Abstract
Endothelial dysfunction and impaired angiogenesis is a hallmark of hypercholesterolemia (HC). This study was designed to examine the effects of resveratrol (R), an antioxidant with lipid lowering properties like statin, on neovascularization along with caveolar interaction with proangiogenic molecules in hypercholesterolemic (HC) rats. Animals were divided into: control (C), maintained on normal diet; rats maintained on 5% high cholesterol diet for 8 weeks (HC); & HC + R (20mg/kg) orally for 2 weeks (HCR). Myocardial infarction was induced by ligating the left anterior descending artery (LAD). Herein we examined a novel method of stimulating myocardial angiogenesis by pharmacological preconditioning with resveratrol at both capillary and arteriolar levels and the potential role of hemeoxygenase-1 (HO-1), e-NOS and Caveolin-1(Cav-1) in mediating such a response. We also investigated the functional relevance of such treatment by assessing whether the induced neovascularization can help preserve LV–contractile functional reserve in the setting of chronic hypercholesterolemic condition. Four weeks after sham surgery and LAD occlusion, rats underwent echocardiographic evaluation which revealed improvement in ejection fraction and fractional shortening in HCR compared to HC. Left ventricular tissue sections displayed increased capillary and arteriolar density in HCR compared to HC. Western blot analysis displayed downregulation of VEGF, HO-1 and increased association of Cav-1 along with eNOS in HC, preventing availability of eNOS to the system which was reversed with resveratrol treatment in HCR. This study was further validated in cardiac specific HO-1 overexpressed mice assuming molecular cross talk between the targets. Hence, our data identified potential regulators that primarily attenuate and blunt endothelial dysfunction by resveratrol therapy in hypercholesterolemic myocardium.
Keywords: Hypercholesterolemia, myocardium, Resveratrol, Angiogenesis, HO-1, Caveolin-1, eNOS, VEGF
Introduction
Endothelial dysfunction characterized by impaired nitric oxide bioactivity and decreased synthesis of endothelial derived nitric oxide has been suggested to contribute to early event in atherosclerotic process [1]. Decreased nitric oxide production as well as increased interaction of caveolin and endothelial nitric oxide synthase (eNOS) has been demonstrated in hypercholesterolemia [2] which is known to be a dominant risk factor of atherosclerosis. Caveolae, the non-clathrin coated vesicles are highly enriched in sphingomyelin, cholesterol and a 21kDa coat protein caveolin [3]. Mammalian caveolin family proteins caveolin-1, 2 and 3 were found to be the major components of caveolae [4]. It has been demonstrated that caveolin-1 is involved in the regulation of cellular cholesterol homeostasis and dowregulation of cholesterol was shown to decrease caveolae numbers [5], Peterson et al have shown that cholesterol treatment increases eNOS association with caveolae [6]. In addition, caveolin-1 has the ability to negatively regulate eNOS activity and hyperactivation of the nitric oxide has been shown in caveolin-1 knockout mice (cav-1−/−) [7]. Kim et al have demonstrated that in the absence of caveolin-1 the activity of HO-1 is markedly increased suggesting the inhibitory regulation by caveloin-1 [8]. HO-1 is a stress inducible cytoprotective enzyme which catalyzes the degradation of Heme to free iron, carbon monoxide (CO) and biliverdin which subsequently gets converted to bilirubin by bilirubin reductase [9]. In addition, the absence of heme oxygenase-1 (HO-1) is known to exacerbate atherosclerotic lesion formation and vascular remodeling [10]. Impairment of angiogenesis during hypercholesterolemia might be due to reduced endothelium-derived nitric oxide which is a critical modulator of angiogenesis [11], moreover eNOS gene delivery is shown to promote angiogenesis in a rat model of hind limb ischemia [12]. HO-1 is also demonstrated as a mediator of VEGF production [13] and also to facilitate angiogenesis in a rat model of hind limb ischemia [14]. Regulatory interactions between the nitric oxide synthase and Heme Oxygenase has been reported [15]. Based on the experimental data it is evident that pharmacological approach to regulate caveolin-1/ HO-1/ eNOS/ VEGF might play an important role in the improvement of endothelial dysfunction and myocardial angiogenic response in hypercholesterolemia. Earlier, cardioprotection with grapes as well as the grape seed proanthocyanidins against ischemia reperfusion injury has been demonstrated [16, 17]. We have also demonstrated that resveratrol (a polyphenol present in red wine) enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor [18]. Moreover we have recently demonstrated that pharmacological preconditioning by resveratrol modulates the lipid levels in the high fat fed rats as well as increases cardiac functions by reducing the infarct size and cardiomyocyte apoptosis [19]. In the same study we have demonstrated that resveratrol in combination with statin induces better cardiac functions as compared to resveratrol or statin alone [19]. Again, resveratrol mediated alleviation of cardiac dysfunction in streptozotocin-induced diabetes and nitric oxide, thioredoxin and heme oxygenase play an important role in the resveratrol mediated cardioprotection [20]. Ischemic preconditioning is a healthy heart phenomenon and Juhasz et al [21] demonstrated significant vulnerability of the myocardium to reperfusion-induced arrhythmias in hypercholesterolemic myocardium indicating that the ischemic preconditioning only works with intact, healthy heart. Therefore we adapted pharmacological preconditioning over ischemic preconditiong to study the Cav-1/eNOS/HO-1 signaling in the ischemic hypercholesterolemic myocardium.
Therefore, based on the data available in the literature, we designed this study to investigate the effect of resveratrol in high fat fed rats to observe whether (1) resveratrol has any effect on caveolin-1 signaling involving eNOS and HO-1 (2) to examine the extent of expression of proangiogenic factor VEGF and the extent of vessel formation (3) to examine the extent of cardiac function and ventricular remodeling. We have further used cardiac specific HO-1 transgenic (over expressed) mice to validate our results.
Our study demonstrates the new insights of therapeutic angiogenic response of resveratrol in the modulation of endothelial dysfunction and other cardiovascular complications induced during hypercholesterolemia.
