---|---|---|
| Increased Ca2+ Clearance | Decreased Ca2+ Clearance | ||
| Bladder |
|
Overflow Incontinence | Urge Incontinence |
| Aorta |
|
Hypotension | Hypertension |
| Vascular Endothelium |
|
Hypertension | Hypotension |
| Trachea |
|
Not Applicable (N/A) | Asthma |
| Portal Vein |
|
Portal Hypertension, Ascites, Esophageal Varices, Gastric Varices, Splenomegaly | N/A |
| Gastric Antrum |
|
Functional Dyspepsia, Gastro- Paresis | Functional Dyspepsia, Rumination |
As might be expected for complex physiological organisms capable of compensation, these predictions are subject to many limitations. For example, blood pressure measurements made in PLN-deficient mice [111] were found to be similar to those of age-matched wild-type controls. One would have predicted a hypotensive mouse based on isolated smooth muscle data, while a hypertensive state would have been hypothesized based on experimental evidence from endothelial tissue. Additionally, disease cannot be readily predicted in all organs, as is the case for the trachea and portal vein (see Table 1). None-the-less, it is of utility to consider, to the best of our knowledge, potential smooth muscle effects in light of PLN mutations. Future studies will likely help to either confirm or refute such predictions. Since these PLN mutations, in effect, lead to either increased or decreased Ca2+ clearance, one might be tempted to make general predictions of their consequences, independent of which Ca2+ clearance systems may be affected. One always must bear in mind, however, that there is significant evidence for compartmentation of the various Ca2+ clearance proteins and isoforms. As a consequence of such subcellular divisions, the subregion cleared may also play a role in altered function.
INTERACTIONS
Though we have treated the Ca2+ clearance systems, PMCA, NCX-NKA and SERCA-PLN as separate entities, they are linked. For example, Ca2+ extrusion by PMCA leads to a counter transport of H+ [112], and thus coupling to NKA via the Na+-H+ exchanger. This is in addition to the coupling of NCX and NKA discussed earlier. SERCA is also interrelated, particularly as proposed to a subsarcolemmal compartmentalization with α2-NKA and NCX.
Mitochondria, also proposed to be involved in the intricate relationships shared among Ca2+ handling proteins, have been suggested to be a major player in Ca2+ homeostasis [113], however their role as a major clearance component rather than as a modulator of the interacting systems of Ca2+-clearance is controversial [114]. Given their role in ATP synthesis, cellular redox potential, and generation of reactive oxygen species (ROS), it would be surprising if mitochondria did not play any role in Ca2+ homeostasis. The literature clearly indicates that mitochondria can not only sense [Ca2+]i, but can also take-up, and release Ca2+. Somlyo and colleagues [12, 115] have argued that the affinity for mitochondria Ca2+ uptake is high, suggesting that it may be important only in pathological conditions. To overcome this problem of low mitochondrial Ca2+ affinity it is postulated that a higher [Ca2+] exists in cytosolic subdomains [14]. There are a number of reports in a variety of cells types, summarized in a recent review [113], which indicate that mitochondrial Ca2+ uptake or release may control a number of parameters, such as Ca2+ influx, the frequency of oscillations or the spatial distribution of Ca2+.
In the field of cardiac muscle research, a definitive role for mitochondria in Ca2+ cycling remains elusive. The pursuit of such a role has stirred considerable controversy and skepticism. Although it has been shown that the mitochondria of cardiomyocytes can, in fact, respond to elevations in cytosolic [Ca2+]i via the accumulation of Ca2+, their role in beat-to-beat Ca2+ cycling is still debated [116]. Interestingly, there is strong evidence supporting a role for mitochondria in the cycling of Ca2+ to and from the SR [117, 118], suggesting an important role for these organelles in the regulation of cellular Ca2+ homeostasis. This role within Ca2+ cycling, however, appears to be limited to the Ca2+ pools of cytosolic subdomains, as the contribution of slow mechanisms, which include the mitochondrial Ca2+ uniporter and the sarcolemmal Ca2+-ATPase, to total cellular Ca2+ uptake during relaxation has been estimated to be only 0.5%, with the SR Ca2+-ATPase and sarcolemmal NCX assuming the major responsibilities [119].
The major importance of Ca2+ uptake and efflux through the mitochondria appears to be related to the modulation of other Ca2+ clearance systems and to the regulation of mitochondrial metabolism. Of particular note is the observation that mitochondrial Ca2+ uptake might very well stimulate the production of NO inside these organelles (for review [116]). Studies have localized nitric oxide synthase (NOS) to the inner mitochondrial membrane of cardiac mitochondria (mtNOS). Furthermore, as NO is a strong regulator of mitochondrial respiration, Ca2+ uptake may act indirectly as a regulator of mitochondrial oxygen consumption, reactive species generation and ATP production. Such regulation would undoubtedly establish a major role for Ca2+ cycling through the mitochondria in overall cellular Ca2+ homeostasis. It should be noted that skepticism exists regarding the existence, origin and functional role of mtNOS. Future studies must aim to clarify such controversy.
