Abstract
The aim of this study was to examine optimal self-management in osteoarthritis and its association with patient-reported outcomes. We recruited a population-based sample of Medicare beneficiaries (n=551) residing in Allegheny County, PA, USA and elicited an expanded set of self-management behaviors using open-ended inquiry. We defined optimal self-management according to clinical recommendations, including use of hot compresses on affected joints, alteration of activity, and exercise. Only 20% practiced optimal self-management as defined by two or more of these criteria. Optimal and suboptimal self-managers did not differ in sociodemographic features. Both white and African–Americans who practiced optimal self-management reported significantly less pain, but the benefit was greatest in severe disease for whites and for mild-moderate disease among African–Americans. This backdrop of naturally occurring self-management behaviors may be important to recognize in planning programs that seek to bolster self-management skills.
Keywords: Osteoarthritis, Self-care, Race, Self-management, Population-based sample
Self-care has long been recognized as critical in chronic disease management (Ory and DeFriese 1998), yet health care professionals continue to lack guidelines for how best to support patient self-management (Sevick et al. 2007). Still, the need to support self-management is likely to become more important, since the number of people with chronic disease is growing. By 2030, as many as 171 million Americans will report at least one chronic disease and half will have more than one condition, or “multimorbidity” (Anderson and Horvath 2004).
Self-management has recently become the focus of randomized clinical trials that seek to determine if patients trained in appropriate exercise, control of fatigue, adequate nutrition, stress reduction, and effective medication use manage symptoms more effectively. The evidence for the value of self-management in arthritis is compelling. These trials support the benefit of self-management training for patient mental health (Buszewicz et al. 2006), physical function (Heuts et al. 2005), and exercise self-efficacy and capacity (Lorig et al. 2000; Hughes et al. 2006). Efforts to promote self-management and train “expert patients” have also become widely adapted in the UK National Health Service as a promising way of reducing morbidity in chronic disease (www.expertpatients.nhs.uk).
Diffusion of arthritis self-management programs in community settings is at an early phase, and introduction of such programs will occur against the backdrop of self-management practices already adopted by patients. Thus, a first step in supporting patient self-management is to determine what patients actually do to manage symptoms and reduce morbidity. We report results from a population-based sample in which older adults with osteoarthritis reported self-management behaviors. We were particularly interested in the co-occurrence of different types of self-management behaviors across different levels of disease severity. We also sought to determine if African–American and white elders differ in self-management and whether such differences are consistent across mild and more severe osteoarthritis. Differences in self-management would be consistent with recent evidence suggesting that African–Americans have lower expectations of receiving joint replacement surgery (Groeneveld et al. 2008). Other research suggests that African–American and white elders with osteoarthritis do not differ in health beliefs or care-seeking related to arthritis (Ang et al. 2008). In addition, African–Americans and whites with similar radiographic evidence of osteoarthritis report differently on pain and disability (Burns et al. 2007), suggesting again that the groups may differ in expectations regarding self-management.
To explore these issues, we examined (1) what proportion of patients could be considered optimal self-managers; (2) whether optimal self-managers differed from other participants in self-reported pain, control, social support, and indicators of mental and physical well-being; and (3) whether the benefits of optimal self-management might differ among African–Americans and whites.
Sample
Community-dwelling adults aged 65 and over who resided in Allegheny County, PA, USA and who met screening criteria for knee or hip osteoarthritis and had no obvious indication of cognitive impairment, were eligible to participate (Albert et al. 2008). Screening was based on a series of self-report questions asked in a telephone interview. Respondents had to report that they had (1) pain in their hip or knee on most days for at least a month, (2) pain when walking or standing for at least half the days during the preceding month, and (3) pain of this severity for a period of at least 6 months. Information on whether participants participated in formal osteoarthritis self-management programs was not available; hence no exclusions were made on this basis.
From June 2001 to May 2002, 5094 community-dwelling older adults in Allegheny County completed telephone screening to identify people with osteoarthritis or ischemic heart disease. The sample was randomly drawn from individuals 65 and over who were included in the Medicare Enrollment File for Allegheny County in April 2001. The Medicare Enrollment File includes 96% or more of adults age 65 and over nationally and thus is broadly representative of older adults. We enrolled 551 participants with osteoarthritis in 2001–2003, 52% of respondents who met criteria for osteoarthritis (an additional 577 participants with heart disease were also enrolled but are not discussed here). The initial screening interview allowed us to assess how representative participants were relative to the entire screened sample of people with osteoarthritis. People participating in the research were more likely to be African–American (57.3% of eligible African–Americans participated, compared to 48.1% of eligible whites, p<.001), younger (54.6% of participants aged 65–74 participated, compared to 48.6% of non-participants in this age range, p<.01), and more educated (57.8% of participants attended college, compared to 42.2% of non-participants, p<.001).