Materials and Methods
Animals
This study was performed in accordance with the principles of laboratory animal care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (Publication No. 85-23, revised 1985). The experimental protocol was examined and approved by the Institutional Animal Care Committee of the Connecticut Health Center (Farmington, CT).
Experimental design
Male Sprague-Dawley rats weighing 200–250 g (starting body weight) were used for the study. The rats were randomized into three groups (n=18 in each group): (1) Control (C), (2) Hypercholesterolemia (HC) and (3) Hypercholesterolemia + Resveratrol (R) (HCR). Rats in HC group were fed with 5% cholesterol diet (CD) for 8 weeks (High cholesterol diet was purchased from Harlan Teklad Company, Madison, Wisconsin 53744, USA). Corresponding control rats were fed with normal diet. Eight weeks later the final body weight in the control group was 375–400g and in the HC group was 450–500g. After 8 weeks of high cholesterol diet the rats in HCR group were treated with resveratrol (R) (20mg/kg/day) for 2 weeks orally. The in vivo myocardial infarction model was (subjected to 4 and 30 days of permanent LAD occlusion) used to measure the protein expression, capillary and arteriolar density respectively. Echocardiogram was used to examine myocardial function 30 days after myocardial infarction.
Determination of lipid levels
Approximately 2–2.5 ml blood was collected from the jugular vein and transferred into heparinized tubes and the plasma was separated after centrifugation. Cholesterol, triglycerides, and HDL-C levels were measured using colorimetric kits for total cholesterol (TC), triglycerides (TC) and HDL-C (Wako Diagnostics, Richmond, VA 23237, USA) following the kit protocol. LDL-C was calculated using the Friedwald Equation LDL-C = TC – (HDL-C + (TG/5)) [11].
Surgical procedure
Male Sprague Dawley rats weighing 375–400g (control group in which rats were fed with normal diet for 8 weeks) and 450–500g (groups 2 & 3 in which rats were fed with high cholesterol diet for 8 weeks) were anesthetized with ketamine HCl (100 mg/kg i.p.) and xylazine (10 mg/kg i.p.). Cefazolin (25 mg/kg i.p.) was administered as a preoperative antibiotic cover. The rats were mechanically ventilated (Harvard Apparatus Rodent ventilator: Model 683) and the hearts were exposed through a left lateral thoracotomy (4th intercostal space). A 6-0 polypropylene suture was passed with tapered needle under the left anterior descending coronary artery (LAD) just below the tip of the left auricle, and the myocardial infarction was produced by permanent LAD occlusion. After completion of all surgical protocols, the chest wall was closed. After application of buprenorphine (0.1 mg/kg s.c.) and weaning from the respirator, the rats were placed on a heating pad while recovering from anesthesia [11, 18, 22].
Echocardiography Measurements
Rats were sedated using isoflurane (3%, inhaled). When adequately sedated, the rats were secured with tape in the supine position in a custom-built mold designed to maintain the rat’s natural body shape after fixation. The hair on the chest wall was removed with a commercially available hair remover from Nair, Princeton, NJ, USA. The hair remover was applied on the chest wall and after 2–3min the hair was removed using a cotton swab. Ultrasound gel was spread over the precordial region, and ultrasound biomicroscopy (Vevo 770, Visual-Sonics Inc., Toronto, ON, Canada) with a 25-MHz transducer was used to visualize the left ventricle. The left ventricle was analyzed in apical, parasternal long axis, and parasternal short axis views for left ventricular (LV) systolic function, LV cavity diameter, wall thickness, diastolic function, and LV end-systolic and end-diastolic volume determination. 2D directed M-mode images of the LV short axis were taken just below the level of the papillary muscles for analyzing ventricular wall thickness and chamber diameter. All left ventricular parameters were measured according to the modified American Society of Echocardiography–recommended guidelines. Ejection fraction (EF) and fractional shortening (FS) were assessed for left ventricular systolic function. Diastolic function was assessed by measuring mitral peak flow velocity of the E-wave and A-wave in centimeters per second (cm/s), as was the ratio between the two waves (E/A). All measurements represent the mean of at least 3 consecutive cardiac cycles. Throughout the procedure ECG, respiratory rate, and heart rate were monitored as described previously [11, 23].
Generation of cardiac specific HO-1 transgenic mice
The cardiac-specific HO-1 transgenic mice in FVB genetic background were generated as described by Yet et al [24]. Litter mate non-transgenic mice were used as the control group. Mice were given an intraperitoneal bolus of heparin (500 IU kg−1) and were anesthetized by the intraperitoneal administration of sodium pentobarbital (100 mg kg−1, Abbot, Baxter Health Care, Deep Field, IL) [25]. The hearts were perfused for 3 min in langendorff mode for baseline samples to clear off the blood. The left ventricular tissue was flash frozen for isolation of membrane and cytosolic protein to perform Western blot analysis.
Western Blot analysis for Caveolin-1, HO-1, phosphorylated-eNOS, eNOS and VEGF
To quantify the caveolin-1, HO-1, p-eNOS, eNOS and VEGF standard SDS/PAGE Western blot technique was performed. The membrane and cytosolic protein was isolated according to the Kit protocol (BioVision Research Products, CA-94043, USA) and the protein concentration was determined using a BCA (bicinchoninic acid) protein assay kit (Pierce, Rockville, IL). The proteins were run on polyacrylamide electrophoresis gels (SDS-PAGE) typically using 7% for eNOS & p-eNOS (the phosphorylation site is specific to serine-1177) & 10% for VEGF & HO-1, 12% for cavelolin-1 (acrylamide to bis ratios). Standard Western blot technique was done as described previously [11, 18]. The antibodies for p-eNOS, eNOS were purchased from Cell Signaling Technology, Danvers, MA and caveolin-1, VEGF, HO-1 were purchased from Santa Cruz Biotechnology, Santa Cruz, CA, USA respectively.
