Abstract
Pregnancy hormones are believed to be involved in the protection against breast cancer conferred by pregnancy. The authors explored the association of maternal breast cancer with human chorionic gonadotropin (hCG) and α-fetoprotein (AFP). In 2001, a case-control study was nested within the Northern Sweden Maternity Cohort, an ongoing study in which blood samples have been collected from first-trimester pregnant women since 1975. Cases (n = 210) and controls (n = 357) were matched for age, parity, and date of blood donation. Concentrations of hCG and AFP were measured by immunoassay. No overall significant association of breast cancer with either hCG or AFP was observed. However, women with hCG levels in the top tertile tended to be at lower risk of breast cancer than women with hCG levels in the lowest tertile in the whole study population and in subgroups of age at sampling, parity, and age at cancer diagnosis. A borderline-significant decrease in risk with high hCG levels was observed in women who developed breast cancer after the median lag time to cancer diagnosis (≥14 years; odds ratio = 0.53, 95% confidence interval: 0.27, 1.03; P = 0.06). These findings, though very preliminary, are consistent with a possible long-term protective association of breast cancer risk with elevated levels of circulating hCG in the early stages of pregnancy.
Keywords: alpha-fetoproteins, breast neoplasms, chorionic gonadotropin, pregnancy, prospective studies
Childbearing affords the mother lifetime protection against breast cancer. Compared with nulliparous women, women with at least 1 pregnancy experience, on average, a 25% reduction in risk of breast cancer (1). An increasing number of pregnancies confers further protection (1), evident for up to 8 births among grand-multiparous women (2). However, the protective effect becomes apparent several years following pregnancy: Initially, there is a transient increase in risk, which peaks about 5 years after a first delivery (3). Furthermore, the protection is strongest when the first pregnancy has been completed early in reproductive life (4).
The biologic reasons for the beneficial effect of pregnancy are not understood. A growing body of literature on chemical carcinogen-induced mammary tumor models in rodents has shown that pregnancy inhibits tumor formation. Administration of the main hormones of pregnancy, such as combined estrogen and progesterone or human chorionic gonadotropin (hCG), to virgin animals either before or after carcinogen exposure also confers strong and lasting protection (5–7). Thus, the benefits of a pregnancy could be related to exposure to specific patterns of endogenous hormones during its course.
Only a few studies—all prospective cohort studies—have addressed the role of pregnancy hormones in maternal breast cancer in humans. Two (8, 9) focused on α-fetoprotein (AFP), a member of the albumin superfamily (10) that has been shown to bind to estradiol and suppress estrogen-dependent growth of breast cancer cells (11, 12). Investigators in both studies reported protective associations of maternal breast cancer with increasing circulating levels of AFP, particularly at younger ages. A third study linked increased third-trimester levels of circulating progesterone, but not estrogen, to a 50% reduction in subsequent maternal breast cancer risk (13). So far, no epidemiologic study has directly related hCG levels during pregnancy to maternal breast cancer.
We utilized data from the Northern Sweden Maternity Cohort, a population-based cohort of more than 83,000 women who gave blood during the early months of pregnancy, to address the hypothesis that long-term maternal breast cancer risk is protectively associated with elevations in circulating hCG and AFP levels during the first trimester of pregnancy.
MATERIALS AND METHODS
Study subjects were part of the Northern Sweden Maternity Cohort, which is based at the University Hospital in Umeå (Umeå, Sweden). This cohort has been described previously (14). The cohort was established in November 1975 with the purpose of preserving for research use serum samples from pregnant women tested for systemic infections. Virtually all pregnant women from the 4 northernmost counties of Sweden (total population, approximately 800,000) visit one of the maternity health-care clinics in the region and donate a blood sample, mostly during the final weeks of the first trimester of pregnancy or the early weeks of the second trimester (weeks 6–18). These samples are periodically shipped, frozen, to a central repository at Umeå University Hospital, where they are analyzed for the presence of infections and the leftovers are stored at −20°C. As of 2001, the biorepository included approximately 110,000 serum specimens from more than 83,000 women.
A case-control study was nested within the Northern Sweden Maternity Cohort. Cohort members whose pregnancy resulted in the delivery of 1 live or stillborn infant were eligible. Miscarriages, induced abortions, and twin pregnancies were reasons for exclusion. Women whose blood had been drawn after the 20th week of gestation, had used hormonal medications to become pregnant, had used hormonal medications during pregnancy, or had had a preceding diagnosis of cancer (except nonmelanoma skin cancers) were also excluded. If a cohort member had donated blood samples during several pregnancies, she was included in the study with the first donated sample.