In smooth muscle, Van Breemen and colleagues have shown that mitochondria, like those found in cardiomyocytes, can modulate Ca2+ homeostasis in a variety of ways [120]. Much of the knowledge gained in the studies above, relied on mitochondrial inhibitors, such as FCCP or CCCP. While these collapse the mitochondrial membrane potential thought to be necessary for Ca2+ uptake, they also alter ROS, redox potential, and ATP supply. This is a limitation blurring the distinction between mitochondrial Ca2+ handling and other pathways. One can also use hypoxia or anoxia to inhibit mitochondrial energy production to help distinguish between Ca2+ clearance per se from a more modulatory role for mitochondrial Ca2+ handling. Non-stimulated systemic vessels in general, do not contract when exposed to hypoxia, as might be anticipated in response to a large mitochondrial Ca2+ release. Pig coronary arteries contracted with an agonist or K+-depolarization relax when exposed to hypoxia. For moderate stimulation, hypoxic relaxation is associated with a decrease in [Ca2+]i [121]. This decrease in Ca2+ is believed to be due to K+- or Ca2+ channel sensitivity to O2 [122]. Coronary arteries under anoxic conditions can reversibly contract and relax to K+ depolarization or agonist stimulation [121]. Thus under anoxic/hypoxic conditions, systemic vessels can maintain low [Ca2+]i and some level of reversible contractile function. These observations can in part be explained by a robust glycolytic capacity [123]. which preferentially supports membrane ion pumps in VSM [124]. Pulmonary arteries do contract in response to low O2 levels. However, this appears to involve an increase in [Ca2+]i via O2-sensitive channels or other signaling molecules capable of modifying Ca2+ sensitivity [125]. The evidence from studies of vascular smooth muscle responses to hypoxia suggests that mitochondria are not a major Ca2+ clearance system, or at least that they can be dominated by other Ca2+ clearance mechanisms.
Measurement of [Ca2+]i in cultured smooth muscle cells has also been ambivalent with respect to the role mitochondrial Ca2+ clearance. In cultured rat femoral artery cells, the time course of [Ca2+]i following treatment with caffeine, a trigger for the release of Ca2+ from internal stores, was affected by mitochondrial inhibition with CCCP. This suggests a role for mitochondrial Ca2+ clearance, however the effects of mitochondrial regulation of other clearance pathways was not studied [126]. On the other hand, with inhibition of NCX by Na+-free media and PMCA with vanadate, [Ca2+]i, after store release with CPA, was not significantly affected by CCCP or ruthenium red in cultured mouse aortic cells [59]. Thus differences in vascular smooth muscle types may obscure our understanding of the role of mitochondria in Ca2+ clearance.
Mitochondria have been estimated to occupy about 5% of the volume of vascular smooth muscle and thus require some Ca2+ buffer in order to significantly clear cytosolic Ca2+. This is generally thought to be PO4. Thus, mitochondria would appear to be a limited Ca2+ sink, for if PO4 bound to significant Ca2+ this might be expected to limit ATP production. Mitochondria would require a way to reduce its Ca2+, which some suggest is via the SR. Thus, as in cardiomyocytes, mitochondria may also participate in SR Ca2+ loading [120].
Based on studies using inhibitors of Ca2+ clearance, mitochondrial uptake does not appear to be a major factor compared to NCX, PMCA or SERCA [30, 127]. On the other hand, mitochondria appear to have a role in the regulation, and, potentially, in the coordination of Ca2+ clearance systems. Further experimentation will be necessary to elucidate the true roles of mitochondria in Ca2+ homeostasis.
SUMMARY AND BEYOND
Gene-altered mouse models have significantly increased our understanding of the Ca2+ clearance systems: PMCA, NCX-NKA and SERCA-PLN. All are important in Ca2+ clearance, contractility and other Ca2+-dependent signaling pathways. Our understanding of how these units are coordinated in Ca2+ extrusion is less well understood. Moreover, there is evidence suggesting a sort of genetic homeostasis through which the expression of these Ca2+ clearance units is coordinated [128, 129]. We anticipate that these aspects will be the next frontier in our knowledge of Ca2+ extrusion. We further anticipate that human mutations will be found in other Ca2+ clearance proteins, similar to those uncovered for PLN. These will likely drive further use of gene-altered mouse models towards the understanding of the pathology of both cardiac and smooth muscle.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].de Lanerolle P, Paul RJ. Myosin phosphorylation/dephosphorylation and regulation of airway smooth muscle contractility. Am J Physiol. 1991 Aug;261(2 Pt 1):L1–14. doi: 10.1152/ajplung.1991.261.2.L1. [DOI] [PubMed] [Google Scholar]
- [2].Ishida Y, Paul RJ. Ca(2+) clearance in smooth muscle: lessons from gene-altered mice. J Smooth Muscle Res. 2005 Oct;41(5):235–45. doi: 10.1540/jsmr.41.235. [DOI] [PubMed] [Google Scholar]
