Abstract
BACKGROUND:
During intra-arterial(IA) revascularization, either guide catheter injections of contrast in the neck or microcatheter contrast injections (MCIs) at or beyond the site of an occlusion, can be used to visualize intracranial vasculature. Neurointerventionalists vary widely in their use of MCIs for a given circumstance. We tested the hypothesis that MCIs are a risk factor for intracranial hemorrhage (ICH) in the Interventional Management of Stroke (IMS) I and II trials of combined IV/IA rt-PA therapy.
METHODS:
All arteriograms with M1, M2, and ICA terminus occlusions were reanalyzed (n=98). The number of MCIs within or distal to the target occlusion was assigned. Post-procedure CTs were reviewed for CEx and ICH. CEx was defined as a hyperdensity suggestive of contrast (Hounsfield unit>90) seen at 24 hours, or present prior to 24 hours and persisting or replaced by ICH at 24 hours.
RESULTS:
In this IMS subset, the rate of any ICH was 58% (57/98). More MCIs were seen in the ICH group (median=2 vs 1; p=0.04). Increased MCIs were associated with higher ICH rates (p=0.03). MCIs remained associated with ICH in multivariable analysis (p=0.01), as did baseline CT edema/mass effect, atrial fibrillation, time to IV rt-PA initiation, and TICI reperfusion score. MCIs were also associated with CEx in unadjusted and adjusted analyses.
CONCLUSIONS:
MCIs may risk ICH in the setting of combined IV/IA rt-PA therapy, possibly due to contrast toxicity or pressure transmission by injections. MCIs should be minimized whenever possible. These findings will be tested prospectively in the IMS III trial.
Keywords: Acute Stroke, Cerebral Infarct, Interventional Neuroradiology, Intracerebral Hemorrhage, Thrombolytic Rx
INTRODUCTION
As the use of intra-arterial (IA) therapy for acute ischemic stroke becomes widespread in the United States,1 the analysis of procedural factors that may have an impact on clinical outcome is critical.
During an IA intracranial revascularization procedure, contrast material can be injected either through a guide catheter in the neck, or through an intracranial microcatheter at or beyond the site of an occlusion. Microcatheter contrast injections (MCIs) are used to identify the proximal thrombus, confirm catheter placement within the thrombus, or visualize the vasculature distal to an occlusive thrombus. Specific circumstances may necessitate at least one MCI for acute revascularization. For example, at least one MCI beyond a proximal internal carotid artery occlusion may be required to determine the location of the distal symptomatic occlusion. Revascularization with the MERCI® device also requires one MCI for identifying the distal aspect of the thrombus prior to its attempted retrieval. On the other hand, some cases require no MCIs for acute thrombolytic revascularization. Neurointerventionalists vary widely in their practice regarding how often they routinely perform MCIs, at times using many MCIs in a single case.
Based on anecdotal experience, we hypothesized that MCIs may be a risk factor for intracranial hemorrhage (ICH), either indirectly by causing contrast extravasation with toxicity to the blood-brain-barrier or directly by causing traumatic injury of the blood-brain-barrier and microvasculature through the pressure effects of these injections. If MCIs are indeed a risk factor for ICH, minimizing their use would be warranted.
Therefore, we reanalyzed cases from the Interventional Management of Stroke (IMS) I and II trials of combined IV/IA rt-PA2,3 to (1) determine if MCIs are an independent risk factor for ICH and (2) explore the association of MCIs with contrast extravasation (CEx).
METHODS
The IMS I and II trials were designed to test the safety of combined low-dose intravenous rt-PA (0.6 mg/kg) followed by delivery of additional IA rt-PA (up to 22 mg) at the site of symptomatic occlusion in patients with moderate-to-large (NIHSS score ≥10) ischemic strokes treated within three hours of symptom onset. In the IMS II trial, IA rt-PA was delivered in the setting of low-energy ultrasound via the EKOS MicroLysis® Micro-infusion Catheter whenever possible. Heparin was administered following identification of a treatable lesion as a 2000 unit IV bolus, followed by a 450 units/hour IV infusion, and discontinued at the end of the IA procedure. Contrast material type and volume were not systematically recorded.
