Abstract
Pharmacological inhibition or genetic deletion of cyclooxygenase (COX)-2, but not COX-1, has been shown to increase susceptibility to kainic acid (KA)-induced excitotoxicity. However, it is unclear if susceptibility to excitotoxins that act through other neurotransmitter receptors is altered by COX-2 inhibition. To further understand the involvement of COX-2 in regulating susceptibility to excitotoxicity, we investigated the effect of COX-2 deletion on excitotoxicity induced by peripheral injection of N-methyl-D-aspartate (NMDA, a specific agonist of the NMDA receptors) or lindane (a GABAA receptor antagonist). COX-2−/− mice injected intraperitoneally with NMDA (50-100 mg/kg) exhibited significantly increased median seizure intensity when compared to COX-2+/+ mice. Further, COX-2−/− mice exposed to NMDA showed neuronal damage, detected by Fluoro Jade B (FJB) staining, in the CA3 region of the hippocampus. There was no FJB staining nor any significant difference in median or maximal seizure intensity in COX-2+/+ and COX-2−/− mice exposed to lindane. LC-MS/MS analysis of brain prostaglandin profile in COX-2−/− mice demonstrated a significant increase in PGF2α, TXB2, PGE2 and PGD2 expression 1 hour after administration of an excitotoxic dose of KA, but not of NMDA. Our findings demonstrate that COX-2 regulates susceptibility to KA and NMDA excitotoxicity, which directly activate glutamatergic neurotransmission, but not to lindane, which indirectly alters glutamatergic neurotransmission. Furthermore, increased levels of prostaglandins after seizures are associated with consistent manifestation of neuronal damage.
Keywords: N-methyl-D-Aspartate, Prostaglandin, Excitotoxicity, Lindane, Kainate, Fluoro Jade B
1. Introduction
Cyclooxygenase (COX) catalyzes the primary step in the metabolism of unesterified arachidonic acid to bioactive prostaglandins (PG) and thromboxanes (TX) (Smith et al. 2000). This metabolic pathway has been shown to play an important role both in physiological processes, such as synaptic transmission, neurotransmitter release and cerebral blood flow regulation as well as in neurological and neurodegenerative diseases, such as stroke, epilepsy, and Alzheimer's disease (Kam and See 2000; Katori and Majima 2000). Recent data suggest that while the COX isoforms, COX-1 and COX-2, catalyze the same metabolic reaction of arachidonic acid to PGH2, they may play differential roles in neuroinflammation and excitotoxicity (Bosetti 2007), a persistent overstimulation of excitatory neurotransmitter receptors that results in neuronal damage. Specifically, progression of the neuroinflammatory response to intracerebroventricular injections of lipopolysaccharide is dampened in mice deficient in COX-1 (Choi et al. 2008) and heightened in mice deficient in COX-2 (Aid et al. 2008). Furthermore, we have recently demonstrated that mice in which COX-2 was genetically deleted or chronically inhibited by celecoxib, expressed augmented kainic acid (KA)-induced excitotoxicity (Toscano et al. 2008). However, COX-1 deficient mice were not differentially susceptible to KA-induced excitotoxicity when compared to their wild-type counterparts. While this and other studies reporting an increased susceptibility to excitotoxicity when COX-2 is inhibited pharmacologically (Baik et al. 1999; Kim et al. 2008) suggest that deletion or inhibition of COX-2, but not COX-1, in the brain would result in increased susceptibility to all excitotoxicants, this has not been systematically tested. Therefore, in this study, we attempted to further our understanding of the role of the COX isoforms in excitotoxicity by examining the susceptibility of mice deficient in COX-2 (COX-2−/−) to N-methyl-D-aspartate (NMDA) and lindane, excitoxicants which act at sites distinctly different from those used in previous studies (Collingridge and Singer 1990; Narahashi 2002).
NMDA is a methylated aspartate and a specific agonist of the NMDA receptor (NMDAR), one of the three types of ionotropic glutamate receptors expressed in the brain (Collingridge and Singer 1990). Exposure to either KA or NMDA results in the generation of limbic seizures which progress to neuronal damage in the cerebral cortex, hippocampus and other limbic structures in the brain (Wang et al. 2005). The exact mechanism of this excitotoxic neuronal damage has not been completely characterized but a prevailing theory suggests that the over excitation of the neurons allows significant concentrations of intracellular calcium to build, resulting in activation of calcium dependent proteases (Sattler and Tymianski 2001).
Lindane, a persistent organic pollutant, exerts its seizurigenic activity in both experimental animals and humans (Telch and Jarvis 1982; Gilbert and Mack 1995) through its ability to antagonize the GABAA receptor (GABAR) and thereby disinhibit the central nervous system (Narahashi 2002). The removal of this inhibitory influence of GABA results in hyperexcitation of excitatory neurotransmitter receptors which may progress to seizures and excitotoxicity.
Activation of the arachidonic acid metabolic pathway is known to occur after exposure to NMDA, KA and lindane (Kroll et al. 1999; Kawaguchi et al. 2005; Takemiya et al. 2006). The production of the bioactive eicosanoids from this process increases stimulation of their cognate receptors which mediate the downstream biological responses (Wright et al. 2001). While previous studies have shown increased production of individual prostaglandins in models of excitotoxicity (Kawaguchi et al. 2005; Takemiya et al. 2006), comprehensive analysis of the prostaglandin profile in the brains of mice after exposure to excitotoxins is lacking. Underlining the need for further understanding of mouse brain prostaglandin profile production in excitotoxicity is the observation that differential roles for the COX isoforms have been shown in the production of brain prostaglandins in response to KA in rats (Yoshikawa et al. 2006). Since we have recently established a role for COX-2 in modulating susceptibility to KA-induced excitotoxicity, it is important to understand how decreased activity of this isoform alters the mouse brain prostaglandin profile.
