Abstract
Several models that develop epileptiform discharges and epilepsy have been associated with a decrease in the activity of calmodulin-dependent kinase II. However, none of these studies has demonstrated a causal relationship between a decrease in calcium/calmodulin kinase II activity and the development of seizure activity. The present study was conducted to determine the effect of directly reducing calcium/calmodulin-dependent kinase activity on the development of epileptiform discharges in hippocampal neurons in culture. Complimentary oligonucleotides specific for the α subunit of the calcium/calmodulin kinase were used to decrease the expression of the enzyme. Reduction in kinase expression was confirmed by Western analysis, immunocytochemistry, and exogenous substrate phosphorylation. Increased neuronal excitability and frank epileptiform discharges were observed after a significant reduction in calmodulin kinase II expression. The epileptiform activity was a synchronous event and was not caused by random neuronal firing. Furthermore, the magnitude of decreased kinase expression correlated with the increased neuronal excitability. The data suggest that decreased calmodulin kinase II activity may play a role in epileptogenesis and the long-term plasticity changes associated with the development of pathological seizure activity and epilepsy.
Epilepsy is a common neurological condition characterized by spontaneous, recurrent synchronous discharges of a population of neurons, unprovoked by a known proximal cause (1, 2). Epilepsy is the second most common neurological pathology in the United States, behind only stroke (2). Symptomatic epilepsy results from a known condition or injury producing spontaneous, recurrent seizures in previously normal brain. Depending on the study criteria used to estimate epilepsy prevalence, symptomatic epilepsies account for one- to two-thirds of new cases (2).
Epileptogenesis is the process responsible for the transformation of normal neuronal populations into neurons that display neurophysiological behaviors associated with epilepsy. Although the processes underlying epileptogenesis are complex, alterations in Ca2+ homeostasis have been suggested as a cellular mechanism in the development of seizure activity and epilepsy (3). In particular, alteration in the function of calcium and calmodulin-dependent kinase II (CaM kinase II), a neuronally enriched Ca2+-dependent second messenger system (4–6), has been associated with a number of models of epilepsy. Wasterlain and Farber (7) initially showed a decrease in CaM kinase II activity was associated with the kindling model of epilepsy. Subsequently, decreased CaM kinase II activity has been demonstrated in numerous models of epileptogenesis and epilepsy (8–10). These findings suggest that decreasing CaM kinase II activity in brain may produce spontaneous epileptiform activity.
Evidence for a direct role in decreased CaM kinase II activity causing epileptiform discharges came from the observation that transgenic mice having a null mutation for the α subunit of CaM kinase II developed epileptiform activity and epileptic seizures from limbic structures (11). Although this observation strongly implicates decreased CaM kinase II activity in the production of epileptiform activity, it does not exclude the possibility that secondary developmental mechanisms, caused by decreased CaM kinase II expression, are involved in the development of limbic seizures. Thus, it is important to further evaluate the effect of decreased CaM kinase II activity on neuronal excitability. This study was designed to directly determine the effect of decreasing α CaM kinase II subunit expression on the production of epileptiform activity in hippocampal neurons in culture. The findings demonstrated that inhibition of the expression of the α subunit of CaM kinase II resulted in increased neuronal excitability and epileptiform discharges.
Materials and Methods
Materials.
All materials are reagent grade and obtained from Sigma or Fisher Scientific unless specifically noted. Antisense and missense oligonucleotides were obtained from Operon Technologies (Alameda, CA). Radiolabeled flunitrazepam was purchased from DuPont/NEN. The monoclonal anti-CaM kinase II α subunit was purchased from Chemicon.
Hippocampal Neuronal Culture.
Primary hippocampal cultures were prepared by a modification of the method of Banker and Cowan (12) as described in detail (13). Briefly, hippocampal cells were prepared from 2-day-old postnatal rats (Harlan Breeders, Indianapolis) and grown on a confluent hippocampal astroglial feeder layer. Astrocytes were prepared from 2-day-old pups by the methods of Abney et al. (14). The glial cultures were maintained for 2 weeks in 60-mm dishes (Costar) and fed twice weekly with MEM/10% FBS/2 mM l-glutamine/10 mM glucose. After confluence (≈4 days), the glial cultures were exposed to 5 μM cytosine arabinoside to inhibit further proliferation. The day before neuronal plating, the glial feed was replaced with N3-supplemented neuronal feed. The N3 supplement contained 25 mM Hepes (pH 7.4), 2 mM glutamine, 5 μg/ml insulin, 100 μg/ml transferrin, 100 μM putrescine, 30 nM sodium selenite, 20 nM progesterone, 1 mM sodium pyruvate, 0.1% ovalbumin, 20 ng/ml T3, and 40 ng/ml corticosterone. Hippocampal cells were plated at a density of 7.5 × 105 cells per 60-mm culture dish onto a confluent glial bed. Cultures were maintained at 37°C under 5% CO2/95% air. Cultures were fed three feedings per week (1/2 half media change) with N3-supplemented Earle's salts containing MEM. The glutamine, MEM, and Hepes buffer were obtained from GIBCO.
