Abstract
The largest part of the primate prefrontal cortex has no homologue in other mammals. Accordingly, it probably confers some advantage that other mammals either lack or attain another way. Yet this advantage remains enigmatic. Not so for other parts of cortex. For example, certain visual areas encode, represent and store knowledge about objects. By analogy, perhaps the primate prefrontal cortex encodes, represents and stores knowledge about behaviors, including the consequences of doing (or not doing) something in complex and challenging situations. The long list of functions often attributed to prefrontal cortex may all contribute to knowing what to do and what will happen when rare risks arise or outstanding opportunities knock.
Keywords: frontal lobe, cytoarchitectonics, comparative neuroanatomy, working memory, cognition
The earliest experiments on the frontal areas were those of the French neurologist Flourens (1824), who, on the basis of ablation studies…attributed to the frontal lobes, acting in harmony with the rest of the brain, the higher perceptual, associative, and executive functions of the mind [1, p. 447].
Flourens got it right, in a way, yet his experiment and conclusions had no validity whatsoever. He studied the effect of cerebral ablations on a hen, and she did not have frontal lobes — either before or after the lesion. Research on the frontal lobes, especially the prefrontal cortex, has always been thus, and every generation seems to reach conclusions much like those that Flourens advocated in 1824 (the year of Broca's birth). The doctrine of the day seems intriguing, and authorities announce the problem solved, or nearly so: for a while, then dissatisfaction develops and the cycle begins anew. When the puzzle of prefrontal cortex is fairly solved, the account will inevitably seem familiar, even tired, if only because every possibility has probably been propounded.
Flourens' misconception underscores an important principle for understanding prefrontal cortex: combining findings from different species requires a serious consideration of the relevant homologies. This opinion piece takes up that topic first, and at heart it involves a fundamental question: What is prefrontal cortex? The second topic concerns the distinction between cognitive processes and knowledge, and it involves a similarly fundamental question: What does prefrontal cortex do? Grafman and his colleagues [2] have noted that most ideas about prefrontal cortex function involve cognitive processes such as working memory, retrieval of long-term memories, top-down attention, and so forth, rather than a particular kind of knowledge stored in long-term memory. This is a strange state of affairs. Other parts of the cerebral cortex encode, represent and store specialized kinds of knowledge, which can be used in several cognitive processes. For example, high-order visual areas such as the inferotemporal and perirhinal cortex store knowledge about objects [3-6]. When we ask — “What knowledge does the prefrontal cortex store?” — an equally straightforward answer might emerge.
What is the Prefrontal Cortex?
Most knowledge about the prefrontal cortex comes from research on rodents, various kinds of monkeys (mainly macaques), and the peculiar primate that has spilled so much ink over its own prefrontal cortex. Synthesis of this knowledge should promote our understanding of the prefrontal cortex, but an important misconception often hampers this undertaking. Although rarely expressed as such, common opinion holds that the frontal cortex of rodents is essentially a replica-in-miniature of the primate frontal cortex. Many experts assume, and some have forcefully argued [7], that rodents have a homologue of the areas collectively called the “granular” prefrontal cortex in monkeys, apes and humans. I find an alternative opinion to be more persuasive. It holds that primates — and primates alone — have evolved certain new areas, and that these new areas dominate their frontal lobe (Figs. 1 and 2).
Figure 1.
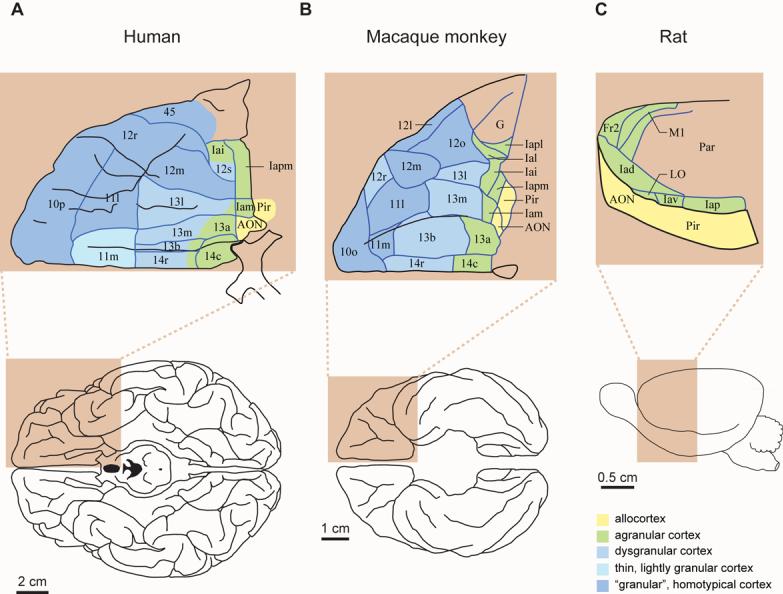
Top: Architectonic maps of the orbitofrontal cortex in humans (A) and macaque monkeys (B), and lateral frontal cortex in rats (C). Bottom: ventral views of a human (A) and monkey (B) brain; lateral view of a rat brain (C). The “granular” areas appear in blue; agranular areas in green; allocortical areas in yellow. Note that the “granular” prefrontal cortex dominates the frontal lobe of primates. Abbreviations: AON, anterior olfactory “nucleus”; Fr2, second frontal area; I, insula; LO, lateral orbital area; M1, primary motor area; Par, parietal cortex; Pir, Piriform cortex; l, lateral; m, medial; o, orbital; r, rostral; c, caudal; i, inferior; p, posterior; s, sulcal; v, ventral. a has two meanings: in Ia, it means agranular; in 13a, it distinguishes that area from area 13b. Architectonics from Öngür et al. [20] (A), Carmichael and Price [21] (B), and Palomero-Gallagher and Zilles [22] (C). All parts of the figure were adapted from their source.
Figure 2.