With our recent demonstration that COX-2 deficient mice exhibit decreased spontaneous inhibitory postsynaptic currents in their CA1 pyramidal neurons along with increased susceptibility to KA-induced excitotoxicity (Toscano et al. 2008), we hypothesized that these mice would also have augmented NMDA and lindane-induced excitotoxicity. In this study, we demonstrate for the first time that COX-2 deficient mice exhibit augmented excitotoxicity to NMDA, but not to lindane. Furthermore, we also demonstrate that altered brain prostaglandin levels in COX-2 deficient mice correlate with neuronal damage.
2. Materials and Methods
2.1 Animal Procedures
All procedures were performed under an approved animal protocol (NICHD #06-001) in accordance with NIH guidelines on the care and use of laboratory animals. Male COX-2 −/− and COX-2 +/+ mice used in this study had identical strain and genetic backgrounds and were obtained from heterozygous by heterozygous breedings, as detailed elsewhere (Toscano et al. 2007; Toscano et al. 2008). Mice were received at our facility at 6-8 weeks of age and were fed and watered ad libitum and maintained on a 12/12 light dark cycle.
Mice (12-14 week-old) were injected intraperitoneally (i.p., injection volume 100-300 μl) with 10 mg/kg KA (Biomol International, Plymouth Meeting, PA; 2 mg/ml in 0.9% saline), 25-100 mg/kg N-methyl-D-aspartate (NMDA, Sigma, St. Louis, MO; 7.5-20 mg/ml in 0.9% saline) or 20-80 mg/kg lindane (γ-hexachlorocylohexane, Sigma; 4-16 mg/ml in corn oil) and then videotaped for 1 h after injection. Seizure intensity was rated using a modified Racine seizure scale for KA and NMDA and the Gilbert seizure scale for lindane (Gilbert and Mack 1995; Schauwecker and Steward 1997; Toscano et al. 2008). Two different seizure ratings scales were used due to the difference in molecular mechanism and in seizure manifestation between the compounds. KA and NMDA cause seizures by directly stimulating the glutamatergic system whereas lindane-induced seizures are due to inhibition of GABA receptors. Therefore, the manifestations of seizures for the two classes of compounds are distinctly different. Specifically, the Racine scale used in this study was 0 = no behavioral alteration; 1 = immobility, mouth and facial movements, facial clonus; 2= head nodding, forelimb and/or tail extension, rigid posture; 3= forelimb clonus, repetitive movements; 4= rearing, forelimb clonus with rearing, rearing and falling; 5= continuous rearing and falling, jumping; 6= severe tonic-clonic seizures. The Gilbert scale used in this study was: 0=no behavioral alteration; 1= jaw clonus, immobility, chewing in absence of boli or bedding, twitching of eyes and ears; 2= myoclonic jerk (MCJ), abrupt flexion or extension of forelimbs, head or neck; 3= multiple MCJ, more than 2 over the observation period; 4= prolonged MCJ, accompanied by rapid burst of chewing, forelimb clonus; 5= clonic seizures, uni- or bilateral forelimb clonus enduring for more than 10 seconds; 6=multiple clonic bouts, more than one clonic seizure episode over the observation period.
Mice were assigned a seizure score (SS) for each 5 min interval over the course of the 1 h session, after which a median and a maximum SS was calculated for the entire 1 h session for each mouse. Mice were euthanized by decapitation 1 hour after injection with the test substances (for prostaglandin (PG) analysis) or 24 hours after injection with the test substances by intracardial perfusion with 4% paraformaldehyde (for histology) followed by overnight fixation of the brain in 4% paraformaldehyde and subsequent cryoprotection in 30% sucrose (Choi et al. 2008).
2.2 Fluoro Jade B Histology
Cryoprotected mouse brains were sectioned coronally (30 μm) on a cryostat (Bright Instrument Company, LTD; Huntingdon, England) and stored at -20°C in antifreeze (30% glycerol, 30% ethylene glycol in 20 mM phosphate buffer at pH 7.2). Before use, sections were briefly rinsed in distilled water and then mounted on gelatin-coated glass specimen slides on which they were allowed to dry overnight. Fluoro Jade B (FJB) (Histo-Chem, Inc., Jefferson, AR) staining was performed as described previously (Schmued and Hopkins 2000; Toscano et al. 2008). Briefly, FJB is a histological stain which detects neuronal damage by staining neurons undergoing necrosis or apoptosis a fluorescent green color. Brain sections from positive (COX-2 −/− mice euthanized 24 hours after i.p. injection with 10 mg/kg KA) and negative (COX-2 −/− mice euthanized 24 hours after injection with 0.9% saline) controls were included in each batch of staining. Cresyl violet was performed as previously described (Toscano et al. 2008).
2.3 PG analysis by LC/MS/MS
Mice were euthanized 1 h after injection with 10 mg/kg KA or 50 mg/kg NMDA by decapitation followed by rapid excision of the brain from the skull. Brains were snap frozen in methylbutane (Sigma) precooled with dry ice. LC-MS/MS analysis of whole brain prostaglandins was performed in a manner similar to that described previously (Kingsley et al. 2005). However, here the prostaglandins were detected by the mass spectrometer in negative ion mode.