Inhibition of CaM Kinase II Expression.
Inhibition of CaM kinase II α subunit expression was executed by the addition of antisense oligonucleotides for 3 days, unless otherwise noted. Antisense (3 μM 5′-GGTAGCCATCCTGGCACT-3′) or missense control (3 μM 5′-GGTCGCCATCAGGTCACT-3′) were added directly to the culture media. The antisense oligonucleotide was complimentary to rat mRNA at nucleotides 33–50 (E value = 0.001; National Center for Biotechnology Information, blast database). Except for a complimentary match of rat DNA for the α subunit of CaM kinase II, there were no other matches for the antisense oligonucleotide found in primary neuronal cultures from the rat (National Center for Biotechnology Information, blast database). The missense nucleotide, comprised of a scrambled sequence of the antisense nucleotide, contained the same content of nucleic acids, and the identical molecular weight and chemical properties to the antisense oligonucleotide sequence. The missense control did not significantly align to any known rat RNA or DNA sequences contained in the National Center for Biotechnology Information blast database. In addition, the missense oligonucleotide did not match any known RNA sequences found in other species with an E value < 4.5 (National Center for Biotechnology Information, blast database). Other control oligonucleotides tested included sense oligonucleotide for the CaM kinase II α subunit, and missense oligonucleotides for γ-aminobutyric acid type A α2 receptor subunit (5′-CAACCGTCGTGGTGGGTCCAC-3′), transcription factors including Δfos B (5′-AGATCTTCTCAAGTGCTT-3′), and serum response factor (5′-TGAGCTACGTCGTAGCGC-3′). These additional missense oligonucleotides were used to control for possible nonspecific effects of oligonucleotide exposure.
To determine the level of decreased CaM kinase II expression, hippocampal cultures were scraped, homogenized, and subjected to Western analysis as described (13, 15). Immunoreactivity against the α subunit of CaM kinase II was measured by using computer-assisted densitometry (Loats Associates, Westminster, MD). To ensure that changes in subunit expression were within the linear range of visualization, immunoreactivity of CaM kinase II expression was compared with a standard regression curve as described (13, 15, 16). Changes in CaM kinase II subunit expression were expressed as percent of control expression. To control for the selectivity of the CaM kinase II α subunit antisense treatment, we evaluated the effect of antisense treatment on the expression of similar molecular weight proteins and proteins with similar turnover rates. CaM kinase II β subunit levels were determined by immunoreactivity on Western and slot blots as described in detail (16). The structural proteins tubulin, microtubule-associated protein 2, and synapsin were determined by densitometric quantification of SDS/PAGE protein patterns as described (13, 15, 16). The antisense oligonucleotide directed against the CaM kinase II α subunit did not affect the expression of the CaM kinase II β subunit, or any of the structural proteins studied.
Determination of CaM Kinase II Activity.
Determination of CaM kinase II substrate phosphorylation was performed exactly as described (15). Standard phosphorylation reaction solutions contained 41 μg of protein, 60 μM Syntide II (Sigma), 10 mM MgCl2, 7 μM [γ-32P]ATP, 10 mM Pipes (pH 7.40), ±5 μM CaCl2, and ± 1 μg of calmodulin. Standard reactions were performed in a shaking water bath at 30°C. The phosphorylation reaction was initiated by the addition of Ca2+, allowed to continue for 1 min, and stopped by the addition of 20 μM EDTA. Assay solution (10 μl) was immediately blotted onto phosphocellulose filter paper, P-81 (Whatman), as described (15). Each reaction was quantitated in triplicate. P-81 filter paper was then washed three times in 50 mM phosphoric acid, rinsed with acetone, and allowed to air dry. Radioactive phosphate was quantitated by scintillation counting as described (15).
Electrophysiological Recording.
Whole-cell voltage clamp analysis was used to determine the effects of decreased CaM kinase II α subunit expression on neuronal excitability (17). For voltage-clamp analysis, cultures were placed on the stage of an inverted microscope (Nikon) and continuously perfused with base-recording solution containing 1 mM tetrodotoxin, 25 mM 2-amino-5-phosphonovaleric acid, 10 mM 6-cyano-7-nitroquinoxaline-2,3-dione, 145 mM NaCl, 2.5 mM KCl, 10 mM Hepes (pH 7.3), 1 mM MgCl2, 2 mM CaCl2, and 10 mM glucose. The osmolarity was adjusted to 325 mOsm with sucrose. Patch electrodes (2–4 MΩ resistance) with pipette solution containing 140 mM CsCl, 1 mM MgCl2, 10 mM Hepes (pH 7.2), and 1.1 mM EGTA were used and the solution was adjusted to 310 mOsm with sucrose. After the patch was established, the membrane potential was clamped at −50 mV and neuronal recording was performed with an Axopatch 1D amplifier.