Potential cases were women diagnosed for the first time with invasive breast cancer after their entry into the cohort. Cases were identified through record linkage with the nationwide Swedish Cancer Registry using the unique 10-digit personal identity number assigned to every person born in or legally resident in Sweden. The registration of newly detected cancers in Sweden is based on mandatory reports from all physicians serving outpatient and inpatient departments in all public and private hospitals. Reporting is also mandatory for all pathologists involved with surgical biopsies, cytologic specimens, and autopsies, including private laboratories. The completeness of cancer registration in Sweden is considered close to 100%. Linkages carried out in 2000 and 2001 led to the identification of 426 potential case subjects.
Potential controls were selected among cohort members who were alive and free of cancer at the time of diagnosis of the index case. Controls were matched to the case (1:2 ratio) on parity at the time of blood sampling (primiparous, nonprimiparous), age at blood sampling (±2.5 years), and date of blood sampling (±3 months). Because information on subjects in the cohort is limited to the personal identity number, name, consecutive serial number of sampling, and place of sampling, identification of appropriately matched controls required a multistep approach. First, lists of up to 8 potential controls were drawn for each case based on approximate dates of blood drawing and birth (part of the personal identity number). We subsequently contacted potential cases and controls individually by letter (up to 2) and, if necessary, by phone, to describe the project and elicit permission to participate in the study and consent to the release of pregnancy medical records. Women willing to participate were asked to return the signed informed consent form along with a brief reproductive history questionnaire to facilitate matching for parity before medical records were requested. For women who consented and for cohort members who were deceased, a full copy of the maternity and delivery records was requested from the 10 hospitals in the region. Records were first abstracted with regard to matching information (parity and date of blood drawing) and eligibility (gestational age at blood drawing). Among eligible women who met the matching criteria for a given case, the remainder of the index pregnancy information was abstracted for 2 controls, chosen randomly.
Of the potential study subjects initially identified (426 cases and 3,408 controls), 1,279 controls were never contacted because of lack of funding. Among the subjects contacted, 2,378 (93%; 415 cases) returned the baseline information and 177 (7%; 11 cases) were nonrespondents. Among the respondents, 547 (58 cases) did not meet the eligibility criteria for various reasons (blood was drawn after 20 weeks of gestation, twin pregnancy, abortion/miscarriage, woman not pregnant, or missing/deteriorated blood sample (n = 57; 18 cases)). Inadequate matching (596 controls) and matched sets lacking either the case or both controls further reduced the number of subjects available for study to 351 cases and 850 controls. Insufficient funding at the end of the study period forced us to cease retrieval of information and selection of study subjects and exclude from laboratory analyses an unselected subset of otherwise-eligible study subjects (139 cases and 481 controls) who were very similar regarding all known characteristics to those included. Excluded from the present analyses were also 2 cases and 12 controls with no information about exact gestational day at blood donation, leaving us a total of 210 cases and 357 controls for the study.
Technicians, who were unaware of the case-control status of the specimens, performed the hormonal assays in the clinical chemistry laboratory of the University Hospital of Umeå. Individually matched case and control serum samples were always included in the same batch. All samples were measured in duplicate. If measurements between duplicates deviated by more than 5%, all samples in that batch were measured anew. Bio-Rad control samples (Lyphochek; Bio-Rad, Irvine, California) at 2 levels of the analyte were included in duplicate in each batch as quality controls. hCG was quantified with the Coat-a-Count hCG immunoradiometric assay (Diagnostic Products Corporation, Los Angeles, California). The antibody used is highly specific for intact hCG, with low cross-reactivity to other glycoprotein hormones present in patient samples and low cross-reactivity to α and β hCG subunits. The intra- and interbatch coefficients of variation based on the undiluted 170 mIU/mL Bio-Rad hCG control were 2.8% and 3.8%, respectively. AFP was quantified by enzyme-linked immunosorbent assay (Genentech, Inc., San Francisco, California). The intra- and interassay coefficients of variation based on the 17 μg/L Bio-Rad AFP control were 3.2% and 6.7% respectively. Probable outliers with hormone concentrations exceeding quartile 1 minus 3 times the interquartile range or quartile 3 plus 3 times the interquartile range were set to missing.
In samples drawn in 1988 and thereafter, there was an evident reduction in measured hCG concentrations to levels incompatible with pregnancy. No such effect on AFP concentrations was observed. Therefore, all subjects whose baseline blood sample had been drawn after January 1, 1988, were excluded from analyses of hCG (34 cases, 56 controls). Further exclusions involved a few other subjects with implausibly low hCG levels (<5,000 mIU/mL) or subjects who were matched to 1 such case (3 cases, 7 controls). Seven AFP measurements exceeding quartile 1 or 3 ± 3 times the interquartile range were set to missing. A total of 173 cases and 294 controls with valid hCG measurements and 204 cases and 349 controls with valid AFP measurements from sets with a case and at least 1 control were included in the statistical analyses.