Brain samples were removed from -80C storage and immediately homogenized in a 1:5 solution of methanol:ethyl acetate with 0.5% acetic acid (v:v:v). Tetra-deuterated internal standards for PGF2α, PGE2, PGD2, Thromboxane B2 (TXB2) and 6-keto-PGF1α (Cayman Chemical, Ann Arbor, MI) were added to the homogenization vessel prior to homogenization. The homogenate was centrifuged and a portion of the supernatant was removed, evaporated to dryness under N2 and reconstituted in 300 μL methanol followed by 3 mL of aqueous 0.5% acetic acid. The reconstituted samples were then loaded onto OASIS HLB solid phase extraction cartridges (3cc 60mg, Waters Corp. Milford, MA) which had been pre-conditioned with 3 mL methanol followed by 3 mL 0.5% acetic acid(aq). The cartridges were washed with 3 mL 0.5% acetic acid(aq) followed by 3 mL 0.5% acetic acid(aq) with 15% methanol. After drying the sorbent bed for 2-3 minutes, the analytes were eluted with 3 mL of acetonitrile. The samples were dried under N2 and reconstituted in 50 μL methanol plus 75 μL H2O. Some reconstituted samples were opaque due to suspended particulate, thus all samples were filtered through 0.22 μm nylon filters. Finally, the samples were injected onto an LC-MS-MS system for analysis.
The LC-MS-MS consisted of a Thermo Surveyor pump and autosampler in-line with a Thermo Quantum triple quadrupole mass spectrometer (Thermo Corp., Waltham, MA). Chromatographic separation was achieved on a C18 column (5.0 × 0.2 cm, 3 μm, Phenomenex, Torrance, CA) via gradient elution. Component B was increased from 25% to 50% over 3.5 minutes and held at 50% for an additional minute. Component B was acetonitrile with 0.5% acetic acid (v:v) while component A was H2O with 0.5% acetic acid (v:v).
Analytes were detected by selected reaction monitoring (SRM) in the negative ion mode. The addition of acetic acid to the mobile phase, which was done to improve chromatographic peak shape, allows a robust response from prostaglandins in negative ion mode. Quantitation was achieved via stable isotope dilution. The analytes were detected with the following reactions (the mass in parentheses represents the mass of the deuterated internal standard): PGF2α(m/z 353(357)∧193(197)); PGE2 & PGD2 (m/z 351(355)∧271(275)); TXB2 (m/z 369(373)∧169(173)) and 6-keto-PGF1α (m/z 369(373)∧163(167)).
2.4 Statistics
Mann-Whitney U-test (OpenStat 4; http://www.statpages.org/miller/openstat) was used for statistical analysis of seizure intensity due to their nature as ordinal measures. Seizure data are expressed as frequency or median and all other data are expressed as mean ± SD. Seizure data from all animals, whether they survived the observation period or not, were used to calculate median and maximal seizure intensity. Results from LC-MS/MS analysis of prostaglandins were analyzed with F test for homogeneity of variance and then, depending on the results of the F test, an unpaired t-test assuming equal variance or an unpaired t-test assuming unequal variance was calculated. p values <0.05 were considered statistically significant.
3. Results
3.1 NMDA-induced excitotoxicity is enhanced in COX-2 −/− mice
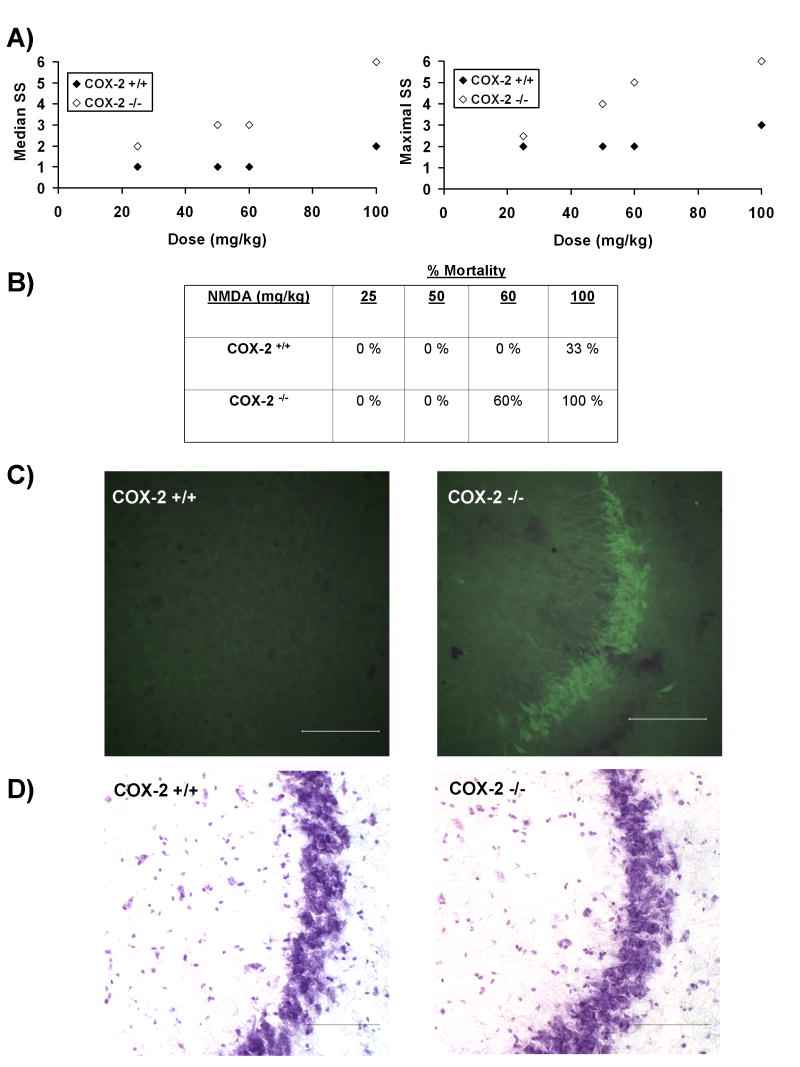