Immunohistochemistry for capillary and arteriolar density
Capillary density (CD-31) and arteriolar density staining was done following 4 and 30 days of the surgical procedure, respectively. The rats were sacrificed; hearts removed and paraffin-embedded sections were made. Endothelial cells were labeled using mouse monoclonal anti-CD31/PECAM-1 (1:100, Pharmingen, San Diego, CA, USA) followed by a biotinylated horse anti-mouse secondary antibody (1:200 dilution). The reaction product (brown) was visualized with 3,3’-diaminobenzidine (DAB) substrate using the Vector ABC Vectastain Elite Kit (Vectorlabs, Burlingame, CA, USA). On separate slides, vascular smooth muscle cells were labeled using mouse monoclonal anti-smooth muscle actin (1:100, Sigma, St. Louis, MO, USA) followed by a FITC conjugated donkey anti-mouse secondary antibody (1: 200 dilution, Jackson Immunoresearch Inc, West Grove, PA, USA). After incubation the sections were again washed in PBS for 3 times and mounted with coverslip using mounting medium (Vector laboratories, Burlingame, CA). Images were captured and stored in digital tiff file format for later image analysis. Counts of capillary density and arteriolar density per mm2 were obtained after superimposing a calibrated morphometric grid on each digital image using Adobe Photoshop Software [11, 22].
Statistical Analysis
Results were expressed as Mean ± Standard Deviation (SD). ANOVA followed by Bonferroni’s correction was carried out to determine any differences between the mean values of all groups. The results were considered significant if p <0.05.
Results
Effect of resveratrol on lipid levels
Plasma lipids were significantly altered after high-cholesterol feeding in the HC group. The levels of plasma cholesterol, triglycerides and LDL-C increased while the level of HDL-C decreased in the HC group as compared to the control group. Treatment with resveratrol significantly lowered the levels of cholesterol, triglyceride and LDLC in the high cholesterol-fed rats as compared to the untreated HC group. The HDL-C level was found to be increased with resveratrol treatment (n=6) as shown in Table-1.
Table_ 1. Effect of Resveratrol on Lipid Levels.
Results are expressed as Mean ± SD.
| S.NO | Groups | Cholesterol (mg/dl) | Triglycerides (mg/dl) | HDL-C (mg/dl) | LDL-C (mg/dl) |
|---|---|---|---|---|---|
| 1 | Control | 90.24 ± 5.36 | 81.92 ± 4.34 | 43.52 ± 3.89 | 28.32 ± 2.93 |
| 2 | Hypercholesterolemia | 151.7 ± 7.14* | 159.5 ± 6.23* | 24.37 ± 2.80* | 83.91 ± 3.89* |
| 3 | Hypercholesterolemia + Resveratrol | 104.1 ± 4.29† | 94.67 ± 4.63† | 37.04 ± 3.12† | 49.04 ± 3.72† |
p<0.05 represents significant difference between HC and control
p<0.05 represents significant difference between HCR and HC. Where HC represents high cholesterol diet fed rats, HCR represents HC rats treated with resveratrol.
Effect of resveratrol on Cav-1, eNOS and HO-1 in the membrane fraction
The Cav-1 and eNOS levels were found to be significantly increased in the HC hearts by 2 and 1.7 fold in the HC group as compared to corresponding normal control. The increase in the Cav-1 expression correlated with the increase in the cholesterol levels. The protein expression of Cav-1 and eNOS was found to be decreased by 0.58 and 0.52 fold respectively as compared to HC group after resveratrol treatment. Conversely the HO-1 level was found to be decreased (0.35 fold) in the untreated HC group as compared to the normal control. Resveratrol treatment in HC animals increased HO-1 expression by 2.2 fold as compared to the untreated HC (n=6) (Figure-1A). These results have demonstrated that HO-1 conversely regulates the Cav-1 and eNOS association.
Figure 1.
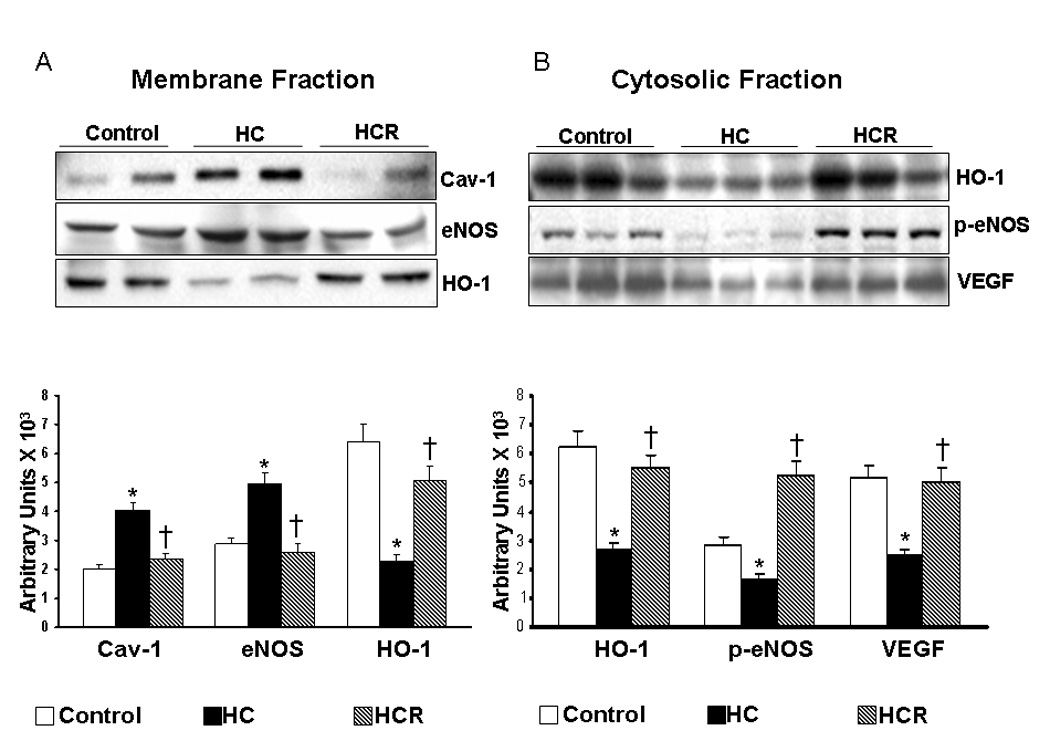
A) Representative Western blots showing the protein expression of Cav-1, eNOS and HO-1 in the membrane fraction. Graph represents the quantitative comparison between the groups. B) Representative Western blots showing the protein expression of HO-1, p-eNOS and VEGF in the cytosolic fraction. Graph represents the quantitative comparison between the groups. *p<0.05 represent HC compared with control, †p<0.05 HCR compared with HC. Where HC represents high cholesterol diet fed rats, HCR represents HC rats treated with resveratrol.