Methods
Derivation of self-management behavior categories
To elicit a complete picture of osteoarthritis self-management, cohort participants completed both an open-ended inquiry and a structured questionnaire on a broad set of osteoarthritis management behaviors derived from review of the literature. Structured items included use of “special diets or dietary supplements,” “herbal or natural remedies,” “copper bracelets, crystals, or magnets,” “salves or ointments,” “hot or cold treatments,” “exercise,” “relaxation techniques,” “acupuncture or needle treatments,” “injections,” “massage therapy,” “chiropractor,” “physical therapy,” and “natural healer or homeopath.” Open-ended questions centered on activities participants had adopted to manage osteoarthritis symptoms over the course of a day, including activities used when first waking up and activities adopted in the evening (Silverman et al. 2008; this volume). Responses were recorded verbatim in face-to-face interviews and later entered into text fields in a computerized database.
In this report, we limit results to the open-ended inquiry. The open-ended inquiry revealed the most prevalent self-management behavior, which was not included in the structured set of self-management behaviors. This is regulation of daily activity and movement, in which participants reported changing the way they performed current activities, avoiding certain activities entirely, or adopting new activities. This critical set of responses to osteoarthritis would have been completely missed if we had not allowed respondents to reply in their own terms to our inquiry on management of symptoms. Aside from its prevalence, this behavior is critical for defining optimal self-management using guidelines provided by Lorig (Lorig et al. 2000), described below.
Each verbatim response was independently coded by two raters. When necessary, codes were reconciled by the research team in regular meetings. We allowed coding at this level to be as granular as possible. That is, we did not restrict categories for initial codes but rather sought highly detailed (and even unique) self-management behaviors that covered use of particular products or responses to symptoms. Indeed, in open-ended inquiry the 551 respondents mentioned 1757 management behaviors.
We used an expanded coding system to categorize self-management behaviors. We defined eight super-categories. These included exercise, weight or diet control, daily activity or movement, hot or cold treatments, use of dietary supplements, natural remedies, relaxation or meditation techniques, and use of ointments or salves. We subdivided the exercise, weight/diet, and activity regulation categories because of the diversity of behaviors mentioned. Exercise included four subtypes: changing lifestyle to include exercise, adopting forms of recreation specifically to burn calories, engaging in exercise that does not require equipment, and exercising with equipment. Weight or diet control included losing weight, reducing intake of harmful ingredients, avoiding certain foods, adding vegetables or fruits, and adding healthy ingredients aside from vegetables or fruits. Activity or movement regulation was subdivided into the categories of changing current activities, avoiding some activities entirely, and adopting new activities. Coding of activity or movement regulation was challenging. We considered “change routines,” and “plan activities” as instances of changing activity. “Avoid almost everything,” “be really still,” and “not bend” were considered cases of avoiding activity. “Be moderate,” “take showers only,” and “lay on one side” were considered cases of adopting activity. We recognize that many of these could go in more than one category, and for some analyses we ultimately grouped these within a single category of activity regulation.
These self-care categories represent a consensus judgment based on review of the literature and overall conceptual similarity, as well as inspection of the kinds of behaviors respondents mentioned in response to the original set of categories. While respondents often mentioned more than one behavior within a category (with some duplication), we counted any mention as an indication of use of a self-management strategy. The coding scheme was reviewed and refined over a number of iterations by the research team.
Defining optimal self-management in osteoarthritis
A popular guide to chronic disease self-management has proposed use of three strategies to manage pain and stiffness in osteoarthritis (Lorig et al. 2000). These include exercise, management of activity, and use of hot compresses on affected joints. While Lorig, et al. include other behaviors as well, such as addressing mental health issues and use of social support, these behaviors are singled out as specific self-management options that have reasonable evidence base for reducing pain and stiffness. Lorig et al. (2000) did not specify amount or type of exercise or activity control.