For this analysis, all arteriograms with M1, M2, and ICA terminus occlusions from these two trials were reanalyzed (n=98). Cases treated with only IV therapy were excluded.
Based on available imaging, the number of MCIs within or distal to the target occlusion was assigned for every case. All available post-procedure CTs were reviewed for CEx and ICH. CEx was defined as a hyperdensity suggestive of contrast (Hounsfield unit>90) seen at 24 hours, or present prior to 24 hours and persisting or replaced by ICH at 24 hours. CEx was considered as distinct from contrast enhancement, defined as a hyperdensity that cleared within 24 hours based on definitions published by Yoon et al.4 It should be noted that these terms (contrast extravasation versus deposition) have been used synonymously in some of the stroke literature.
Statistical analyses of the locked IMS I and II databases were performed using SAS® statistical software, version 9.1. Two primary multivariable analyses were performed: (1) an ICH prediction model and (2) a CEx prediction model. For each model, variables were tested in univariate analysis using either t-tests or Wilcoxon Rank Sum test for continuous variables or Chi-Square/Fisher's Exact tests for categorical variables. Tested covariables were: number of MCIs, baseline CT with edema or mass effect, baseline NIHSS score, history or new diagnosis of atrial fibrillation, time to IV treatment, age, baseline blood glucose level (continuous), Thrombolysis in Cerebral Infarction (TICI) reperfusion grades, baseline systolic blood pressure, history of diabetes mellitus, arterial occlusive lesion (AOL) recanalization score,5 proximal ICA occlusion, IA procedure duration, baseline INR, total IA rt-PA dose, site of symptomatic occlusion (ICA, MCA1, MCA2, MCA3) and ultrasound delivery. Variables significant (p<0.25) in univariate analysis were considered as potential covariables in logistic regression analyses. A backward elimination approach was used to find the most parsimonious model, and then variables were reentered into the model individually to assess their effects on the independent variable of interest.
Upon further consideration of the results, we also developed a multivariable logistic model to assess the association of MCIs and three-month clinical outcome. The same covariables were assessed using the same criteria for inclusion as used above models, and good clinical outcome was defined as mRS ≤2.
RESULTS
In this subset of cases from the IMS I and II trials, the rate of any intracranial (ICH) hemorrhage was 58% (57/98). The rate of PH2s in this subset was 10% (10/98). The median number of MCIs was 2, and the frequency of their use varied widely between cases (range 0-12).
The Relationship between MCIs and ICH
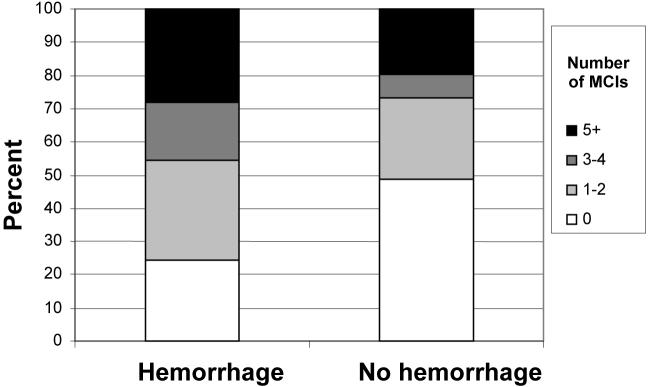
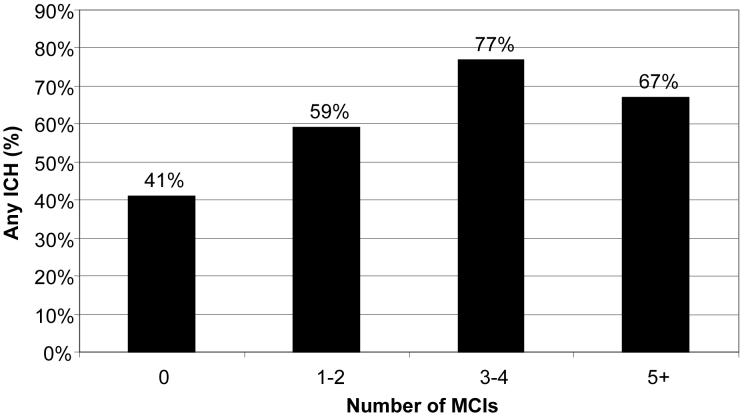
Unadjusted analyses showed more MCIs in the ICH group (median=2) compared to the non-ICH group (median=1; p=0.04), as displayed in Figure 1. Cases with increased numbers of MCIs were associated with higher rates of ICH (p=0.03; Figure 2). After excluding proximal ICA occlusions (n=7), which often necessitate at least one MCI, increased MCIs still showed a trend towards an association with increased ICH rates (p=0.08).