Results
Inhibition of CaM Kinase II Expression.
Hippocampal neurons in culture were exposed to either antisense oligonucleotide specific for the mRNA for the CaM kinase II α subunit or missense (scrambled antisense) oligonucleotide. To determine the level of CaM kinase II α subunit expression, Western analysis of homogenates isolated from cultures after specific periods of time for exposure to either control or antisense oligonucleotides was performed (Fig. 1). In agreement with reports (13, 18), the brain maintains a high level of CaM kinase II protein level expression (Fig. 1A). Treatment of neuronal cultures with antisense oligonucleotide directed against the CaM kinase II α subunit resulted in a significant decrease (53.4 ± 6.0%, n = 4) in α subunit expression after 3 days of exposure (Fig. 1 A and B). In addition, inhibition of CaM kinase II α subunit expression resulted in a 35.4 ± 12.3% decrease in calcium-dependent substrate phosphorylation activity when compared with missense control (Fig. 1C; n = 4, P < 0.001, Student's t test).
Figure 1.
Inhibition of CaM kinase II α subunit mRNA translation resulted in decreased α CaM kinase II activity and subunit protein expression. Hippocampal neurons in culture were exposed to missense (scrambled antisense) or antisense oligonucleotides complementary to the mRNA to the α subunit of CaM kinase II for 3 days. Cultures were then harvested, homogenized, and resolved on SDS/PAGE (see Materials and Methods). (A) Representative Western analysis with a mAb directed against the α subunit shows a high level of expression under missense (control) treatment. Exposure to antisense oligonucleotides resulted in a significant decrease in CaM kinase α subunit expression. (B) Densitometry quantification of CaM kinase II α subunit expression. Western blots were digitized and compared with a standard CaM kinase II expression curve (see Materials and Methods). Western analysis showed that treatment with antisense oligonucleotides resulted in a significant decrease in subunit protein expression. Antisense oligonucleotide exposure for 3 days resulted in a 53.4 ± 6.0% inhibition in CaM kinase II α subunit expression when compared with missense-treated control cultures. (C) Inhibition of CaM kinase II α subunit expression resulted in decreased substrate phosphorylation. CaM kinase II-dependent phosphorylation of exogenously added Syntide II was significantly reduced (35.4 ± 12.3% compared with a missense control) after treatment with antisense oligonucleotide when compared with missense-treated control. **, P < 0.001, Student's t test, n = 4.
To determine the specificity of the antisense oligonucleotide treatment, we evaluated the effect of antisense oligonucleotide exposure on other proteins. Under the conditions used to inhibit CaM kinase II α subunit expression, we did not observe significant inhibition in protein expression of the CaM kinase II β subunit, nor to structural proteins including tubulin, microtubule-associated protein 2, or synapsin (see Materials and Methods). The data demonstrate a selective inhibition in CaM kinase II α subunit expression with antisense oligonucleotide exposure. To test for nonspecific effects of control oligonucleotide exposure, sense oligonucleotide to CaM kinase II α subunit and missense oligonucleotides to other protein sequences were tested. None of the missense oligonucleotides tested resulted in significant effects on CaM kinase II α subunit expression.
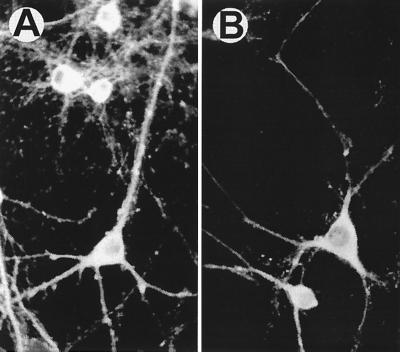
To determine the cellular location of the decrease in α subunit expression, immunocytochemical analysis was performed (Fig. 2). Immunoreactivity with a mAb directed against the α subunit demonstrated a high neuronal level of CaM kinase II expression and a low to nondetectable glial expression of the enzyme (Fig. 2A) (13). Expression of the α CaM kinase II subunit was observed in the neuronal soma, dendrites, dendritic spines, and nuclear regions. There was also a punctate level of expression along neuronal dendrites which most likely represented the location of synaptic contacts between neurons. Treatment with antisense CaM kinase II oligonucleotides resulted in a substantial decrease in α subunit immunoreactivity (Fig. 2B) that was observed throughout the neuronal soma and in the dendritic processes. The decrease in CaM kinase II α subunit expression observed in the neuronal dendrites demonstrated that the inhibition of kinase expression was observed in areas closely associated with synaptic contacts.