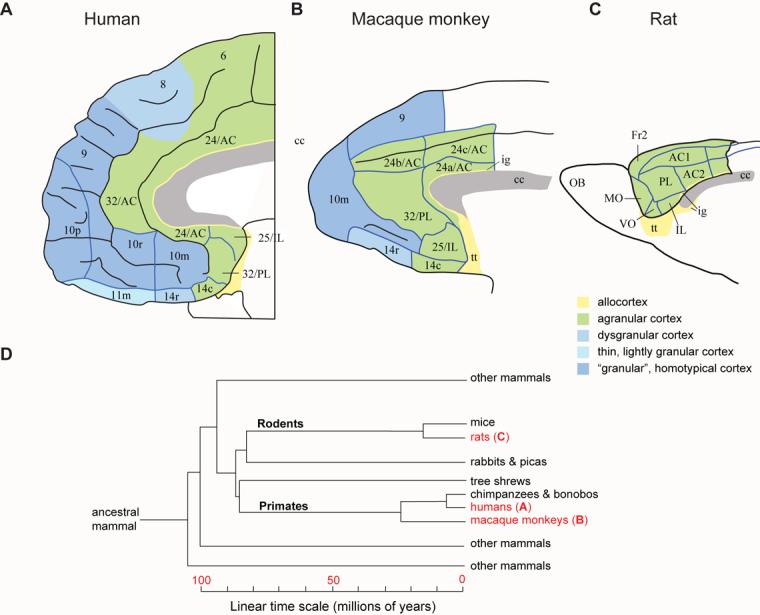
A–C: Architectonic maps of the medial frontal cortex, corresponding to Figure 1A–C. Abbreviations and color code as in Fig. 1, plus: AC, anterior cingulate area; cc, corpus callosum; IL, infralimbic cortex; MO, medial orbital area; PL, prelimbic cortex; VO, ventral orbital area; numbers indicate cortical fields, except that after certain areas, such as Fr2 and AC1, they indicate subdivisions of cortical fields. D: Simplified cladogram of mammals, indicating the divergence times of selected groups. The letters (A-C) after the groups in red refer to the corresponding parts of Figures 1 and 2. Time scale in millions of years before the present. Architectonics from Öngür et al. [20] (A), Carmichael and Price [21] (B), and Palomero-Gallagher and Zilles [22] (C). A-C were adapted from their source.
In a sense, this idea has been around for a long time, but Todd Preuss first dealt with this issue convincingly by placing it in a contemporary, comparative perspective [8,9]. In essence, Preuss concluded that rodents lack a “granular” prefrontal cortex and have no areas that need be called prefrontal. First published in 1995, his thesis has distressed neuroscientists who sometimes suppose that Preuss's perspective devalues or demeans rodent research. Although I understand that sentiment, there is no need to think of his conclusions that way. If rodents lack certain areas that appeared during primate evolution, they nevertheless share many important frontal areas that evolved earlier, in ancestors common to both rodents and primates (Fig. 2D); if some parts of the frontal lobe lie beyond the reach of rodent research, much remains within its grasp [8,10]; and, finally, labeling the shared areas prefrontal does no harm when authors acknowledge additional, unshared areas in primates.
Before presenting a picture of prefrontal cortex in a comparative context, let me dispense with a few red herrings. Traditionally, three arguments have underpinned the view that the rodent frontal cortex is essentially a replica-in-miniature of the primate frontal cortex: both have inputs from the mediodorsal (MD) nucleus of the thalamus, both receive especially prominent dopamine projections, and lesions of both cause comparable deficits in spatial working memory. The first argument fails, however, because MD's outputs extend far beyond the “granular” prefrontal cortex in primates [11]. MD inputs are a feature of “granular” prefrontal cortex, but not a diagnostic feature. The second argument falls short for a similar reason: dopamine inputs distribute to much of the primate cerebral cortex and are by no means confined to — or even notably prominent in — the “granular” prefrontal cortex [12-14]. As for lesion-induced spatial-memory deficits, in my opinion the deficits in rodents and primates resemble each other only superficially. In the most common task, called the spatial delayed-response (DR) task, animals must choose a transiently cued location after an imposed delay interval. Complete removal of a monkey's prefrontal cortex consistently causes performance to persist at chance level for a protracted period. In a study that used a 5-s delay interval, two of four monkeys performed at chance level for 1000 trials, when testing stopped [15]. In another, which used a 4-s interval, three of four monkeys remained at chance levels for 320 trials. This complete failure persisted in subsequent testing with 2-s intervals [16]. Monkeys trained after prefrontal lesions failed to learn the DR task at all, even after 500 trials with a brief, 1-s interval [17]. In contrast, rats trained after medial frontal lesions reached 90% correct performance at a median interval of 2 s, and they did so in just 140 trials: a respectable, if subpar, achievement [18]. In another test of spatial memory, rats returned to normal performance just 60 trials after medial frontal lesions [19]. Thus, none of the three traditional arguments stand up to scrutiny.
Cytoarchitectonics
Figures 1 and 2 show the basic, architectonic types of cerebral cortex, from Price and Zilles [20-22]. Architectonics seems a dark art to many, but the distinction between the basic types of cortex is not so difficult to discern. In the primate frontal lobe, the main types are agranular cortex, which lacks an internal granular layer (layer 4), homotypical cortex, which has a conspicuous layer 4, and dysgranular cortex, which has a subtle layer 4. Although neuroanatomists often refer to certain cortical areas in primates as “granular” prefrontal cortex, this is a misnomer: these areas have either homotypical or dysgranular architecture. They are “granular” only in a frontal context because frontal cortex lacks a true granular cortex, also known as konicortex (e.g., striate visual cortex). Hence the quotation marks around “granular” throughout this article.
As Figures 1 and 2 illustrate, the rodent frontal cortex lacks any “granular” frontal areas. In primates, the “granular” areas include dorsolateral prefrontal cortex (area 46), dorsal and medial prefrontal areas (different parts of area 9), ventral prefrontal cortex (areas 12, 44, 45 and 47), frontal pole cortex (area 10), and rostral orbitofrontal cortex (parts of areas 11, 13 and 14). The agranular parts of frontal cortex are, unlike “granular” ones, shared by mammals generally. Figures 1 and 2 thus illustrate a parsimonious notion: the agranular frontal cortex (green) of rodents is homologous to the agranular frontal cortex of primates and not to the “granular” prefrontal cortex (blue), which appeared some time during primate evolution. At a finer level, they yield an equally parsimonious view: the infralimbic (IL), prelimbic (PL), agranular insular (Ia), agranular orbital, and anterior cingulate (AC) areas of rodents each have a homologue in primates. Beyond architectonics, both topology and connectivity point to the same conclusions.