Both hCG and AFP concentrations vary throughout pregnancy; thus, it was essential to take into consideration gestational day at blood donation. Exact matching for gestational day would have imposed unwarranted constraints for control selection and would have not been feasible even in a much larger cohort with gestational age data available in electronic format. Instead, to account for the hormonal variation with gestational age, we applied the statistical approach described by Richardson et al. (8). In brief, mean curves for hCG and AFP variation during pregnancy were estimated on the basis of all available hormonal data using local linear regression (15), a nonparametric smoothing technique that employs weighted regression and uses varying subsets of the data to estimate the curve at each point. Prior to analysis, original hormone levels were natural log-transformed to limit heteroscedasticity. Concentrations for each woman were characterized as the difference (residual) between her assay value and the estimated mean hormone value determined for the day of gestation on which the blood sample was donated. In the remainder of this paper, the term “hormone concentrations” refers to residuals.
A paired t test was used to compare the mean hormone concentrations (residuals) of cases and controls (case value vs. mean of the matched controls). The conditional logistic regression model, which is appropriate for matched data, was used to estimate odds ratios and corresponding 95% confidence intervals associated with increasing hormone concentrations. Subjects were classified in tertiles using the frequency distribution of the controls. Tests for trend were conducted by treating the hormone variables as ordered categorical variables. Likelihood ratio tests were used to assess statistical significance. Hormone-disease associations were explored within strata of age at blood donation, parity (primiparous vs. nonprimiparous), and age at cancer diagnosis (below and above the median), as well as by time between blood donation and cancer diagnosis (below and above the median (<14 years vs. ≥14 years for hCG) and the period of interest (≤10 years vs. >10 years) suggested by research on the transient increase in breast cancer risk following a pregnancy (3)). Formal tests of heterogeneity between the odds ratios in different subgroups were based on chi-square statistics, calculated as the deviations of logistic β coefficients observed in each of the subgroups, relative to the overall β coefficient (16). None of the tested covariates (baby's height, weight, or gender, age at first birth, parity by index date (cancer diagnosis), maternal smoking at blood donation, and placental weight) influenced the point estimates substantially (>10%). Smoking was retained as an adjusting variable in all hCG models, since smoking was related to both breast cancer and hCG concentrations.
The study protocol, including the consent form and access to human subjects, was approved annually by the ethics committees of the University of Umeå and New York University School of Medicine.
RESULTS
Selected baseline, pregnancy, and reproductive characteristics of the study population are summarized in Table 1. In brief, mean age at blood donation was 30.9 years for the cases (range, 19–44) and 31.0 years (range, 18–44) for the controls. The mean age at breast cancer diagnosis was 43.3 years, ranging from 25 to 59 years. The mean lag time between blood drawing and date of diagnosis was 12.3 years, ranging from 1 to 23 years. Forty-nine percent of study subjects were primiparous, with a mean age of 29 years. The mean age of multiparous women was 34 years. Age at first birth, parity by date of diagnosis (index date for the controls), maternal weight and height at enrollment, baby's weight, baby's length, placental weight, and proportion of boys were similar between cases and controls. Case women reported smoking at blood drawing more often (32%) than did control women (25%); the odds ratio for breast cancer in smokers versus nonsmokers was 1.47 (95% confidence interval: 1.02, 2.13; P < 0.04). For the majority of study subjects (72%), blood samples were drawn between the 8th and 14th weeks of gestation; 13% were drawn before the 8th week and 15% were drawn after the 14th week. The mean day of gestation was 76.1 for cases and 77.8 for controls.
Table 1.
Selected Baseline, Pregnancy, and Reproductive Characteristics of Northern Sweden Maternity Cohort Participants Included in the Current Study, 1975–2001
| Characteristic | Cases (n = 210) |
Controls (n = 357) |
||||
| Mean (SD) | No. | % | Mean (SD) | No. | % | |
| Age at blood donation, years | 30.9 (4.8) | 31.0 (4.8) | ||||
| Age at diagnosis, years | 43.3 (6.8) | |||||
| Lag time to diagnosis, years | 12.3 (5.5) | |||||
| Primiparous at blood donation | 102 | 49 | 176 | 49 | ||
| Age at first birth (all women), years | 26.7 (4.8) | 26.4 (4.8) | ||||
| Parity by diagnosis (index) date | ||||||
| 1 | 27 | 13 | 49 | 14 | ||
| 2 | 102 | 49 | 148 | 42 | ||
| 3 | 61 | 29 | 111 | 31 | ||
| >3 | 20 | 10 | 49 | 14 | ||
| Gestational day at blood donation | 76.1 (21.5) | 77.8 (19.8) | ||||
| Gestational week at blood donation | ||||||
| <8 | 30 | 14 | 43 | 12 | ||
| 8–14 | 149 | 71 | 259 | 73 | ||
| >14 | 31 | 15 | 55 | 15 | ||
| Child's birth weight, g | 3,432 (512) | 3,461 (564) | ||||
| Child's birth length, cm | 50 (2.3) | 50 (2.5) | ||||
| Placental weight, ga | 575 (117) | 595 (137) | ||||
| Male gender | 100 | 48 | 164 | 46 | ||
| Maternal weight at enrollment, kg | 61.8 (8.6) | 62.5 (8.7) | ||||
| Maternal height, cm | 165 (5.7) | 165 (5.9) | ||||
| Smoking at blood donation | ||||||
| Yes | 68 | 32 | 90 | 25 | ||
| No | 136 | 65 | 262 | 73 | ||
| Missing data | 6 | 3 | 5 | 1 | ||
| Human chorionic gonadotropin residualsb | −0.07 (0.55) | 0.05 (0.49)c | ||||
| α-fetoprotein residualsd | 0.04 (0.80) | 0.01 (0.85)c | ||||
Abbreviation: SD, standard deviation.