Figure 1.
MCIs in ICH versus Non-ICH Groups
Figure 2.
Rates of ICH based on Frequency of MCIs
Covariates with potential significance in univariate testing (and therefore considered in the model) were baseline CT with edema or mass effect, baseline NIHSS score, history or new diagnosis of atrial fibrillation, time to IV treatment, age, glucose (continuous), TICI reperfusion score, baseline systolic blood pressure, history of diabetes mellitus, and site of symptomatic occlusion (Table 1). As there was only one subject with an M3 occlusion, this case was excluded from the multivariable analysis. MCIs remained associated with ICH after adjustment for significant ICH risk factors (Table 2).
Table 1.
Odd Ratios of ICH for each Covariable (Univariate Analysis)
| Covariable | OR (95% CI) | p-value |
|---|---|---|
| CT Edema/ Mass Effect | 3.92 (1.64, 9.37) | 0.002 |
| Vessel of occlusion ICA-T MCA-M1 branch MCA-M2 branch |
10.45 (2.81, 38.8) 2.79 (1.05, 7.47) reference |
0.002 0.04 |
| # of MCIs (categorized as 0, 1-2, 3-4, 5+) |
1.48 (1.03, 2.12) | 0.03 |
| Time to IV rt-PA Initiation (Increasing15-minute intervals) |
1.02 (1.00, 1.03) | 0.03 |
| Baseline NIHSS score | 1.10 (1.00, 1.20) | 0.05 |
| Age (years) | 1.03 (1.00, 1.07) | 0.05 |
| Atrial Fibrillation | 2.43 (0.91, 6.49) | 0.08 |
| Degree of Reperfusion Success (Scored as TICI 0, 1, 2, 3) |
0.65 (0.40, 1.06) | 0.09 |
| Baseline blood glucose (mg/dL) | 1.01 (1.00, 1.02) | 0.16 |
| Baseline systolic blood pressure (mmHg) |
1.01 (0.99, 1.03) | 0.17 |
| Diabetes mellitus | 2.01 (0.58, 6.93) | 0.27 |
| Proximal ICA occlusion | 1.87 (0.34, 10.17) | 0.47 |
| Ultrasound Delivery | 1.28 (0.51, 3.18) | 0.60 |
| IA procedure duration | 1.00 (0.99, 1.01) | 0.67 |
| Total IA rt-PA Dose | 1.01 (0.94, 1.08) | 0.75 |
| Baseline INR | 0.78 (0.02, 37.58) | 0.90 |
| Degree of Recanalization Success (scored as AOL 0, 1, 2, 3) |
1.00 (0.71, 1.40) | 0.98 |
Table 2.