Topology
An examination of the spatial arrangement of cortical areas confirms the homology of agranular areas in rodents and primates. Note the topological relationship of agranular insular area (Ia) with the allocortex, a three-layered kind of cortex (yellow in Fig. 1). Ia borders allocortical areas composed of piriform (olfactory) cortex and the anterior olfactory “nucleus” (another allocortical area) in both rodents and primates. Figure 2 shows two small, obscure allocortical areas (yellow), which also adjoin agranular cortex. The agranular frontal cortex can be thus understood as a group of transition areas adjacent to allocortex [22]. In primates, the “granular” prefrontal cortex borders these transition areas, but does not adjoin allocortex. Additional agranular areas include motor areas (e.g., M1 and Fr2 in Figs. 1 and 2).
Corticostriatal projections
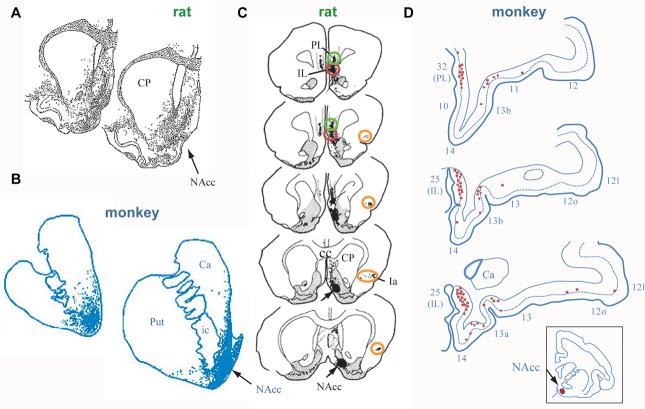
Corticostriatal organization provides further support for the idea that the frontal agranular areas in rodents are homologous to those in primates [23-28]. Homologous areas likely (although not certainly) project to homologous parts of the striatum. If the “granular” prefrontal cortex of primates lacks a homologue in rodent cortex, then these areas likely project to parts of the striatum that rodents also lack. In accord with this idea, neuroanatomists have recognized a “ventral shift” of corticostriatal projections (from a given area) in primates relative to rodents [28]. So an area that projects, for example, about midway between the dorsal and ventral limits of the striatum in rats, as some orbitofrontal areas do, sends its corticostriatal projections relatively more ventrally in primates. Summary diagrams do not always capture this feature, so Figure 3 uses primary data to illustrate the projections from infralimbic (IL) and prelimbic (PL) cortex to the nucleus accumbens, a major part of the ventral striatum. IL (roughly area 25) and PL (roughly area 32) of both rats and monkeys project to the shell of the nucleus accumbens. The agranular insular cortex, Ia, also sends a corticostriatal projection to and near the ventral striatum in both rats and monkeys, as do other agranular frontal areas in monkeys, such as areas 14c and 13a of the orbital frontal cortex (see Fig. 1B). In contrast, the “granular” prefrontal areas send little or no projection to the ventral striatum, targeting more dorsal parts of the striatum instead [23,25,26].
Figure 3.
Primary data on certain corticostriatal projections. The infralimbic cortex (roughly area 25 in primates) sends projections to the shell of the nucleus accumbens and nearby striatum in both rats (A, C) and macaque monkeys (B, D), as demonstrated by both anterograde (A, B) and retrograde (C, D) axonal tracing methods. A, B. Stippling indicates the termination zones of corticostriatal projections. C. Red ovals surround infralimbic corticostriatal cells (filled black circles) that project to the shell of the nucleus accumbens, with the arrow pointing to the injection site for the tracer. Prelimbic cortex (green ovals), roughly subgenual area 32 in primates, and agranular insular cortex (orange ovals) also send a corticostriatal projection to the shell of nucleus accumbens. D. Red circles show the locations of corticostriatal cells projecting to the nucleus accumbens, with the injection site shown by the red spot in the inset. The numbers indicate cortical areas as in Figures 1 and 2. All parts of the figure were adapted from their source. A from Berendse, H.W. et al., Journal of Comparative Neurology, Vol. 316, No. 3, 1992, pp. 314-347, Copyright 1992, reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc. [27]. B from Haber, S.N. et al., Journal of Neuroscience, Vol. 26, 2006, pp. 8368-8376, reprinted with permission of the Journal of Neuroscience [26]; C from Brog, J.S. et al., Journal of Comparative Neurology, Vol. 338, No. 2, 1993, pp. 255-278, Copyright 1993, reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc. [24]. D from Haber, S.N. et al, Journal of Neuroscience, Vol. 15, 1995, pp. 4851-4867, reprinted with permission of the Journal of Neuroscience [25].
The shared frontal areas
For the rest of the story, see Preuss [8,9], but an argument based on cortical types, topology and corticostriatal organization should do. These three lines of evidence have high reliability because they depend on fundamental features of the neocortex, its location relative to other kinds of cortex, and its topographic projections to a consistent target of cortical projections, the striatum. Others disagree [7,10], but in my opinion rodents, monkeys, humans and other mammals share part of their frontal cortex, but only the agranular part (Figs. 1 and 2). Attaching the name prefrontal to the shared part does not present a problem, as long as authors resist using ideas about the primate “granular” prefrontal cortex to interpret results from species that lack such areas. Changing the question from ‘Do rodents have a prefrontal cortex?’ to ‘What kinds of frontal cortex do rodents and primates share?’ should advance our understanding these areas, an inheritance from early mammals (Fig. 2D). Although their long, separate evolutionary history means that the shared areas will have diverged in organization and function, in both rodents and primates they receive relatively direct olfactory, gustatory and visceral inputs, and many of their outputs control the autonomic nervous system. These characteristics further support the homologies outlined here and suggest that the shared frontal areas function in deep-seated mammalian behaviors. Systems neuroscience can anticipate no greater triumph than a comprehensive understanding of their contribution to mammalian fitness.
What does the Prefrontal Cortex Do?
So if the “granular” prefrontal cortex appeared some time during the evolution of primates, what does it do? This question has been asked for a long time, and reviews abound. Two are especially useful in my opinion: one based mainly on neuroimaging and clinical neuropsychology, the other on neurophysiology and neuroanatomy [2,29]. The former focuses on the knowledge that various parts of “granular” prefrontal cortex store, and both reject the popular idea that the chief function of prefrontal cortex involves a particular cognitive process, working memory.