Effect of resveratrol on HO-1, p-eNOS and VEGF in the cytosol
The protein expression profile of HO-1, p-eNOS and VEGF were found to be decreased significantly by 0.43, 0.58 and 0.47 fold respectively in the HC group as compared to normal control. The increased association of Cav-1 and eNOS in the membrane might have resulted in decreased phosphorylation of eNOS in the cytosolic fraction in the HC group as compared to control. The decrease in phosphorylation / activation of eNOS might have resulted in decreased expression of its regulator VEGF. Resveratrol treatment increased the expression of HO-1, p-eNOS and VEGF by 2, 3.1 and 2.05 fold as compared to the untreated HC (n=6) (Figure-1B). p-eNOS was normalized using eNOS as the loading control and VEGF, HO-1 were normalized using GAPDH as the loading control.
Cardiac specific HO-1 transgenic mice
Cav-1, HO-1 and eNOS expression in the membrane fraction
Cav-1 expression significantly decreased by 0.7 fold in the HO-1 transgenic mice as compared to the litter mate controls. Conversely as expected HO-1 expression was significantly increased in the HO-1 transgenic mice by 4.6 fold as compared to the normal control (Figure-2A). The membrane bound eNOS expression was decreased by 0.7 fold in the transgenic group as compared to the litter mate controls (n=6). Increased HO-1 expression correlated with decreased Cav-1 and eNOS association demonstrating that HO-1 conversely regulates the Cav-1 expression.
Figure 2.
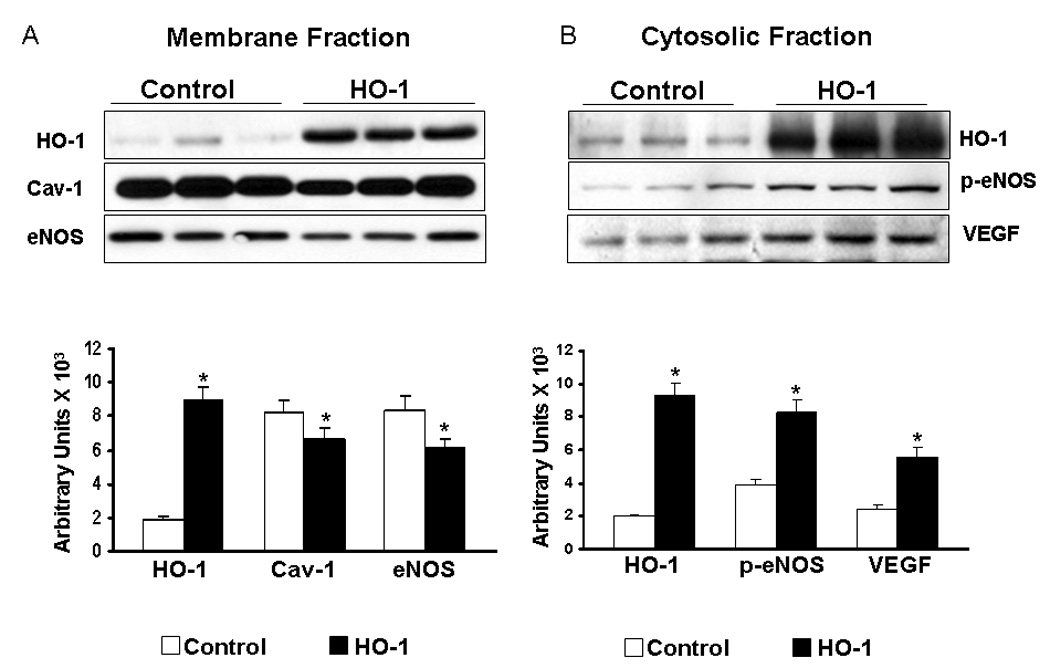
A) Representative Western blots showing the protein expression of HO-1, Cav-1, eNOS in the membrane fraction of HO-1 Tg mice. Graph represents the quantitative comparison between the groups. B) Representative Western blots showing the protein expression of HO-1, p-eNOS and VEGF in the cytosolic fraction of HO-1 Tg mice. Graph represents the quantitative comparison between the groups. *p<0.05 represents HO-1 Tg mice compared with control.
HO-1, p-eNOS and VEGF expression in the cytosolic fraction
HO-1 expression significantly increased in the HO-1 transgenic mice (4.5 fold) as compared to the non-transgenic control. The phosphorylation of eNOS (p-eNOS) significantly increased by 2.13 fold in the transgenic group as compared to the litter mate controls. In addition, the expression of VEGF was also increased by 2.31 fold in the HO-1 transgenic group as compared to the corresponding litter mate controls (Figure-2B) (n=6).
Effect of resveratrol on the extent of capillary (CD-31) and arteriolar density
Immunohistochemical staining of PECAM-1 and anti-smooth muscle actin were used to assess the capillary density (CD-31) and arteriolar density, respectively. At 400X magnification, 8 non-overlapping random fields, each selected from a non-infarcted risk area, were used for counting. Four sections from each heart were examined. Increased capillary density was observed in the HCR treated group (2756 ± 217 vs 1888 ± 98 counts/mm2) as compared to the untreated HC group (Figure-3A). Similarly, increased arteriolar density was also observed in the HCR group (2.5 ± 0.4 vs 1.58 ± 0.21 counts/mm2) as compared to the HC group (n=6) (Figure-3B). The results correlated positively with the increased HO-1, p-eNOS and VEGF expression thus supporting the notion that the increased capillary and arteriolar density on resveratrol treatment might be HO-1/eNOS/VEGF mediated.
