Abstract
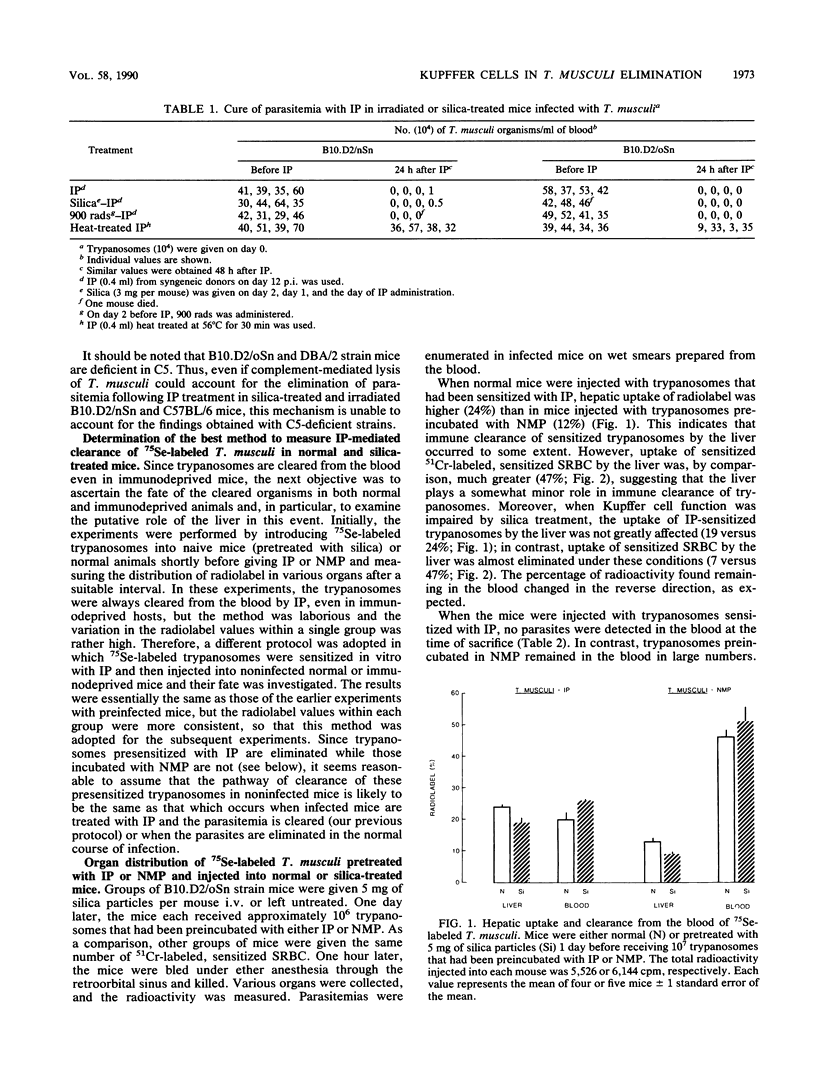
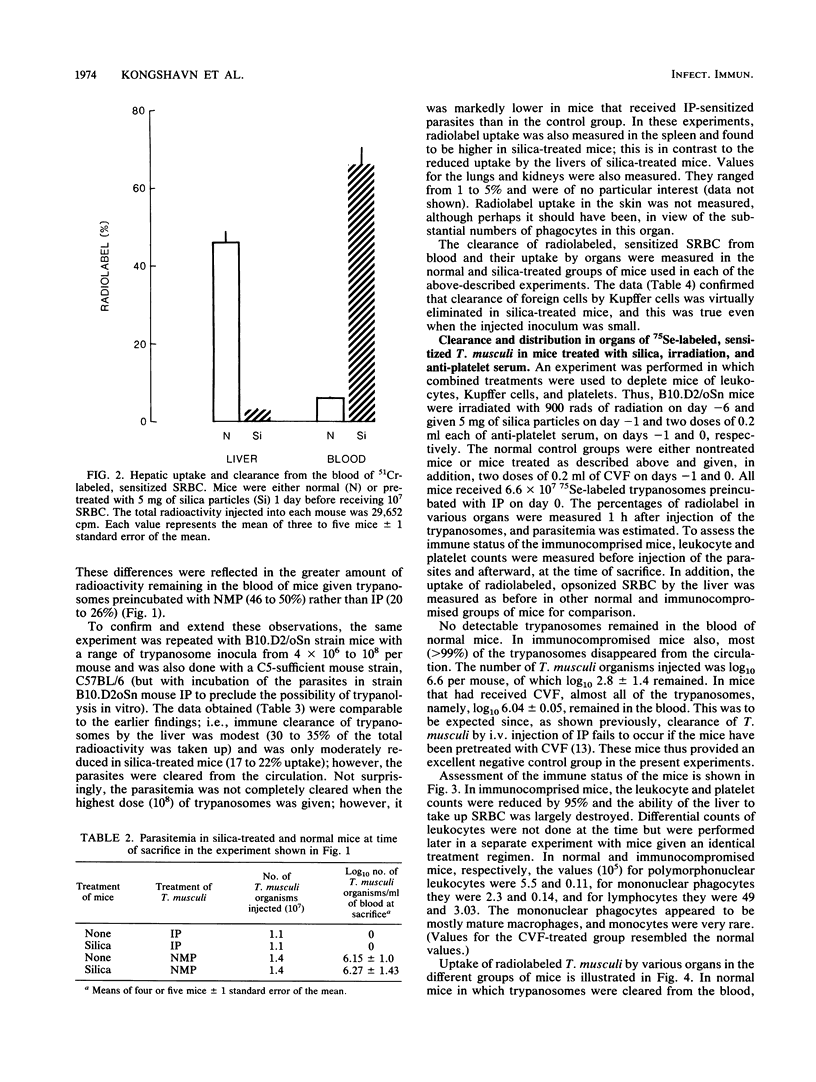
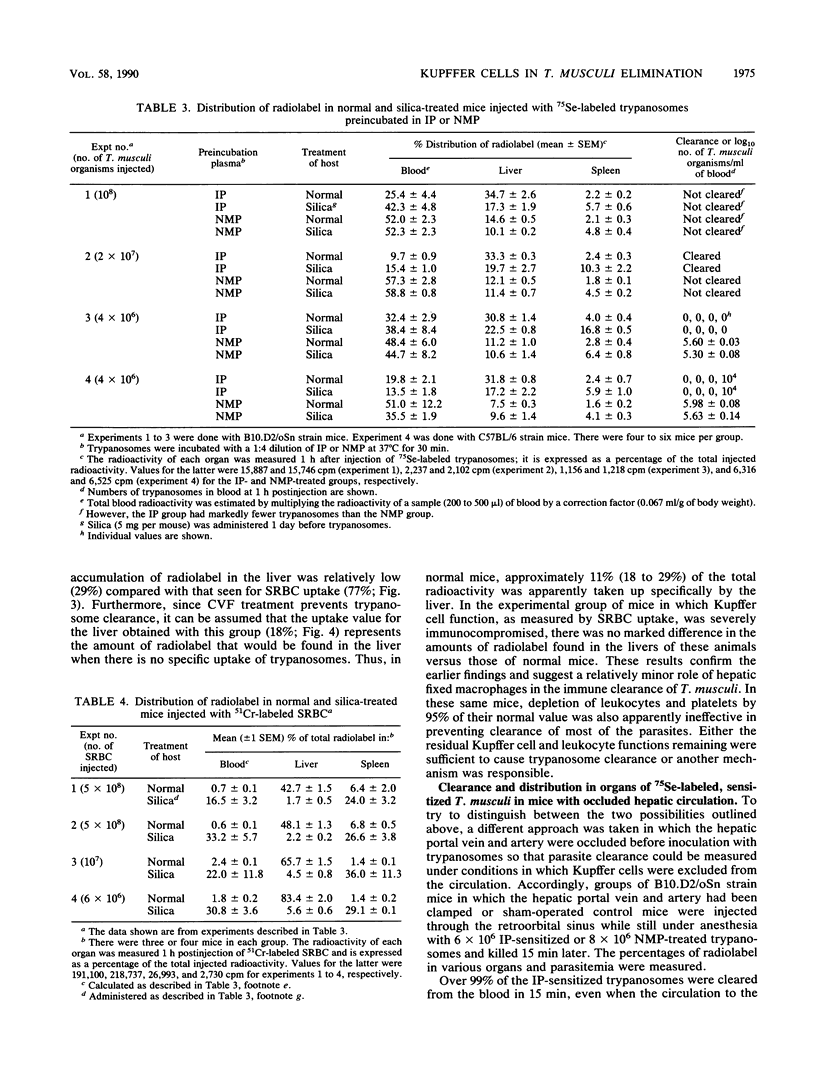
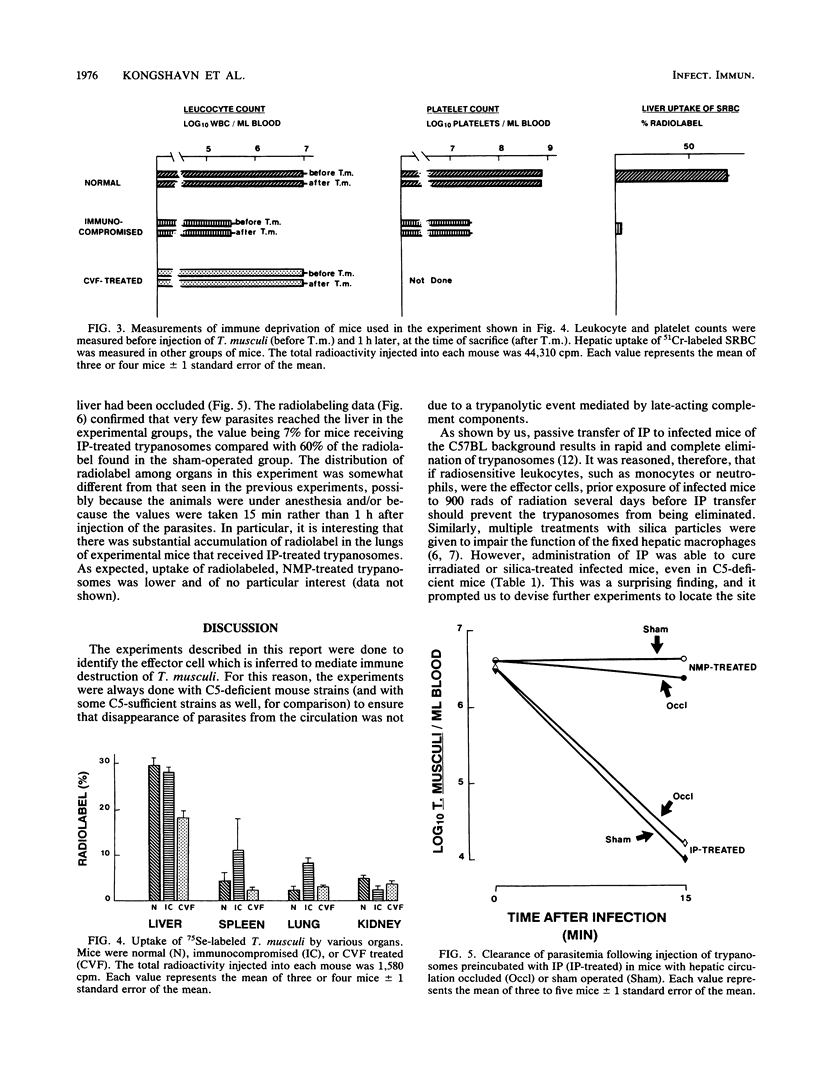
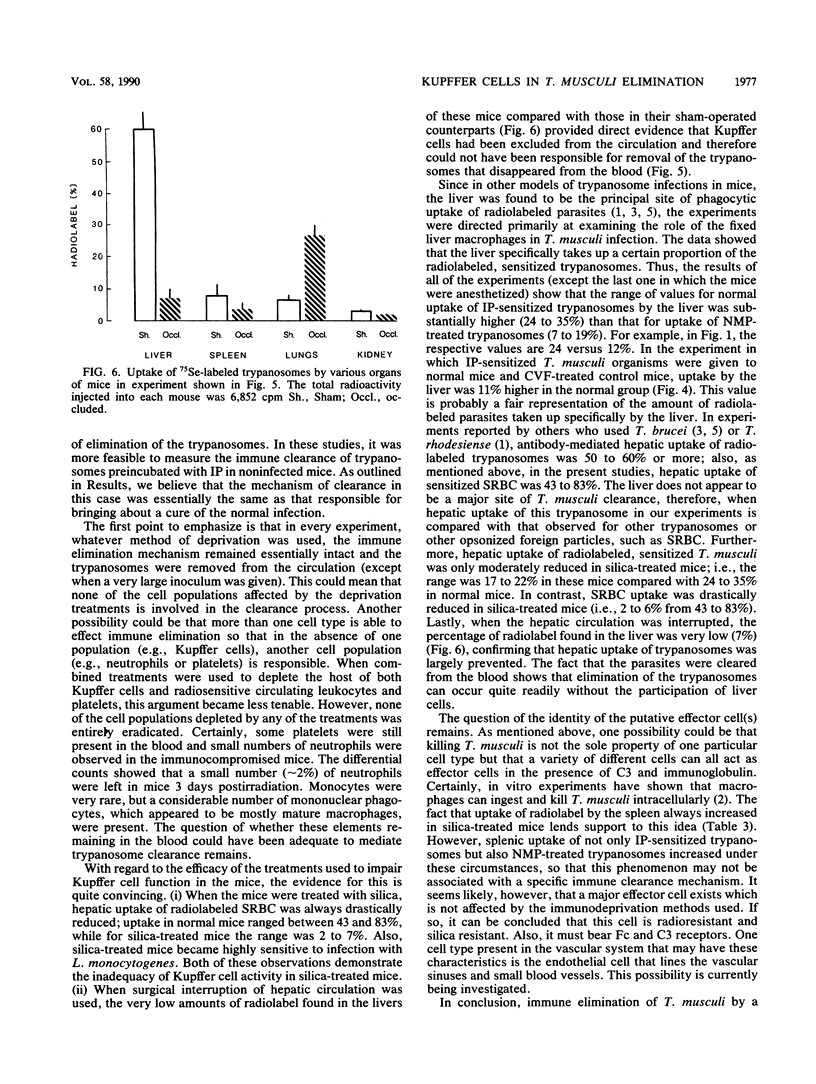
Previous studies have indicated that elimination of parasitemia in Trypanosoma musculi infection is brought about by immunoglobulin G2a antibodies, C3, and an effector cell. Experiments were designed to identify the putative effector cell by using several approaches. Infected C5-deficient or C5-sufficient mice treated with silica particles or given 900 rads of radiation 3 days earlier effectively eliminated trypanosomes following administration of immune plasma (IP). Silica-treated, noninfected mice given T. musculi preincubated with IP also cleared the parasites. Radiolabeling studies revealed that uptake of the cleared trypanosomes by the liver in normal mice was relatively low (24%) and fell only slightly (19%) in silica-treated mice. In contrast, uptake of radiolabeled sheep erythrocytes by the liver was normally much higher (47%) and fell drastically (7%) in silica-treated mice. Mice were then immunocompromised by 900 rads of