Independent Risk Factors of ICH in Multivariable Analysis
| Risk Factor | OR (95% CI) | p-value |
|---|---|---|
| Vessel of occlusion ICA-T MCA-1 MCA-2 |
12.10 (2.12, 69.19) 4.41 (1.26, 15.44) reference |
0.005 0.02 |
| Atrial Fibrillation | 6.64 (1.60-27.58) | 0.009 |
| CT Edema/ Mass Effect | 3.98 (1.27-12.41) | 0.02 |
| # of MCIs (categorized as 0, 1-2, 3-4, 5+) |
1.63 (1.02-2.58) | 0.04 |
| Time to IV rt-PA Initiation (Increasing15-minute intervals) |
1.58 (1.19-2.19) | 0.003 |
| Degree of Reperfusion Success (Scored as TICI 0, 1, 2, 3) |
0.54 (0.30-1.05) | 0.07 |
The Relationship between MCIs and CEx
Median MCIs were 3.5 in the CEx group (n=18) and 1.0 in the non-CEx group (n=80; p=0.03). MCIs were associated with CEx after adjusting for the only additional significant covariate of baseline INR (OR 1.60; 95% CI 1.0-2.60; p=0.02). All sites in IMS I and II, except for one, reported using only non-ionic contrast agents.
The Relationship between CEx and ICH
As per our definitions, all CEx cases (18/18) developed ICH, compared to 48% (38/80) of non-CEx cases (p<0.0001). In addition, 28% (5/18) of CEx cases developed PH2s, compared to 9% (7/80) of the non-CEx cases (p=0.02).
The Relationship between MCIs to Clinical Outcome
Upon consideration of number of MCIs and its association to good clinical outcome (defined as mRS 0-2), no significant association was seen in univariate analysis OR =0.84 95%CI (0.60, 1.20, p=0.34). Therefore, further multivariable models of clinical outcome were not developed.
DISCUSSION
MCIs were significantly associated with total ICH risk in the setting of IV/IA thrombolysis in this retrospective analysis. The relationship was robust. It was seen when comparing the median number of MCIs among ICH and non-ICH cases. In addition, cases with higher numbers of MCIs had higher rates of ICH, suggesting a “dose-dependent” effect. This relationship remained significant after controlling for additional factors that may affect hemorrhage rates.
Potential confounders of the relationship between MCIs and ICH may include a higher frequency of MCIs performed in cases of greater complexity with larger clots, or clots more resistant to thrombolysis. We attempted to address these possibilities by controlling for baseline stroke severity (NIHSS score), the presence of a concurrent extracranial ICA occlusion, IA procedure duration, total rt-PA dose, and final revascularization status. The association of MCIs and ICH remained significant after adjustment for these variables.
It should be noted that we considered risk factors of total ICH, rather than those of only the most severe radiologically subtypes of ICH (parenchymal hematoma types I and II), inorder to have a sample of adequate size for multivariable analyses. Whether milder forms of ICH should be avoided has not been determined, and requires further study.
Additional methodological limitations should be noted, although these would not be expected to lead to a systematic bias. Specifically, the number of MCIs for each case may have been undercounted if all the angiographic runs were not recorded and provided to the central reader. In addition, this analysis cannot account for variable techniques used for MCIs in individual cases, including different injection pressures, syringe sizes, contrast bolus volumes, contrast types, and contrast dilutions.