- [3].Floyd R, Wray S. Calcium transporters and signalling in smooth muscles. Cell Calcium. 2007 Oct-Nov;42(45):467–76. doi: 10.1016/j.ceca.2007.05.011. [DOI] [PubMed] [Google Scholar]
- [4].Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999 Jul;79(3):763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- [5].Blaustein MP. Physiological effects of endogenous ouabain: control of intracellular Ca2+ stores and cell responsiveness. Am J Physiol. 1993;264(6 Pt 1):C1367–87. doi: 10.1152/ajpcell.1993.264.6.C1367. [DOI] [PubMed] [Google Scholar]
- [6].Blaustein MP, Juhaszova M, Golovina VA, Church PJ, Stanley EF. Na/Ca exchanger and PMCA localization in neurons and astrocytes: functional implications. Ann N Y Acad Sci. 2002 Nov;976:356–66. doi: 10.1111/j.1749-6632.2002.tb04762.x. [DOI] [PubMed] [Google Scholar]
- [7].Hilgemann DW, Nicoll DA, Philipson KD. Charge movement during Na+ translocation by native and cloned cardiac Na+/Ca2+ exchanger. Nature. 1991 Aug 22;352(6337):715–8. doi: 10.1038/352715a0. [DOI] [PubMed] [Google Scholar]
- [8].Carafoli E. The Ca2+ pump of the plasma membrane. J Biol Chem. 1992 February 5;267(4):2115–8. 1992. [PubMed] [Google Scholar]
- [9].Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994 Jul;74(3):595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- [10].Verboomen H, Wuytack F, De Smedt H, Himpens B, Casteels R. Functional difference between SERCA2a and SERCA2b Ca2+ pumps and their modulation by phospholamban. Biochem J. 1992;286(Pt 2):591–5. doi: 10.1042/bj2860591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Szewczyk MM, Davis KA, Samson SE, Simpson F, Rangachari PK, Grover AK. Ca2+-pumps and Na2+-Ca2+-exchangers in coronary artery endothelium versus smooth muscle. J Cell Mol Med. 2007 Jan-Feb;11(1):129–38. doi: 10.1111/j.1582-4934.2007.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Broderick R, Somlyo AP. Calcium and magnesium transport by in situ mitochondria: electron probe analysis of vascular smooth muscle. Circ Res. 1987;61(4):523–30. doi: 10.1161/01.res.61.4.523. [DOI] [PubMed] [Google Scholar]
- [13].Meisheri KD, Palmer RF, Van Breemen C. The effects of amrinone on contractility, Ca2+ uptake and cAMP in smooth muscle. Eur J Pharmacol. 1980 Jan 25;61(2):159–65. doi: 10.1016/0014-2999(80)90158-2. [DOI] [PubMed] [Google Scholar]
- [14].Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008 Apr;23(2):84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- [15].Daniel EE, El-Yazbi A, Cho WJ. Caveolae and calcium handling, a review and a hypothesis. J Cell Mol Med. 2006 Apr-Jun;10(2):529–44. doi: 10.1111/j.1582-4934.2006.tb00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Noble K, Zhang J, Wray S. Lipid rafts, the sarcoplasmic reticulum and uterine calcium signalling: an integrated approach. J Physiol. 2006 Jan 1;570(Pt 1):29–35. doi: 10.1113/jphysiol.2005.098475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cho WJ, Daniel EE. Proteins of interstitial cells of Cajal and intestinal smooth muscle, colocalized with caveolin-1. Am J Physiol Gastrointest Liver Physiol. 2005 Mar;288(3):G571–85. doi: 10.1152/ajpgi.00222.2004. [DOI] [PubMed] [Google Scholar]
- [18].Liu L, Mohammadi K, Aynafshar B, Wang H, Li D, Liu J, et al. Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am J Physiol Cell Physiol. 2003 Jun;284(6):C1550–60. doi: 10.1152/ajpcell.00555.2002. [DOI] [PubMed] [Google Scholar]
- [19].Moore ED, Etter EF, Philipson KD, Carrington WA, Fogarty KE, Lifshitz LM, et al. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature. 1993 Oct 14;365(6447):657–60. doi: 10.1038/365657a0. [DOI] [PubMed] [Google Scholar]
- [20].Juhaszova M, Blaustein MP. Na+ pump low and high ouabain affinity alpha subunit isoforms are differently distributed in cells. Proc Natl Acad Sci U S A. 1997 Mar 4;94(5):1800–5. doi: 10.1073/pnas.94.5.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McCarron JG, Olson ML. A single luminally continuous sarcoplasmic reticulum with apparently separate Ca2+ stores in smooth muscle. J Biol Chem. 2008 Mar 14;283(11):7206–18. doi: 10.1074/jbc.M708923200. [DOI] [PubMed] [Google Scholar]
- [22].Ebashi S, Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–83. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- [23].MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003 Jul;4(7):566–77. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- [24].Schatzmann HJ. ATP-dependent Ca++-extrusion from human red cells. Experientia. 1966 Jun 15;22(6):364–5. doi: 10.1007/BF01901136. [DOI] [PubMed] [Google Scholar]
- [25].Shull GE, Greeb J. Molecular cloning of two isoforms of the plasma membrane Ca2+-transporting ATPase from rat brain. Structural and functional domains exhibit similarity to Na+,K+- and other cation transport ATPases. J Biol Chem. 1988 Jun 25;263(18):8646–57. [PubMed] [Google Scholar]
- [26].Strehler EE. Plasma membrane Ca2+ pumps and Na+/Ca2+ exchangers. Semin Cell Biol. 1990 Aug;1(4):283–95. [PubMed] [Google Scholar]