Figure 3.
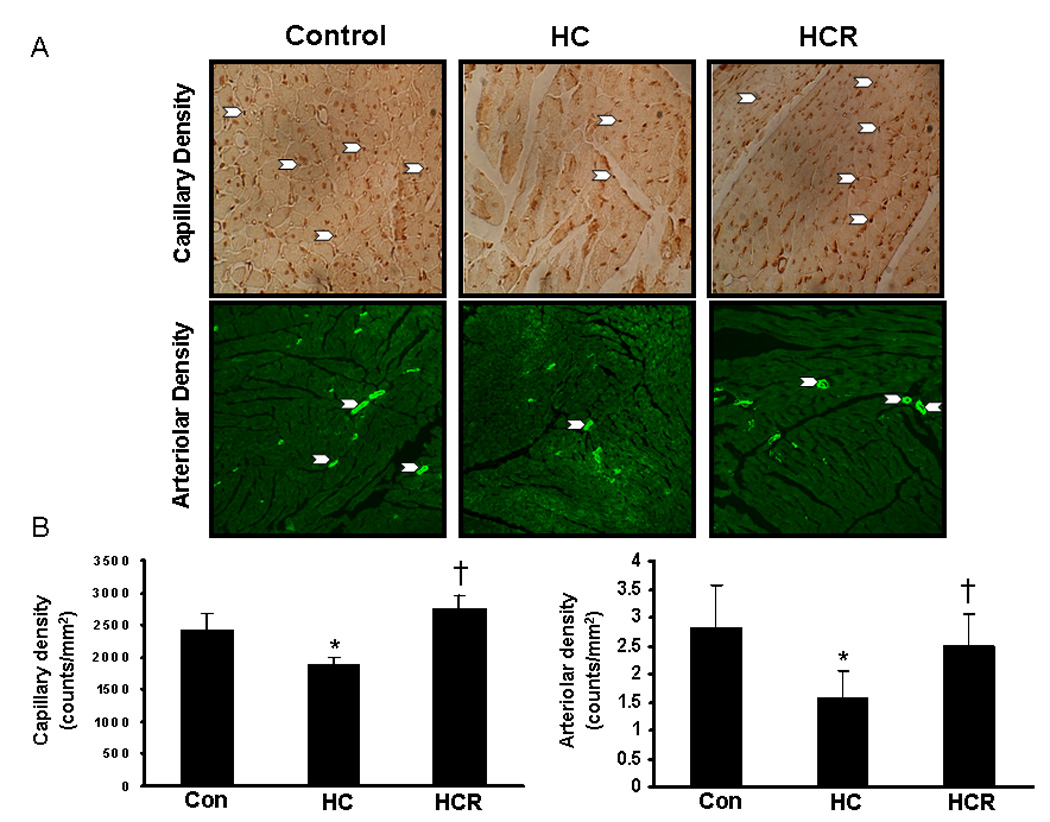
A)_Figures represent the capillary density and arteriolar density between the comparative groups in the peri-infarct area 4 and 30 days after myocardial infarction respectively. B) Graphs represent the quantitative analysis of capillary and arteriolar density between the comparative groups. *p<0.05 represent HC compared with control, †p<0.05 HCR compared with HC. Where HC represents high cholesterol diet fed rats, HCR represents HC rats treated with resveratrol.
Effect of resveratrol on echocardiographic evaluation after MI in HC rats
The left ventricular functional parameters were studied by echocardiography 30 days after LAD ligation. The cardiac output parameters which demonstrate the functionality of the left ventricle such as ejection fraction and fractional shortening were significantly decreased in the HC group compared to the control. The ejection fraction (46 vs 39 %) and fractional shortening (24 vs 20 %) increased significantly in the HCR as compared to the HC group. A significant increase in the chamber dilation was observed in the HC as compared to the control. By comparison, HC rats showed a progressive increase in systolic Left Ventricular Internal Diameter (LVIDs) and resveratrol treatment has significantly decreased the LVIDs (7.8 vs 8.6 mm) as compared to HC. The left intraventricular septal thickeness (IVSs) decreased in the HC rats significantly as compared to control. Resveratrol treatment significantly increased the IVSs (2.4 vs 2mm) compared to untreated HC rats. Significant decrease in left ventricular posterior wall thickness (LVPW) was observed in HC which was significantly increased on treatment with resveratrol in the HCR compared to HC. The LVPWs was found to be decreased to 2mm in the HC group as compared to 2.5 and 2.3 mm in control and HCR groups respectively. There was a compensatory increase in the posterior (LVPW) and lateral wall systolic thickness in the HCR group compared to the HC (n=6) (Figure-4). Taken together, resveratrol treatment has demonstrated progressive, significant increase in left ventricular function as compared to untreated HC group.
Figure 4.