This observed relationship between MCIs and ICH raises the question of its mechanism. We considered the possibility that MCIs may lead to ICH by causing contrast extravasation. Several studies have examined the issue of contrast deposition (extravasation or enhancement) in the brain. It has long been acknowledged that contrast deposition may be identified on post-thrombolysis CT scans and misclassified as ICH, and may have no untoward effect.6 However, the literature suggests that ICH in the absence of contrast deposition after IA therapy is less common.7,8 Nakano reported that 37 of 77 (48%) stroke patients exhibited “hyperdense” areas after IA tPA treatment. Eleven of 37 (29.7%) developed symptomatic ICH, and no patient developed ICH in the absence of a hyperdense area. Yokogami et al also suggested a significant correlation between post-procedure CT scans with hyperdensity and hemorrhagic complications.9 Yoon et al formally distinguished CEx from contrast deposition using Hounsfield units, and found that extravasation portended a higher SICH rate and poorer outcome than did enhancement. They proposed that contrast extravasation may be due to degradation of the basal lamina, whereas contrast enhancement may be due to increased blood-brain-barrier permeability.4 The former disruption would likely be required for hemorrhagic transformation.10 It is possible that different contrast materials may have different blood-brain permeability effects and tendencies to cause ICH.11,12
Among the contrast deposition studies described above, only that of Nakano et al described local microcatheter injection as part of the IA technique. Their group subsequently reported a relationship between early venous filling demonstrated by MCI arteriography, arising particularly from the lenticulostriate distribution, and both contrast extravasation and hemorrhagic transformation.13 They pointed out that early veins were primarily seen when contrast material was injected into the ischemic area using MCI rather than guide catheter arteriography. However, a causal link between MCIs and both CEx and ICH was not proposed by the authors.
We found a statistically significant association between MCI use and contrast extravasation in both adjusted and unadjusted analyses. However, our exploration of the relationship between MCIs and CEx has key limitations. Specifically, Hounsfield units could not be measured in several of the cases due to the availability of only hard copy films. Furthermore, GRE MR imaging was not available to make these distinctions. Therefore, some cases may have been inaccurately categorized as ICH rather than contrast extravasation, and some cases of CEx may have been overlooked. In addition, cases of contrast extravasation preceding ICH may have been missed because the IMS I and II protocols did not mandate CT imaging prior to 24 hours from treatment unless clinically indicated. It is well-known that contrast hyperdensity diminishes in the first 24 hours, and may be reduced to less than 90 Hounsfield units beyond this time. Despite this potential for underestimating CEx, MCIs were associated with this phenomenon in this analysis. It remains possible that CEx may cause ICH through direct toxicity, or may be an epiphenomenon of pressure transmission from injections causing blood-brain-barrier disruption. If the former is the primary cause, then washing injected contrast with a saline infusion immediately afterwards may be beneficial. This may particularly avoid prolonged contrast stagnation in an artery without flow.
Inherent in our definition of contrast extravasation was the likelihood that all cases of contrast extravasation would ultimately be classified as ICH. However, it is notable that significantly more PH2s were seen in the contrast extravasation group compared to the non-CEx group, suggesting that contrast extravasation is a clinically relevant and potentially detrimental phenomenon.
This analysis of ICH risk factors is notable for two additional findings. First, time to initiation of IV rt-PA therapy (alteplase) was independently associated with ICH in our cohort, replicating prior time observations from the pilot study of duteplase regarding total ICH 14 and from the ATLANTIS Part A trial of alteplase regarding symptomatic ICH.15 Second, we show that the extent of reperfusion on angiography (TICI score) was inversely associated with ICH. These cases with no or minimal reperfusion by the end of the procedure may actually be those with better revascularization at a later time. If so, this would be consistent with prior studies by Molina et al suggesting that late recanalization (beyond 6 hours) is associated with higher rates of total ICH among proximal MCA stroke cases.16,17
Our findings are in contrast to the ICH model developed based on the IMS I trial alone, which suggested that any ICA stenosis or occlusion (versus isolated MCA occlusion) and atrial fibrillation were the only independent risk factors for total ICH.18 Potential explanations for this different result include the smaller sample size (n=80) and the inclusion of all patients in intention-to-treat group in the IMS I hemorrhage analysis (as opposed to only M1, M2, and ICA terminus occlusions from both IMS I and II in the current analysis).