COX-2 −/− mice injected with 50, 60 or 100 mg/kg NMDA had significantly enhanced median and maximum seizure intensity, assessed by Racine Seizure Scale (RSS) scores (Figure 1A). No difference in median or maximal seizure intensity was observed between COX-2−/− and COX-2+/+ mice at 25 mg/kg NMDA. Mortality was only observed in doses greater than 50 mg/kg NMDA (Figure 1B). Since 50 mg/kg significantly increased seizure intensity in COX-2 −/− in the absence of mortality, all remaining experiments were performed using 50 mg/kg NMDA. In COX-2−/− mice exposed to 50 mg/kg NMDA, Fluoro Jade B (FJB), positive neurons were limited to the CA3 region of the hippocampus in 20% of the mice observed (Figure 1C). Previously, we have demonstrated that COX-2−/− mice treated with 10 mg/kg KA express extensive FJB staining of neurons in the CA1, CA3, thalamus and amygdala (Toscano et al. 2008). No FJB positive neurons were observed in the brains of COX-2 +/+ mice exposed to 50 mg/kg NMDA.
Figure 1. NMDA-induced excitotoxicity is enhanced in COX-2 −/− mice.
A) Dose response analysis of median and maximal NMDA-induced seizure intensity demonstrates that COX-2 −/− mice (open symbols) exposed to 50, 60 and 100 mg/kg had significantly higher median (z=2.16, p=0.01; z=2.08, p=0.02; z=2.18, p=0.01, respectively; n=3-5/group) and maximal (z=2.30, p=0.01, z=2.38, p=0.0085; z=2.18, p=0.01, respectively; n=3-5/group) seizure score (SS) when compared to COX-2 +/+ mice (filled symbols). No significant effect of genotype on median (z=1.15, p=0.124; n=5/group) or maximal seizure (z=1.15, p=0.124; n=5/group) score was observed at 25 mg/kg.
B) Mortality rates for mice injected with NMDA. Since, no significant mortality was observed at 50 mg/kg, which is the lowest observed effect level for increased NMDA-induced seizure intensity in COX-2−/− mice, this dose was used for the remaining experiments (25 mg/kg, n=5/group; 50 mg/kg, n=5/group; 60 mg/kg, n=5/group; 100 mg/kg, n=3/group.
C) Twenty-four hours after injection with 50 mg/kg NMDA, Fluoro Jade B positive neurons were observed in the CA3 region of the hippocampus of 20% of COX-2 −/− mice (n=5) examined. No FJB positive neurons were observed in COX-2 +/+ mice injected with 50 mg/kg NMDA (n=5). Magnification is 40×; reference bar = 100 μm.
D) Cresyl violet stained sections are provided for reference. Magnification is 40×; reference bar = 100 μm.
3.2 Lindane-induced seizure intensity is not affected by COX-2 deletion
Although lindane-induced seizures did occur in both COX-2 +/+ and COX-2 −/− mice during the 1 hour period after injection, no difference in median (z=0.14, p=0.44; z=0.0001, p=0.50; z=0.87, p=0.19, respectively; n= 4-5/group) or maximal (z=0.2887, p=0.38; z=0.57; p=0.28; z=0.22, p=0.41, respectively; n=4-5/group) seizure intensity was observed at 20, 60 or 80 mg/kg. Furthermore, lindane-induced mortality occurred in a dose dependent fashion (20 mg/kg= 0%, 60 mg/kg = 20%, 80 mg/kg = 50%) and mortality rates were identical in COX-2+/+ and COX-2−/− mice. Additionally, no FJB positive neurons were observed in COX-2+/+ or COX-2−/− mice twenty-four hours after injection with 20, 60 or 80 mg/kg lindane. Since a non-lethal dose which caused differential seizure intensity in COX-2 +/+ and COX-2 −/− mice could not be identified and no FJB staining was observed at any dose tested, we concluded that COX-2 deficiency was not a significant determinant of lindane-induced excitotoxicity severity.