Mnemonic monomania
Jacobsen discovered that damage to the prefrontal cortex produces problems with what we now call working memory. In his experiments, monkeys and chimpanzees with bilateral removals of the “granular” prefrontal cortex failed to retrieve food from one of two opaque cups if the food had been hidden for just a few seconds. Normal animals could find the food after delays of 5 minutes or more. In time, this finding and its many replications [e.g., 15-17] came to dominate thinking about prefrontal cortex function. During the 1980s and 1990s, most studies on the prefrontal cortex focused on working memory to the exclusion of its other functions: short-term mnemonic monomania took hold. Although some experts held more nuanced views, very few studies tested the working-memory theory of prefrontal cortex critically, and one that did contradicted it fairly convincingly [30]. Neuroimaging studies at first emphasized working memory, then other ideas began to intrude [31]. Specifically, neuroimaging experiments pitting attentional selection against the maintenance of information in working memory showed a predominance of attentional functions for the dorsolateral prefrontal cortex [32], and other studies also stressed attentional functions, such as monitoring items in short-term memory [33].
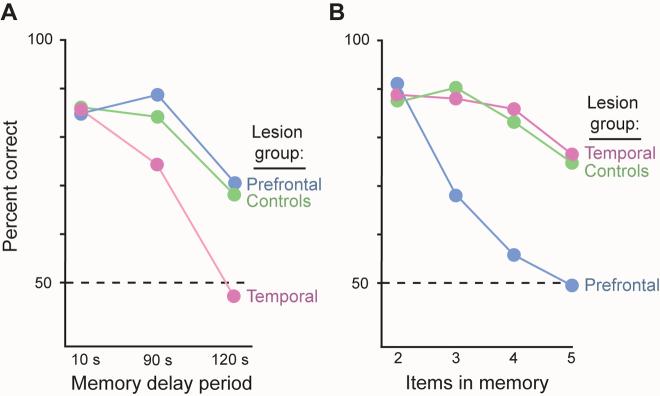
Petrides [34] did an especially crucial experiment in macaque monkeys. He manipulated two variables, the duration of a memory period and the number of items in working memory. After bilateral removal of the dorsal prefrontal cortex, the monkeys showed large impairments in remembering a list of items (Fig. 4B, blue), but showed no deficit when a prolonged memory period increased working-memory demands (Fig. 4A, blue). After bilateral removals of the anterior inferotemporal cortex, Petrides obtained the opposite result (Fig. 4A, B, pink). The latter finding shows, sensibly enough, that a sensory area known to represent and store object information in long-term memory — high-order visual cortex [3-6] — plays a crucial role in maintaining information about objects in short-term memory. The “granular” prefrontal cortex performs a very different function. Petrides [34, p. 7496] concluded that its role “does not lie in the maintenance of information per se” and that sensory areas, not prefrontal ones, make the most important contribution to working memory.
Figure 4.
A. Percent correct choices in a memory task with three delay intervals. Monkeys with lesions of the dorsal prefrontal cortex (blue) perform like intact monkeys (green) when they must remember a sample object for 2 minutes. Monkeys with lesions of the anterior inferotemporal cortex (pink), a high-order visual area, show profound deficits in working memory, declining to chance levels of performance (dashed line) on this memory test at 120 s delays. B. Monkeys with lesions of the anterior inferotemporal cortex perform like intact monkeys when required to remember 2–5 objects. Monkey with dorsal prefrontal lesions, in contrast, show profound deficits, declining to chance performance when required to remember 4 or 5 objects. Adapted from Petrides [34].
Petrides' findings for dorsal parts of “granular” prefrontal cortex, along with the results of Rushworth and Passingham for ventral parts [30], intensified doubts about interpretations of neurophysiological results from the “granular” prefrontal cortex in terms of working memory. Neurophysiological results that had been interpreted as memory signals [35] allowed other interpretations, usually because crucial variables remained uncontrolled and often unmonitored. No one doubted that prefrontal cortex cells show sustained activity changes during memory periods, a finding that dates from the beginning of behavioral neurophysiology. Many cognitive processes, however, operate simultaneously during such memory periods, and these tasks typically call upon many kinds of knowledge. Cues to remember a given location, for example, also capture attention and call up stored knowledge. Figure 5 shows our experimental approach to this problem. On each trial, monkeys looked at a fixation point on a video screen, and later a circle appeared either at the top, bottom, left or right of the screen. The monkeys had to maintain continuous central fixation even after the circle began to move and came to rest elsewhere (Fig. 5A). This task required that the monkeys remember the circle's initial location on each trial because a later cue could require a saccadic eye movement there. Simultaneously, the monkey had to attend to the circle's current location because a change in its brightness triggered a saccade. By independently controlling attention and memory in this way, we found that attentional signals predominate over memory signals in the dorsolateral prefrontal cortex (Fig. 5B,C) [36]. Neuroimaging [33,37-39] and other [40] research in humans yield the same conclusion.
Figure 5.
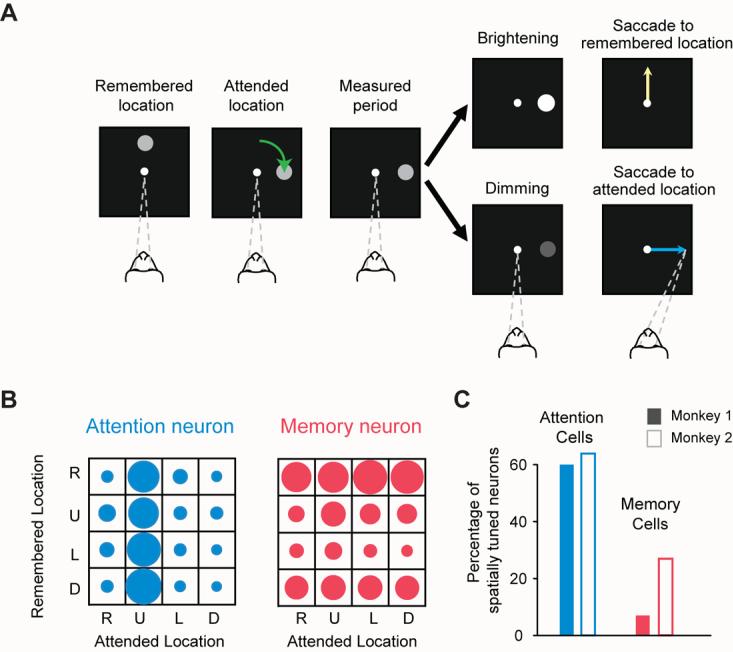
Predominance of attention coding over working-memory coding in the dorsolateral prefrontal cortex. A. Task use to distinguish attention signals from memory signals. The monkey began each trial by fixating a central spot on a video screen (dashed lines). Next, a circle appeared in one of four places: right (R), up (U), left (L), or down (D) from the fixation spot. As the monkey maintained central fixation, the circle revolved around the central spot (green arrow), coming to rest at one of those same four locations. The original location of the circle needed to be remembered because it could be the target of a future eye movement. Later, the circle either brightened or dimmed as a “go” cue, which triggered a saccadic eye movement. If it brightened (top fork), then the monkeys had to make a saccadic eye movement to fixate the remembered location (yellow arrow). In this example, the monkey had to remember the “up” location. If the circle dimmed as the “go” cue (bottom fork), then the saccade had to be made to the current location of the circle (blue arrow). The circle's current location had to be attended because its change in brightness not only instructed the monkey about which place to look next, but also when to do so. B. The activity of two neurons during the measured period, an 800-ms interval before the “go” cue. In each 4 × 4 display, the diameter of each filled circle is proportional to a neuron's firing rate for one combination of attended and remembered locations. The neuron in blue showed its greatest activity when the monkey attended to the “up” location (second column), regardless of the location stored in working memory (rows). The neuron in red showed its highest activity when the monkey remembered the R location (top row), regardless of where the monkey attended (columns). C. Most cells in the dorsolateral prefrontal cortex are like the blue one in B. Adapted from Lebedev et al. [36].