Figure 2.
Immunocytochemical evaluation of the decreased CaM kinase II α subunit expression. Neuronal cells in culture were treated with missense control or antisense oligonucleotides for 3 days. Indirect immunocytochemistry was performed with a mAb directed against the CaM kinase II α subunit (see Materials and Methods). Exposure to antisense oligonucleotides resulted in a substantial decrease in CaM kinase II α subunit immunocytochemical staining in cell soma, dendrites, dendritic spines, and nuclear regions. The figure shown was representative of 15 different experiments. Although immunocytochemical studies are not best suited for quantitative determinations, exposure of neurons in culture to antisense oligonucleotides produced a decrease in the α subunit of CaM kinase II protein expression in all cultures studied.
Effect of Inhibition of CaM Kinase II α Subunit Expression on Neuronal Excitability.
To test the effect of decreased CaM kinase II α subunit expression on neuronal activity, intracellular recordings were obtained in hippocampal cultures subjected to naive (no treatment), missense control, or antisense oligonucleotides. Recordings from naive or missense control cultures revealed a resting potential of ≈−60 mV (n = 10). Both naive and missense control neurons displayed normal synaptic activity, with intermittent action potentials (≈0.4 Hz) (Fig. 3A). The synaptic activity level observed in control cultures was not significantly different between missense-treated sham and naive cultures (17). To further evaluate the selectivity of oligonucleotide exposure to hippocampal neurons in culture, the sense sequence for the α subunit of CaM kinase II and missense sequences for other proteins including the γ-aminobutyric acid type A α2 receptor subunit, and transcription factors including Δfos B and SRF were tested with no significant effect on neuronal survival or membrane excitability (see Materials and Methods).
Figure 3.
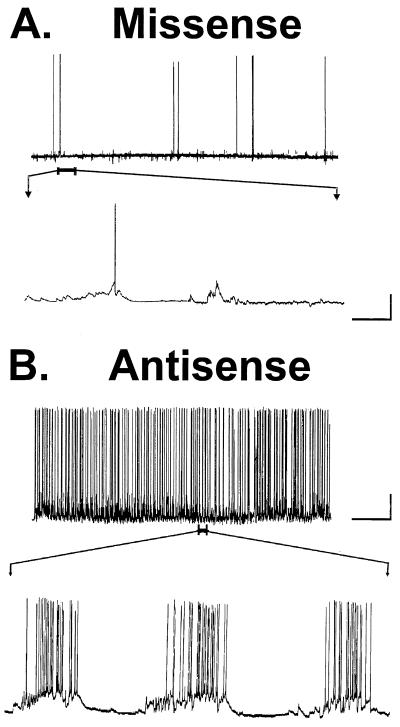
Inhibition of CaM kinase II α subunit expression results in epileptiform activity in hippocampal neurons in culture. (A) Representative intracellular recording from a missense-treated (control) neuron displaying background spontaneous synaptic activity (n = 10). An expanded record of spontaneous spike activity shows a single spike and no alteration of baseline polarity. (B) Representative intracellular recording from a neuron exposed to antisense oligonucleotides demonstrated epileptiform discharges and a high level of spontaneous synaptic activity (n = 10). An expanded record of three bursts shows numerous spikes superimposed on a large paroxysmal depolarization shift. [Standard bars: horizontal bar represents 2 min (slow sweep) and 2 s (fast sweep); and vertical bar represents 40 mV.]
In contrast to cultures treated with missense control oligonucleotides, neuronal cultures treated with antisense oligonucleotides against the CaM kinase II α subunit showed increased neuronal activity and epileptiform discharges (Fig. 3B). The frequency of neuronal firing increased to a maximum level averaging 15.8 ± 0.44 Hz for intermittent periods of time during epileptiform discharges in the antisense-treated cultures. These epileptiform discharges manifested a neuronal firing greater than 3 Hz and ranged in duration from 20 s to 1–2 min. Neuronal spike activity of a frequency greater than 3 Hz and lasting greater than 20 s was consistent with the definition of electrographic seizure activity. The increased neuronal excitability associated with inhibition of CaM kinase II α subunit expression was also associated with the development of paroxysmal depolarization shifts (21 ± 3 mV, range 15–28 mV) and multiple spike firing. The paroxysmal depolarization shifts were not observed in naive and missense-treated control cultures.
Inhibition of CaM Kinase II α Subunit Expression Correlates with Increased Neuronal Excitability.