radiation, silica particles, and anti-platelet serum combined before IP-sensitized trypanosomes were given. Leukocyte and platelet counts were both reduced by 95% and sheep erythrocyte uptake by the liver fell from 77 to 5%; however, greater than 99% of the injected trypanosomes were cleared in these mice and uptake of radiolabeled trypanosomes by the liver was similar to that of normal mice. Lastly, in anesthetized mice in which Kupffer cells were excluded surgically from the circulation, greater than 99% of the IP-sensitized trypanosomes disappeared rapidly from the blood. Only 7% of the radiolabel was found in the liver versus 60% in sham-operated mice. The results are interpreted as showing that hepatic Kupffer cells play a minor role in the immune elimination of T. musculi. Likewise, radiosensitive leukocytes and platelets are unlikely to be sole candidates for the putative effector cell that mediates a cure of murine trypanosomiasis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dempsey W. L., Mansfield J. M. Lymphocyte function in experimental African trypanosomiasis. V. Role of antibody and the mononuclear phagocyte system in variant-specific immunity. J Immunol. 1983 Jan;130(1):405–411. [PubMed] [Google Scholar]
- Ferrante A. The role of the macrophage in immunity to Trypanosoma musculi. Parasite Immunol. 1986 Mar;8(2):117–127. doi: 10.1111/j.1365-3024.1986.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Holmes P. H., MacAskill J. A., Whitelaw D. D., Jennings F. W., Urquhart G. M. Immunological clearance of 75Se-labelled Trypanosoma brucei in mice. I. Aspects of the radiolabelling technique. Immunology. 1979 Mar;36(3):415–420. [PMC free article] [PubMed] [Google Scholar]
- Jarvinen J. A., Dalmasso A. P. Trypanosoma musculi infections in normocomplementemic, C5-deficient, and C3-depleted mice. Infect Immun. 1977 May;16(2):557–563. doi: 10.1128/iai.16.2.557-563.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaskill J. A., Holmes P. H., Whitelaw D. D., McConnell I., Jennings F. W., Urquhart G. M. Immunological clearance of 75Se-labelled Trypanosoma brucei in mice. II. Mechanisms in immune animals. Immunology. 1980 Aug;40(4):629–635. [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Formal S. B. Effect of silica on the innate resistance of inbred mice to Salmonella typhimurium infection. Infect Immun. 