Instead of pointing to a single function such as working memory, neurophysiological studies indicate that the “granular” prefrontal areas, when considered collectively, perform many functions. They contribute to prospective coding [41,42], sensory working memory [43,44], attention to objects, places, actions and intentions [36,45,46], response inhibition [47], the categorization of objects, sounds and event sequences [48-50], the selection, generation and sequencing of multiple actions [51-53], problem-solving strategies [54], and the selection of behavior-guiding rules or task sets [55-57]. These concepts include preparatory set, processing events across time gaps [58-60], decision making [61], and goal selection [62], as well as the ideas emphasized in contemporary neuroeconomics [63] such as reward expectation, the valuation and revaluation of actions and predicted outcomes, and the effect of reinforcement history on decisions, choices, and actions.
Although that list — by no means exhaustive — comes from behavioral neurophysiology in monkeys, research on humans leads to a similar view. Dorsal and rostral prefrontal areas are thought to guide behavior based on rule and strategy representations, including rule-based task management and action selection [64]. Orbital areas seem to function more in value- and object-based action selection [65,66]. Ventral areas (often termed ventrolateral) have been linked to retrieval of semantic knowledge, speech and language. Research on the “granular” prefrontal cortex of humans emphasizes such processes as updating, manipulating and strategic control of information stored in short-term memory [33,67], establishing task sets and rules [68], and mediating hierarchies of behavioral control, behavioral inhibition, cognitive control in social situations [69], and other processes too numerous to mention. Thus, the prefrontal cortex seems to contribute to a bewildering array of functions, some closely related to each other, others less so. Yet this formulation lacks parsimony, popularity, and, worst of all, any real usefulness.
E pluribus unum
In Douglas Adams' four-part “trilogy”, The Hitchhiker's Guide to the Galaxy, people asked a computer named Deep Thought the Great Question of Life, the Universe and Everything. After it churned for 7.5 million years, the answer turned out to be 1010102, and — as Deep Thought itself foresaw — the people did not like the answer. Our question is simpler, although it might actually have something to do with the Great Question after all: What does the “granular” prefrontal cortex do? The answer is often a long list like the ones compiled here, and, like 1010102, the people do not like that answer. The obverse of American coins states the goal clearly enough: E pluribus unum, From many, one.
In the spirit of E pluribus unum, the strategy task (Fig. 6A) that we devised might help us understand how several kinds of knowledge combine to produce a sophisticated behavior [54,62,70]. Our task required several kinds of long-term knowledge and short-term memory, including which cue had occurred on the previous trial and which goal had been selected, rule knowledge about what to do if the cue on the current trial matched that on the previous one, and, depending on that, which of two strategies to use in selecting the next (future) goal. This task thus required the processes invoked by several theories of the “granular” prefrontal cortex: the maintenance of information in working memory [71], adaptive coding, which confers the ability to guide behavior whenever problems exceed routine levels of difficulty [72,73], and attentional selection [74,75], including the selection of items in working memory [32,33,75]. In addition to these processes, our task required many kinds of knowledge, including knowledge about the most salient input, the goal given a context and rule, and the outcomes that should follow, perhaps along with alternative goals and outcomes — suspended for the moment, but not forever. We showed that neurons in the prefrontal cortex encoded these abstract behavioral strategies [54,70], along with both previous and future goals [62,70] (Fig. 6B, C). It seems likely that the “granular” prefrontal cortex stores much of the knowledge needed for this task and participates in the many cognitive processes underlying its performance. It therefore stands to reason that studies focused on any of these kinds of knowledge or processes, in isolation or in small combinations, would find evidence for all of them.
Figure 6.
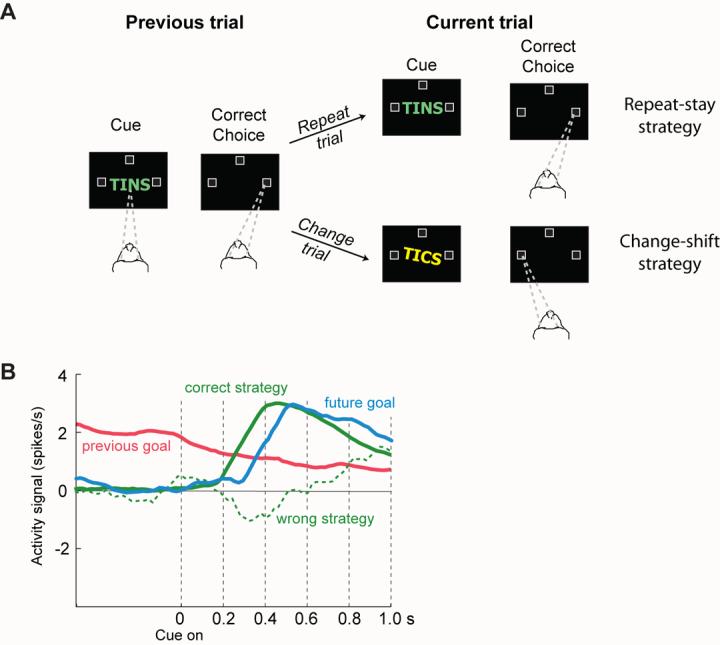
A. The strategy task of Genovesio et al. [54]. Each trial began when the monkey fixated a spot at the center of a video screen (dashed lines). Later, a cue appeared, and when it disappeared, the monkey had to make a saccadic eye movement to one of three potential goals (unfilled squares). The monkey needed to remember the cue and goal from the previous trial because if the cue repeated from that trial (called a repeat trial, top fork), then the same goal (the rightward one in this example) had to be fixated by a saccadic eye movement. If the cue changed (called a change trial, bottom fork), then the monkey had to select one of the other two goals (the leftward or upward goals in this example, with the left one illustrated). B. Neural signals reflecting the previous goal (red), the future goal (blue) and the strategy (green), when the monkeys chose the correct strategy (solid line) or the wrong one (dashed line). Note that the previous-goal signal decreased after cue onset, as the signals for the correct strategy and future goal increased. When the monkey chose the wrong strategy (dashed line), a weak or absent strategy signal occurred during the time of goal selection. B adapted from Genovesio et al. [70].