To operationalize this approach to self-management, we adopted the following algorithm. We considered optimal self-management to include at least two of the three behaviors. Further, we required that participants report at least two of the four types of exercise and at least two of the three types of activity change. We considered any use of hot compresses as successful use of the strategy. This definition of optimal self-management requires that respondents adopt a majority or the behaviors singled out by Lorig et al. (2000), with at least two of the different types of exercise and activity change mentioned by respondents. We considered a more stringent definition, in which respondents would be considered optimal self-managers only if they adopted all three behavior types. However, as reported below, very few people met this criterion.
Measures
We sought to examine the benefits of optimal self-management in osteoarthritis. To do so, we identified a series of self-reported outcomes that might plausibly be altered or reduced with effective self-management. These included the following:
Pain
Respondents reported pain in the last month on a visual analogue scale (0=no pain, 10=maximum pain).
Depression
Respondents completed the ten-item short form of the Center for Epidemio-logic Studies-Depression measure (Andresen et al. 1994; Radloff 1977). Reliability, as indicated by internal consistency for the measure, was adequate (α=0.78).
Functional status
Respondents were asked if they had difficulty performing eight instrumental activities (managing money, using the telephone, shopping, cooking, handling light and heavy housework, getting to physicians’ offices, filling prescriptions) because of health problems; these were summed for a range of 0–8. Reliability was adequate (α=0.68).
Health engagement control strategies
Respondents completed the Optimal Primary and Secondary Control Scale, a measure of personal control over health (Wrosch et al. 2002; Heckhausen and Schulz 1998). The measure assesses use of control strategies to moderate affective response to symptoms or health conditions. The nine scale items include “I do whatever is necessary to be as healthy as I possibly can be” and “If I have a health problem that gets worse, I put in even more effort to get better.” Participants report how true they think these statements are for themselves. Scores for questions range from 1 (almost never true) to 5 (almost always true). Scale reliability was good as indicated by α=0.81.
Social participation
Social support was assessed using the Lubben Social Network Scale (Lubben 1988; Lubben and Gironda 2004). The ten-item scale, score range 0–50, assesses perceived social support as well as the size, closeness and frequency of social contacts. Questions ask how many friends or contacts respondents have had over the past month and range from none (0) to nine or more (5). Reliability for the sample was low, with α=0.56.
Physical and mental health
Participants completed the SF-36v2 and summary scores were computed for the physical (PCS) and mental health (MCS) composite. The summary measures capture more than 80% of the variance in the eight SF-36 subscales (McHorney et al. 1993). These were rescaled against population norms following scoring guidelines (Ware and Kosinski 2001). A score of 50 represents the population average for each measure. Subscales used in construction of the composites had adequate internal consistency, with reliability coefficients (α) ranging from 0.70 (vitality) to 0.90 (role function).
Covariates used in analyses included the following:
Osteoarthritis severity
was determined by the ten-item Lequesne Index (LI) for hip and knee osteoarthritis (Lequesne 1997). The index measures such symptoms as pain, stiffness, performance in activities of daily living, and the need for assistive devices. Internal reliability for subscales was good, with α ranging from 0.84 to 0.88. The Index generates five severity groups, which range from mild to extremely severe. We defined a state of mild to moderate (composite LI, 1–3) and more severe (LI, 4–5) disease.
Number of comorbid conditions
Respondents were asked if a physician had ever diagnosed any of 17 medical conditions. These were summed.
Sociodemographics
Respondents provided information on age, gender, race, education, and marital status.
Analyses
We examined differences in self-management behaviors and optimal self-management by severity of osteoarthritis and race. We compared these proportions using the χ2 test. We examined correlates of self-management behaviors within osteoarthritis severity categories, again using the χ2 test. Stratifying analyses by disease severity is important because African–Americans in the sample were more likely to report severe disease (see below). Also, optimal self-management may offer different degrees of benefit in participants with mild as opposed to severe disease.
To examine the value of optimal self-management to control osteoarthritis symptoms, we developed two-way analysis of covariance models for each of the seven outcomes described earlier. Separate models were developed for each outcome (pain, depression, IADL disability, perceived control, social network involvement, physical function, and mental health). Between-subjects factors in the models included osteoarthritis severity (mild-moderate, severe) and self-management (suboptimal, optimal). Covariates models included age, gender, education, race, and number of comorbid conditions. To examine the effect of race in greater detail, we developed three-way ANCOVA models, again for each of the seven outcomes of interest, with race, disease severity, and optimal self-management as factors.