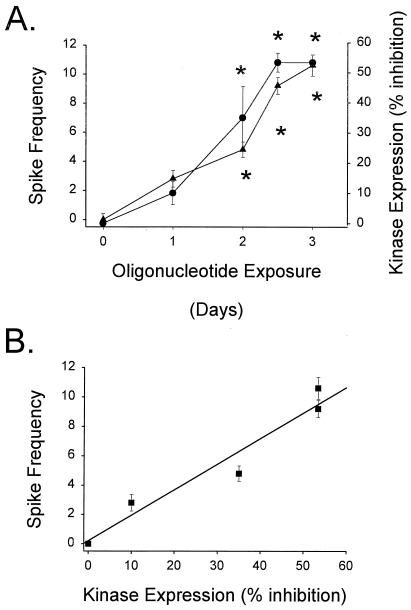
A significant decrease in CaM kinase II protein expression was not observed when tested within 24 h of antisense oligonucleotide exposure (Fig. 4A). After 24 h of oligonucleotide exposure, the CaM kinase II α subunit enzyme expression level was 90 ± 4.0% of control values (n = 4). Significant loss of CaM kinase II α subunit enzyme expression was not observed until 2 days of antisense oligonucleotide exposure (65 ± 10.8% control, n = 4). Maximal inhibition of CaM kinase II α subunit enzyme expression was not observed until after 60 h of antisense oligonucleotide exposure (46.6 ± 3.2% control). Approximately 53.4% inhibition of CaM kinase II α subunit enzyme expression was observed at all time points measured after 2.5 days of exposure. No significant decrease in α subunit expression was observed between missense (control)-treated and naive cultures.
Figure 4.
Inhibition of CaM kinase II α subunit expression correlates with increased neuronal excitability. (A) CaM kinase II α subunit antisense oligonucleotide exposure resulted in inhibition of CaM kinase II α subunit expression and increased spike frequency. Both spike frequency (●) and decreased immunoreactivity of CaM kinase II α subunit protein (▴) were measured as a function of time of CaM kinase II α subunit antisense oligonucleotide exposure in the same cultures. A significant increase in spike frequency and inhibition of CaM kinase II α subunit expression was not observed until 2 days of antisense oligonucleotide exposure, and maximal inhibition of kinase expression and spike frequency were observed after 2.5–3 days of oligonucleotide exposure. (*, P < 0.001 different from missense control, n = 4, one-way ANOVA). (B) Correlation between inhibition of CaM kinase II α subunit enzyme expression and increased neuronal excitability. Linear regression analysis contrasting inhibition of CaM kinase II α subunit expression and increased spike frequency was performed. Both inhibition of CaM kinase II α subunit expression and spike frequency were measured in the same cultures (data are expressed as means ± SEM for an n = 4). Inhibition of CaM kinase II α subunit expression correlated with increased spike frequency with an r2 = 0.945.
To determine whether or not inhibition of the CaM kinase II α subunit expression correlated with increased neuronal excitability, subunit expression was compared with multiple measures of neuronal excitability. Epileptiform activity did not appear until day 2 of antisense oligonucleotide exposure. Once observed, burst duration averaged 2.5 ± 0.2 s per episode. However, spike frequency during an epileptiform episode increased from no epileptiform discharges (day 0) to a maximal frequency of 10.62 spikes/s after 3 days of oligonucleotide exposure (Fig. 4A). The percent inhibition of CaM kinase II α subunit expression correlated with increased spike frequency with an r2 = 0.945 (Fig. 4B).
To further correlate the inhibition of CaM kinase II α subunit expression with increased neuronal activity, the CaM kinase II α subunit was compared with percent seizure duration and frequency of seizure episodes. The total epileptiform activity observed per unit time and the frequency of seizure episodes increased as increased inhibition of the CaM kinase II α subunit expression was observed (r2 = 0.837, each). Percent seizure duration of recorded time increased from 4% (day 1 of oligonucleotide exposure) to a maximum of 37.3% (day 2.5 of oligonucleotide exposure). After 3 days of antisense oligonucleotide exposure, percent seizure time was 34.9. The frequency of observed seizure episodes increased as the increased inhibition of CaM kinase II α subunit expression was observed. Seizure frequency increased from 1.2–1.4 seizure burst/min after 1–2 days of oligonucleotide exposure to 8–10 seizure burst/min after 2.5 days of oligonucleotide treatment.
Inhibition of CaM Kinase II α Subunit Expression Produces Synchronous Epileptiform Activity.