The idea presented here, then, is that the “granular” prefrontal cortex confers a subtle but important adaptive advantage on the primates that have one. Its various areas accumulate different kinds of knowledge about nonroutine behaviors, as well as their contexts and outcomes. In a phrase, the prefrontal cortex learns about what to do, and it does so mainly in unusual or complicated situations, when the usually dominant behavior will not do. This knowledge enables better, more sophisticated choices and decisions, strategies, and hierarchically nested rules, along with a remarkable ability to defer goals, often to pursue different ones. The idea that the knowledge stored by the “granular” prefrontal cortex concerns aspects of behavior agrees with previous proposals about its role in behavioral flexibility and the diversity of its cognitive contributions, as propounded for example by Duncan and his colleagues [72, 73,76] and by Shallice and Norman as part of their supervisory attention system, which encompasses a role in behavioral strategies [77,78].
This idea could bring thinking about the “granular” prefrontal cortex into line with that for other parts of the cerebral cortex. For example, the inferotemporal and perirhinal areas of cortex encode, represent and store knowledge about objects [3-6]. Likewise, the “granular” prefrontal areas may encode, represent and store knowledge about behaviors, with the proviso that this knowledge includes the likely consequences of these behaviors and that these are not run-of-the-mill behaviors, but nonroutine ones, including high-order rules and rarely deployed behavioral strategies, along with complex event sequences played out over a particularly long time-horizon [2]. Consider, for example, using a subway or underground in a foreign land. Even without the ability to read the signs or converse with anyone, we can still deploy considerable knowledge about what to do. Enter a hole in the ground, get a token or ticket, go through a gate, avoid standing on the tracks, wait for a train, and get on it when the doors open (not before). Strategies for reaching a goal could draw on the knowledge that colors often label commuter lines, that termini could indicate directions, and that system maps often disregard scale, but usually indicate compass directions fairly accurately. Such prefrontal knowledge contributes to a multitude of cognitive processes, such as attention, working memory, goal selection, outcome prediction, and so forth. These notions could also encompass the knowledge underlying our social behavior, including speech.
According to this view of the “granular” prefrontal cortex, its areas contribute collectively to behaviors particularly important to our lives as primates, an order noted for our braininess and longevity. The “granular” prefrontal areas provide an adaptive advantage by storing knowledge [2] — knowledge about how to behave under a wide range of nonroutine and complicated circumstances, all of which require attention to our actions and the diverse behavioral contexts encountered over an extended lifetime. The use of this knowledge in various cognitive processes gives rise to the long list of functions usually attributed to the prefrontal cortex. In routine and relatively simple situations, the behavioral knowledge stored by the “granular” prefrontal cortex is more-or-less dispensable, which explains why other mammals — and even some unfortunate people [79] — get along so well without one. Most mammals get along splendidly without a “granular” prefrontal cortex, not because they engage exclusively in simple or routine behaviors, but because the additional behavioral flexibility gained through prefrontal knowledge confers only a subtle advantage, albeit one with enormous consequences. Rodents, among the other mammals, show substantial flexibility in their behavior, and the frontal areas they share with primates — which have many similarities to and connections with the unshared ones — may be responsible for this capacity. But when we primates recognize a rare risk in a complex pattern of events or an outstanding opportunity knocks, we have an advantage over other mammals: We are especially good at knowing what to do and what is likely to happen in the event.
Acknowledgements
I thank Drs. Sarah Rhodes, Peter Rudebeck, Todd Preuss and Elisabeth Murray for their comments on a previous version of this opinion piece, and Drs. David Bousfield, Gavin Swanson and Sian Lewis for permitting me to participate in Trends in Neurosciences for more than two decades.
Footnotes
‘Teaser’
The prefrontal cortex remains enigmatic, but two trends in neurosciences will help. First, comparative neuroanatomy indicates that the frontal areas shared by rodents and primates compose only part of the primate frontal lobe, which should facilitate research on these areas. Second, investigations of unshared areas have begun to focus on stored knowledge rather than cognitive processes.
Conflicts of interest: The author declares no competing interests in association with this article.
