Abstract
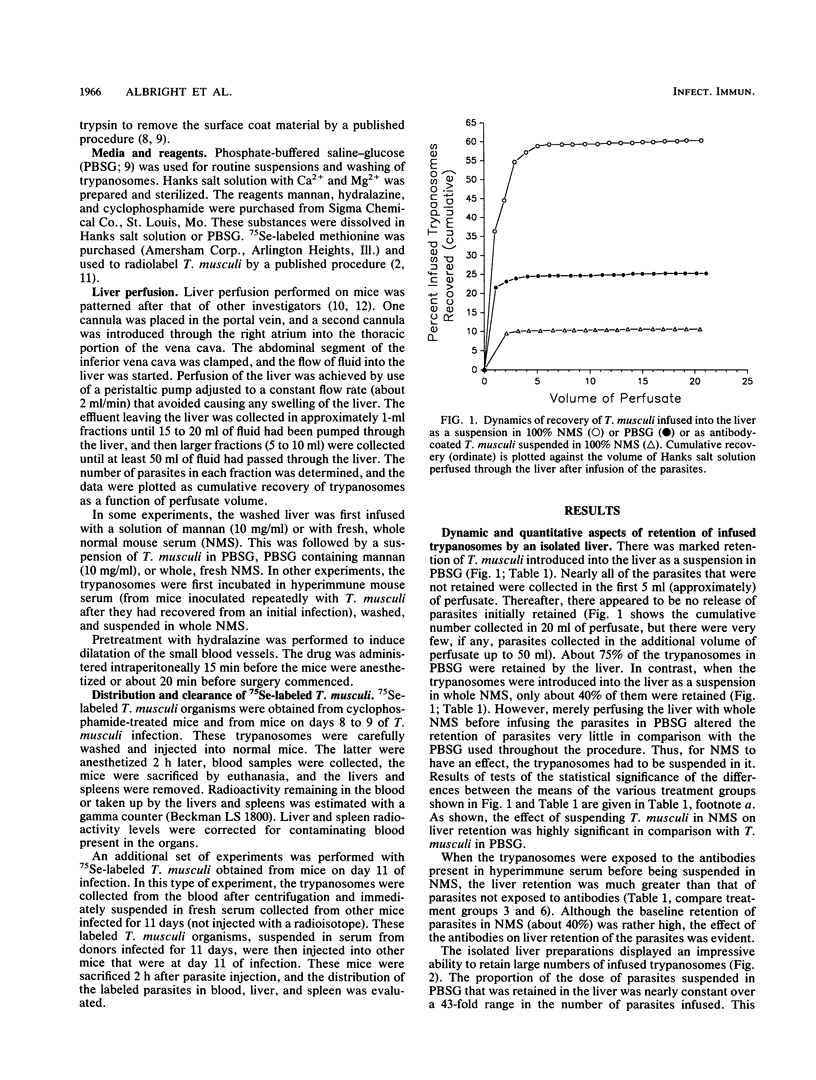
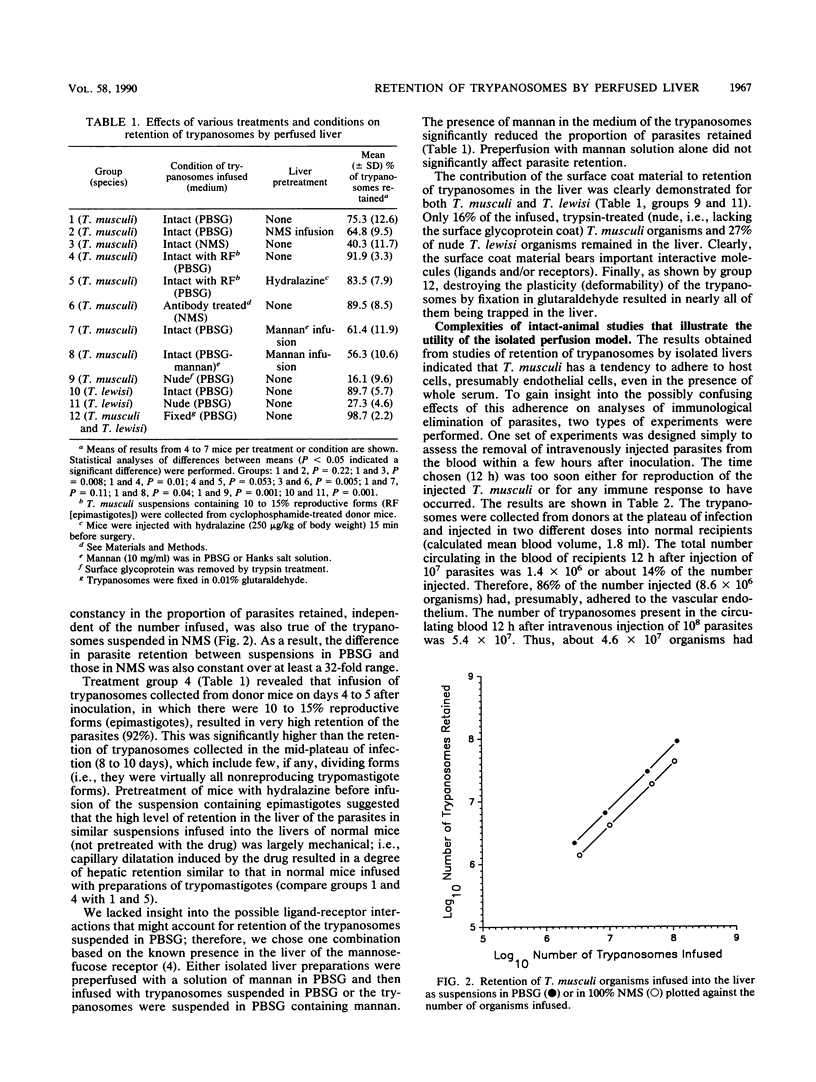
The isolated liver perfusion model has been used to investigate immunological elimination of bacteria and yeasts but not for analysis of mechanisms of immunological destruction of extracellular parasitic protozoa. Extracellular trypanosomes are eliminated primarily through antibody (and complement?)-promoted hepatic (Kupffer cell) uptake and destruction. We studied the suitability of the isolated liver model system for analyzing the mechanism of immune elimination of mouse-specific Trypanosoma musculi and identified several factors which can complicate such analyses: (i) mechanical trapping of trypanosomes that are quite large (for example, reproducing forms or epimastigotes) or are nonviable and, therefore, nondeformable; (ii) variable species and concentrations of cytadhesive molecules; and (iii) the integrity and composition of the trypanosomal surface coat. There was a substantial difference between hepatic retention of infused T. musculi organisms coated with a specific antibody and those devoid of antibody when both were suspended in normal mouse serum. The difference appeared sufficient to allow accurate quantitative studies of immune destruction in the liver. Studies of whole mice indicated that quantitative investigations of immunological elimination of trypanosomes from the bloodstream are likely to be complicated by problems such as cytadherence of parasites to host endothelial cells and mechanical trapping. Uptake by the liver and spleen appeared more reliable. Thus, the isolated liver perfusion model should significantly benefit studies to elucidate the mechanisms of immune elimination of extracellular trypanosomes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright J. W., Albright J. F., Dusanic D. G. Mechanisms of trypanosome-mediated suppression of humoral immunity in mice. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3923–3927. doi: 10.1073/pnas.75.8.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright J. W., Matusewicz N. M., Albright J. F. Aging of the murine immune system is reflected by declining ability to generate antibodies that promote elimination of Trypanosoma musculi. J Immunol. 1988 Aug 15;141(4):1318–1325. [PubMed] [Google Scholar]
- Banks K. L. Binding of Trypanosoma congolense to the walls of small blood vessels. J Protozool. 1978 May;25(2):241–245. doi: 10.1111/j.1550-7408.1978.tb04405.x. [DOI] [PubMed] [Google Scholar]
- Chakraborty P., Das P. K. Role of mannose/N-acetylglucosamine receptors in blood clearance and cellular attachment of Leishmania donovani. Mol Biochem Parasitol. 1988 Feb;28(1):55–62. doi: 10.1016/0166-6851(88)90180-6. [DOI] [PubMed] [Google Scholar]