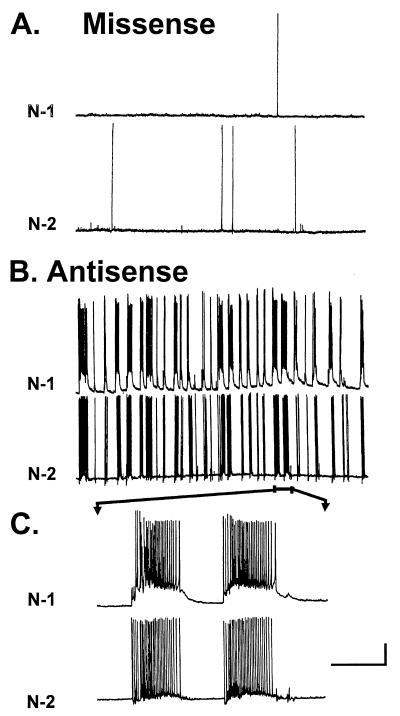
To demonstrate whether decreased CaM kinase II α subunit expression produced synchronous epileptiform discharges, multiple, simultaneous recordings from two individual pyramidal neurons were performed. Whole-cell voltage clamp recording was performed simultaneously on two pyramidal neurons in the field of vision (≈1 mm). In missense control cultures, background intrinsic activity analogous to naive neurons in culture was observed in both neurons (Fig. 5A). In addition, neuronal activity was random. Inhibition of CaM kinase II α subunit expression resulted in synchronous, epileptiform activity in numerous recordings (n = 15). Two-cell recordings demonstrated multiple, seizure-like events similar to that observed in single-cell recordings (Fig. 5B). However, the seizure-like events and synchronized spike firing were found to occur simultaneously in both pyramidal neurons demonstrating a network effect of inhibition of CaM kinase II α subunit expression-dependent increased neuronal excitability (Fig. 5C). Inhibition of CaM kinase II α subunit expression resulted in increased synchronous epileptiform discharges analogous to epileptiform seizure activity characteristic of models of epilepsy.
Figure 5.
Synchronous epileptiform discharges in simultaneous voltage-clamp recordings from two neurons. Whole-cell voltage-clamp recordings were obtained from two neurons by dual electrodes. (A) Representative simultaneous recordings from missense oligonucleotide-treated neurons in culture displayed nonsynchronous background neuronal activity (n = 10). (B) Representative simultaneous recordings from neurons treated with antisense α CaM kinase II oligonucleotides displayed synchronous, epileptiform discharges (n = 10). (C) Faster sweep speed of simultaneous recordings from B revealed a prolonged duration of synchronous discharge activity, lasting between 1–3 s and demonstrating a high degree of synchronization. N-1, neuron 1; and N-2, neuron 2. (Standard bars: horizontal bar represents 20 s (slow sweep) and 2 mV (fast sweep); and vertical bar represents 20 mV.)
Discussion
The studies presented in this manuscript describe the net effect of inhibition of CaM kinase II α subunit expression on neuronal excitability. CaM kinase II activity was reduced by inhibition of protein expression of the α subunit. Treatment with oligonucleotides directed against the CaM kinase II α subunit was selective and did not affect the expression of either the CaM kinase II β subunit or other similar molecular weight proteins studied. Once the decrease in CaM kinase II α subunit expression was manifest, neuronal excitability increased. The increased neuronal excitability was characterized by repeated, synchronous epileptiform activity. Furthermore, the magnitude of inhibition of CaM kinase II α subunit expression correlated with three measures of increased neuronal excitability. Missense oligonucleotide exposure did not have any effect on neuronal excitability at any point studied. Thus, selectively decreasing CaM kinase II activity resulted in increased neuronal excitability and spontaneous, recurrent epileptiform discharges.
The data presented in this study demonstrate that exposure of neurons in culture to oligonucleotides complimentary to the CaM kinase II α subunit mRNA (at nucleotides 33–50) resulted in inhibition of the subunit expression. The decreased expression of CaM kinase II was observed immunocytochemically in the soma, neuronal processes, and synaptic regions (Fig. 2). CaM kinase II is highly expressed in neurons relative to glial cells (4, 19, 20). The α subunit of CaM kinase II is highly enriched also in the synaptic regions of excitatory neurons, and is homologous to the major postsynaptic density protein (21–23). Thus, CaM kinase II is highly expressed in synaptic regions, in close vicinity to neurotransmitter receptors. To establish such a high level of dendritic expression, it has been shown that neurons synthesize the α subunit of CaM kinase II in the dendritic processes in situ (24). Therefore, inhibition of CaM kinase II α subunit expression by inhibition of mRNA translation would be expected to be observed in the dendritic regions. In addition, loss of CaM kinase II activity in this region of high neurotransmitter receptor expression would be expected to significantly alter neuronal excitability (25).