3.3 Prostaglandin levels are increased in the brains of COX-2 −/− mice in response to KA but not NMDA-induced seizures
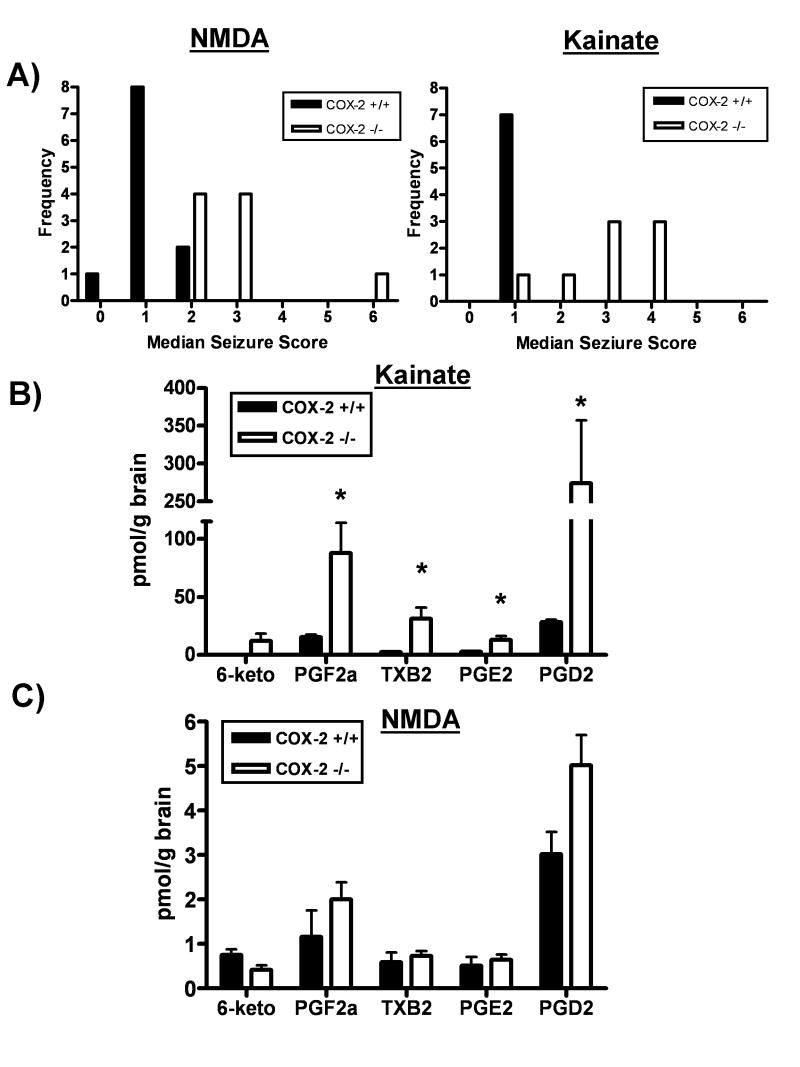
COX-2 −/− mice exhibited increased median seizure intensity in response to 50 mg/kg NMDA (COX-2 +/+ median= 1, COX-2 −/− median = 3; Fig 2A) and 10 mg/kg KA (COX-2 +/+ median= 1, COX-2 −/− median = 3; Figure 2A) when compared to COX-2+/+ mice. However, the median seizure intensity in COX-2 −/− mice induced by KA or NMDA were not significantly different from each other (COX-2 −/− KA median seizure=3; COX-2 −/− NMDA median seizure=3; z=0.769, p=0.22).
Figure 2. Prostaglandin production is enhanced in COX-2 deficient mice in response to KA but not NMDA.
A) Frequency histogram of median seizure score demonstrates increased seizure intensity in COX-2 −/− mice exposed to 50 mg/kg NMDA (open bars; z=3.49, p=0.0002; n=11 for COX-2+/+ mice and n=9 for COX-2−/− mice) or 10 mg/kg KA (z=2.83, p=0.0023; n=7 for COX-2+/+ mice and n=9 for COX-2−/− mice) when compared to COX-2+/+ (filled bars). Furthermore, the median seizure intensity in COX-2 −/− mice exposed to 50 mg/kg NMDA was no different from that of COX-2−/− mice exposed to 10 mg/kg KA (Mann Whitney U-test; z=0.769, p=0.22)
B) Prostaglandin profile in whole brains from mice exposed to KA. PG profile measured one hour after injection (mice from Fig. 2A) demonstrate an increase in prostaglandin (PG) F2α (t=2.79, p=0.02), thromboxane (TX) B2 (t=2.91, p=0.02), PGE2 (t=2.91, p=0.02) and PGD2 (t=2.97, p=0.02) in COX-2 −/− mice exposed to 10 mg/kg KA when compared to COX-2 +/+ mice. 6-keto-PGF1α (6-keto), a metabolite of prostacyclin, was only detected in COX-2 −/− mice that achieved a maximal seizure score of 6. This prostaglandin metabolite was not detected in any COX-2 +/+ mice exposed to KA.
C) Prostaglandin profile in whole brains from mice exposed to NMDA. Whole brain prostaglandin profile measured one hour after injection (mice from Fig. 2A) demonstrates no significant effect of genotype on 6-keto-PGF1α (t=2.22, p=0.06), PGF2α (t=1.75, p=0.12), TXB2 (t=0.473, p=0.64), PGE2 (t=0.50, p=0.62) and PGD2 (t=1.63, p=0.17) expression in COX-2 −/− mice exposed to 50 mg/kg NMDA when compared to COX-2 +/+ mice.
Prostaglandin levels in the brains of mice injected with NMDA or KA were assessed by LC-MS/MS at 1 hour after injection. In COX-2 −/− mice injected with 10 mg/kg KA, brain levels of PGF2α, TXB2, PGE2 and PGD2 were all significantly elevated (Figure 2B). While 6-keto-PGF1α was not detected in every sample, it was consistently detected only in the brains of COX-2 −/− mice which attained a maximal KA-induced seizure intensity of 6 on the Racine Seizure Scale at some point during their observation session (Figure 2B). Although they achieved the same median seizure intensity as KA-exposed COX-2−/− mice, COX-2−/− mice injected with 50 mg/kg NMDA did not show increased levels of brain prostaglandins (PGF2α, TXB2, PGE2, PGD2, 6-keto-PGF1α) when compared to COX-2 +/+ mice (Figure 2C).