Results
The osteoarthritis cohort, by design, included roughly equal numbers of white (n=267) and African–American (n=284) elders. Women were overrepresented among African–Americans (206 women, 78 men) compared to whites (131 women, 136 men). Age did not significantly differ across race by gender groups. For men, the mean age (SD) was 73.5 (6.1) among whites and 73.9 (6.3) among African–Americans. For women, it was 72.6 (5.7) among whites and 73.6 (5.6) among African–Americans. African–Americans completed fewer years of education than whites. Among women, 23.3% of African–Americans did not complete high school, compared to 10.7% of whites (p=.001). 46.2% of African–American men and 11.0% of white men did not complete high school (p<.001).
African–American men and women were less likely than whites to rate their health as “excellent” or “very good” (10.3% vs. 24.3% among men, p<.05; 14.2% vs. 27.5% among women, p<.001), but the four groups did not differ in number of self-reported chronic conditions (median of 2 in addition to arthritis in all groups).
Severity of osteoarthritis differed across racial groups. 60.3% of African–American men and 70.4% of African–African women were categorized as “severe” or “very severe” by Lequesne Index classification; among whites, only 40.4% of men and 53.4% of women met this level of severity (p<.01; Albert et al. 2008).
Prevalence of self-care behaviors
The prevalence of self-reported osteoarthritis management behaviors in participants with mild-moderate and severe disease did not differ across most categories (Table 1). Participants with more severe disease were more likely to report adoption of new activities (70.7% vs. 62.4%, p<.05) and greater use of ointments and salves (10.7% vs. 6.0%, p<.05). Overall, 20.1% of the mild-moderate group and 18.6% of the more severe osteoarthritis groups practiced optimal self-management by our algorithm.
Table 1.
Frequency of Self-Management Behaviors, by Osteoarthritis Severity
| Mild–mild OA | Severe OA | |
|---|---|---|
| Exercises/activities, % | ||
| Lifestyle change to include exercise | 12.0 | 9.1 |
| Recreational activities to burn calories | 0.4 | 0.3 |
| Exercise without equipment | 35.5 | 39.7 |
| Exercise with equipment | 6.0 | 9.5 |
| Exercise, 2+ | 9.0 | 12.6 |
| Weight/diet control, % | ||
| Lose weight | 2.1 | 0.6 |
| Reduce intake of harmful ingredients | 2.1 | 0.6 |
| Avoid certain foods/drinks | 0.0 | 0.0 |
| Add vegetables/fruits/juices | 0.9 | 1.6 |
| Add healthy ingredients | 1.3 | 1.9 |
| Daily activity/movement, % | ||
| Change activities | 42.7 | 47.0 |
| Avoid activities | 59.8 | 64.0 |
| Adopt new activities | 62.4 | 70.7* |
| Activity change, 2+ | 58.5 | 63.7 |
| Heat and cold treatment, % | ||
| Heat treatment | 23.5 | 19.6 |
| Cold treatment | 0.9 | 0.6 |
| Dietary supplements, % | 2.6 | 3.5 |
| Natural remedies, % | 3.4 | 2.8 |
| Relaxation techniques, % | 18.4 | 21.5 |
| Ointment/salves, % | 6.0 | 10.7* |
| Optimal self-management, % (exercise 2+, activity 2+, heat) | 20.1 | 18.6 |
n=551
p<.05
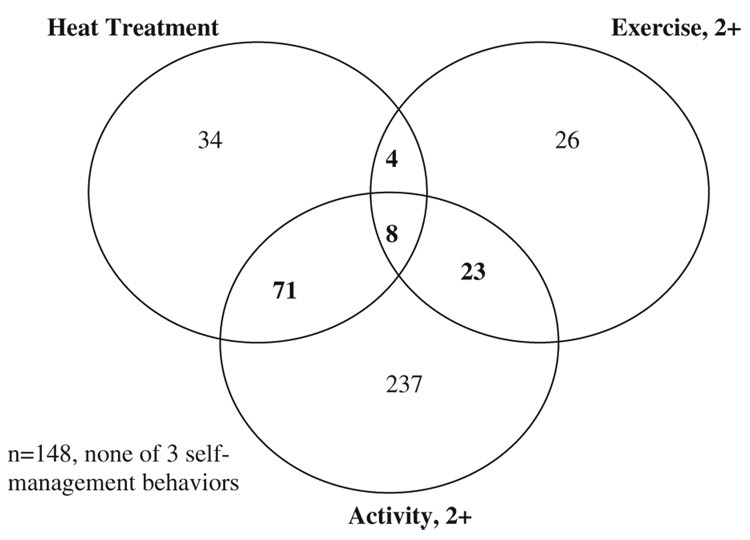
The co-occurrence of behaviors used to define optimal self-management is shown in a Venn diagram (Fig. 1). Only 8/551 participants, 1.5%, met all three indicators used to define optimal self-management. 26.9% (149/551) reported none of the three behaviors. A majority reported activity change in response to osteoarthritis.