Transgenic mice that express a null mutation for the α subunit of CaM kinase II, and manifest decreased enzyme activity, demonstrate a decreased level of long-term potentiation and decreased performance on behavioral learning models (26, 27). The original observations described a decrease in synaptic potentiation and spatial learning in genetically mutated mice when compared with nonmutated mice. These mice also exhibited an aberrantly high neuronal excitation level and displayed limbic epilepsy (11). The findings that α CaM kinase II knockout mice displayed increased neuronal excitability and limbic epilepsy is supported by the findings in the present study. However, in the whole-animal knockout model, it is more difficult to determine whether the knockout of CaM kinase II expression directly results in seizure activity in the limbic structures or whether lack of CaM kinase II subunit expression alters the formation of correctly functioning synaptic connections during development. The present study demonstrates that significant seizure-like events and epileptiform activity occurs in neurons with direct inhibition of CaM kinase II α subunit expression. The epileptiform activity produced by inhibition of CaM kinase II α subunit expression occurred after a stable level of neuronal development in culture was achieved and after synaptic contacts were established. Thus, in whole-animal models as well as the present study, genetically induced decreased CaM kinase II α subunit expression resulted in increased neuronal excitability and epileptiform discharges.
The present investigation, along with studies in the null mutant mouse model (11), demonstrate that loss of CaM kinase II activity may be involved in epileptogenesis. A decrease in CaM kinase II activity has been observed in multiple models of increased neuronal excitability including kindling (28, 29), spontaneous seizure activity (30), status epilepticus (9, 10, 31), and excitotoxicity (13, 15). In addition, studies have demonstrated a high correlation between inhibition of CaM kinase II activity and increased severity of seizure activity in a model of status epilepticus (9). In the present study, inhibition of CaM kinase II α subunit expression correlated with three measures of neuronal excitability: spike frequency during seizure activity, frequency of seizure episodes, and percent seizure duration. As inhibition of CaM kinase II α subunit expression increased, so did the severity of each of these measures of neuronal excitability. Thus, CaM kinase II may play a regulatory role and act to modulate neuronal excitability (32). In this capacity, loss of CaM kinase II activity would result in increased neuronal excitation which would eventually result in seizure activity and the development of epileptiform discharges.
The present study demonstrates that long-lasting, significant inhibition of CaM kinase II activity produced by selective inhibition of kinase mRNA translation results in increased neuronal excitability in hippocampal neuronal cells in culture. Because the hippocampus plays an important role in regulating brain excitability, the data support the hypothesis that modulation of CaM kinase II activity will result in significant alteration of neuronal excitability. The precise cellular and molecular mechanisms whereby inhibition of CaM kinase II activity plays a role in the long-term plasticity changes associated with epileptogenesis will be the subject of future studies.
Acknowledgments
We thank Drs. J. Travis Parsons and Robert Tombes for their helpful discussions in the preparation of the manuscript, and Aniruddha Rana for his assistance in the figure production for this study. This work was supported by National Institute of Neurological Disorders and Stroke Awards R01-NS23350 and PO1-NS25630 to R.J.D., the Sophie and Nathan Gumenick Neuroscience Research Fund, and the Milton L. Markel Alzheimer and Neuroscience Research Fund.
Abbreviation
- CaM kinase II
calcium- and calmodulin-dependent kinase II
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.080071697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.080071697
References
- 1.Lothman E W, Bertram E H, III, Stringer J L. Prog Neurobiol. 1991;37:1–82. doi: 10.1016/0301-0082(91)90011-o. [DOI] [PubMed] [Google Scholar]