- Danforth H. D., Moon R. J., Jensen J. B., Vrable R. G., Beaudoin R. L. Retention of Plasmodium berghei sporozoites within perfused mouse livers. Acta Trop. 1982 Mar;39(1):5–10. [PubMed] [Google Scholar]
- Decker T., Kiderlen A. F., Lohmann-Matthes M. L. Liver macrophages (Kupffer cells) as cytotoxic effector cells in extracellular and intracellular cytotoxicity. Infect Immun. 1985 Nov;50(2):358–364. doi: 10.1128/iai.50.2.358-364.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. L., Mansfield J. M. Lymphocyte function in experimental African trypanosomiasis. V. Role of antibody and the mononuclear phagocyte system in variant-specific immunity. J Immunol. 1983 Jan;130(1):405–411. [PubMed] [Google Scholar]
- Desai B. B., Albright J. W., Albright J. F. Cooperative action of complement component C3 and phagocytic effector cells in innate murine resistance to Trypanosoma lewisi. Infect Immun. 1987 Feb;55(2):358–363. doi: 10.1128/iai.55.2.358-363.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer D. M. Cell surface saccharides of Trypanosoma lewisi. I. Polycation-induced cell agglutination and fine-structure cytochemistry. J Cell Sci. 1975 Dec;19(3):621–644. doi: 10.1242/jcs.19.3.621. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Moon R. J. Role of Kupffer cells, complement, and specific antibody in the bactericidal activities of perfused livers. Infect Immun. 1980 Jul;29(1):152–157. doi: 10.1128/iai.29.1.152-157.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD J. G., WARDLAW A. C. The opsonic effect of normal serum on the uptake of bacteria by the reticulo-endothelial system; perfusion studies with isolated rat liver. Immunology. 1958 Oct;1(4):338–352. [PMC free article] [PubMed] [Google Scholar]
- Holmes P. H., MacAskill J. A., Whitelaw D. D., Jennings F. W., Urquhart G. M. Immunological clearance of 75Se-labelled Trypanosoma brucei in mice. I. Aspects of the radiolabelling technique. Immunology. 1979 Mar;36(3):415–420. [PMC free article] [PubMed] [Google Scholar]
- Lowrance J. H., Hasty D. L., Simpson W. A. Adherence of Streptococcus sanguis to conformationally specific determinants in fibronectin. Infect Immun. 1988 Sep;56(9):2279–2285. doi: 10.1128/iai.56.9.2279-2285.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaskill J. A., Holmes P. H., Whitelaw D. D., McConnell I., Jennings F. W., Urquhart G. M. Immunological clearance of 75Se-labelled Trypanosoma brucei in mice. II. Mechanisms in immune animals. Immunology. 1980 Aug;40(4):629–635. [PMC free article] [PubMed] [Google Scholar]