Reference List
- 1.Fulton JF. Physiology of the Nervous System. Oxford University Press; 1949. [Google Scholar]
- 2.Wood JN, Grafman J. Human prefrontal cortex: Processing and representational perspectives. Nat. Rev. Neurosci. 2003;4:139–147. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- 3.Buckley MJ, Gaffan D. Perirhinal cortex ablation impairs visual object identification. J. Neurosci. 1998;18:2268–2275. doi: 10.1523/JNEUROSCI.18-06-02268.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bussey TJ, et al. Impairments in visual discrimination after perirhinal cortex lesions: testing ‘declarative’ vs. ‘perceptual-mnemonic’ views of perirhinal cortex function. Eur. J. Neurosci. 2003;17:649–660. doi: 10.1046/j.1460-9568.2003.02475.x. [DOI] [PubMed] [Google Scholar]
- 5.Murray EA, et al. Visual perception and memory: A new view of medial temporal lobe function in primates and rodents. Annu. Rev. Neurosci. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka K. Inferotemporal cortex and object vision. Annu. Rev. Neurosci. 1996;19:109–139. doi: 10.1146/annurev.ne.19.030196.000545. [DOI] [PubMed] [Google Scholar]
- 7.Kolb B. Do all mammals have a prefrontal cortex? In: Kaas JH, Krubitzer L, editors. The Evolution of Primate Nervous Systems. Elsevier; 2007. pp. 443–450. [Google Scholar]
- 8.Preuss TM. Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J. Cogn. Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Preuss TM. Evolutionary specializations of primate brain systems. In: Ravosa MJ, Dagasto M, editors. Primate origins: Adaptations and evolution. Springer; New York: 2007. pp. 625–675. [Google Scholar]
- 10.Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25:340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- 11.Matelli M, Luppino G. Thalamic input to mesial and superior area 6 in the macaque monkey. J. Comp. Neurol. 1996;372:59–87. doi: 10.1002/(SICI)1096-9861(19960812)372:1<59::AID-CNE6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 12.Gaspar P, et al. Topography and collateralization of the dopaminergic projections to motor and lateral prefrontal cortex in owl monkeys. J. Comp. Neurol. 1992;325:1–21. doi: 10.1002/cne.903250102. [DOI] [PubMed] [Google Scholar]
- 13.Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb. Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- 14.Lewis DA, et al. The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. J. Neurosci. 1987;7:279–290. doi: 10.1523/JNEUROSCI.07-01-00279.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishkin M. Effects of small frontal lesions on delayed alternation in monkeys. J. Neurophysiol. 1957;20:615–622. doi: 10.1152/jn.1957.20.6.615. [DOI] [PubMed] [Google Scholar]
- 16.Kojima S, et al. Operant behavioral analysis of memory loss in monkeys and prefrontal lesions. Brain Research. 1982;248:51–59. doi: 10.1016/0006-8993(82)91146-5. [DOI] [PubMed] [Google Scholar]
- 17.Battig K, et al. Comparison of the effects of frontal and caudate lesions on delayed response and alternation in monkeys. J. Comp. Physiol. Psychol. 1960;53:400–404. doi: 10.1037/h0047392. [DOI] [PubMed] [Google Scholar]
- 18.Kolb B, et al. Double dissociation of spatial impairments and perseveration following selective prefrontal lesions in rats. J. Comp. Physiol. Psychol. 1974;87:772–780. doi: 10.1037/h0036970. [DOI] [PubMed] [Google Scholar]
- 19.Kolb B, et al. Dissociation of the medial prefrontal, posterior parietal, and posterior temporal cortex for spatial navigation and recognition memory in the rat. Cereb. Cortex. 1994;4:664–680. doi: 10.1093/cercor/4.6.664. [DOI] [PubMed] [Google Scholar]
- 20.Öngür, et al. Architectonic subdivision of the human orbital and medial prefrontal cortex. J. Comp. Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 21.Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial. J. Comp. Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- 22.Palomero-Gallagher N, Zilles K. Isocortex. In: Paxinos G, editor. The Rat Nervous System. 3rd edition Elsevier Academic Press; San Diego CA: 2004. pp. 729–757. [Google Scholar]
- 23.Ferry AT, et al. Prefrontal cortical projections to the striatum in macaque monkeys: Evidence for an organization related to prefrontal networks. J. Comp. Neurol. 2000;425:447–470. doi: 10.1002/1096-9861(20000925)425:3<447::aid-cne9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 24.Brog JS, et al. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: Immunhistochemical detection of retrogradely transported fluoro- gold. J. Comp. Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- 25.Haber SN, et al. The orbital and medial prefrontal circuit through the primate basal ganglia. J. Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haber SN, et al. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J. Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berendse HW, et al. Topographical organization and relationship with ventral striatal compartmentts of prefrontal corticostriatal projections in the rat. J. Comp. Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- 28.Schilman EA, et al. The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci. Lett. 2008;432:40–45. doi: 10.1016/j.neulet.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 29.Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol. Rev. 2008;88:37–57. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- 30.Rushworth MFS, et al. Ventral prefrontal cortex is not essential for working memory. J. Neurosci. 1997;17:4829–4838. doi: 10.1523/JNEUROSCI.17-12-04829.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Postle BR, et al. Seeking the neural substrates of visual working memory storage. Cortex. 2003;39:927–946. doi: 10.1016/s0010-9452(08)70871-2. [DOI] [PubMed] [Google Scholar]
- 32.Rowe JB, et al. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- 33.Petrides M, et al. Differential activation of the human orbital, midventrolateral, and middorsolateral prefrontal cortex during the processing of visual stimuli. Proc. Nat. Acad. Sci. USA. 2002;99:5649–5654. doi: 10.1073/pnas.072092299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrides M. Dissociable roles of mid-dorsolateral prefrontal and anterior inferotemporal cortex in visual working memory. J. Neurosci. 2000;20:7496–7503. doi: 10.1523/JNEUROSCI.20-19-07496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Funahashi S, et al. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J. Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 36.Lebedev MA, et al. Representation of attended versus remembered locations in prefrontal cortex. PLoS Biol. 2004;2:1919–1935. doi: 10.1371/journal.pbio.0020365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowe JB, Passingham RE. Working memory for location and time: activity in prefrontal area 46 relates to selection rather than maintenance in memory. Neuroimage. 2001;14:77–86. doi: 10.1006/nimg.2001.0784. [DOI] [PubMed] [Google Scholar]
- 38.Manly T, et al. Enhancing the sensitivity of a sustained attention task to frontal damage: convergent clinical and functional imaging evidence. Neurocase. 2003;9:340–349. doi: 10.1076/neur.9.4.340.15553. [DOI] [PubMed] [Google Scholar]