1979 Aug;25(2):513–520. doi: 10.1128/iai.25.2.513-520.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrangeli C., Pang K. C., Skamene E., Kongshavn P. A. Characteristics of mononuclear phagocytes mediating antilisterial resistance in splenectomized mice. Infect Immun. 1983 Feb;39(2):742–749. doi: 10.1128/iai.39.2.742-749.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skamene E., Kongshavn P. A. Phenotypic expression of genetically controlled host resistance to Listeria monocytogenes. Infect Immun. 1979 Jul;25(1):345–351. doi: 10.1128/iai.25.1.345-351.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viens P., Dubois R., Kongshavn P. A. Platelet activity in immune lysis of Trypanosoma musculi. Int J Parasitol. 1983 Dec;13(6):527–530. doi: 10.1016/s0020-7519(83)80023-x. [DOI] [PubMed] [Google Scholar]
- Viens P., Targett G. A., Leuchars E., Davies A. J. The immunological response of CBA mice to Trypanosoma musculi. I. Initial control of the infection and the effect of T-cell deprivation. Clin Exp Immunol. 1974 Feb;16(2):279–294. [PMC free article] [PubMed] [Google Scholar]
- Vincendeau P., Daëron M., Daulouede S. Identification of antibody classes and Fc receptors responsible for phagocytosis of Trypanosoma musculi by mouse macrophages. Infect Immun. 1986 Sep;53(3):600–605. doi: 10.1128/iai.53.3.600-605.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. S., Kongshavn P. A. Characterization of antibodies mediating protection and cure of Trypanosoma musculi infection in mice. Infect Immun. 1985 Jun;48(3):787–794. doi: 10.1128/iai.48.3.787-794.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. S., Kongshavn P. A. Cure of Trypanosoma musculi infection by heat-labile activity in immune plasma. Infect Immun. 1984 Jun;44(3):756–759. doi: 10.1128/iai.44.3.756-759.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. S., Kongshavn P. A. Further characterization of the curative antibodies in Trypanosoma musculi infection. Infect Immun. 1988 Sep;56(9):2379–2384. doi: 10.1128/iai.56.9.2379-2384.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. S., Kongshavn P. A. Heat-labile IgG2a antibodies affect cure of Trypanosoma musculi infection in C57BL/6 mice. J Immunol. 1986 Nov 1;137(9):2968–2972. [PubMed] [Google Scholar]