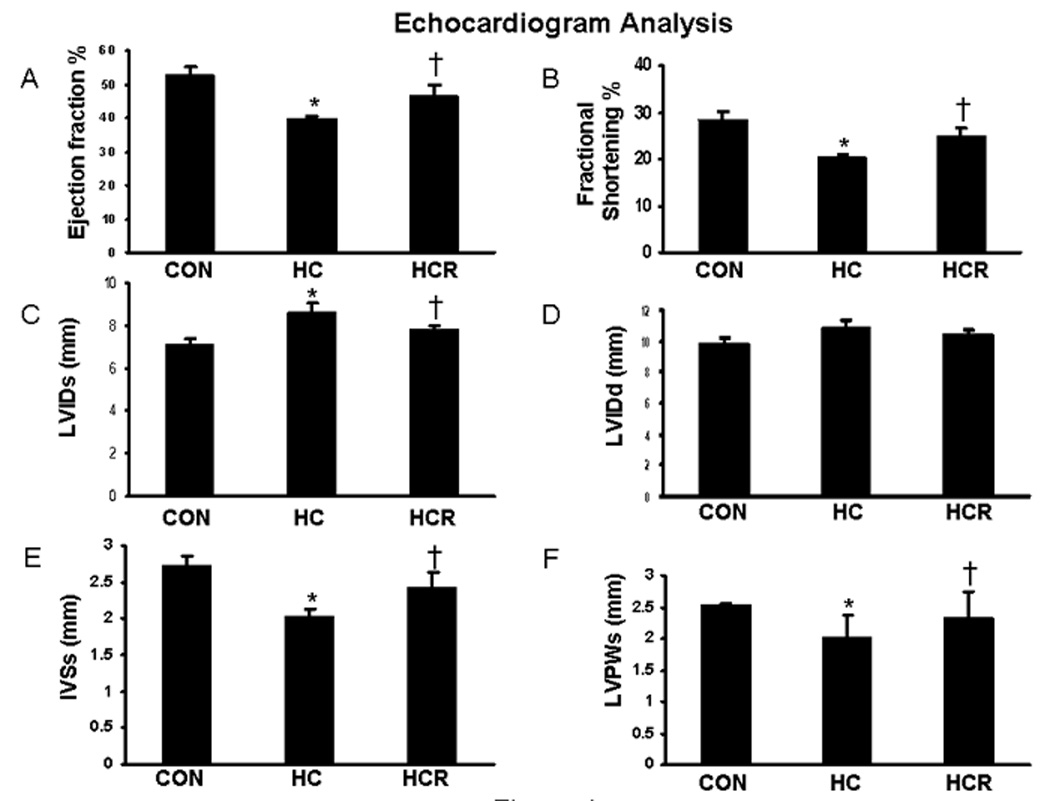
Echocardiographic measurements between the comparative groups after 30 days of myocardial infarction. A) Ejection fraction B) Fractional Shortening C) LVIDs D) LVIDd E) IVSs and F) LVPWs. Graphical representation shows the quantitative comparison between the groups. *p<0.05 represent HC compared with control, †p<0.05 HCR compared with HC. Where HC represents high cholesterol diet fed rats, HCR represents HC rats treated with resveratrol.
Discussion
In the present study we demonstrate for the first time that HC induced complications such as increased lipid levels, caveolin-1/eNOS association and decreased HO-1 expression as well as reduction in myocardial functions could be normalized with resveratrol therapy. We also documented that resveratrol regulates HO-1 conversely for the disruption of caveolin-1/eNOS association in HC myocardium. We further validated our results using HO-1 Tg mice. HO-1 over expression resulted in a significant decrease of caveolin-1/eNOS association thus showing HO-1 regulates cav-1/eNOS conversely.
Studies have suggested that caveolin-1 is involved in the regulation of cholesterol homeostasis and promotes cellular cholesterol efflux [26, 27]. Fielding et al have demonstrated that increased LDL and thus cell free cholesterol levels results in transport of the caveolin from the intracellular pools to the cell surface. They have also demonstrated that caveolin synthesis is upregulated at the transcriptional level resulting in increased caveolin-1 expression [28]. Frank and Lisanti have shown the role of caveolin in atherosclerosis and have demonstrated that a marked decrease in atherosclerotic lesion is observed in ApoE−/− caveolin-1−/− double knock out mice indicating that lack of caveolin-1 inhibits atherogenesis [29]. We have observed increased cholesterol level as well as caveolin-1 level in the HC group which were reduced on resveratrol treatment. The reduced cholesterol along with reduced LDL levels might have resulted in decreased caveolin-1 expression in the resveratrol treated group. Feron et al has shown that caveolin-1 negatively regulate the eNOS activity [2]. Moreover it is demonstrated that endothelial dysfunction which is characterized by decreased synthesis and activity of endothelial derived nitric oxide is an important factor leading to atherosclerosis [30]. As expected we have observed decreased phosphorylation of eNOS along with increased caveolin-1 in the HC group which was positively reversed on resveratrol treatment. Kim et al have also shown caveolae compartmentalization of HO-1 in endothelial cells [8] they have demonstrated that endothelial HO-1 localize in part to detergent resistant fractions that contained caveolin-1 [8]. Interestingly they have also shown that downregulation of caveolin-1 by antisense expression increased HO-1 activity and overexpression of caveolin-1 downregulated LPS-inducible HO activity. In addition, Ziche et al [31], have shown that biological processes modulated by NO might play a role in angiogenesis. NO released from cultured human endothelial cells as well as isolated vascular strips confirmed that VEGF stimulates endothelial nitric oxide release in vitro [32]. VEGF mediated angiogenesis was found to be attenuated by inhibitors of NOS [33]. It is known that VEGF administration restores normal vascular responsiveness in ischemically injured tissue [34]. Furthermore, VEGF administration does not restore normal collateral development in eNOS–deficient mice, indicating that eNOS may be a downstream effector molecule of VEGF [35]. Other studies showed that eNOS is required for endothelial cell proliferation and/or migration; the essential steps required for neovascularization [36].
In our present study in addition to decreased caveolin-1 expression in HO-1 transgenic mice we have also observed increased myocardial phosphorylation of eNOS along with increased VEGF expression. This demonstrates that over expression of HO-1 might lead to caveolin-1 down regulation which in turn increases eNOS availability and increased activity resulting in increased expression of VEGF. We have also found that HO-1, p-eNOS as well as VEGF expression is downregulated in the HC group which were upregulated on treatment with resveratrol. Moreover we have demonstrated earlier in a rat MI model that increased HO-1 and VEGF activation with resveratrol treatment resulted in cardioprotection [18]. HO-1 mediated cardioprotection has been demonstrated earlier and the antiatherogenic properties of HO-1 are attributed to its enzymatic production of carbon monoxide (CO) and bilirubin which act as a vasodilator and antioxidant respectively [37]. In addition, HO-1 mediated production of CO was shown to prevent ventricular fibrillation in ischemia-reperfused mouse myocardium [38, 39]. Ishikawa et al have shown that modulation of HO-1 expression in LDL-receptor knockout mice fed with high fat diet leads to atherosclerotic lesion formation [40]. From the results obtained we interpreted that regulation of HO-1/caveolin-1/ eNOS/ VEGF by resveratrol might result in increased angiogenesis leading to cardioprotection in the hypercholesterolemic myocardium. As expected, the extent of capillary and arteriolar density was found to be increased in resveartrol treated group as compared to HC. Echocardiography has also revealed a significant reduction in ischemic ventricular remodeling and showed an improved diastolic and systolic thickness of the ventricular wall in the HCR, showing the efficacy of resveratrol in maintaining the left ventricular systolic function. The increase in capillary and arteriolar density in resveratrol treated group might have resulted in a compensatory response leading to sustained myocardial perfusion along with increased cardiac functions after MI leading to cardioprotection.