4. Discussion
In this study, we have supported our previous findings that COX-2 −/− mice are more vulnerable to KA-induced excitotoxicity (Toscano et al. 2008) and further characterized the susceptibility of COX-2 −/− mice to excitotoxicity by describing an increase in seizure intensity induced by the glutamatergic agonist, NMDA (Figure 1). We also demonstrate that lindane, an excitotoxicant which exerts its action by enhancing disinhibition via antagonism of the GABA receptor (Narahashi 2002), a distinctly different mechanism than NMDA, does not result in augmented seizure activity in COX-2−/− mice. Furthermore, we show for the first time that COX-2 −/− mice, while lacking one of the two COX isoforms, still posses the ability to produce a full complement of brain prostaglandins (Fig 2B and 2C) and that increased brain prostaglandin levels correlated with the occurrence of neuronal damage. That is, in treatment groups expressing identical median seizure intensity (Figure 2A), increased production of brain prostaglandins was a predictor of neuronal damage with KA-exposed COX-2 −/− mice expressing high levels of PG and widespread neuronal damage (Fig 2B, (Toscano et al. 2008)) and NMDA-exposed COX-2 −/− mice expressing PG levels similar to that of COX-2 +/+ mice (Fig 2C) in the presence of limited neuronal damage. Our observations suggest that deletion of COX-2 results in a generalized susceptibility to glutamate agonist-induced seizure. Furthermore, it also supports our previously proposed mechanism that GABA inhibition in the hippocampus of COX-2 −/− mice decreases the threshold for seizure-induction by excitotoxins (Toscano et al. 2008). Finally, our results also suggest that elevation of prostaglandin levels after excitotoxin-induced seizure is a predictor of neuronal damage.
While it is not completely understood why lindane did not enhance seizure intensity in COX-2 −/− mice, it is possible that their increased susceptibly to excitotoxicity is only unmasked upon direct stimulation of the glutamatergic system. Since lindane evokes seizures through indirect stimulation of the glutamatergic system via disinhibition of the GABAA receptor and is less potent than other antagonists such as pentylenetetrazole (Tusell et al. 1992; Narahashi 2002), the magnitude of the disinhibition of the glutamatergic system may not have been sufficient, upon lindane exposure, for the mechanism of increased seizure susceptibility to be unmasked in the COX-2 −/− mice. Unfortunately, our ability to expand the dose range for lindane in our experiments was limited by significant mortality in groups exposed to the highest level of lindane used in this study.
An emerging explanation of the role of COX-2 in exacerbation of seizures and mediation of neuronal damage due to brain insult involves the possibility that COX-2 plays a role in regulating neuronal activity since its inhibition before the introduction of a seizurigenic stimulus results in exacerbation of the seizure intensity (Gong et al. 2008; Toscano et al. 2008). However, it has also been suggested that COX-2 mediates the production of neuronal damage and inflammation in the brain since its inhibition after an insult to the brain seems to reduce subsequent neuronal damage (Gobbo and O'Mara 2004). When considering the latter point, it is important to remember that COX itself does not produce the final bioactive product or biological response but it is the downstream synthases and PG receptors, respectively, which perform these functions. Our data suggests that while deletion of COX-2 has profound and important effects on the intensity of seizure that manifests due to an excitotoxic stimulus (Toscano et al. 2008), it also suggests that the prostaglandin production and the subsequent stimulation of PG receptors is associated with manifestation of neuronal damage, a concept consistent with the results of similar experiments (Kim et al. 2008). It is unclear from our study whether the increased production of PGs in response to KA but not NMDA in COX-2 −/− is due to a release of the mediators from neurons as a chemical marker of damage (Pepicelli et al. 2002) or if the excitotoxic process stimulates the release of PGs from an alternative cellular compartment, such as glia (Tham et al. 2007). Therefore, we are unable to determine if the increase in PG production in our study plays a causative role in the neuronal damage observed 24 h after injection of KA, but not NMDA, or if they are strictly biomarkers of effect. What is clear from our study is that the prostaglandin profile produced during seizures does not influence the severity of the seizure since KA and NMDA injected COX-2 −/− mice exhibited identical median seizure intensity but very different quantities of PG production (Figure 2).