Data were available for 173 cases and 290 controls.
Data were available for 173 cases and 294 controls.
Paired t test for difference between cases and controls: P = 0.04 for human chorionic gonadotropin and P = 0.83 for α-fetoprotein.
Data were available for 204 cases and 349 controls.
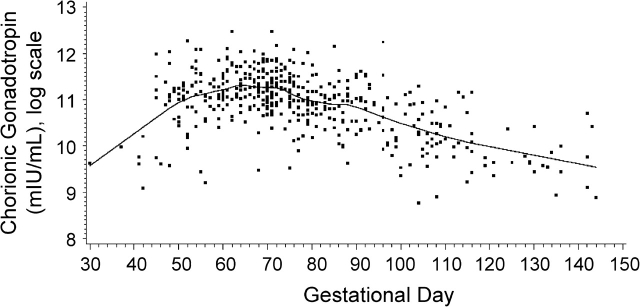
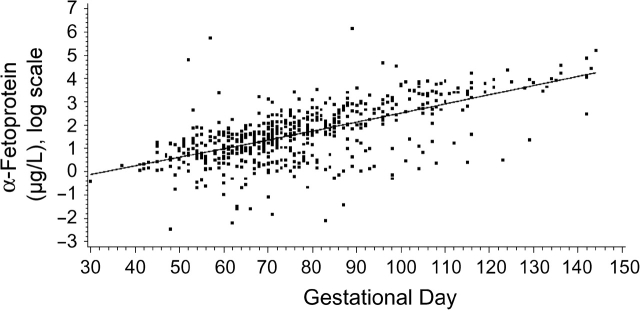
Concentrations of hCG increased markedly during the first part of the first trimester, peaked around gestation week 10, and then started to decline (Figure 1). Among the cases and controls combined, median hCG concentrations were 72,274 mIU/mL before the 70th gestational day, 65,645 mIU/mL between the 70th and 84th days, and 34,396 mIU/mL after day 84. This pattern is in agreement with clinical data (17, 18). As expected (19, 20), AFP concentrations increased progressively with increasing gestational age (Figure 2). Among the cases and controls combined, median AFP concentrations were 2.6 μg/L before the 70th gestational day, 5.5 μg/L between the 70th and 84th days, and 16.0 μg/L after day 84. As was observed in other studies, both cases and controls who donated blood during their first full-term pregnancy had higher hCG concentrations than those who already had at least 1 child (0.06 vs. −0.04; P < 0.05), while levels of AFP were similar (21–23). hCG residuals were lower in smokers than in nonsmokers (−0.19 vs. 0.08; P < 0.0001), but smoking during pregnancy did not affect AFP concentrations. hCG and AFP were not correlated with each other or with maternal age, weight, height, placental weight, or baby's height or weight (all correlations for hCG and AFP were weaker than ±0.13 and ±0.08, respectively).
Figure 1.
Distribution of log human chorionic gonadotropin values by gestational day for all study subjects, Northern Sweden Maternity Cohort, 1975–2001. The distribution follows a pattern consistent with what is expected on the basis of the physiology of human chorionic gonadotropin in early pregnancy (16). The solid line shows the progression of human chorionic gonadotropin during pregnancy, estimated by local linear regression.
Figure 2.
Distribution of log α-fetoprotein values by gestational day for all study subjects, Northern Sweden Maternity Cohort, 1975–2001. Alpha-fetoprotein levels increase linearly with gestational age, as expected (16). The solid line shows the progression of α-fetoprotein during pregnancy, estimated by local linear regression.