- [27].Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev. 2001 Jan;81(1):21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- [28].Hammes A, Oberdorf S, Strehler EE, Stauffer T, Carafoli E, Vetter H, et al. Differentiation-specific isoform mRNA expression of the calmodulin-dependent plasma membrane Ca(2+)-ATPase. FASEB J. 1994 Apr 1;8(6):428–35. doi: 10.1096/fasebj.8.6.8168693. [DOI] [PubMed] [Google Scholar]
- [29].Hammes A, Oberdorf-Maass S, Rother T, Nething K, Gollnick F, Linz KW, et al. Overexpression of the sarcolemmal calcium pump in the myocardium of transgenic rats. Circ Res. 1998 Nov 2;83(9):877–88. doi: 10.1161/01.res.83.9.877. [DOI] [PubMed] [Google Scholar]
- [30].Liu L, Ishida Y, Okunade G, Shull GE, Paul RJ. Role of plasma membrane Ca2+-ATPase in contraction-relaxation processes of the bladder: evidence from PMCA gene-ablated mice. Am J Physiol Cell Physiol. 2006 Apr;290(4):C1239–47. doi: 10.1152/ajpcell.00440.2005. [DOI] [PubMed] [Google Scholar]
- [31].Liu L, Ishida Y, Okunade G, Pyne-Geithman GJ, Shull GE, Paul RJ. Distinct roles of PMCA isoforms in Ca2+ homeostasis of bladder smooth muscle: evidence from PMCA gene-ablated mice. Am J Physiol Cell Physiol. 2007 Jan;292(1):C423–31. doi: 10.1152/ajpcell.00313.2006. [DOI] [PubMed] [Google Scholar]
- [32].Taggart MJ, Wray S. Agonist mobilization of sarcoplasmic reticular calcium in smooth muscle: functional coupling to the plasmalemmal Na+/Ca2+ exchanger? Cell Calcium. 1997;22(5):333–41. doi: 10.1016/s0143-4160(97)90018-x. [DOI] [PubMed] [Google Scholar]
- [33].Matthew A, Shmygol A, Wray S. Ca2+ entry, efflux and release in smooth muscle. Biol Res. 2004;37(4):617–24. doi: 10.4067/s0716-97602004000400017. [DOI] [PubMed] [Google Scholar]
- [34].Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O’Connor KT, Neumann JC, et al. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem. 2004 Aug 6;279(32):33742–50. doi: 10.1074/jbc.M404628200. [DOI] [PubMed] [Google Scholar]
- [35].Fujimoto T. Calcium pump of the plasma membrane is localized in caveolae. J Cell Biol. 1993 Mar;120(5):1147–57. doi: 10.1083/jcb.120.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schuh K, Quaschning T, Knauer S, Hu K, Kocak S, Roethlein N, et al. Regulation of vascular tone in animals overexpressing the sarcolemmal calcium pump. J Biol Chem. 2003 Oct 17;278(42):41246–52. doi: 10.1074/jbc.M307606200. [DOI] [PubMed] [Google Scholar]
- [37].Gros R, Afroze T, You XM, Kabir G, Van Wert R, Kalair W, et al. Plasma membrane calcium ATPase overexpression in arterial smooth muscle increases vasomotor responsiveness and blood pressure. Circ Res. 2003 Oct 3;93(7):614–21. doi: 10.1161/01.RES.0000092142.19896.D9. [DOI] [PubMed] [Google Scholar]
- [38].Philipson KD, Nicoll DA. Sodium-calcium exchange: a molecular perspective. Annu Rev Physiol. 2000;62:111–33. doi: 10.1146/annurev.physiol.62.1.111. [DOI] [PubMed] [Google Scholar]
- [39].Quednau BD, Nicoll DA, Philipson KD. Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am J Physiol. 1997 Apr;272(4 Pt 1):C1250–61. doi: 10.1152/ajpcell.1997.272.4.C1250. [DOI] [PubMed] [Google Scholar]
- [40].Lingrel JB, Kuntzweiler T. Na+,K(+)-ATPase. J Biol Chem. 1994 Aug 5;269(31):19659–62. [PubMed] [Google Scholar]
- [41].Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol. 1998 Nov;275(5 Pt 2):F633–50. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- [42].Shelly DA, He S, Moseley A, Weber C, Stegemeyer M, Lynch RM, et al. Na(+) pump alpha 2-isoform specifically couples to contractility in vascular smooth muscle: evidence from gene-targeted neonatal mice. Am J Physiol Cell Physiol. 2004 Apr;286(4):C813–20. doi: 10.1152/ajpcell.00389.2003. [DOI] [PubMed] [Google Scholar]
- [43].Orlowski J, Lingrel JB. Tissue-specific and developmental regulation of rat Na,K-ATPase catalytic alpha isoform and beta subunit mRNAs. J Biol Chem. 1988;263(21):10436–42. [PubMed] [Google Scholar]
- [44].Juhaszova M, Blaustein MP. Distinct distribution of different Na+ pump alpha subunit isoforms in plasmalemma. Physiological implications. Ann N Y Acad Sci. 1997;834:524–36. doi: 10.1111/j.1749-6632.1997.tb52310.x. [DOI] [PubMed] [Google Scholar]
- [45].Lingrel J, Moseley A, Dostanic I, Cougnon M, He S, James P, et al. Functional roles of the alpha isoforms of the Na,K-ATPase. Ann N Y Acad Sci. 2003 Apr;986:354–9. doi: 10.1111/j.1749-6632.2003.tb07214.x. [DOI] [PubMed] [Google Scholar]
- [46].Reuter H, Philipson KD. Sodium-calcium exchanger overexpression in the heart--insights from a transgenic mouse model. Basic Res Cardiol. 2002;97(Suppl 1):I31–5. doi: 10.1007/s003950200026. [DOI] [PubMed] [Google Scholar]
- [47].Henderson SA, Goldhaber JI, So JM, Han T, Motter C, Ngo A, et al. Functional adult myocardium in the absence of Na+-Ca2+ exchange: cardiac-specific knockout of NCX1. Circ Res. 2004 Sep 17;95(6):604–11. doi: 10.1161/01.RES.0000142316.08250.68. [DOI] [PubMed] [Google Scholar]
- [48].James PF, Grupp IL, Grupp G, Woo AL, Askew GR, Croyle ML, et al. Identification of a specific role for the Na,K-ATPase alpha 2 isoform as a regulator of calcium in the heart. Mol Cell. 1999 May;3(5):555–63. doi: 10.1016/s1097-2765(00)80349-4. [DOI] [PubMed] [Google Scholar]