The fact that significantly increased levels of prostaglandins were detected in COX-2−/− mice that demonstrated increased neuronal damage at 24 hours has several important implications. First, this observation suggests that COX-1 alone and through coupling with specific terminal synthases can produce prostaglandins in the brain. However, this does not suggest that the isoforms are physiologically equivalent since differential sensitivity to both seizure intensity and neuroinflammation have been demonstrated in mice lacking one or the other cyclooxygenase isoform (Bosetti et al. 2008; Choi et al. 2008; Toscano et al. 2008). Second, increased levels of prostaglandins correlate with the occurrence of neuronal damage in KA-induced excitotoxic effects. While slight differences in the total amounts of specific prostaglandin species produced in the brains of rats exposed to KA in a study by Yoshikawa et al and the brains of mice exposed to KA in our study may exist ((Yoshikawa et al. 2006); Figure 2B), both studies demonstrate that PGF2α and PGD2 are the prostaglandin species that exhibit the greatest dynamic response to KA injections. Additionally, both studies also agree that the early production of brain prostaglandins in response to KA-induced seizures is independent of the ability to up regulate COX-2 expression ((Yoshikawa et al. 2006); Figure 2B). Furthermore, our suggestion that there is a relationship between the production of widespread neuronal damage in mouse brain and elevated levels of prostaglandins is supported by previous studies (Iadecola et al. 2001; Kawaguchi et al. 2005; Takadera and Ohyashiki 2006). That is, both our study and that of Kawaguchi and colleagues demonstrate that when levels of PGE2 are increased in the rodent brain in response to KA-induced seizures, elevated levels of neuronal damage can be detected ((Kawaguchi et al. 2005), Figures 1 and 2). This is reflected in our inability to detect widespread and consistent neuronal damage in the COX-2−/− mice exhibiting NMDA-induced seizures without a concomitant increase in prostaglandin levels. (Figure 2C). However, in mice exhibiting increased neuronal damage after KA-induced seizures, significantly elevated prostaglandin levels were observed suggesting a role for prostaglandins in the neuronal damage process (Figure 2B). Furthermore, Iadecola and colleagues demonstrate that COX-2−/− mice exhibit decreased levels of PGE2 in response to direct intracortical injections of NMDA and also exhibit enhanced protection from the massive cortical lesions resulting from this procedure (Iadecola et al. 2001). Supporting this concept is the observation that NMDA-induced neuronal toxicity is potentiated by the addition of PGE2 (Takadera and Ohyashiki 2006).
While it is uncertain at this point why KA exposure but not NMDA results in increased FJB staining (Toscano et al. 2008) and PG production in the presence of similar levels of seizure exacerbation in COX-2 −/− mice, our observations support the concept that seizure intensity may not be predictive of the extent of neuronal damage in models of excitotoxicity (Benkovic et al. 2004). However, it is possible that a variable other than seizure intensity, such as prostaglandin production or distinct stimulation of specific combinations of PG receptors, may be a better predictor of neuronal damage. If true, this would further emphasize the importance of eicosanoid metabolism and its physiological responses that occur downstream of the initial metabolic step of COX metabolism of arachidonic acid.
Overall, our observations in this study further support the emerging body of data that suggests a role for COX-2 in regulating neuronal activity (Gong et al. 2008; Toscano et al. 2008). Furthermore, these observations continue to underscore the need for an epidemiological investigation to examine if the chronic usage of COX-2 selective inhibitors, poses an increased risk of seizure in susceptible populations, such as epileptics.
Acknowledgments
The authors would like to thank Dr. Robert Langenbach for supplying the cyclooxygenase-2 knockout mice and Drs. Saba Aid and Sang-Ho Choi for their helpful discussions and technical advice. This research was supported by the Intramural Research Program of the NIH, National Institute on Aging (Z01AG000423-04; CDT, FB) and by NIH grant # GM15431 to Dr. Lawrence Marnett.
References
- Aid S, Langenbach R, Bosetti F. Neuroinflammatory response to lipopolysaccharide is exacerbated in mice genetically deficient in cyclooxygenase-2. J Neuroinflammation. 2008;5:17. doi: 10.1186/1742-2094-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik EJ, Kim EJ, Lee SH, Moon C. Cyclooxygenase-2 selective inhibitors aggravate kainic acid induced seizure and neuronal cell death in the hippocampus. Brain Res. 1999;843:118–29. doi: 10.1016/s0006-8993(99)01797-7. [DOI] [PubMed] [Google Scholar]
- Benkovic SA, O'Callaghan JP, Miller DB. Sensitive indicators of injury reveal hippocampal damage in C57BL/6J mice treated with kainic acid in the absence of tonic-clonic seizures. Brain Res. 2004;1024:59–76. doi: 10.1016/j.brainres.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Bosetti F. Arachidonic acid metabolism in brain physiology and pathology: lessons from genetically altered mouse models. J Neurochem. 2007;102:577–86. doi: 10.1111/j.1471-4159.2007.04558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosetti F, Choi SH, Aid S. Differential Roles of Cyclooxygenase-1 and -2 in Lipopolysaccharide-Induced Neuroinflammation. J Neurochem. 2008;104:13. [Google Scholar]
- Choi SH, Langenbach R, Bosetti F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. Faseb J. 2008;22:1491–501. doi: 10.1096/fj.07-9411com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci. 1990;11:290–6. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Mack CM. Seizure thresholds in kindled animals are reduced by the pesticides lindane and endosulfan. Neurotoxicol Teratol. 1995;17:143–50. doi: 10.1016/0892-0362(94)00065-l. [DOI] [PubMed] [Google Scholar]