Fig. 1.
Distribution of behaviors: optimal self-management
Correlates of optimal self-management
In this sample, likelihood of optimal self-management was not associated with demographic differences, such as race, gender, education, or marital status (Table 2). Neither duration of disease nor severity increased the likelihood of optimal self-management.
Table 2.
Correlates of Optimal Self-Management, by Osteoarthritis Severity
| Optimal OA self-management (%) | ||
|---|---|---|
| Low OA severity | ||
| White (n=142) | 18.3 | |
| Black (n=92) | 22.8 | |
| Male (n=112) | 16.1 | |
| Female (n=122) | 23.8 | |
| Age | 65–74 (n=158) | 18.4 |
| 75+ (n=76) | 23.7 | |
| Education | <HS (n=43) | 25.6 |
| HS (n=75) | 24.0 | |
| Some college (n=70) | 12.9 | |
| BA/BS+ (n=46) | 19.6 | |
| Marital status | Single (n=108) | 23.1 |
| Married (n=126) | 17.5 | |
| Duration of disease, year | ≤5 (n=106) | 20.8 |
| 6+ (n=128) | 19.5 | |
| High OA severity | ||
| White (n=125) | 14.4 | |
| Black (n=192) | 21.4 | |
| Male (n=102) | 25.5 | |
| Female (n=215) | 15.3 | |
| Age | 65–74 (n=191) | 19.4 |
| 75+ (n=126) | 17.5 | |
| Education | <HS (n=70) | 18.6 |
| HS (n=123) | 16.3 | |
| Some college (n=86) | 18.6 | |
| BA/BS+ (n=38) | 26.3 | |
| Marital status | Single (n=168) | 17.9 |
| Married (n=149) | 19.5 | |
| Duration of disease, year | ≤5 (n=108) | 17.6 |
| 6+ (n=209) | 19.1 |
Outcomes associated with optimal self-management
Within disease severity categories, optimal self-management was associated with less reported pain (4.3 vs. 5.1 in mild-moderate disease, 6.0 vs. 6.5 in severe disease; p<.05) in two-way ANCOVA adjusting for demographic indicators and number of comorbid conditions. Participants adopting optimal self-management were also likely to report greater perceived control over health, though this association achieved only marginal significance. Optimal self-management was not associated with any of the other outcomes (Table 3).
Table 3.
Disease Outcomes, by Severity of Osteoarthritis and Self-Management Status
| Range | Mild OA |
Severe OA |
|||
|---|---|---|---|---|---|
| Sub-optimal (n=162) | Optimal (n=41) | Sub-optimal (n=231) | Optimal (n=58) | ||
| Pain* | 0–10 | 5.1 | 4.3 | 6.5 | 6.0 |
| CESD | 0–29 | 6.2 | 6.3 | 7.2 | 6.6 |
| N-IADL | 0–5 | .7 | .7 | 1.4 | 1.1 |
| OPS** | 2–5 | 4.0 | 4.1 | 3.9 | 4.1 |
| Networks | 2–50 | 31.5 | 31.7 | 32.2 | 32.9 |
| PCS | 11.5–60.8 | 39.7 | 39.9 | 32.3 | 30.3 |
| MCS | 9.5–79.3 | 54.2 | 54.6 | 53.8 | 51.8 |
Means adjusted for age, gender, education, race, and number of comorbid conditions
CESD Center for Epidemiological Studies-Depression, N-IADL number of difficulties with instrumental activities, OPS Optimal Primary and Secondary Control Scale, Networks Lubben Social Network Scale, PCS physical component score: SF-36, MCS mental component scale, SF-36
p<.05
p<.10 in two-way analysis of covariance (OA severity, OA management)
To determine if the relation between optimal self-management and pain was uniform across race groups, we examined the interaction of race and optimal self-management in ANCOVA models, again stratifying by disease severity. Results are shown in Table 4 and Fig. 2. Pain reports were significantly related to OA severity (F=29.2, p<.001), with greater severity associated with higher levels of reported pain. Optimal self-management was also a significant main effect (F=5.2, p=.02), with optimal self-management associated with lower pain ratings within each category of disease severity. The model also showed important interactions between OA severity and race (F=6.0, p=.015): African–Americans reported less pain than whites in mild OA but more pain than whites in severe OA. Figure 2 shows that this pattern is explained by optimal self-management. Lower ratings of pain in mild OA among African–Americans were apparent only in the optimal self-management group. Similarly, lower pain ratings among whites with more severe OA were evident only in the optimal self-management group.