Compared with control subjects, cases had lower mean hCG concentrations (P < 0.04) but similar AFP concentrations (Table 1). Table 2 summarizes the results of conditional logistic regression analyses. For hCG, the overall analysis and most subgroup analyses (by age at blood donation, parity, and age at cancer diagnosis) showed a nonsignificant decrease in breast cancer risk for subjects with hCG levels in the top tertile in comparison with women with hCG levels in the first tertile. Risk estimates varied by lag time between blood donation and cancer diagnosis. High hCG levels conferred borderline statistically significant protection against breast cancer only in women diagnosed after the median lag time to cancer diagnosis (14 years), but the odds ratios for hCG before and after the median time to diagnosis were not significantly different (P = 0.10). Analyses limited to cancers diagnosed more than 10 years after blood donation yielded similar results (data not shown). Adjustment for any potential confounders had only a minimal effect on point estimates; however, since smoking was related to both breast cancer and hCG levels, it was retained in all hCG models. Limiting the analyses to subjects who donated blood on gestational days 49–98 (weeks 7–14 of gestation) or to nonsmokers yielded very similar risk estimates for the top tertiles: Odds ratios were 0.58 (95% confidence interval: 0.33, 1.01; P < 0.06) and 0.73 (95% confidence interval: 0.37, 1.44; P < 0.33), respectively.
Table 2.
Odds Ratios for Breast Cancer by Tertile of Human Chorionic Gonadotropin and α-Fetoprotein Concentrations in Pregnancy, Northern Sweden Maternity Cohort, 1975–2001
| Tertile |
P-Trend | |||||||||||
| 1 |
2 |
3 |
||||||||||
| OR | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | ||
| Human Chorionic Gonadotropina | ||||||||||||
| All women | 1.00 | 63 | 98 | 1.03 | 0.63, 1.69 | 61 | 98 | 0.86 | 0.52, 1.42 | 49 | 98 | 0.54 |
| Age at sampling, years | ||||||||||||
| <30 | 1.00 | 30 | 45 | 0.92 | 0.44, 1.92 | 27 | 45 | 0.77 | 0.37, 1.61 | 22 | 45 | 0.49 |
| ≥30 | 1.00 | 31 | 53 | 1.19 | 0.63, 2.24 | 36 | 53 | 0.91 | 0.46, 1.81 | 27 | 53 | 0.84 |
| Parity | ||||||||||||
| Primiparous | 1.00 | 35 | 44 | 0.68 | 0.32, 1.44 | 21 | 44 | 0.68 | 0.33, 1.41 | 22 | 45 | 0.29 |
| Multiparous | 1.00 | 34 | 53 | 0.817 | 0.46, 1.66 | 31 | 54 | 0.92 | 0.48, 1.75 | 30 | 54 | 0.78 |
| Lag time to diagnosis, yearsb | ||||||||||||
| <14 | 1.00 | 21 | 47 | 2.20 | 0.95, 5.10 | 35 | 48 | 1.67 | 0.72, 3.86 | 26 | 48 | 0.25 |
| ≥14 | 1.00 | 41 | 50 | 0.58 | 0.30, 1.15 | 27 | 50 | 0.53 | 0.27, 1.03 | 23 | 51 | 0.06 |
| Age at cancer diagnosis, years | ||||||||||||
| <45 | 1.00 | 31 | 50 | 1.25 | 0.63, 2.46 | 33 | 50 | 0.82 | 0.40, 1.70 | 24 | 51 | 0.66 |
| ≥45 | 1.00 | 34 | 47 | 0.71 | 0.35, 1.44 | 26 | 48 | 0.72 | 0.36, 1.44 | 25 | 48 | 0.36 |
| Nonsmoker at blood donation | 1.00 | 38 | 43 | 0.68 | 0.33, 1.38 | 25 | 43 | 0.73 | 0.37, 1.44 | 26 | 44 | 0.33 |
| α-Fetoprotein | ||||||||||||
| Full data set | 1.00 | 67 | 116 | 1.08 | 0.67, 1.73 | 73 | 116 | 0.91 | 0.55, 1.50 | 64 | 117 | 0.63 |
| Restricted datac | 1.00 | 54 | 99 | 1.21 | 0.75, 1.95 | 66 | 99 | 0.96 | 0.58, 1.58 | 54 | 99 | 0.80 |
Abbreviations: CI, confidence interval; OR, odds ratio.
All models for human chorionic gonadotropin were adjusted for smoking.
Test for homogeneity of results below and above the median lag time to cancer diagnosis: P = 0.10.
Women who donated blood before January 1, 1988 (174 cases and 297 controls).
AFP was not related to maternal risk of breast cancer overall (Table 2) or in any of the subgroup analyses (data not shown). Excluding subjects who donated blood after January 1, 1988, also yielded similar risk estimates (Table 2).
DISCUSSION
In this study, no significant association between breast cancer and hCG or AFP concentrations measured during the first half of pregnancy was observed. However, in the total study population and in subgroups based on age at blood donation, parity, and median age at cancer diagnosis, women with hCG levels in the top tertile consistently had lower risks of breast cancer than women with hCG levels in the lowest tertile. Borderline-significant protection was observed only among women with lag times between blood donation and cancer diagnosis that exceeded the median. The latter observation fits epidemiologic data showing a dual effect of pregnancy on risk of breast cancer—a transient increase in risk lasting 5–10 years, followed by lifelong protection (3).