- Moon R. J., Vrable R. A., Broka J. A. In situ separation of bacterial trapping and killing functions of the perfused liver. Infect Immun. 1975 Aug;12(2):411–418. doi: 10.1128/iai.12.2.411-418.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noisin E. L., Villalta F. Fibronectin increases Trypanosoma cruzi amastigote binding to and uptake by murine macrophages and human monocytes. Infect Immun. 1989 Apr;57(4):1030–1034. doi: 10.1128/iai.57.4.1030-1034.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenhouse C. F., Tandon N. N., Magowan C., Jamieson G. A., Chulay J. D. Identification of a platelet membrane glycoprotein as a falciparum malaria sequestration receptor. Science. 1989 Mar 17;243(4897):1469–1471. doi: 10.1126/science.2467377. [DOI] [PubMed] [Google Scholar]
- Pick-Kober K. H., Münker D., Gressner A. M. Fibronectin is synthesized as an acute phase reactant in rat hepatocytes. J Clin Chem Clin Biochem. 1986 Aug;24(8):521–528. doi: 10.1515/cclm.1986.24.8.521. [DOI] [PubMed] [Google Scholar]
- Quaissi M. A., Kusnierz J. P., Gras-Masse H., Drobecq H., Velge P., Cornette J., Capron A., Tartar A. Fluorescence-activated cell-sorting analysis of fibronectin peptides binding to Trypanosoma cruzi trypomastigotes. J Protozool. 1988 Feb;35(1):111–114. doi: 10.1111/j.1550-7408.1988.tb04087.x. [DOI] [PubMed] [Google Scholar]
- Samarawickrema N. A., Howell M. J. Interactions between peritoneal cells and Trypanosoma musculi in mice. Int J Parasitol. 1988 Feb;18(1):69–73. doi: 10.1016/0020-7519(88)90038-0. [DOI] [PubMed] [Google Scholar]
- Sawyer R. T., Moon R. J., Beneke E. S. Hepatic clearance of Candida albicans in rats. Infect Immun. 1976 Dec;14(6):1348–1355. doi: 10.1128/iai.14.6.1348-1355.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. S., Kongshavn P. A. Characterization of antibodies mediating protection and cure of Trypanosoma musculi infection in mice. Infect Immun. 1985 Jun;48(3):787–794. doi: 10.1128/iai.48.3.787-794.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth J. J., Kierszenbaum F. Fibronectin enhances macrophage association with invasive forms of Trypanosoma cruzi. J Immunol. 1984 Jul;133(1):460–464. [PubMed] [Google Scholar]
- Wyler D. J., Sypek J. P., McDonald J. A. In vitro parasite-monocyte interactions in human leishmaniasis: possible role of fibronectin in parasite attachment. Infect Immun. 1985 Aug;49(2):305–311. doi: 10.1128/iai.49.2.305-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kreuter B. F., Sadigursky M., Santos-Buch C. A. Complementary surface epitopes, myotropic adhesion and active grip in Trypanosoma cruzi-host cell recognition. Mol Biochem Parasitol. 1988 Sep;30(3):197–208. doi: 10.1016/0166-6851(88)90088-6. [DOI] [PubMed] [Google Scholar]