- [49].Moseley AE, Cougnon MH, Grupp IL, El Schultz J, Lingrel JB. Attenuation of cardiac contractility in Na,K-ATPase alpha1 isoform-deficient hearts under reduced calcium conditions. J Mol Cell Cardiol. 2004 Nov;37(5):913–9. doi: 10.1016/j.yjmcc.2004.06.005. [DOI] [PubMed] [Google Scholar]
- [50].Yamamoto T, Su Z, Moseley AE, Kadono T, Zhang J, Cougnon M, et al. Relative abundance of alpha2 Na(+) pump isoform influences Na(+)-Ca(2+) exchanger currents and Ca(2+) transients in mouse ventricular myocytes. J Mol Cell Cardiol. 2005 Jul;39(1):113–20. doi: 10.1016/j.yjmcc.2005.03.023. [DOI] [PubMed] [Google Scholar]
- [51].Berry RG, Despa S, Fuller W, Bers DM, Shattock MJ. Differential distribution and regulation of mouse cardiac Na+/K+-ATPase alpha1 and alpha2 subunits in T-tubule and surface sarcolemmal membranes. Cardiovasc Res. 2007 Jan 1;73(1):92–100. doi: 10.1016/j.cardiores.2006.11.006. [DOI] [PubMed] [Google Scholar]
- [52].Cougnon MH, Moseley AE, Radzyukevich TL, Lingrel JB, Heiny JA. Na,K-ATPase alpha- and beta-isoform expression in developing skeletal muscles: alpha(2) correlates with t-tubule formation. Pflugers Arch. 2002 Oct;445(1):123–31. doi: 10.1007/s00424-002-0898-6. [DOI] [PubMed] [Google Scholar]
- [53].Radzyukevich TL, Moseley AE, Shelly DA, Redden GA, Behbehani MM, Lingrel JB, et al. The Na(+)-K(+)-ATPase alpha2-subunit isoform modulates contractility in the perinatal mouse diaphragm. Am J Physiol Cell Physiol. 2004 Nov;287(5):C1300–10. doi: 10.1152/ajpcell.00231.2004. [DOI] [PubMed] [Google Scholar]
- [54].He S, Shelly DA, Moseley AE, James PF, James JH, Paul RJ, et al. The alpha(1)- and alpha(2)-isoforms of Na-K-ATPase play different roles in skeletal muscle contractility. Am J Physiol Regul Integr Comp Physiol. 2001 Sep;281(3):R917–25. doi: 10.1152/ajpregu.2001.281.3.R917. [DOI] [PubMed] [Google Scholar]
- [55].Karashima E, Nishimura J, Iwamoto T, Hirano K, Hirano M, Kita S, et al. Involvement of Na+-Ca2+ exchanger in cAMP-mediated relaxation in mice aorta: evaluation using transgenic mice. Br J Pharmacol. 2007 Feb;150(4):434–44. doi: 10.1038/sj.bjp.0707119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ruknudin A, He S, Lederer WJ, Schulze DH. Functional differences between cardiac and renal isoforms of the rat Na+-Ca2+ exchanger NCX1 expressed in Xenopus oocytes. J Physiol. 2000 Dec 15;529(Pt 3):599–610. doi: 10.1111/j.1469-7793.2000.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Xie Z, Cai T. Na+-K+--ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv. 2003 May;3(3):157–68. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- [58].Edwards A, Pallone TL. Ouabain modulation of cellular calcium stores and signaling. Am J Physiol Renal Physiol. 2007 Nov;293(5):F1518–32. doi: 10.1152/ajprenal.00251.2007. [DOI] [PubMed] [Google Scholar]
- [59].Lynch RM, Weber CS, Nullmeyer KD, Moore ED, Paul RJ. Clearance of Store Released Ca2+ by the Na+-Ca2+ Exchanger is Diminished in Aortic Smooth Muscle from Na+-K+ ATPase {alpha}2-isoform Gene-Ablated Mice. Am J Physiol Heart Circ Physiol. 2008 Jan 11; doi: 10.1152/ajpheart.00855.2007. [DOI] [PubMed] [Google Scholar]
- [60].Zhang J, Lee MY, Cavalli M, Chen L, Berra-Romani R, Balke CW, et al. Sodium pump alpha2 subunits control myogenic tone and blood pressure in mice. J Physiol. 2005 Nov 15;569(Pt 1):243–56. doi: 10.1113/jphysiol.2005.091801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Pritchard TJ, Parvatiyar M, Bullard DP, Lynch RM, Lorenz JN, Paul RJ. Transgenic mice expressing Na+-K+-ATPase in smooth muscle decreases blood pressure. Am J Physiol Heart Circ Physiol. 2007 Aug;293(2):H1172–82. doi: 10.1152/ajpheart.00279.2007. [DOI] [PubMed] [Google Scholar]
- [62].Pritchard Ta, Paul RJ. Na+-K+ ATPase and Ca2+ Clearance Proteins in Smooth Muscle: A Functional Unit. Am J Physiol (in review) 2008 doi: 10.1152/ajpheart.00527.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Missiaen L, Wuytack F, Raeymaekers L, De Smedt H, Droogmans G, Declerck I, et al. Ca2+ extrusion across plasma membrane and Ca2+ uptake by intracellular stores. Pharmacol Ther. 1991;50(2):191–232. doi: 10.1016/0163-7258(91)90014-d. [DOI] [PubMed] [Google Scholar]
- [64].Periasamy M, Huke S. SERCA pump level is a critical determinant of Ca(2+)homeostasis and cardiac contractility. J Mol Cell Cardiol. 2001 Jun;33(6):1053–63. doi: 10.1006/jmcc.2001.1366. [DOI] [PubMed] [Google Scholar]
- [65].Zarain-Herzberg A, MacLennan DH, Periasamy M. Characterization of rabbit cardiac sarco(endo)plasmic reticulum Ca2(+)-ATPase gene. J Biol Chem. 1990 Mar 15;265(8):4670–7. [PubMed] [Google Scholar]
- [66].Burk SE, Lytton J, MacLennan DH, Shull GE. cDNA cloning, functional expression, and mRNA tissue distribution of a third organellar Ca2+ pump. J Biol Chem. 1989 Nov 5;264(31):18561–8. [PubMed] [Google Scholar]
- [67].Wu KD, Lytton J. Molecular cloning and quantification of sarcoplasmic reticulum Ca(2+)-ATPase isoforms in rat muscles. Am J Physiol. 1993 Feb;264(2 Pt 1):C333–41. doi: 10.1152/ajpcell.1993.264.2.C333. [DOI] [PubMed] [Google Scholar]