In conclusion, MCIs were significantly associated with ICH risk in this retrospective analysis of a cohort of patients treated with combined IV/IA therapy. This may be due to contrast extravasation and its toxicity, or possibly pressure transmission from the injections. Our observations regarding contrast extravasation had several limitations and, thereby, cannot be conclusive about the mechanism by which MCIs are associated with ICH. Nevertheless, we provide compelling data to suggest that MCIs should be minimized during IV/IA revascularization procedures whenever possible. This relationship will be tested in additional acute revascularization trial cohorts, including prospectively in the IMS III trial.
Acknowledgments
Clinical Coordinating Center: University of Cincinnati
Canadian Coordinating Center: University of Calgary
Statistical Coordinating Center: Medical University of South Carolina
- Clinical Sites, Investigators and Coordinators
- – University of Cincinnati, University of Calgary, University of Pittsburgh, Oregon Health Sciences, University of Texas-Houston, Case Western Reserve University, University of California-SF, University of California-LA, Wayne State University, St. Luke's Hospital of Kansas City, Vancouver General Hospital, University of Alberta-Edmonton, Erlanger Health Care System, University of British Columbia, Henry Ford Hospital, Emory University, St. Luke's Hospital-Mayo, One Site Sutter General Hospital, Queens University
Funding: NIH/NINDS R01NS39160, EKOS Corporation in partnership with NIHH R44HL64434, Genentech supplied study drug
Footnotes
Publisher's Disclaimer: (This is an un-copyedited author manuscript that was accepted for publication in Stroke, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at http://stroke.ahajournals.org/. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.)
Disclosures: JPB and TAT receive drugs and devices from Genentech, Concentric, EKOS, and Johnson & Johnson as co-PIs for the ongoing NIH-funded IMS III trial. PK and JAC also serve on the Executive Committee of this trial.
REFERENCES
- 1.Suzuki S, Saver JL, Scott P, Jahan R, Duckwiler G, Starkman S, Su Y, Kidwell CS. Access to Intra-Arterial Therapies for Acute Ischemic Stroke: An Analysis of the US Population. AJNR Am J Neuroradiol. 2004 November 1;25(10):1802–1806. [PMC free article] [PubMed] [Google Scholar]
- 2.IMS Study Investigators Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke. 2004 Apr;35(4):904–911. doi: 10.1161/01.STR.0000121641.77121.98. [DOI] [PubMed] [Google Scholar]
- 3.IMS II Trial Investigators The Interventional Management of Stroke (IMS) II Study. Stroke. 2007 Jul;38(7):2127–2135. doi: 10.1161/STROKEAHA.107.483131. [DOI] [PubMed] [Google Scholar]
- 4.Yoon W, Seo JJ, Kim JK, Cho KH, Park JG, Kang HK. Contrast enhancement and contrast extravasation on computed tomography after intra-arterial thrombolysis in patients with acute ischemic stroke. Stroke. 2004 Apr;35(4):876–881. doi: 10.1161/01.STR.0000120726.69501.74. [DOI] [PubMed] [Google Scholar]
- 5.Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T, IMS-I Investigators Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke. 2005 Nov;36(11):2400–2403. doi: 10.1161/01.STR.0000185698.45720.58. [DOI] [PubMed] [Google Scholar]
- 6.Wildenhain SL, Jungreis CA, Barr J, Mathis J, Wechsler L, Horton JA. CT after intracranial intraarterial thrombolysis for acute stroke. AJNR Am.J.Neuroradiol. 1994 Mar;15(3):487–492. [PMC free article] [PubMed] [Google Scholar]