The study was inspired by the hypothesis of Russo and Russo (24), who proposed that hormonally induced differentiation of the breast during pregnancy, characterized by profound albeit transient morphologic changes, limits the population of cancer-susceptible cells present in an immature gland and underlies the protective effect of childbearing. These same authors recently showed that a full-term pregnancy induces in breast epithelial cells a permanent genomic signature, characterized by altered expression of genes related to basic processes such as immune surveillance, DNA repair, cell proliferation, cell differentiation, and metabolism (reviewed in detail by Russo et al. (25)). Experimental work suggests that hCG may play an important role in this differentiation process, since, in rodents, pregnancy and short treatment with recombinant hCG induce a very similar genomic signature (25). Furthermore, treatment with hCG either before or after the administration of a chemical carcinogen resulted in a significant decrease in the incidence of mammary carcinoma in comparison with controls, similar to that conferred by pregnancy (26, 27). Alternatively, it has been proposed that the protection related to hCG administration in experimental models is mediated through an increase in estrogen and progesterone synthesis (28).
AFP has been shown to bind to estradiol and to suppress estrogen-dependent growth of breast cancer cells (11, 12). In contrast to our null findings, so far, 2 studies have indicted that a high AFP level during pregnancy is associated with reduced risk of breast cancer (8, 9). However, in these studies, AFP was measured later in the course of pregnancy, during the second or third trimester, when its concentrations in maternal circulation increase substantially and may be more relevant as determinants of breast cancer risk than the relatively low levels seen during early pregnancy.
The major limitations of our study are the inability to complete the recruitment of study subjects as initially intended and the unexpected drop in hCG concentrations in samples collected after 1988. Exclusion of potentially eligible subjects because of insufficient funding was random and was not related to any particular characteristics of these women; thus, it is unlikely that selection bias was introduced. However, we have no explanation for the observed drop in hCG levels in samples collected after 1988, since there were no differences in sample handling, storage, or analysis of which we are aware. Furthermore, no similar effect of storage time on hCG concentrations has been observed in another Scandinavian cohort study with an identical design (29). The combined effect of these problems was to greatly reduce study size and ability to detect hormone-breast cancer associations. The study had 80% power to detect an odds ratio of 0.52 (30).
It is likely that several other factors decreased our ability to detect a hormone-disease association. The long-term storage of the Northern Sweden Maternity Cohort serum samples at −20°C could have caused analyte deterioration. However, no consistent effect of storage time on concentrations of either pre-1988 hCG or AFP or insulin-like growth factors I and II, measured in the same samples, was evident (14). Hormonal variations with gestational age closely followed what is reported in the literature (17–20), and the expected effects of parity and smoking on hCG and AFP concentrations were observed (21–23, 31). Other studies (including one of the studies on AFP in pregnancy and maternal breast cancer) have shown that samples stored for many years at −20°C can be used in epidemiologic research on hormones and cancer, and the expected hormone-cancer risk associations were observed (8, 13, 29, 32).
It is unknown how well a single hormone measurement taken during early pregnancy is likely to represent exposure throughout gestation, as such data are difficult to collect. In successive pregnancies, correlations between total estradiol levels and between free estradiol levels measured between weeks 8 and 17 in both pregnancies were found to be as high as 0.78 and 0.73, respectively (33). Correlations of hCG and AFP in successive pregnancies (measured during the first/second trimester) have been lower—about 0.40 for both hormones (34). Although it is plausible to assume that the correlation between hormone measurements taken within a given pregnancy would be higher than that between hormone levels in successive pregnancies, a single measurement may still not be sufficient to characterize overall hormonal exposure during pregnancy. On the positive side, the samples of the Northern Sweden Maternity Cohort were donated around the time of peak hCG concentrations during pregnancy—between the 7th and 10th weeks of gestation, which is likely to be the most relevant period of exposure to this hormone.
In conclusion, although the results of the current study must be regarded as suggestive and should be interpreted with caution, they are consistent with a protective effect of elevated hCG levels during pregnancy on long-term risk of breast cancer.
Acknowledgments
Author affiliations: Department of Obstetrics and Gynecology, New York University School of Medicine, New York, New York (Annekatrin Lukanova, Alan A. Arslan, Paolo Toniolo); Hormone and Cancer Group, Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (Annekatrin Lukanova, Laure Dossus); Department of Medical Biosciences, University of Umeå, Umeå, Sweden (Ritu Andersson, Kjell Grankvist, Eva Lundin); Department of Clinical Sciences, University of Umeå, Umeå, Sweden (Marianne Wulff); Department of Environmental Medicine, New York University School of Medicine, New York, New York (Anne Zeleniuch-Jacquotte, Yelena Afanasyeva, Alan A. Arslan, Paolo Toniolo); Department of Radiation Sciences, University of Umeå, Umeå, Sweden (Robert Johansson, Per Lenner); Department of Clinical Microbiology, University of Umeå, Umeå, Sweden (Göran Wadell); and Department of Public Health and Clinical Medicine, University of Umeå, Umeå, Sweden (Göran Hallmans).