- 39.Lau HC, et al. Willed action and attention to the selection of action. Neuroimage. 2004;21:1407–1415. doi: 10.1016/j.neuroimage.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 40.Rushworth MFS, et al. Role of the human medial frontal cortex in task switching: A combined fMRI and TMS study. J. Neurophysiol. 2002;87:2577–2592. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- 41.Rainer G, et al. Prospective coding for objects in primate prefrontal cortex. J. Neurosci. 1999;19:5493–5505. doi: 10.1523/JNEUROSCI.19-13-05493.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Browning PG, et al. Frontal-temporal disconnection abolishes object discrimination learning set in macaque monkeys. Cereb. Cortex. 2007;17:859–864. doi: 10.1093/cercor/bhk039. [DOI] [PubMed] [Google Scholar]
- 43.Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- 44.Rainer G, et al. Memory fields of neurons in the primate prefrontal cortex. Proc. Natl. Acad. Sci. USA. 1998;95:15008–15013. doi: 10.1073/pnas.95.25.15008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 46.Miller EK, et al. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J. Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konishi S, et al. Neural mechanism in anterior prefrontal cortex for inhibition of prolonged set interference. Proc. Nat. Acad. Sci. USA. 2005;102:12584–12588. doi: 10.1073/pnas.0500585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freedman DJ, et al. Visual categorization and the primate prefrontal cortex: Neurophysiology and behavior. J. Neurophysiol. 2002;88:929–941. doi: 10.1152/jn.2002.88.2.929. [DOI] [PubMed] [Google Scholar]
- 49.Cohen YE, et al. Spontaneous processing of abstract categorical information in the ventrolateral prefrontal cortex. Biol. Lett. 2006;2:261–265. doi: 10.1098/rsbl.2005.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shima K, et al. Categorization of behavioural sequences in the prefrontal cortex. Nature. 2007;445:315–318. doi: 10.1038/nature05470. [DOI] [PubMed] [Google Scholar]
- 51.Averbeck BB, et al. Parallel processing of serial movements in prefrontal cortex. Proc. Natl. Acad. Sci. USA. 2002;99:13172–13177. doi: 10.1073/pnas.162485599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ninokura Y, et al. Integration of temporal order and object information in the monkey lateral prefrontal cortex. J. Neurophysiol. 2004;91:555–560. doi: 10.1152/jn.00694.2003. [DOI] [PubMed] [Google Scholar]
- 53.Hoshi E, Tanji J. Area-selective neuronal activity in the dorsolateral prefrontal cortex for information retrieval and action planning. J. Neurophysiol. 2004;91:2707–2722. doi: 10.1152/jn.00904.2003. [DOI] [PubMed] [Google Scholar]
- 54.Genovesio A, et al. Prefrontal cortex activity related to abstract response strategies. Neuron. 2005;47:307–320. doi: 10.1016/j.neuron.2005.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoshi E, et al. Neuronal activity in the primate prefrontal cortex in the process of motor selection based on two behavioral rules. J. Neurophysiol. 2000;83:2355–2373. doi: 10.1152/jn.2000.83.4.2355. [DOI] [PubMed] [Google Scholar]
- 56.Wallis JD, Miller EK. From rule to response: Neuronal processes in the premotor and prefrontal cortex. J. Neurophysiol. 2003;90:1790–1806. doi: 10.1152/jn.00086.2003. [DOI] [PubMed] [Google Scholar]
- 57.Barraclough DJ, et al. Prefrontal cortex and decision making in a mixed-strategy game. Nat. Neurosci. 2004;7:404–410. doi: 10.1038/nn1209. [DOI] [PubMed] [Google Scholar]
- 58.Quintana J, Fuster JM. From perception to action: Temporal integrative functions of prefrontal and parietal neurons. Cereb. Cortex. 1999;9:213–221. doi: 10.1093/cercor/9.3.213. [DOI] [PubMed] [Google Scholar]
- 59.Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 60.Browning PG, Gaffan D. Prefrontal cortex function in the representation of temporally complex events. J. Neurosci. 2008;28:3934–3940. doi: 10.1523/JNEUROSCI.0633-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim JN, Shadlen MN. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat. Neurosci. 1999;2:176–185. doi: 10.1038/5739. [DOI] [PubMed] [Google Scholar]
- 62.Genovesio A, et al. Representation of future and previous spatial goals by separate neural populations in prefrontal cortex. J. Neurosci. 2006;26:7281–7292. doi: 10.1523/JNEUROSCI.0699-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Padoa-Schioppa C, Assad JA. The representation of economic value in the orbitofrontal cortex is invariant for changes of menu. Nat. Neurosci. 2008;11:95–102. doi: 10.1038/nn2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sakai K, Passingham RE. Prefrontal set activity predicts rule-specific neural processing during subsequent cognitive performance. J. Neurosci. 2006;26:1211–1218. doi: 10.1523/JNEUROSCI.3887-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu. Rev. Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- 66.Murray EA, Izquierdo A. Orbitofrontal cortex and amygdala contributions to affect and action in primates. Ann. N. Y. Acad. Sci. 2007;1121:273–296. doi: 10.1196/annals.1401.021. [DOI] [PubMed] [Google Scholar]
- 67.Owen AM, et al. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rowe J, et al. Rule-selection and action-selection have a shared neuroanatomical basis in the human prefrontal and parietal cortex. Cereb. Cortex. 2008 doi: 10.1093/cercor/bhm249. doi:10.1093/cercor/bhm249, ( http://cercor.oxfordjournals.org) [DOI] [PMC free article] [PubMed]
- 69.Weissman DH, et al. Cognitive control in social situations: A role for the dorsolateral prefrontal cortex. Neuroimage. 40:955–962. doi: 10.1016/j.neuroimage.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Genovesio A, et al. Encoding problem-solving strategies in prefrontal cortex: activity during strategic errors. Eur. J. Neurosci. 2008;27:984–990. doi: 10.1111/j.1460-9568.2008.06048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behaviour by representational memory. In: Plum F, Mountcastle VB, editors. Handbook of Physiology: the Nervous System, Vol. 5. American Physiological Society; Bethesda: 1987. pp. 373–417. [Google Scholar]
- 72.Duncan J, et al. Intelligence and the frontal lobe: the organization of goal-directed behavior. Cogn. Psychol. 1996;30:257–303. doi: 10.1006/cogp.1996.0008. [DOI] [PubMed] [Google Scholar]
- 73.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 74.Rowe JB, Passingham RE. Working memory for location and time: activity in prefrontal area 46 relates to selection rather than maintenance in memory. Neuroimage. 2001;14:77–86. doi: 10.1006/nimg.2001.0784. [DOI] [PubMed] [Google Scholar]
- 75.Rowe J, et al. Attention to action: Specific modulation of corticocortical interactions in humans. Neuroimage. 2002;17:988–998. [PubMed] [Google Scholar]
- 76.Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nat. Rev. Neurosci. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- 77.Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- 78.Norman DA, Shallice T. Attention to action: Willed and automatic control of behavior. In: Davidson RJ, et al., editors. Consciousness and Self-Regulation: Advances in Research and Theory, Vol. 4. Plenum; New York: 1986. pp. 1–18. [Google Scholar]
- 79.Al-hai J. The lobotomist: A maverick medical genius and his tragic quest to rid the world of mental illness. John Wiley and Sons; 2005. [Google Scholar]