In conclusion, our present study provides evidence for the first time that, resveratrol therapy ameliorates endothelial dysfunction during hypercholesterolemia by regulating the biomarkers like cav-1, HO-1, eNOS and VEGF. Resveratrol treatment in hypercholesterolemic rats enhanced the expression of HO-1 which plays an important role in the targeted disruption of cav-1 and eNOS association resulting in phosphorylation of eNOS and increased VEGF expression leading to neovascularization of the HC myocardium.
Acknowledgement
This study was supported by National Institutes of Health Grants HL 56803, HL 69910 and HL 85804.
List of Abbreviations
- HC
Hypercholesterolemia
- Cav-1
Caveolin-1
- HO-1
Heme Oxygenase-1
- CO
Carbon Monoxide
- eNOS
endothelial Nitric Oxide Synthase
- p-eNOS
phosphorylated endothelial Nitric Oxide Synthase
- VEGF
Vascular Endothelial Growth Factor
- LAD
Left Anterior Descending Artery
- EF
Ejection Fraction
- FS
Fractional Shortening
- LDL
Low Density Lipoprotein
- HDL
High Density Lipoprotein
- LVPW
Left Ventricular Posterior Wall
- IVS
IntraVentricular Septal thickeness
- LVID
Left Ventricular Internal Diameter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 2.Feron O, Dessy C, Moniotte S, Desager JP, Balligand JL. Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J Clin Invest. 1999;103:897–905. doi: 10.1172/JCI4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 4.Parton RG. Caveolae and caveolins. Curr Opin Cell Biol. 1996;8:542–548. doi: 10.1016/s0955-0674(96)80033-0. [DOI] [PubMed] [Google Scholar]
- 5.Chang WJ, Rothberg KG, Kamen BA, Anderson RG. Lowering the cholesterol content of MA104 cells inhibits receptor-mediated transport of folate. J Cell Biol. 1992;118:63–69. doi: 10.1083/jcb.118.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson TE, Poppa V, Ueba H, Wu A, Yan C, Berk BC. Opposing effects of reactive oxygen species and cholesterol on endothelial nitric oxide synthase and endothelial cell caveolae. Circ Res. 1999;85:29–37. doi: 10.1161/01.res.85.1.29. [DOI] [PubMed] [Google Scholar]
- 7.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 8.Kim HP, Wang X, Galbiati F, Ryter SW, Choi AM. Caveolae compartmentalization of heme oxygenase-1 in endothelial cells. Faseb J. 2004;18:1080–1089. doi: 10.1096/fj.03-1391com. [DOI] [PubMed] [Google Scholar]
- 9.Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1029–L1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 10.Yet SF, Layne MD, Liu X, Chen YH, Ith B, Sibinga NE, Perrella MA. Absence of heme oxygenase-1 exacerbates atherosclerotic lesion formation and vascular remodeling. Faseb J. 2003;17:1759–1761. doi: 10.1096/fj.03-0187fje. [DOI] [PubMed] [Google Scholar]
- 11.Penumathsa SV, Koneru S, Zhan L, John S, Menon VP, Prasad K, Maulik N. Secoisolariciresinol diglucoside induces neovascularization-mediated cardioprotection against ischemia-reperfusion injury in hypercholesterolemic myocardium. J Mol Cell Cardiol. 2008;44:170–179. doi: 10.1016/j.yjmcc.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith RS, Jr, Lin KF, Agata J, Chao L, Chao J. Human endothelial nitric oxide synthase gene delivery promotes angiogenesis in a rat model of hindlimb ischemia. Arterioscler Thromb Vasc Biol. 2002;22:1279–1285. doi: 10.1161/01.atv.0000026613.18742.67. [DOI] [PubMed] [Google Scholar]
- 13.Jozkowicz A, Huk I, Nigisch A, Weigel G, Dietrich W, Motterlini R, Dulak J. Heme oxygenase and angiogenic activity of endothelial cells: stimulation by carbon monoxide and inhibition by tin protoporphyrin-IX. Antioxid Redox Signal. 2003;5:155–162. doi: 10.1089/152308603764816514. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki M, Iso-o N, Takeshita S, Tsukamoto K, Mori I, Sato T, Ohno M, Nagai R, Ishizaka N. Facilitated angiogenesis induced by heme oxygenase-1 gene transfer in a rat model of hindlimb ischemia. Biochem Biophys Res Commun. 2003;302:138–143. doi: 10.1016/s0006-291x(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 15.Datta PK, Lianos EA. Nitric oxide induces heme oxygenase-1 gene expression in mesangial cells. Kidney Int. 1999;55:1734–1739. doi: 10.1046/j.1523-1755.1999.00429.x. [DOI] [PubMed] [Google Scholar]
- 16.Cui J, Juhasz B, Tosaki A, Maulik N, Das DK. Cardioprotection with grapes. J Cardiovasc Pharmacol. 2002;40:762–769. doi: 10.1097/00005344-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Pataki T, Bak I, Kovacs P, Bagchi D, Das DK, Tosaki A. Grape seed proanthocyanidins improved cardiac recovery during reperfusion after ischemia in isolated rat hearts. Am J Clin Nutr. 2002;75:894–899. doi: 10.1093/ajcn/75.5.894. [DOI] [PubMed] [Google Scholar]