Table 4.
Self-Reported Pain, by Race, Severity of Osteoarthritis, and Self-Management Status
| OA severity | Self-management | Race (n) | Pain rating (SE) |
|---|---|---|---|
| Mild–moderate OA | Sub-optimal | White (116) | 5.25 (.23) |
| African–American (71) | 5.01 (.30) | ||
| Optimal | White (26) | 5.05 (.47) | |
| African–American (21) | 3.49 (.58) | ||
| Severe OA | Sub-optimal | White (107) | 6.51 (.23) |
| African–American (151) | 6.49 (.24) | ||
| Optimal | White (18) | 5.51 (.60) | |
| African–American(41) | 6.57 (.41) |
Adjusted for age, gender, number of medical conditions (p<.001), and education (p=.03); significant main effects: OA severity (F=29.2, p<.001), optimal self-management (F=5.2, p=.02); significant interaction effects: OA severity × gender (p=.006), OA severity × race (p=.015), OA severity × optimal × race (p=.039)
Fig. 2.
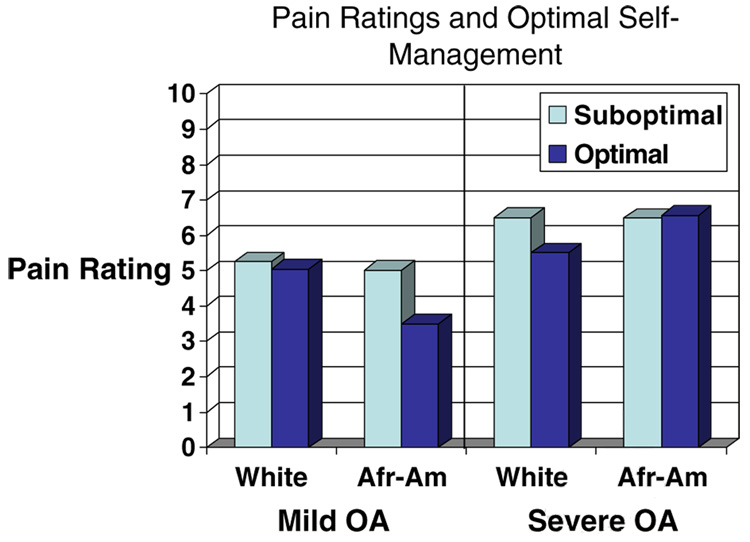
Pain ratings and optimal self-management
To explore this pattern of differences, we examined perceived control in a similar ANCOVA model. African–Americans reported greater control than whites across severity and optimal self-management categories (F=4.45, p=.035). Perceived control was associated with optimal self-management (F=3.1, p=.079), but this relationship was evident only among whites. Thus, differences in perceived control may account for some of the differences in pain ratings between the race groups.
Discussion
In this population-based sample of older people with osteoarthritis only about 20% practiced optimal self-management of symptoms. White and African–American elders did not significantly differ in the proportion practicing optimal self-management.
To define optimal self-management we used clinical criteria recommended by Lorig and colleagues (2000). These include changes in activity, use of hot compresses on affected joints, and exercise. We recognize that optimal self-management can be defined in other ways and that no consensus recommendation is available. Given our open-ended elicitation of these behaviors, we were able to define a number of subtypes within the activity and exercise categories. We considered people to meet the activity or exercise criterion if they performed two or more of the different subtypes, and we required that people report at least two of the three broad types of behavior to be considered an optimal self-manager. Is 20% prevalence for optimal self-management high or low? One reasonable touchstone is the proportion of Americans practicing a healthy lifestyle, as defined by exercise (30 min day/five times each week), fruit and vegetable consumption (five servings per day), non-obesity (body mass index between 18.5 and 25), and absence of smoking. Results from the Behavioral Risk Factors Surveillance System suggest that only 3% of Americans meet this criterion (MMWR 2001). Thus, the 20% prevalence of optimal self-management suggests that people with chronic disease are likely to change lifestyles to respond to symptoms. People are much less likely to change lifestyles for prevention.