- [68].MacLennan DH, Brandl CJ, Korczak B, Green NM. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985 Aug 22-28;316(6030):696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- [69].Lytton J, Zarain-Herzberg A, Periasamy M, MacLennan DH. Molecular cloning of the mammalian smooth muscle sarco(endo)plasmic reticulum Ca2+-ATPase. J Biol Chem. 1989 Apr 25;264(12):7059–65. [PubMed] [Google Scholar]
- [70].Sutliff RL, Hoying JB, Kadambi VJ, Kranias EG, Paul RJ. Phospholamban is present in endothelial cells and modulates endothelium-dependent relaxation. Evidence from phospholamban gene-ablated mice. Circ Res. 1999 Feb 19;84(3):360–4. doi: 10.1161/01.res.84.3.360. [DOI] [PubMed] [Google Scholar]
- [71].Anger M, Samuel JL, Marotte F, Wuytack F, Rappaport L, Lompre AM. The sarco(endo)plasmic reticulum Ca(2+)-ATPase mRNA isoform, SERCA 3, is expressed in endothelial and epithelial cells in various organs. FEBS Lett. 1993 Nov 8;334(1):45–8. doi: 10.1016/0014-5793(93)81677-r. [DOI] [PubMed] [Google Scholar]
- [72].Anger M, Samuel JL, Marotte F, Wuytack F, Rappaport L, Lompre AM. In situ mRNA distribution of sarco(endo)plasmic reticulum Ca(2+)-ATPase isoforms during ontogeny in the rat. J Mol Cell Cardiol. 1994 Apr;26(4):539–50. doi: 10.1006/jmcc.1994.1064. [DOI] [PubMed] [Google Scholar]
- [73].Wuytack F, Papp B, Verboomen H, Raeymaekers L, Dode L, Bobe R, et al. A sarco/endoplasmic reticulum Ca(2+)-ATPase 3-type Ca2+ pump is expressed in platelets, in lymphoid cells, and in mast cells. J Biol Chem. 1994 Jan 14;269(2):1410–6. [PubMed] [Google Scholar]
- [74].Kao J, Fortner CN, Liu LH, Shull GE, Paul RJ. Ablation of the SERCA3 gene alters epithelium-dependent relaxation in mouse tracheal smooth muscle. Am J Physiol. 1999 Aug;277(2 Pt 1):L264–70. doi: 10.1152/ajplung.1999.277.2.L264. [DOI] [PubMed] [Google Scholar]
- [75].Lee MG, Xu X, Zeng W, Diaz J, Kuo TH, Wuytack F, et al. Polarized expression of Ca2+ pumps in pancreatic and salivary gland cells. Role in initiation and propagation of [Ca2+]i waves. J Biol Chem. 1997 Jun 20;272(25):15771–6. doi: 10.1074/jbc.272.25.15771. [DOI] [PubMed] [Google Scholar]
- [76].Muller OJ, Lange M, Rattunde H, Lorenzen HP, Muller M, Frey N, et al. Transgenic rat hearts overexpressing SERCA2a show improved contractility under baseline conditions and pressure overload. Cardiovasc Res. 2003 Aug 1;59(2):380–9. doi: 10.1016/s0008-6363(03)00429-2. [DOI] [PubMed] [Google Scholar]
- [77].Antoons G, Ver Heyen M, Raeymaekers L, Vangheluwe P, Wuytack F, Sipido KR. Ca2+ uptake by the sarcoplasmic reticulum in ventricular myocytes of the SERCA2b/b mouse is impaired at higher Ca2+ loads only. Circ Res. 2003 May 2;92(8):881–7. doi: 10.1161/01.RES.0000069032.81501.98. [DOI] [PubMed] [Google Scholar]
- [78].Weber C, Sutliff RL, Lynne LH, Periasamy M, Shull GE, Paul RJ. Vascular smooth muscle function in SERCA2 gene-ablated mice. Biophys J. 1999;76:A286. [Google Scholar]
- [79].Gomez-Pinilla PJ, Pozo MJ, Baba A, Matsuda T, Camello PJ. Ca2+ extrusion in aged smooth muscle cells. Biochem Pharmacol. 2007 Sep 15;74(6):860–9. doi: 10.1016/j.bcp.2007.06.037. [DOI] [PubMed] [Google Scholar]
- [80].Nazer MA, Van Breemen C. A role for the sarcoplasmic reticulum in Ca2+ extrusion from rabbit inferior vena cava smooth muscle. Am J Physiol. 1998 Jan;274(1 Pt 2):H123–31. doi: 10.1152/ajpheart.1998.274.1.H123. [DOI] [PubMed] [Google Scholar]
- [81].Liu LH, Paul RJ, Sutliff RL, Miller ML, Lorenz JN, Pun RY, et al. Defective endothelium-dependent relaxation of vascular smooth muscle and endothelial cell Ca2+ signaling in mice lacking sarco(endo)plasmic reticulum Ca2+-ATPase isoform 3. J Biol Chem. 1997 Nov 28;272(48):30538–45. doi: 10.1074/jbc.272.48.30538. [DOI] [PubMed] [Google Scholar]
- [82].Kiriazis H, Kranias EG. Genetically engineered models with alterations in cardiac membrane calcium-handling proteins. Annu Rev Physiol. 2000;62:321–51. doi: 10.1146/annurev.physiol.62.1.321. [DOI] [PubMed] [Google Scholar]
- [83].Simmerman HK, Jones LR. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol Rev. 1998 Oct;78(4):921–47. doi: 10.1152/physrev.1998.78.4.921. [DOI] [PubMed] [Google Scholar]
- [84].Paul RJ, Shull GE, Kranias EG. The sarcoplasmic reticulum and smooth muscle function: evidence from transgenic mice. Novartis Found Symp. 2002;246:228–38. doi: 10.1002/0470853050.ch17. discussion 38-43, 72-6. [DOI] [PubMed] [Google Scholar]
- [85].Haghighi K, Gregory KN, Kranias EG. Sarcoplasmic reticulum Ca-ATPase-phospholamban interactions and dilated cardiomyopathy. Biochem Biophys Res Commun. 2004 Oct 1;322(4):1214–22. doi: 10.1016/j.bbrc.2004.07.164. [DOI] [PubMed] [Google Scholar]
- [86].Schmidt AG, Edes I, Kranias EG. Phospholamban: a promising therapeutic target in heart failure? Cardiovasc Drugs Ther. 2001 Sep;15(5):387–96. doi: 10.1023/a:1013381204658. [DOI] [PubMed] [Google Scholar]