- 18.Kaga S, Zhan L, Matsumoto M, Maulik N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol. 2005;39:813–822. doi: 10.1016/j.yjmcc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Penumathsa SV, Thirunavukkarasu M, Koneru S, Juhasz B, Zhan L, Pant R, Menon VP, Otani H, Maulik N. Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. J Mol Cell Cardiol. 2007;42:508–516. doi: 10.1016/j.yjmcc.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thirunavukkarasu M, Penumathsa SV, Koneru S, Juhasz B, Zhan L, Otani H, Bagchi D, Das DK, Maulik N. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic Biol Med. 2007;43:720–729. doi: 10.1016/j.freeradbiomed.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juhasz B, Der P, Turoczi T, Bacskay I, Varga E, Tosaki A. Preconditioning in intact and previously diseased myocardium: laboratory or clinical dilemma? Antioxid Redox Signal. 2004;6:325–333. doi: 10.1089/152308604322899396. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda S, Kaga S, Sasaki H, Zhan L, Zhu L, Otani H, Kalfin R, Das DK, Maulik N. Angiogenic signal triggered by ischemic stress induces myocardial repair in rat during chronic infarction. J Mol Cell Cardiol. 2004;36:547–559. doi: 10.1016/j.yjmcc.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Koneru S, Penumathsa SV, Thirunavukkarasu M, Vidavalur R, Zhan L, Singal PK, Engelman RM, Das DK, Maulik N. Sildenafil Mediated Neovascularization And Protection Against Myocardial Ischemia Reperfusion Injury In Rats: Probable Role Of Vegf/Angiopoietin-1. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, Solovyeva M, Ith B, Melo LG, Zhang L, Ingwall JS, Dzau VJ, Lee ME, Perrella MA. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res. 2001;89:168–173. doi: 10.1161/hh1401.093314. [DOI] [PubMed] [Google Scholar]
- 25.Addya S, Shiroto K, Turoczi T, Zhan L, Kaga S, Fukuda S, Surrey S, Duan LJ, Fong GH, Yamamoto F, Maulik N. Ischemic preconditioning-mediated cardioprotection is disrupted in heterozygous Flt-1 (VEGFR-1) knockout mice. J Mol Cell Cardiol. 2005;38:345–351. doi: 10.1016/j.yjmcc.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 26.Frank PG, Galbiati F, Volonte D, Razani B, Cohen DE, Marcel YL, Lisanti MP. Influence of caveolin-1 on cellular cholesterol efflux mediated by high-density lipoproteins. Am J Physiol Cell Physiol. 2001;280:C1204–C1214. doi: 10.1152/ajpcell.2001.280.5.C1204. [DOI] [PubMed] [Google Scholar]
- 27.Fu Y, Hoang A, Escher G, Parton RG, Krozowski Z, Sviridov D. Expression of caveolin-1 enhances cholesterol efflux in hepatic cells. J Biol Chem. 2004;279:14140–14146. doi: 10.1074/jbc.M311061200. [DOI] [PubMed] [Google Scholar]
- 28.Fielding CJ, Fielding PE. Intracellular cholesterol transport. J Lipid Res. 1997;38:1503–1521. [PubMed] [Google Scholar]
- 29.Frank PG, Lisanti MP. Caveolin-1 and caveolae in atherosclerosis: differential roles in fatty streak formation and neointimal hyperplasia. Curr Opin Lipidol. 2004;15:523–529. doi: 10.1097/00041433-200410000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest. 1997;100:2153–2157. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest. 1997;99:2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Zee R, Murohara T, Luo Z, Zollmann F, Passeri J, Lekutat C, Isner JM. Vascular endothelial growth factor/vascular permeability factor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation. 1997;95:1030–1037. doi: 10.1161/01.cir.95.4.1030. [DOI] [PubMed] [Google Scholar]
- 33.Uneda S, Hata H, Matsuno F, Nagasaki A, Harada N, Mitsuya Y, Matsuzaki H, Mitsuya H. A nitric oxide synthase inhibitor, N(G)-nitro-l-arginine-methyl-ester, exerts potent antiangiogenic effects on plasmacytoma in a newly established multiple myeloma severe combined immunodeficient mouse model. Br J Haematol. 2003;120:396–404. doi: 10.1046/j.1365-2141.2003.04078.x. [DOI] [PubMed] [Google Scholar]
- 34.Matsunaga T, Weihrauch DW, Moniz MC, Tessmer J, Warltier DC, Chilian WM. Angiostatin inhibits coronary angiogenesis during impaired production of nitric oxide. Circulation. 2002;105:2185–2191. doi: 10.1161/01.cir.0000015856.84385.e9. [DOI] [PubMed] [Google Scholar]
- 35.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 37.Siow RC, Sato H, Mann GE. Heme oxygenase-carbon monoxide signalling pathway in atherosclerosis: anti-atherogenic actions of bilirubin and carbon monoxide? Cardiovasc Res. 1999;41:385–394. doi: 10.1016/s0008-6363(98)00278-8. [DOI] [PubMed] [Google Scholar]
- 38.Bak I, Szendrei L, Turoczi T, Papp G, Joo F, Das DK, de Leiris J, Der P, Juhasz B, Varga E, Bacskay I, Balla J, Kovacs P, Tosaki A. Heme oxygenase-1-related carbon monoxide production and ventricular fibrillation in isolated ischemic/reperfused mouse myocardium. Faseb J. 2003;17:2133–2135. doi: 10.1096/fj.03-0032fje. [DOI] [PubMed] [Google Scholar]
- 39.Bak I, Papp G, Turoczi T, Varga E, Szendrei L, Vecsernyes M, Joo F, Tosaki A. The role of heme oxygenase-related carbon monoxide and ventricular fibrillation in ischemic/reperfused hearts. Free Radic Biol Med. 2002;33:639–648. doi: 10.1016/s0891-5849(02)00913-9. [DOI] [PubMed] [Google Scholar]
- 40.Ishikawa K, Sugawara D, Wang X, Suzuki K, Itabe H, Maruyama Y, Lusis AJ. Heme oxygenase-1 inhibits atherosclerotic lesion formation in ldl-receptor knockout mice. Circ Res. 2001;88:506–512. doi: 10.1161/01.res.88.5.506. [DOI] [PubMed] [Google Scholar]