This study was supported by research grant BCTR 2000 505 from the Susan G. Komen Breast Cancer Foundation; US National Cancer Institute grants CA16087, CA114329, and CA120061; and the Lion’s Cancer Foundation of Northern Sweden University Hospital, Umeå, Sweden.
The authors thank Lena Marklund, Helena Schock, Hubert Sjödin, and Le Thu Trinh for their excellent technical assistance in the conduct of the study.
Conflict of interest: none declared.
Glossary
Abbreviations
- AFP
α-fetoprotein
- hCG
human chorionic gonadotropin
References
- 1.Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2(3):133–140. doi: 10.1016/S1470-2045(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 2.Hinkula M, Pukkala E, Kyyronen P, et al. Grand multiparity and the risk of breast cancer: population-based study in Finland. Cancer Causes Control. 2001;12(6):491–500. doi: 10.1023/a:1011253527605. [DOI] [PubMed] [Google Scholar]
- 3.Liu Q, Wuu J, Lambe M, et al. Transient increase in breast cancer risk after giving birth: postpartum period with the highest risk (Sweden) Cancer Causes Control. 2002;13(4):299–305. doi: 10.1023/a:1015287208222. [DOI] [PubMed] [Google Scholar]
- 4.Ewertz M, Duffy SW, Adami HO, et al. Age at first birth, parity and risk of breast cancer: a meta-analysis of 8 studies from the Nordic countries. Int J Cancer. 1990;46(4):597–603. doi: 10.1002/ijc.2910460408. [DOI] [PubMed] [Google Scholar]
- 5.Russo IH, Russo J. Role of hormones in mammary cancer initiation and progression. J Mammary Gland Biol Neoplasia. 1998;3(1):49–61. doi: 10.1023/a:1018770218022. [DOI] [PubMed] [Google Scholar]
- 6.Medina D. Mammary developmental fate and breast cancer risk. Endocr Relat Cancer. 2005;12(3):483–495. doi: 10.1677/erc.1.00804. [DOI] [PubMed] [Google Scholar]
- 7.Guzman RC, Yang J, Rajkumar L, et al. Hormonal prevention of breast cancer: mimicking the protective effect of pregnancy. Proc Natl Acad Sci U S A. 1999;96(5):2520–2525. doi: 10.1073/pnas.96.5.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson BE, Hulka BS, Peck JL, et al. Levels of maternal serum alpha-fetoprotein (AFP) in pregnant women and subsequent breast cancer risk. Am J Epidemiol. 1998;148(8):719–727. doi: 10.1093/oxfordjournals.aje.a009691. [DOI] [PubMed] [Google Scholar]
- 9.Melbye M, Wohlfahrt J, Lei U, et al. Alpha-fetoprotein levels in maternal serum during pregnancy and maternal breast cancer incidence. J Natl Cancer Inst. 2000;92(12):1001–1005. doi: 10.1093/jnci/92.12.1001. [DOI] [PubMed] [Google Scholar]
- 10.Deutsch HF. Chemistry and biology of alpha-fetoprotein. Adv Cancer Res. 1991;56:253–312. doi: 10.1016/s0065-230x(08)60483-2. [DOI] [PubMed] [Google Scholar]
- 11.Attardi B, Ruoslahti E. Foetoneonatal oestradiol-binding protein in mouse brain cytosol is alpha foetoprotein. Nature. 1976;263(5579):685–687. doi: 10.1038/263685a0. [DOI] [PubMed] [Google Scholar]
- 12.Bennett JA, Semeniuk DJ, Jacobson HI, et al. Similarity between natural and recombinant human alpha-fetoprotein as inhibitors of estrogen-dependent breast cancer growth. Breast Cancer Res Treat. 1997;45(2):169–179. doi: 10.1023/a:1005841032371. [DOI] [PubMed] [Google Scholar]
- 13.Peck JD, Hulka BS, Poole C, et al. Steroid hormone levels during pregnancy and incidence of maternal breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11(4):361–368. [PubMed] [Google Scholar]
- 14.Lukanova A, Toniolo P, Zeleniuch-Jacquotte A, et al. Insulin-like growth factor I in pregnancy and maternal risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2489–2493. doi: 10.1158/1055-9965.EPI-06-0625. [DOI] [PubMed] [Google Scholar]
- 15.Cleveland WS, Loader C. Smoothing by local regression: principles and methods. In: Schimek MG, editor. Statistical Theory and Computational Aspects of Smoothing. New York, NY: Springer Publishing Company; 1996. pp. 113–120. [Google Scholar]
- 16.Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med. 1991;10(11):1665–1677. doi: 10.1002/sim.4780101105. [DOI] [PubMed] [Google Scholar]