- [87].Haghighi K, Kolokathis F, Pater L, Lynch RA, Asahi M, Gramolini AO, et al. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest. 2003 Mar;111(6):869–76. doi: 10.1172/JCI17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Haghighi K, Kolokathis F, Gramolini AO, Waggoner JR, Pater L, Lynch RA, et al. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc Natl Acad Sci U S A. 2006 Jan 31;103(5):1388–93. doi: 10.1073/pnas.0510519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].DeWitt MM, MacLeod HM, Soliven B, McNally EM. Phospholamban R14 deletion results in late-onset, mild, hereditary dilated cardiomyopathy. J Am Coll Cardiol. 2006 Oct 3;48(7):1396–8. doi: 10.1016/j.jacc.2006.07.016. [DOI] [PubMed] [Google Scholar]
- [90].Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, et al. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science. 2003 Feb 28;299(5611):1410–3. doi: 10.1126/science.1081578. [DOI] [PubMed] [Google Scholar]
- [91].Eggermont JA, Wuytack F, Verbist J, Casteels R. Expression of endoplasmic-reticulum Ca2(+)-pump isoforms and of phospholamban in pig smooth-muscle tissues. Biochem J. 1990;271(3):649–53. doi: 10.1042/bj2710649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Lalli J, Harrer JM, Luo W, Kranias EG, Paul RJ. Targeted ablation of the phospholamban gene is associated with a marked decrease in sensitivity in aortic smooth muscle. Circ Res. 1997 Apr;80(4):506–13. doi: 10.1161/01.res.80.4.506. [DOI] [PubMed] [Google Scholar]
- [93].Chacko S, DiSanto M, Wang Z, Zderic SA, Wein AJ. Contractile protein changes in urinary bladder smooth muscle during obstruction-induced hypertrophy. Scand J Urol Nephrol Suppl. 1997;184:67–76. [PubMed] [Google Scholar]
- [94].Nobe K, Sutliff RL, Kranias EG, Paul RJ. Phospholamban regulation of bladder contractility: evidence from gene-altered mouse models. J Physiol. 2001 Sep 15;535(Pt 3):867–78. doi: 10.1111/j.1469-7793.2001.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].McGraw DW, Fogel KM, Kong S, Litonjua AA, Kranias EG, Aronow BJ, et al. Transcriptional response to persistent beta2-adrenergic receptor signaling reveals regulation of phospholamban, which alters airway contractility. Physiol Genomics. 2006 Oct 11;27(2):171–7. doi: 10.1152/physiolgenomics.00044.2006. [DOI] [PubMed] [Google Scholar]
- [96].Sutliff RL, Conforti L, Weber CS, Kranias EG, Paul RJ. Regulation of the spontaneous contractile activity of the portal vein by the sarcoplasmic reticulum: evidence from the phospholamban gene-ablated mouse. Vascul Pharmacol. 2004 Jul;41(6):197–204. doi: 10.1016/j.vph.2004.11.004. [DOI] [PubMed] [Google Scholar]
- [97].Kim M, Cho SY, Han IS, Koh SD, Perrino BA. CaM kinase II and phospholamban contribute to caffeine-induced relaxation of murine gastric fundus smooth muscle. Am J Physiol Cell Physiol. 2005 Jun;288(6):C1202–10. doi: 10.1152/ajpcell.00299.2004. [DOI] [PubMed] [Google Scholar]
- [98].Kim M, Han IS, Koh SD, Perrino BA. Roles of CaM kinase II and phospholamban in SNP-induced relaxation of murine gastric fundus smooth muscles. Am J Physiol Cell Physiol. 2006 Aug;291(2):C337–47. doi: 10.1152/ajpcell.00397.2005. [DOI] [PubMed] [Google Scholar]
- [99].Kim M, Hennig GW, Smith TK, Perrino BA. Phospholamban knockout increases CaM kinase II activity, intracellular Ca2+ wave activity, and alters contractile responses of murine gastric antrum. Am J Physiol Cell Physiol. 2007 Nov 28; doi: 10.1152/ajpcell.00418.2007. [DOI] [PubMed] [Google Scholar]
- [100].Kim M, Perrino BA. CaM kinase II activation and phospholamban phosphorylation by SNP in murine gastric antrum smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2007 Apr;292(4):G1045–54. doi: 10.1152/ajpgi.00203.2006. [DOI] [PubMed] [Google Scholar]
- [101].Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, et al. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995 Oct 27;270(5236):633–7. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- [102].Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–4. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- [103].Santana LF, Kranias EG, Lederer WJ. Calcium sparks and excitation-contraction coupling in phospholamban-deficient mouse ventricular myocytes. J Physiol. 1997 Aug 15;503(Pt 1):21–9. doi: 10.1111/j.1469-7793.1997.021bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Smith GD, Keizer JE, Stern MD, Lederer WJ, Cheng H. A simple numerical model of calcium spark formation and detection in cardiac myocytes. Biophys J. 1998 Jul;75(1):15–32. doi: 10.1016/S0006-3495(98)77491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Wellman GC, Santana LF, Bonev AD, Nelson MT. Role of phospholamban in the modulation of arterial Ca(2+) sparks and Ca(2+)-activated K(+) channels by cAMP. Am J Physiol Cell Physiol. 2001 Sep;281(3):C1029–37. doi: 10.1152/ajpcell.2001.281.3.C1029. [DOI] [PubMed] [Google Scholar]
- [106].ZhuGe R, Tuft RA, Fogarty KE, Bellve K, Fay FS, Walsh JV., Jr. The influence of sarcoplasmic reticulum Ca2+ concentration on Ca2+ sparks and spontaneous transient outward currents in single smooth muscle cells. J Gen Physiol. 1999 Feb;113(2):215–28. doi: 10.1085/jgp.113.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Zucchi R, Ronca-Testoni S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: modulation by endogenous effectors, drugs and disease states. Pharmacol Rev. 1997 Mar;49(1):1–51. [PubMed] [Google Scholar]