The behaviors we recorded represent lay responses to the challenge of living with osteoarthritis. These give an indication of daily management. While we do not have information on how people learned to use hot compresses or decided to exercise despite arthritis, it is likely that people learn these strategies from a variety of sources, including physician and allied health personnel, social networks, and the media. This is the likely backdrop for any programmatic effort to teach self-management. People may be more or less receptive to formal arthritis self-management training (such as the Arthritis Self-Management Course [Osborne et al. 2007] or Fit and Strong! [Hughes et al. 2006] according to their current self-management practices, but we are unaware of research that has investigated this factor explicitly.
Optimal self-managers reported significantly less pain, the only one of the symptom management outcomes to show significant differences. This finding is robust across severity of disease and remained significant after adjustment for many factors associated with reports of pain. Pain may show a stronger association with optimal self-management than the other outcomes because it is a symptom directly related to osteoarthritis and because self-management behaviors are likely undertaken directly to reduce pain. The other outcomes are more diffuse and likely to be related to many factors besides disease severity or self-management. Still, it would be incorrect to say that optimal self-management results in less pain. It may be that people with less pain are better able to practice optimal self-management. Longitudinal analyses are required to investigate causal relationships.
The association between self-management and pain varied by race groups. Optimal self-management was associated with lower pain ratings for both whites and African–Americans, but the benefit was clearest for severe disease among whites and for mild-moderate disease among African–Americans. This interaction was unexpected and requires further investigation. The reasons for this difference are not completely clear. Our data suggest that the race difference may involve control expectancies. Perceived control was associated with optimal self-management only among whites. The relationship between perceived control and self-management needs further investigation.
We conclude that naturally-occurring self-management behaviors can be grouped according to clinical recommendations to define groups that manage disease more or less effectively. Support for the validity of clinical recommendations can be seen in the lower ratings of pain reported by optimal self-managers. The differential benefit of optimal self-management among race or ethnic groups suggests potentially important cross-cultural differences. However, longitudinal studies are required to examine the association between adoption of particular self-care behaviors and patient outcomes.
Acknowledgments
Financial support was provided by the National Institute on Health to Dr. Silverman (R01 AG 18308), Dr. Albert (R01 AG18234), and the University of Pittsburgh Claude D. Pepper Older Americans Independence Center (P30 AG024827). The authors thank Chongyi Wei for helping with data management and coding.
Contributor Information
Steven M. Albert, Department of Behavioral and Community Health Sciences, Graduate School of Public Health, University of Pittsburgh, A211 Crabtree, 130 DeSoto St., Pittsburgh, PA 15261, USA, e-mail: smalbert@pitt.edu
Donald Musa, University Center for Social and Urban Research, University of Pittsburgh, Pittsburgh, PA 15261, USA.
Kent Kwoh, Department of Rheumatology, School of Medicine, University of Pittsburgh, Pittsburgh, PA 15261, USA.
Myrna Silverman, Department of Behavioral and Community Health Sciences, Graduate School of Public Health, University of Pittsburgh, A211 Crabtree, 130 DeSoto St., Pittsburgh, PA 15261, USA.