- 2.Hauser W A. In: Status Epilepticus: Frequency, Etiology and Neurological Sequela. Delgado-Escueta A V, Wasterlain C, Treiman D M, Porter R J, editors. New York: Raven; 1983. pp. 3–14. [PubMed] [Google Scholar]
- 3.Perlin J B, DeLorenzo R J. In: Calcium and Epilepsy. Pedley T A, Meldrum B S, editors. Vol. 5. New York: Churchill Livingstone; 1999. pp. 15–36. [Google Scholar]
- 4.Churn S B. Adv Neuroimmunol. 1995;5:241–259. doi: 10.1016/0960-5428(95)00016-u. [DOI] [PubMed] [Google Scholar]
- 5.Chin J H, Buckholz T M, DeLorenzo R J. Prog Brain Res. 1985;63:169–184. doi: 10.1016/S0079-6123(08)61982-2. [DOI] [PubMed] [Google Scholar]
- 6.Braun A P, Schulman H. Annu Rev Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 7.Wasterlain C G, Farber D B. Proc Natl Acad Sci USA. 1984;81:1253–1257. doi: 10.1073/pnas.81.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Churn S B, Anderson W W, DeLorenzo R J. Epilepsy Res. 1991;9:211–217. doi: 10.1016/0920-1211(91)90054-j. [DOI] [PubMed] [Google Scholar]
- 9.Perlin J B, Churn S B, Lothman E W, DeLorenzo R J. Epilepsy Res. 1992;11:111–118. doi: 10.1016/0920-1211(92)90045-u. [DOI] [PubMed] [Google Scholar]
- 10.Bronstein J, Farber D, Wasterlain C. Neurochem Res. 1988;13:83–86. doi: 10.1007/BF00971859. [DOI] [PubMed] [Google Scholar]
- 11.Butler L S, Silva A J, Abeliovich A, Watanabe Y, Tonegawa S, McNamara J O. Proc Natl Acad Sci USA. 1995;92:6852–6855. doi: 10.1073/pnas.92.15.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banker G A, Cowan W M. J Comp Neurol. 1977;187:469–494. doi: 10.1002/cne.901870302. [DOI] [PubMed] [Google Scholar]
- 13.Churn S B, Sombati S, Taft W C, DeLorenzo R J. Stroke (Dallas) 1993;24:271–278. doi: 10.1161/01.str.24.2.271. [DOI] [PubMed] [Google Scholar]
- 14.Abney E R, Bartlett P P, Raff M C. Dev Biol. 1981;83:301–310. doi: 10.1016/0012-1606(81)90476-0. [DOI] [PubMed] [Google Scholar]
- 15.Churn S B, Limbrick D, Sombati S, DeLorenzo R J. J Neurosci. 1995;15:3200–3214. doi: 10.1523/JNEUROSCI.15-04-03200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Churn S B, Yaghmai A, Povlishock J, Rafiq A, DeLorenzo R J. J Cereb Blood Flow Metab. 1992;12:784–793. doi: 10.1038/jcbfm.1992.109. [DOI] [PubMed] [Google Scholar]
- 17.Sombati S, DeLorenzo R J. J Neurophysiol. 1995;73:1706–1711. doi: 10.1152/jn.1995.73.4.1706. [DOI] [PubMed] [Google Scholar]
- 18.Erondu N E, Kennedy M B. J Neurosci. 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulman H. Curr Opin Cell Biol. 1993;5:247–253. doi: 10.1016/0955-0674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 20.Baitinger C, Alderton J, Poenie M, Schulman H, Steinhardt R A. J Cell Biol. 1990;111:1763–1773. doi: 10.1083/jcb.111.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho K O, Hunt C A, Kennedy M B. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- 22.Kelly P T, McGuinness T L, Greengard P. Proc Natl Acad Sci USA. 1984;81:945–949. doi: 10.1073/pnas.81.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldenring J R, McGuire J S, Jr, DeLorenzo R J. J Neurochem. 1984;42:1077–1084. doi: 10.1111/j.1471-4159.1984.tb12713.x. [DOI] [PubMed] [Google Scholar]
- 24.Torre E R, Steward O. J Neurosci. 1992;12:762–772. doi: 10.1523/JNEUROSCI.12-03-00762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeLorenzo R J. Ann Neurol. 1984;16,Suppl.:S104–S114. doi: 10.1002/ana.410160716. [DOI] [PubMed] [Google Scholar]
- 26.Silva A J, Paylor R, Wehner J M, Tonegawa S. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- 27.Silva A J, Stevens C F, Tonegawa S, Wang Y. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- 28.Goldenring J R, Wasterlain C G, Oestreicher A B, de Graan P N, Farber D B, Glaser G, DeLorenzo R J. Brain Res. 1986;377:47–53. doi: 10.1016/0006-8993(86)91189-3. [DOI] [PubMed] [Google Scholar]
- 29.Bronstein J M, Micevych P, Popper P, Huez G, Farber D B, Wasterlain C G. Brain Res. 1992;584:257–260. doi: 10.1016/0006-8993(92)90903-m. [DOI] [PubMed] [Google Scholar]
- 30.Blair R E, Churn S B, Sombati S, Lou J K, DeLorenzo R J. Brain Res. 2000;851:54–65. doi: 10.1016/s0006-8993(99)02100-9. [DOI] [PubMed] [Google Scholar]
- 31.Kochan L D, Churn S B, Omojokun O, Rice A, DeLorenzo R J. Neuroscience. 2000;95:735–743. doi: 10.1016/s0306-4522(99)00462-5. [DOI] [PubMed] [Google Scholar]
- 32.Chapman P F, Frenguelli B G, Smith A, Chen C M, Silva A J. Neuron. 1995;14:591–597. doi: 10.1016/0896-6273(95)90315-1. [DOI] [PubMed] [Google Scholar]