- [108].Karczewski P, Hendrischke T, Wolf WP, Morano I, Bartel S, Schrader J. Phosphorylation of phospholamban correlates with relaxation of coronary artery induced by nitric oxide, adenosine, and prostacyclin in the pig. J Cell Biochem. 1998;70(1):49–59. doi: 10.1002/(sici)1097-4644(19980701)70:1<49::aid-jcb6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [109].Lynch RM, Nullmeyer KD, Pritchard T, Paul RJ. Ca2+ homeostasis in aortic smooth muscle cells from mice expressing a Na+-K+ ATPase (NKA) alpha 2-isoform transgene specifically in smooth muscle. Faseb Journal. 2007 Mar 7;20(5):A1291–a2. submitted. [Google Scholar]
- [110].van Breemen C, Chen Q, Laher I. Superficial buffer barrier function of smooth muscle sarcoplasmic reticulum. Trends Pharmacol Sci. 1995;16(3):98–105. doi: 10.1016/s0165-6147(00)88990-7. [DOI] [PubMed] [Google Scholar]
- [111].Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, et al. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res. 1994;75(3):401–9. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- [112].Naderali EK, Buttell N, Taggart MJ, Bullock AJ, Eisner DA, Wray S. The role of the sarcolemmal Ca(2+)-ATPase in the pH transients associated with contraction in rat smooth muscle. J Physiol (Lond) 1997;505(Pt 2):329–36. doi: 10.1111/j.1469-7793.1997.329bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Duchen MR, Verkhratsky A, Muallem S. Mitochondria and calcium in health and disease. Cell Calcium. 2008 Mar 29; doi: 10.1016/j.ceca.2008.02.001. [DOI] [PubMed] [Google Scholar]
- [114].Franzini-Armstrong C. ER-mitochondria communication. How privileged? Physiology (Bethesda) 2007 Aug;22:261–8. doi: 10.1152/physiol.00017.2007. [DOI] [PubMed] [Google Scholar]
- [115].Vallieres J, Scarpa A, Somlyo AP. Subcellular fractions of smooth muscle. Isolation, substrate utilization and Ca++ transport by main pulmonary artery and mesenteric vein mitochondria. Archives of Biochemistry & Biophysics. 1975;170(2):659–69. doi: 10.1016/0003-9861(75)90162-9. [DOI] [PubMed] [Google Scholar]
- [116].Dedkova EN, Blatter LA. Mitochondrial Ca(2+) and the heart. Cell Calcium. 2008 Jan 4; doi: 10.1016/j.ceca.2007.11.002. [DOI] [PubMed] [Google Scholar]
- [117].Bassani RA, Bassani JW, Bers DM. Mitochondrial and sarcolemmal Ca2+ transport reduce [Ca2+]i during caffeine contractures in rabbit cardiac myocytes. J Physiol. 1992;453:591–608. doi: 10.1113/jphysiol.1992.sp019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Bassani JW, Bassani RA, Bers DM. Ca2+ cycling between sarcoplasmic reticulum and mitochondria in rabbit cardiac myocytes. J Physiol. 1993 Jan;460:603–21. doi: 10.1113/jphysiol.1993.sp019489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Li L, Chu G, Kranias EG, Bers DM. Cardiac myocyte calcium transport in phospholamban knockout mouse: relaxation and endogenous CaMKII effects. Am J Physiol. 1998 Apr;274(4 Pt 2):H1335–47. doi: 10.1152/ajpheart.1998.274.4.H1335. [DOI] [PubMed] [Google Scholar]
- [120].Poburko D, Lee CH, van Breemen C. Vascular smooth muscle mitochondria at the cross roads of Ca(2+) regulation. Cell Calcium. 2004 Jun;35(6):509–21. doi: 10.1016/j.ceca.2004.01.020. [DOI] [PubMed] [Google Scholar]
- [121].Shimizu S, Bowman PS, Thorne G, 3rd, Paul RJ. Effects of hypoxia on isometric force, intracellular Ca(2+), pH, and energetics in porcine coronary artery. Circ Res. 2000;86(8):862–70. doi: 10.1161/01.res.86.8.862. [DOI] [PubMed] [Google Scholar]
- [122].Lopez-Barneo J, del Toro R, Levitsky KL, Chiara MD. Ortega-Saenz P. Regulation of oxygen sensing by ion channels. J Appl Physiol. 2004 Mar;96(3):1187–95. doi: 10.1152/japplphysiol.00929.2003. discussion 70-2. [DOI] [PubMed] [Google Scholar]
- [123].Hardin CD, Allen TJ, Paul RJ, editors. Metabolism and energetics of vascular smooth muscle. 4th ed. Academic Press; San Diego, CA: 2000. [Google Scholar]
- [124].Campbell JD, Paul RJ. The nature of fuel provision for the Na+,K(+)-ATPase in porcine vascular smooth muscle. J Physiol. 1992 Feb;447:67–82. doi: 10.1113/jphysiol.1992.sp018991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol. 2005 Jan;98(1):390–403. doi: 10.1152/japplphysiol.00733.2004. [DOI] [PubMed] [Google Scholar]
- [126].Kamishima T, Quayle JM. Mitochondrial Ca2+ uptake is important over low [Ca2+]i range in arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2002 Dec;283(6):H2431–9. doi: 10.1152/ajpheart.00865.2001. [DOI] [PubMed] [Google Scholar]
- [127].Shmygol A, Wray S. Functional architecture of the SR calcium store in uterine smooth muscle. Cell Calcium. 2004 Jun;35(6):501–8. doi: 10.1016/j.ceca.2004.01.006. [DOI] [PubMed] [Google Scholar]
- [128].Pritchard T, Paul RJ. Pritchard Thesis. 2007. Na+-K+ ATPase and Ca2 transport proteins are coordinately regulated in smooth muscle. [Google Scholar]
- [129].Berridge MJ. Remodelling Ca2+ signalling systems and cardiac hypertrophy. Biochem Soc Trans. 2006 Apr;34(Pt 2):228–31. doi: 10.1042/BST20060228. [DOI] [PubMed] [Google Scholar]