References
- Albert SM, Musa D, Kwoh CK, Hanlon JT, Silverman M. Self-care and professionally-guided care in osteoarthritis: Racial differences in a population-based sample. Journal of Aging and Health. 2008;20:198–216. doi: 10.1177/0898264307310464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G, Horvath J. The growing burden of chronic disease in American. Public Health Reports. 2004;119:263–270. doi: 10.1016/j.phr.2004.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) American Journal of Preventive Medicine. 1994;10:77–84. [PubMed] [Google Scholar]
- Ang J, Shen P, Monahan D. Factorial invariance found in survey instrument measuring arthritis-related health beliefs among African–Americans and Whites. Journal of Clinical Epidemiology. 2008;61:289–294. doi: 10.1016/j.jclinepi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Burns R, Graney MJ, Lummus AC, Nichols LO, Martindale-Adams J. Differences of self-reported osteoarthritis disability and race. Journal of the National Medical Association. 2007;99:1046–1051. [PMC free article] [PubMed] [Google Scholar]
- Buszewicz M, Rait G, Griffin M, Nazareth I, Patel A, Atkinson A, et al. Self management of arthritis in primary care: randomised controlled trial. BMJ (Clinical Research Ed.) 2006;333(7574):879. doi: 10.1136/bmj.38965.375718.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneveld PW, Kwoh CK, Mor MK, Appelt CJ, Geng M, Gutierrez JC, et al. Racial differences in expectations of joint replacement surgery outcomes. Arthritis and Rheumatism. 2008;59:730–737. doi: 10.1002/art.23565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckhausen J, Schulz R. Developmental regulation in adulthood: Selection and compensation via primary and secondary control. In: Heckhausen J, Skinner E, editors. Motivation and control over the life course. New York: Cambridge University Press; 1998. pp. 50–77. [Google Scholar]
- Heuts PH, de Bie R, Drietelaar M, Aretz K, Hopman-Rock M, Bastiaenen CH, et al. Self-management in osteoarthritis of hip or knee: a randomized clinical trial in a primary healthcare setting. The Journal of Rheumatology. 2005;32:543–549. [PubMed] [Google Scholar]
- Hughes SL, Seymour RB, Campbell RT, Huber G, Pollak N, Sharma L, et al. Long-term impact of Fit and Strong! on older adults with osteoarthritis. The Gerontologist. 2006;46:801–814. doi: 10.1093/geront/46.6.801. [DOI] [PubMed] [Google Scholar]
- Jordan JM, Bernard SL, Callahan LF, Kincade JE, Konrad JE, Defriese GH. Self-reported arthritis-related disruptions in sleep and daily life and the use of medical, complementary, and self-care strategies for arthritis. Archives of Family Medicine. 2000;9:143–149. doi: 10.1001/archfami.9.2.143. [DOI] [PubMed] [Google Scholar]
- Lequesne MG. The algofunctional indices for hip and knee osteoarthritis. The Journal of Rheumatology. 1997;24:779–781. [PubMed] [Google Scholar]
- Lorig K, Holman H, Sobel D, Laurent D, Gonzalez V, Minor M. Living a healthy life with chronic conditions. 2nd ed. Boulder: Bull; 2000. [Google Scholar]
- Lubben JE. Assessing social networks among elderly populations. Family & Community Health. 1988;11:42–52. [Google Scholar]
- Lubben J, Gironda M. Measuring social networks and assessing their benefits. In: Phillipson C, Allan G, Morgan D, editors. Social networks and social exclusion: sociological and policy perspectives. Hampshire: Ashgate; 2004. [Google Scholar]
- McHorney CA, Ware JE, Raczek AE. The MOS 36-itern short-form health survey (SF-36): II, Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- MMWR. Prevalence of healthy lifestyle characteristics—Michigan, 1998 and 2000. Morbidity and Mortality Weekly Review. 2001;50(35):758–761. 9/7/01. [PubMed] [Google Scholar]
- Ory M, DeFriese G. Self-care in later life: Research, program, and policy issues. Thousand Oaks: Sage; 1998. [Google Scholar]
- Osborne RH, Wilson T, Lorig KR, McColl GJ. Does self-management lead to sustainable health benefits in people with arthritis? A 2-year transition study of 452 Australians. The Journal of Rheumatology. 2007;34:1112–1117. [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Sevick MA, Trauth JM, Ling BS, Anderson RT, Piatt GA, Kilbourne AM, et al. Patients with complex chronic diseases: Perspectives on supporting self-management. Journal of General Internal Medicine. 2007;22:438–444. doi: 10.1007/s11606-007-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M, Musa D, Nutini J, King J, Albert SM. I stretch when I get up and rest in the afternoon: Daily temporal responses to osteoarthritis symptoms by older adults. Journal of Cross-Cultural Gerontology. 2008;This volume doi: 10.1007/s10823-008-9082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Kosinski M. SF-36® physical and mental health summary scales: a manual for users of version 1. 2nd ed. Lincoln: QualityMetric; 2001. [Google Scholar]
- Wrosch C, Schulz R, Heckhausen J. Health stresses and depressive symptomatology in the elderly: The importance of health engagement control strategies. Health Psychology. 2002;21:340–348. doi: 10.1037//0278-6133.21.4.340. [DOI] [PubMed] [Google Scholar]