- 17.Speroff L, Fritz M, editors. Clinical Gynecologic Endocrinology and Infertility. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. The endocrinology of pregnancy; pp. 259–318. [Google Scholar]
- 18.Stenman UH, Tiitinen A, Alfthan H, et al. The classification, functions and clinical use of different isoforms of HCG. Hum Reprod Update. 2006;12(6):769–784. doi: 10.1093/humupd/dml029. [DOI] [PubMed] [Google Scholar]
- 19.Leek AE, Ruoss CF, Kitau MJ, et al. Maternal plasma alphafetoprotein levels in the second half of normal pregnancy: relationship to fetal weight, and maternal age and parity. Br J Obstet Gynaecol. 1975;82(8):669–673. doi: 10.1111/j.1471-0528.1975.tb00704.x. [DOI] [PubMed] [Google Scholar]
- 20.Olajide F, Kitau MJ, Chard T. Maternal serum AFP levels in the first trimester of pregnancy. Eur J Obstet Gynecol Reprod Biol. 1989;30(2):123–128. doi: 10.1016/0028-2243(89)90058-0. [DOI] [PubMed] [Google Scholar]
- 21.Wald NJ, Watt HC. Serum markers for Down's syndrome in relation to number of previous births and maternal age. Prenat Diagn. 1996;16(8):699–703. doi: 10.1002/(SICI)1097-0223(199608)16:8<699::AID-PD919>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 22.Barkai G, Goldman B, Ries L, et al. Effect of gravidity on maternal serum markers for Down's syndrome. Prenat Diagn. 1996;16(4):319–322. doi: 10.1002/(SICI)1097-0223(199604)16:4<319::AID-PD859>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 23.Tislaric D, Brajenovic-Milic B, Ristic S, et al. The influence of smoking and parity on serum markers for Down's syndrome screening. Fetal Diagn Ther. 2002;17(1):17–21. doi: 10.1159/000047999. [DOI] [PubMed] [Google Scholar]
- 24.Russo J, Russo IH. Biological and molecular bases of mammary carcinogenesis. Lab Invest. 1987;57(2):112–137. [PubMed] [Google Scholar]
- 25.Russo J, Balogh GA, Heulings R, et al. Molecular basis of pregnancy-induced breast cancer protection. Eur J Cancer Prev. 2006;15(4):306–342. doi: 10.1097/00008469-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Russo IH, Koszalka M, Russo J. Human chorionic gonadotropin and rat mammary cancer prevention. J Natl Cancer Inst. 1990;82(15):1286–1289. doi: 10.1093/jnci/82.15.1286. [DOI] [PubMed] [Google Scholar]
- 27.Russo IH, Russo J. Primary prevention of breast cancer by hormone-induced differentiation. Recent Results Cancer Res. 2007;174:111–130. doi: 10.1007/978-3-540-37696-5_11. [DOI] [PubMed] [Google Scholar]
- 28.Sivaraman L, Medina D. Hormone-induced protection against breast cancer. J Mammary Gland Biol Neoplasia. 2002;7(1):77–92. doi: 10.1023/a:1015774524076. [DOI] [PubMed] [Google Scholar]
- 29.Holl K, Lundin E, Kaasila M, et al. Effect of long-term storage on hormone measurements in samples from pregnant women: the experience of the Finnish Maternity Cohort. Acta Oncol. 2008;47(3):406–412. doi: 10.1080/02841860701592400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Closas M, Lubin JH. Power and sample size calculations in case-control studies of gene-environment interactions: comments on different approaches. Am J Epidemiol. 1999;149(8):689–692. doi: 10.1093/oxfordjournals.aje.a009876. [DOI] [PubMed] [Google Scholar]
- 31.Crossley JA, Aitken DA, Waugh SM, et al. Maternal smoking: age distribution, levels of alpha-fetoprotein and human chorionic gonadotrophin, and effect on detection of Down syndrome pregnancies in second-trimester screening. Prenat Diagn. 2002;22(3):247–255. doi: 10.1002/pd.313. [DOI] [PubMed] [Google Scholar]
- 32.Allen NE, Roddam AW, Allen DS, et al. A prospective study of serum insulin-like growth factor-I (IGF-I), IGF-II, IGF-binding protein-3 and breast cancer risk. Br J Cancer. 2005;92(7):1283–1287. doi: 10.1038/sj.bjc.6602471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernstein L, Lipworth L, Ross RK, et al. Correlation of estrogen levels between successive pregnancies. Am J Epidemiol. 1995;142(6):625–628. doi: 10.1093/oxfordjournals.aje.a117685. [DOI] [PubMed] [Google Scholar]
- 34.Wald NJ, Huttly WJ, Rudnicka AR. Prenatal screening for Down syndrome: the problem of recurrent false-positives. Prenat Diagn. 2004;24(5):389–392. doi: 10.1002/pd.890. [DOI] [PubMed] [Google Scholar]