- 7.Nakano S, Iseda T, Kawano H, Yoneyama T, Ikeda T, Wakisaka S. Parenchymal hyperdensity on computed tomography after intra-arterial reperfusion therapy for acute middle cerebral artery occlusion: incidence and clinical significance. Stroke. 2001 Sep;32(9):2042–2048. doi: 10.1161/hs0901.095602. [DOI] [PubMed] [Google Scholar]
- 8.Mericle RA, Lopes DK, Fronckowiak MD, Wakhloo AK, Guterman LR, Hopkins LN. A grading scale to predict outcomes after intra-arterial thrombolysis for stroke complicated by contrast extravasation. Neurosurgery. 2000 Jun;46(6):1307–14. doi: 10.1097/00006123-200006000-00005. discussion 1314-5. [DOI] [PubMed] [Google Scholar]
- 9.Yokogami K, Nakano S, Ohta H, Goya T, Wakisaka S. Prediction of hemorrhagic complications after thrombolytic therapy for middle cerebral artery occlusion: value of pre- and post-therapeutic computed tomographic findings and angiographic occlusive site. Neurosurgery. 1996 Dec;39(6):1102–1107. doi: 10.1097/00006123-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Lo EH. Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol.Neurobiol. 2003 Dec;28(3):229–244. doi: 10.1385/MN:28:3:229. [DOI] [PubMed] [Google Scholar]
- 11.Doerfler A, Engelhorn T, von Kummer R, Weber J, Knauth M, Heiland S, Sartor K, Forsting M. Are iodinated contrast agents detrimental in acute cerebral ischemia? An experimental study in rats. Radiology. 1998 Jan;206(1):211–217. doi: 10.1148/radiology.206.1.9423675. [DOI] [PubMed] [Google Scholar]
- 12.Wilcox J, Wilson AJ, Evill CA, Sage MR. A comparison of blood-brain barrier disruption by intracarotid iohexol and iodixanol in the rabbit. AJNR Am.J.Neuroradiol. 1987 Sep-Oct;8(5):769–772. [PMC free article] [PubMed] [Google Scholar]
- 13.Ohta H, Nakano S, Yokogami K, Iseda T, Yoneyama T, Wakisaka S. Appearance of early venous filling during intra-arterial reperfusion therapy for acute middle cerebral artery occlusion: a predictive sign for hemorrhagic complications. Stroke. 2004 Apr;35(4):893–898. doi: 10.1161/01.STR.0000119751.92640.7F. [DOI] [PubMed] [Google Scholar]
- 14.del Zoppo G, Poeck K, Pessin M, Wolpert S, Furlan A, Ferbert A, Alberts M, Zivin J, Wechsler L, Busse O, Greenlee R, Jr, Brass L, Mohr J, Feldman E, Hacke W, Kase C, Biller J, Gress D, Otis S. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann of Neurol. 1992;32:78–85. doi: 10.1002/ana.410320113. [DOI] [PubMed] [Google Scholar]
- 15.Clark WM, Albers GW, Madden KP, Hamilton S. The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g) : results of a double-blind, placebo-controlled, multicenter study. Thrombolytic therapy in acute ischemic stroke study investigators. Stroke. 2000 Apr;31(4):811–816. doi: 10.1161/01.str.31.4.811. [DOI] [PubMed] [Google Scholar]
- 16.Molina CA, Montaner J, Abilleira S, Ibarra B, Romero F, Arenillas JF, Alvarez-Sabin J. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke. 2001 May;32(5):1079–1084. doi: 10.1161/01.str.32.5.1079. [DOI] [PubMed] [Google Scholar]
- 17.Molina CA, Alvarez-Sabin J, Montaner J, Abilleira S, Arenillas JF, Coscojuela P, Romero F, Codina A. Thrombolysis-related hemorrhagic infarction: a marker of early reperfusion, reduced infarct size, and improved outcome in patients with proximal middle cerebral artery occlusion. Stroke. 2002 Jun;33(6):1551–1556. doi: 10.1161/01.str.0000016323.13456.e5. [DOI] [PubMed] [Google Scholar]
- 18.IMS Study Investigators Hemorrhage in the Interventional Management of Stroke study. Stroke. 2006 Mar;37(3):847–851. doi: 10.1161/01.STR.0000202586.69525.ae. [DOI] [PubMed] [Google Scholar]