- Gobbo OL, O'Mara SM. Post-treatment, but not pre-treatment, with the selective cyclooxygenase-2 inhibitor celecoxib markedly enhances functional recovery from kainic acid-induced neurodegeneration. Neuroscience. 2004;125:317–27. doi: 10.1016/j.neuroscience.2004.01.045. [DOI] [PubMed] [Google Scholar]
- Gong N, Zhang M, Zhang XB, Chen L, Sun GC, Xu TL. The aspirin metabolite salicylate enhances neuronal excitation in rat hippocampal CA1 area through reducing GABAergic inhibition. Neuropharmacology. 2008;54:454–63. doi: 10.1016/j.neuropharm.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Niwa K, Nogawa S, Zhao X, Nagayama M, Araki E, Morham S, Ross ME. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci U S A. 2001;98:1294–9. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam PC, See AU. Cyclo-oxygenase isoenzymes: physiological and pharmacological role. Anaesthesia. 2000;55:442–9. doi: 10.1046/j.1365-2044.2000.01271.x. [DOI] [PubMed] [Google Scholar]
- Katori M, Majima M. Cyclooxygenase-2: its rich diversity of roles and possible application of its selective inhibitors. Inflamm Res. 2000;49:367–92. doi: 10.1007/s000110050605. [DOI] [PubMed] [Google Scholar]
- Kawaguchi K, Hickey RW, Rose ME, Zhu L, Chen J, Graham SH. Cyclooxygenase-2 expression is induced in rat brain after kainate-induced seizures and promotes neuronal death in CA3 hippocampus. Brain Res. 2005;1050:130–7. doi: 10.1016/j.brainres.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Chung JI, Lee SH, Jung YS, Moon CH, Baik EJ. Involvement of endogenous prostaglandin F(2alpha) on kainic acid-induced seizure activity through FP receptor: The mechanism of proconvulsant effects of COX-2 inhibitors. Brain Res. 2008;1193C:153–161. doi: 10.1016/j.brainres.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Kingsley PJ, Rouzer CA, Saleh S, Marnett LJ. Simultaneous analysis of prostaglandin glyceryl esters and prostaglandins by electrospray tandem mass spectrometry. Anal Biochem. 2005;343:203–11. doi: 10.1016/j.ab.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Kroll B, Kunz S, Klein T, Schwarz LR. Effect of lindane and phenobarbital on cyclooxygenase-2 expression and prostanoid synthesis by Kupffer cells. Carcinogenesis. 1999;20:1411–6. doi: 10.1093/carcin/20.8.1411. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Nerve membrane ion channels as the target site of insecticides. Mini Rev Med Chem. 2002;2:419–32. doi: 10.2174/1389557023405927. [DOI] [PubMed] [Google Scholar]
- Pepicelli O, Fedele E, Bonanno G, Raiteri M, Ajmone-Cat MA, Greco A, Levi G, Minghetti L. In vivo activation of N-methyl-D-aspartate receptors in the rat hippocampus increases prostaglandin E(2) extracellular levels and triggers lipid peroxidation through cyclooxygenase-mediated mechanisms. J Neurochem. 2002;81:1028–34. doi: 10.1046/j.1471-4159.2002.00897.x. [DOI] [PubMed] [Google Scholar]
- Sattler R, Tymianski M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol. 2001;24:107–29. doi: 10.1385/MN:24:1-3:107. [DOI] [PubMed] [Google Scholar]
- Schauwecker PE, Steward O. Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc Natl Acad Sci U S A. 1997;94:4103–8. doi: 10.1073/pnas.94.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–30. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–82. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Takadera T, Ohyashiki T. Prostaglandin E2 deteriorates N-methyl-D-aspartate receptor-mediated cytotoxicity possibly by activating EP2 receptors in cultured cortical neurons. Life Sci. 2006;78:1878–83. doi: 10.1016/j.lfs.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Takemiya T, Maehara M, Matsumura K, Yasuda S, Sugiura H, Yamagata K. Prostaglandin E(2) produced by late induced COX-2 stimulates hippocampal neuron loss after seizure in the CA3 region. Neurosci Res. 2006;56:103–110. doi: 10.1016/j.neures.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Telch J, Jarvis DA. Acute intoxication with lindane (gamma benzene hexachloride) Can Med Assoc J. 1982;126:662–3. [PMC free article] [PubMed] [Google Scholar]
- Tham CS, Whitaker J, Luo L, Webb M. Inhibition of microglial fatty acid amide hydrolase modulates LPS stimulated release of inflammatory mediators. FEBS Lett. 2007;581:2899–904. doi: 10.1016/j.febslet.2007.05.037. [DOI] [PubMed] [Google Scholar]
- Toscano CD, Prabhu VV, Langenbach R, Becker KG, Bosetti F. Differential gene expression patterns in cyclooxygenase-1 and cyclooxygenase-2 deficient mouse brain. Genome Biol. 2007;8:R14. doi: 10.1186/gb-2007-8-1-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano CD, Ueda Y, Tomita YA, Vicini S, Bosetti F. Altered GABAergic neurotransmission is associated with increased kainate-induced seizure in prostaglandin-endoperoxide synthase-2 deficient mice. Brain Res Bull. 2008;75:598–609. doi: 10.1016/j.brainresbull.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusell JM, Vendrell M, Serratosa J, Trullas R. Lindane-induced convulsions in NMRI and OF1 mice: antagonism with (+)MK-801 and voltage-dependent calcium channel blockers. Brain Res. 1992;593:209–14. doi: 10.1016/0006-8993(92)91309-3. [DOI] [PubMed] [Google Scholar]
- Wang Q, Yu S, Simonyi A, Sun GY, Sun AY. Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol Neurobiol. 2005;31:3–16. doi: 10.1385/MN:31:1-3:003. [DOI] [PubMed] [Google Scholar]
- Wright DH, Abran D, Bhattacharya M, Hou X, Bernier SG, Bouayad A, Fouron JC, Vazquez-Tello A, Beauchamp MH, Clyman RI, Peri K, Varma DR, Chemtob S. Prostanoid receptors: ontogeny and implications in vascular physiology. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1343–60. doi: 10.1152/ajpregu.2001.281.5.R1343. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, Kita Y, Kishimoto K, Shimizu T. Profiling of eicosanoid production in the rat hippocampus during kainic acid-induced seizure: dual phase regulation and differential involvement of COX-1 and COX-2. J Biol Chem. 2006;281:14663–9. doi: 10.1074/jbc.M511089200. [DOI] [PubMed] [Google Scholar]